Abstract
Microbial life permeates Earth's critical zone and has likely inhabited nearly all our planet's surface and near subsurface since before the beginning of the sedimentary rock record. Given the vast time that Earth has been teeming with life, do astrobiologists truly understand what geological features untouched by biological processes would look like? In the search for extraterrestrial life in the Universe, it is critical to determine what constitutes a biosignature across multiple scales, and how this compares with “abiosignatures” formed by nonliving processes. Developing standards for abiotic and biotic characteristics would provide quantitative metrics for comparison across different data types and observational time frames. The evidence for life detection falls into three categories of biosignatures: (1) substances, such as elemental abundances, isotopes, molecules, allotropes, enantiomers, minerals, and their associated properties; (2) objects that are physical features such as mats, fossils including trace-fossils and microbialites (stromatolites), and concretions; and (3) patterns, such as physical three-dimensional or conceptual n-dimensional relationships of physical or chemical phenomena, including patterns of intermolecular abundances of organic homologues, and patterns of stable isotopic abundances between and within compounds. Five key challenges that warrant future exploration by the astrobiology community include the following: (1) examining phenomena at the “right” spatial scales because biosignatures may elude us if not examined with the appropriate instrumentation or modeling approach at that specific scale; (2) identifying the precise context across multiple spatial and temporal scales to understand how tangible biosignatures may or may not be preserved; (3) increasing capability to mine big data sets to reveal relationships, for example, how Earth's mineral diversity may have evolved in conjunction with life; (4) leveraging cyberinfrastructure for data management of biosignature types, characteristics, and classifications; and (5) using three-dimensional to n-D representations of biotic and abiotic models overlain on multiple overlapping spatial and temporal relationships to provide new insights.
Key Words: Astrobiology, Biosignatures, Taphonomy, Extraterrestrial life, Extremophile.
1. Introduction
The search for extraterrestrial life is fundamentally referenced to Earth as the only known and accessible benchmark for comparison (a sample size of n = 1 problem), from the microscopic level up to the scale of our planet and its atmosphere, where life has perturbed planetary environments over long timescales (Judson, 2017). “Life” is a complex phenomenon, and here we refer to it as we know it today—a self-organized, self-replicating, and metabolically active molecular system that is carbon based (Pace, 2001). All of Earth's surface and subsurface waters have likely been in contact with microbes or their by-products since at least 3.5 Ga, when the first widely accepted traces of life appear in the geological record (cf., Schopf et al., 2018 and references therein). That traces of life are preserved within very ancient remnants of the crust indicates that perhaps life had already colonized the entire planet. Accordingly, over the eons during which water-rich Earth has been teeming with life, it is difficult to determine how an “uninhabited habitable planet” would appear when sampled directly or observed remotely.
It has often been generally assumed that substances or objects in Earth's near-surface environment might be abiotic unless there is definitive evidence of biological activity. However the pervasiveness of life in Earth's near-surface and subsurface environments indicates that, conversely, perhaps virtually everything might be biologically influenced unless an abiotic origin can be definitively established.
2. Biosignature Definitions
A biosignature is an object, substance, and/or pattern whose origin specifically requires a biological agent (Des Marais et al., 2008). The usefulness of a biosignature is determined not only by the probability that life produced it, but also by the improbability that nonbiological processes produced it. Biosignatures can be any observable phenomena such as elemental abundances, molecules, objects, isotopic abundance patterns, or processes that provide evidence of past or present life. Biosignatures include heteroatoms in graphitic carbon or isotopic patterns between reduced carbon and carbonates in ancient rocks (e.g., Bernard and Papineau, 2014), molecular biomarkers or their fragments (Summons et al., 2008; Jolley and Douglas, 2012), fossil-like cellular structures (e.g., Schopf and Kudryavtsev, 2012), possible biogenic structures in diagenetic concretions, granules, and rosettes (Berner, 1968; Coleman, 1993; Papineau et al., 2016, 2017), and microbially influenced structures such as stromatolite-like morphologies (e.g., Grotzinger and Knoll, 1999; Berelson et al., 2011; Pepe-Ranney et al., 2012) and Microbially Induced Sedimentary Structures (abbreviated as ‘MISS,’ Noffke et al., 1996). It is important to note that biosignatures typically include some objective measure or indicator of normal biological processes (e.g., pathogenesis or photosynthesis) (Mata et al., 2012), and these factors can be difficult to define, let alone measure (Cady et al., 2003). One example of this challenge is identifying particular biological processes associated with potential global-scale biosignatures in exoplanets (Des Marais et al., 2002).
An ‘abiosignature’ is a substance, object, or pattern that has a nonbiological origin. The usefulness of an abiosignature is determined not only by the probability that an abiotic process produced it, but also by the improbability that biological processes produced it. Definitive abiosignatures could provide insights about how an uninhabited habitable planet would appear when sampled directly or observed remotely. Characterizing abiosignatures should enhance our capacity to delineate and confirm biosignatures.
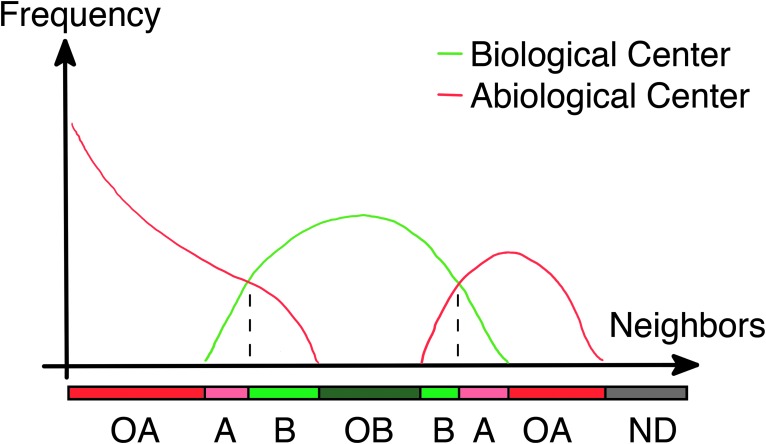
An ‘ambiguous biosignature’ (termed a ‘potential biosignature,’ Des Marais et al., 2008) is a feature that occupies the ‘gray zone’ of uncertainty between biosignatures and abiosignatures. An ambiguous biosignature might compel investigators to gather more data before reaching a conclusion as to the presence or absence of life. Navigating this ‘gray zone’ is a central challenge for astrobiology life detection efforts.
‘Agnostic biosignatures’ are substances, objects, and/or patterns whose origins specifically require biological agents and also include features that might not have originated on Earth. Agnostic biosignatures compel us to envision attributes of life that are more fundamental and widespread in the cosmos than attributes that are apparent in our own biosphere (Johnson et al., 2018; Exoplanet Science Strategy, 2018).
We address the challenges of differentiating between biosignatures and abiosignatures for astrobiology, and searching for the origins of life, by beginning within the perspective of the earthly bias that shapes present science. The interdependent linkages of biology, chemistry, and geology are fundamental to defining the following: (1) what constitutes a biosignature or a biomarker, or conversely an abiosignature; (2) how extant life would be recognized, preserved, and identified; and (3) whether fossil life-forms exist and can be detected and recognized elsewhere in the Universe.
Aside from identifying and measuring biosignatures across multiple disciplines, including biology, geology, engineering, and environmental science (Cady and Noffke, 2009), we need to define what constitutes a biosignature to search for evidence of life on other planets and moons, and attempt to constrain the timing of the origins of life on Earth. Extraterrestrial exploration for life is extremely challenging due to the technical demands of making remote measurements in the Solar System and beyond. Similarly, searches for the earliest traces of life on Earth are challenging due to ongoing disruption by the formation of tectonic/metamorphic belts, the constant recycling of the crust, Earth's active hydrologic cycle and consequent weathering and erosion, and the ubiquity of modern life.
3. Life Limits and Uncertainties
Beyond the challenges described above, there is a further complication in evaluating biosignatures. Science is presently unable to explain satisfactorily how terrestrial life originated, namely, whether early life and extraterrestrial life were or are compositionally or functionally similar with modern terrestrial life and had the same effects on the environment as modern life that we can observe directly. Biological evolution is undoubtedly influenced and constrained by larger scale planetary and Solar System processes, some of which are beyond biology's influence, such as tectonics (Lindsay and Brasier, 2002), solar activity (Ribas et al., 2005), and impacts (Kring, 2000). Yet, in other large-scale processes, such as the terrestrial nitrogen cycle (Stüeken et al., 2016; Laneuville et al., 2018), biology may have become a major factor very early on. As an example of the intertwining of planetary and biological processes, it is widely assumed that the buildup of molecular oxygen in the Earth's atmosphere is due to biology (Des Marais et al., 2002). However, the pacing of the rise of oxygen depended on parameters inherent in Earth's formation, for example, its size and elemental composition, which were inherited from stochastic processes during formation of the Solar System.
The structural complexity of organisms appears to have increased during the biological evolution on Earth, although it is acknowledged that there are multiple criteria by which complexity can be gauged (Emmeche, 1997; Hazen et al., 2007). The earliest organisms were unicellular (Woese, 1998) and perhaps even preceded by acellular ones for which we have no fossil record. There was then a development from single-celled bacteria, archaea, and eucarya, to multicellular organisms. As a result of this progression, biochemical complexity has also evolved over time according to various metrics (Woese, 1998; Böttcher, 2018), with certain metabolic capabilities arising sequentially (e.g., oxygenic photosynthesis or oxidative metabolism). It further seems logical that, however, life began, it started in a “simpler” state that included less compositional, morphological, and functional capabilities (Woese, 1998). These differences could naturally affect the types of biosignatures a planet would be capable of producing at any given point in its history once life began.
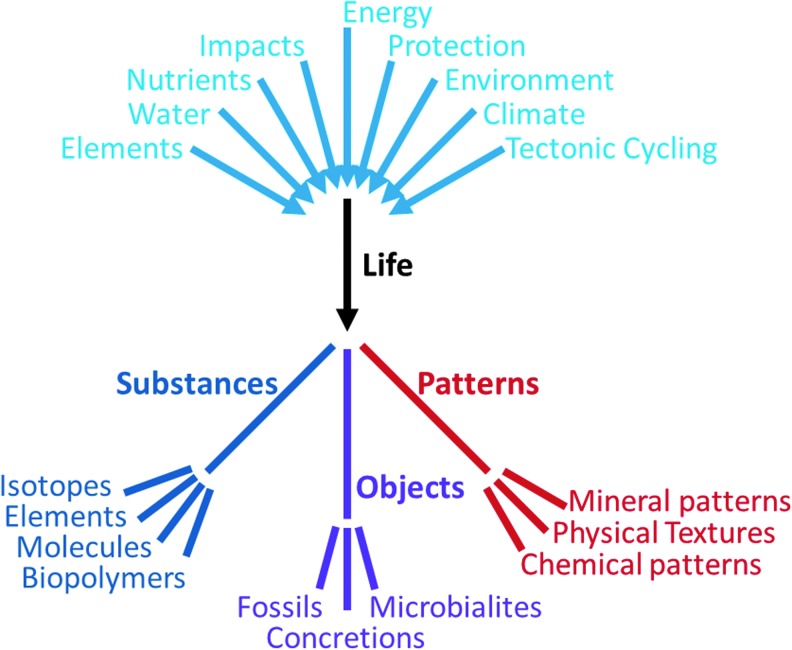
We presently lack a universal definition of life, which contributes to making the search for unambiguous biosignatures a central unifying challenge of astrobiology (see e.g., Smith, 2016). In this work, we assert that life uses environmentally available energy and matter (Fig. 1) to reproduce itself as both a structure and a process in a state of chemical and thermodynamic disequilibrium relative to the surrounding environment, and that life's processes generate waste and alter the environment. This alteration of the environment can result in simply changing it more rapidly than would occur in the absence of the catalytic properties of organisms or can produce phenomena that abiogenic substances, objects, and patterns may mimic.
FIG. 1.
Tree diagram of the relationship of variables affecting the formation and development of life, and the resulting biosignatures in the three categories of substances, objects, and patterns. These major components and products capture the current state of opinion in astrobiology. Within each category, there are challenges to identify and measure the type of biosignatures, evaluate the fluxes that may be relevant to enhancing life, understand the context of scales and relationships, and evaluate importance, applicability, and confidence in the signature.
The mutual interactions between life and its host planet may be self-reinforcing or self-amplifying. Indeed, it has been suggested that perhaps the entirety of the planet behaves as one large organism in some sense, with both biotic and abiotic spheres of influence overlapping across all planetary environments (Lovelock and Giffin, 1969). Direct evidence of such a complexity is not yet robustly in hand.
4. Cosmic Perspective
Our understanding of terrestrial life strongly clouds but uniquely informs the search for life beyond Earth. Terrestrial life has managed to obscure or overwhelm abiotic planetary processes to the point that Earth life is readily observable even from space (Sagan et al., 1993). It is possible that a common stable outcome of the evolution of planetary biospheres is that a given biosphere may not be capable of entirely saturating its environment to the extent that life has on Earth. This idea was argued against by Lovelock and Margulis (1974) who favored all or nothing outcomes with regard to life dominating its host planet. Nevertheless, there may be transient periods during the development of biospheres in which the impact of biology on planetary processes is relatively feeble.
5. Biosignature Phenomena
Based on our current knowledge, we discuss biosignature phenomena (including biomarkers) in three main categories: substances, objects, and patterns (Figs. 1 and 2). Each section includes a definition of the classification, the scales and methods of evaluation, and how the biosignatures can be validated or verified, with application to astrobiology. These three categories of biosignatures are complexly interrelated in that substances are present in objects and both can contribute to patterns. The challenges are to measure multiple types of biosignatures; to evaluate the fluxes that may be relevant to enhancing life (e.g., mass fluxes of elements, water, and nutrients as well as energy fluxes of protection, environment, and climate); and to understand the context of scales and relationships, with weightings of importance, applicability, and confidence in the signature.
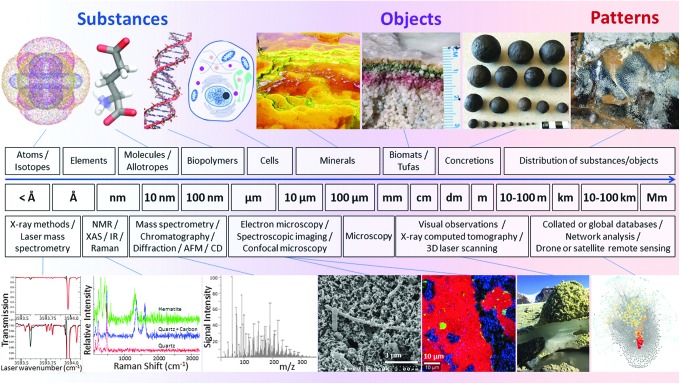
FIG. 2.
Biosignatures and life detection methods range from microscopic (left) to planetary scales (right). A nested astrobiological approach will provide context for the physicochemical parameters and processes governing the preservation of biosignatures. Images created by R.J.G. using VMD software (Humphrey et al., 1996). Top row images: carbonate formation in Río Tinto (Fernández-Remolar et al., 2012); biomats (Des Marais, 2003); concretions (M.A.C.); and biovermiculation patterns, Cueva de Villa Luz, Tabasco, Mexico (P.J.B.). Life detection analytical techniques in bottom row (left to right) are laser spectroscopy (modified with permission from Leshin et al., 2013); Raman spectra from 3.49 Ga Dresser Formation chert (D.M.B.); high-resolution mass spectrometry (Parker et al., 2016); scanning electron microscopy (Chivian et al., 2008); Raman spectra map (D.M.B.), photograph of sulfur deposits on the Borup Fiord Pass glacier (Lau et al., 2017) computational network analysis (http://dtdi.carnegiescience.edu) (Morrison et al., 2017).
5.1. Substances
Substances are materials, or combinations of materials, with structures that are fixed by chemical and physical constraints. Examples include elemental abundances, molecules, allotropes, enantiomers, minerals, and their associated properties. Geotemporal context can also help distinguish a biosignature from an abiotic substance. In this section, we explore criteria for unambiguous biosignatures and/or abiosignatures (or antibiosignatures of Walker et al., 2018) stored in substances by the following: (1) examining substances or associations of substances that provide strong evidence for biological activity and therefore qualify as biosignatures; (2) addressing physical, chemical, and biological processes that preserve or degrade substances over time; (3) determining the spatial scaling and/or distributional relationships of substances required to map and validate biosignatures; (4) quantifying uncertainty in substance-based biosignatures to define a framework for their interpretation over time and space; and (5) exploring case examples.
5.1.1. Substances as biosignatures
The search for life beyond Earth or in ancient rocks remains an exceedingly difficult problem (Tashiro et al., 2017), in part, because of challenges in appropriately defining unambiguous biosignatures or abiosignatures (Westall et al., 2015). Presently there is no fundamental framework or theory to evaluate the best substances or combinations of substances that might constitute a biosignature.
Many scientists have examined criteria required for life as we know it and then attempted to relate them to specific substances that might constitute direct or indirect evidence for life. Biogeochemical assemblages span scales from the least complex, most immutable units, such as elemental abundances, that can preserve evidence for metabolic processes, continuing up through greater levels of complexity. Alternatively, minerals and rocks constitute the base abiogenic matrix in planetary systems, yet minerals themselves and their morphology or diversity may be biosignatures themselves. Strong arguments can be made for substances as biosignatures (e.g., Catling et al., 2018) especially when they occur in combinations, for example—minerals and isotopic patterns, minerals and morphology, organic molecules, and isotopic patterns. Hence multipronged approaches for biosignature detection are needed to address this complexity and interrelatedness.
5.1.2. Preserving substances as biosignatures
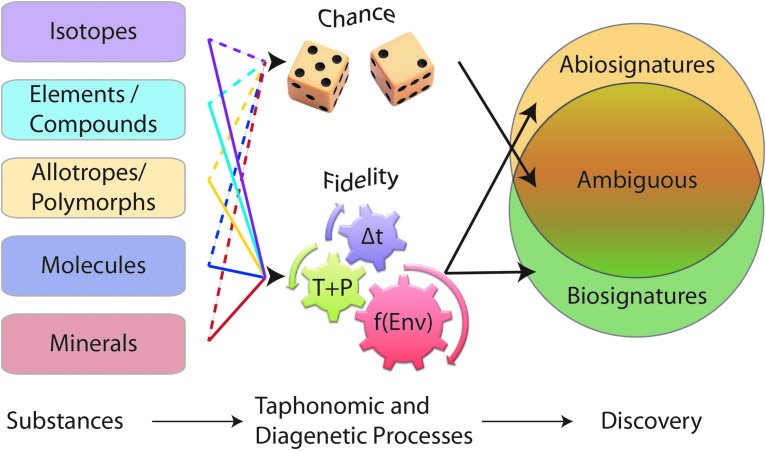
Changes in physicochemical conditions over time and space can produce significant ambiguity in identifying biosignatures (Fig. 3) (e.g., Farmer, 1999a).
FIG. 3.
Most biosignatures in the geologic record are ambiguous, with discovery highly dependent on taphonomic and diagenetic processes. When these processes are not well understood, the resulting signatures are ambiguous, appearing to be the result of chance events (dashed lines). When processes are well understood, they function as high-fidelity representations of the original substances, biotic or not (solid lines). Multiple independent observations can distinguish the biosignature and abiosignature fields, reducing the size of the ambiguous signature field. Δt = time since formation of the substance. T+P = temperature and pressure changes the substance experiences over time. “f(Env)” = environmental conditions under which diagenetic and taphonomic processes occur, specifically, chemical reactions and the presence and movement of fluids or absence of fluids over time.
To increase certainty, a purported biosignature is ideally interpreted in its original depositional and preservational context. In many cases, the original mineral or biological content of a depositional event is not preserved. Determining the extent and timing of preservational effects is not trivial and requires extensive comparison with natural systems and laboratory experiments (Grosch and McLoughlin, 2014, 2015). Unfortunately, there is often incomplete information about depositional and subsequent diagenetic conditions, and it is not always possible to accurately and experimentally model diagenetic processes. Hence, the likelihood of biosignature preservation, discovery, and accurate interpretation is limited, and a systematic approach to document the appropriate physicochemical conditions that best allow preservation is critical.
Radiolytic processing may be an important factor in biosignature preservation in planetary environments (Dartnell et al., 2012; Pavlov et al., 2012). Such a process can be part of the “f(Env)” term shown in Fig. 3, which broadly refers to environmental conditions under which diagenetic and taphonomic processes occur, especially chemical reactions and the presence or absence of fluids and their movements over time. Considering the multitude of potential mineral/organic combinations, how can the degradation products of radiation damage to organic molecules and minerals be recognized? Basic chemical principles can be applied to candidate combinations to identify potential reactions and develop tests for specific chemical, physical, and mineralogical products. The geologic and environmental context is important in all cases.
5.1.3. Spatial scales and distributions to validate substances as biosignatures
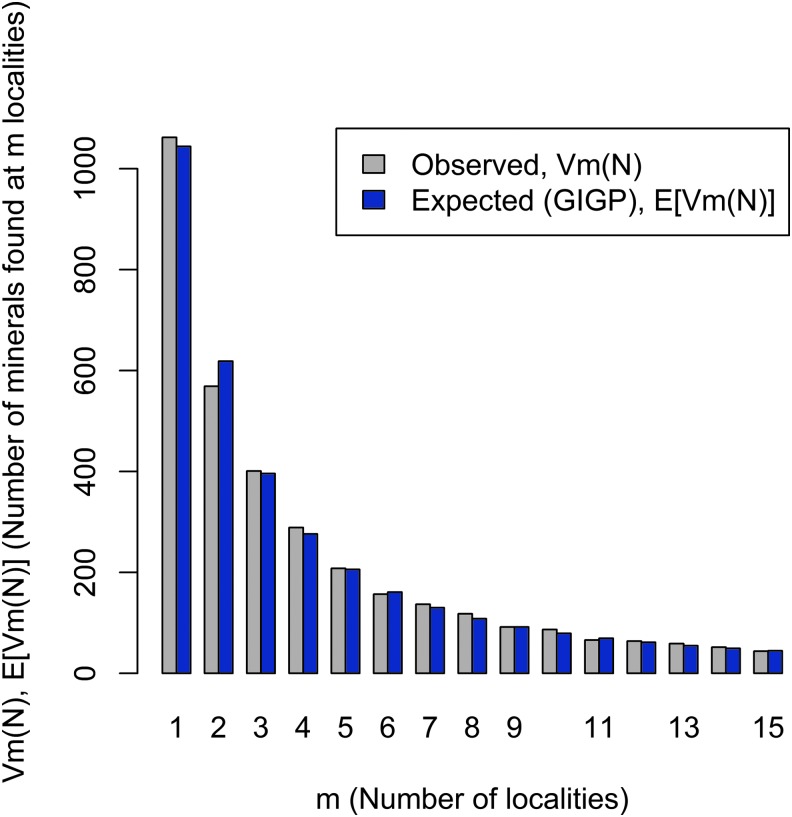
The diversity and distribution of minerals at a planetary scale (i.e., a Large Number of Rare Events [LNRE] frequency spectrum) could itself be a biosignature. Recent analyses of large mineralogical data resources reveal that Earth's mineralogy conforms to a distinctive LNRE distribution (Fig. 4). This type of distribution arises when a few species are commonly found but most species are rare. In the case of Earth minerals, over half of all species are known from five or fewer localities (Hazen et al., 2015; Hystad et al., 2015).
FIG. 4.
Earth's mineral inventory follows an LNRE distribution. Observed (gray) and modeled (blue) frequency distribution for rare minerals on Earth (Hazen et al., 2015; Hystad et al., 2015). Most of Earth's >5300 known mineral species are rare, occurring at ≤5 localities, and changes in Earth's environment caused by biology may contribute to this phenomenon. Statistical expected relationships GIGP means generalized inverse Gauss–Poisson distribution. LNRE, Large Number of Rare Events. Image: R.M.H.
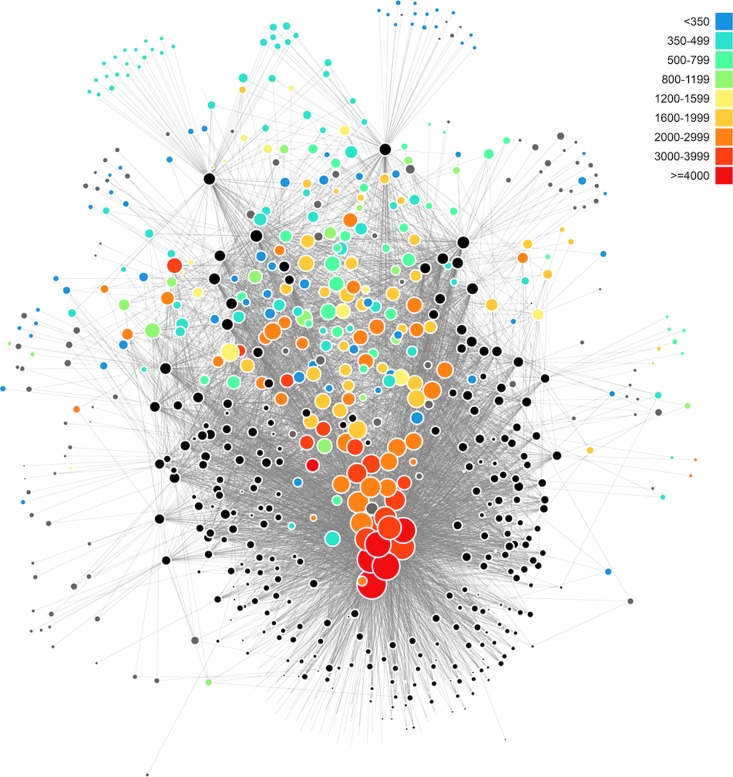
The LNRE distribution is also manifest in network analyses of mineral systems (Morrison et al., 2017). Consider the bipartite network for 400+ carbon-bearing minerals (Fig. 5). Large red nodes positioned near the center of the “U”-shaped array of black locality nodes indicate the most common species, whereas the “halo” of small blue nodes represents the large number of rare minerals found at only one or two localities. The topology of this network diagram is a visual representation of an LNRE mineral distribution.
FIG. 5.
A bipartite network diagram for all carbon-bearing mineral species reveals relationships among mineral localities (represented by black circles), connected to mineral species (represented by colored nodes) that occur at those localities. Sizes of locality nodes indicate how many mineral species occur at that locality. Sizes and colors of mineral species nodes reflect mineral abundances. The topological distribution of mineral nodes represents an LNRE frequency spectrum (Fig. 4). Image: R.M.H.
Although data are available for >5300 minerals found in hundreds of thousands of locations on Earth (mindat.org), we lack comprehensive data for LNRE analyses of any other solar system body. Preliminary analysis suggests that the Mars and Moon have much lower mineral diversity than Earth, along with spatial distributions that do not conform to an LNRE model (Hazen et al., 2015). Thus, while an LNRE distribution might constitute a global scale biosignature (Hazen et al., 2015; Hystad et al., 2015), for the foreseeable future, this hypothesis will be testable for only a few bodies in our Solar System.
5.1.4. Energy production as a biosignature
The recent discovery of subsurface chemolithotrophic microorganisms living in a relatively low-radiation biosphere (cf. Colman et al., 2017) suggests evolution of certain genes (e.g., hydrogenases, acetyl-CoA synthases, and CO-dehydrogenases) that are more prevalent in subsurface chemolithotrophic organisms than surface organisms (Colman et al., 2017). Thus, the rock matrix supplies multivalent elements that can transfer electrons via reduction/oxidation (redox) reactions to produce energy in the system, and metabolism is accelerated with mobilization of these redox substrates by groundwater.
5.1.5. Uncertainties in evaluating substances as biosignatures
Defining biosignatures must be weighed against the null hypothesis, which states that every known nonbiological process must be rejected before a biological conclusion can be adopted. For example, graphite in sedimentary rocks from hydrothermally influenced environments could have sourced nonbiological carbon formed from Fischer–Tropsch Type (FTT) synthesis during serpentinization. Consequently, we need to adopt uniform principles for evaluating biosignatures and consider analogs of biosignatures in younger rocks where biology has left a stronger trace (e.g., Dodd et al., 2017). It may also be useful to develop methods to assign confidence to the variables described in Fig. 1. Such an approach occurs in ore deposit exploration, where expected formation processes are combined into a model with weights or ranks (e.g., Wyborn et al., 1994; Skirrow et al., 2009).
5.1.5.1. Importance of abiotic chemistry
Searches for extraterrestrial life often focus on biomarkers but determining whether a specific compound is of extraterrestrial biological origin and not a terrestrial contaminant is the crux of this problem. Abiotic chemistry must be understood sufficiently in detail such that when a signal is observed, there is a reasonable certainty as to whether it is biotically or abiotically produced versus being a terrestrial contaminant (Fox and Strasdeit, 2017). Extensive work in abiotic chemistry shows that a variety of biochemicals can be generated by abiological processes, for example, through atmospheric (Miller, 1953), hydrothermal (Hennet et al., 1992; Amend and Shock, 1998), or interstellar chemistry (Bernstein et al., 2005). These studies caution that many seemingly complex or uniquely biogenic compounds may be at best ambiguous biosignatures and they could be called dubio-biosignatures (e.g., Cady et al., 2003). Indeed, environmental chemistry that generates organic complexity is widely viewed as being a stage in the emergence of life (Cleaves, 2012), and thus, it is possible that such compounds could be markers of transitional stages in biogenesis, although not of life per se.
Increased knowledge of abiotic chemistry is also synergistic with biomarker detection. That is, knowledge of the conditions required for the emergence of life can inform the types of extraterrestrial environments where life should be sought. Conversely, extraterrestrial signals determined to be abiotic rather than biotic can also point to potentially novel naturally occurring abiotic chemistries and can provide insight into prebiotic chemical processes.
The transition from nonlife to life must have included a means to produce and complexify organic molecules. As an example of an organic-generating abiotic process, FTT synthesis involves the abiotic metal-catalyzed reduction of CO or CO2 by H2 to produce reduced carbon compounds. Depending on the availability of other compounds, these abiotic organic molecules can include methane, short-chain alkanes, carboxylic acids, and nitrogenous and sulfurous organic molecules, which may contribute to the synthesis of other prebiotic molecules (Rushdi and Simoneit, 2004; McCollom, 2016).
Once abiotically synthesized, other abiotic reactions may further process organic compounds. For example, the dicarboxylic acid, malonate, is oxidized by sulfate and bromate through a chemically oscillating, Belousov–Zhabotinsky (B-Z) reaction (Zaikin and Zhabotinsky, 1970). Such reactions are somewhat similar with metabolic reactions. Chemically oscillating reactions have been proposed as stimulants for the development of early metabolic pathways (Russell, 2003). In contrast with FTT reactions, chemically oscillating reactions are not known to produce organic compounds with ∂13C values similar with those of metabolism.
On modern Earth, there are few examples of unambiguously abiotic organic synthesis supported by carbon isotope data (e.g., Prokurowski et al., 2008) (also see Section 5.3.2). The occurrence or prevalence of chemically oscillating or other types of abiotic reactions in nature and their potential for isotopic fractionation of carbon or other elements remain largely unknown, although they may be more common and widespread than currently recognized (Papineau et al., 2016, 2017).
5.1.6. Substance case examples
5.1.6.1. Elements and compounds
Biology affects most major and minor elements on Earth's surface with respect to their abundance in various reservoirs and incorporation into molecular and mineral species. We explore the nitrogen and carbon cycles here, but effects are also evident in other biogeochemically active elements. For example, the enormous quantity of O2 in the modern atmosphere is almost entirely due to biological activity.
5.1.6.1.1. The nitrogen cycle as a biosignature
Nitrogen is abundant in Earth's atmosphere as N2, which is difficult to fix abiotically (due to the strength of the N-N triple bond). The evolution of metabolic pathways to fix atmospheric nitrogen for use in biomolecules such as DNA and proteins has allowed life to thrive despite the relatively low flux of abiotically fixed nitrogen (Falkowski, 1997). As the oceans and atmosphere became suffused in O2, life developed a variety of pathways to cycle nitrogen back to the atmosphere, the most efficient being biological denitrification, which displays marked isotopic fractionation (Nielsen, 1992; Sigman et al., 2009). As N is recycled in ecosystems, life greatly augments the amount of N2 drawn down from the atmosphere and alters the way that fixed higher and lower oxidation state N-species can be passed into the mantle by subduction (Zerkle and Mikhail, 2017; Laneuville et al., 2018).
In Precambrian sedimentary rocks, N isotopes in graphite, kerogen, ammonium-bearing phyllosilicate minerals, and bulk rock are interpreted variably. They may be seen as possible signatures of either biological nitrogen fixation or ammonium assimilation when 15N-depletions occur or attributed to denitrification when 15N-enrichments occur (Thomazo and Papineau, 2013). However, nonbiological processes such as diagenesis, metamorphism, fluid/rock interactions, and possibly varying atmospheric N-isotope composition can add significant uncertainty to the interpretation of the fractionation origin (Ader et al., 2016).
5.1.6.1.2. Carbon cycle
Transfers between air and other reservoirs, such as the biosphere, the oceans, and Earth's interior, control the concentration of CO2 in the atmosphere (Fig. 6). During oxygenic photosynthesis, plants, photosynthetic algae, and bacteria use energy from sunlight to combine CO2 with H2O to form carbohydrates (CH2O). These carbohydrates are used as an energy source, and O2 is released as a by-product. Some of the carbohydrate is stored as biomass. Consumers such as animals, fungi, and bacteria get their energy from this excess biomass via respiration, in which O2 is combined with carbohydrates to liberate energy, with water and CO2 as by-products.
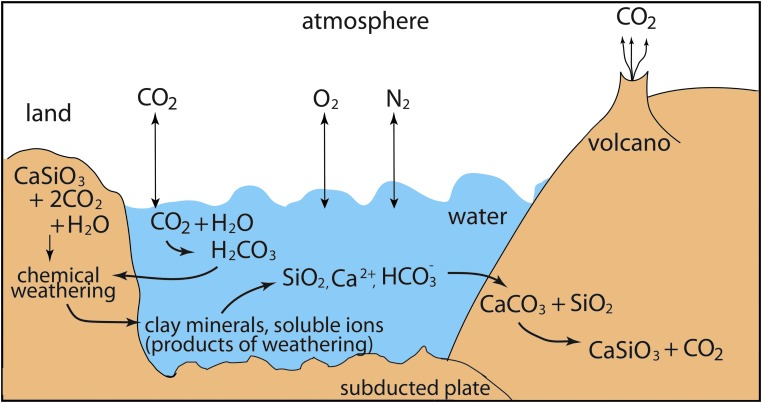
FIG. 6.
Complex linkages of the C and Si cycles (Kasting and Catling, 2003). Atmospheric CO2 dissolves in surface waters. The dissolved and atmospheric CO2 is in equilibrium. Dissolved CO2 reacts with water to form H2CO3 (carbonic acid, a weak acid). H2CO3 dissociates into H+ and HCO3−. Ultimately, H+ and water react with most common minerals, silicates, and carbonates, altering those minerals. The predominant weathering products are clay minerals (silicates) and soluble ions (Ca2+, Fe2+, Na+, K+). HCO3- also remains in solution. Image: M.S.-R.
5.1.6.1.3. Carbonate versus silicate formation on Earth
Carbon and silicon cycles are linked by chemical weathering and biological stoichiometry (Wang et al., 2016) (Fig. 6). Some geochemical factors (e.g., thermodynamics, chemical kinetics, hydrology, host-rock mineralogy, and texture) affect silicate and carbonate mineral formation and degradation. The microbial carbon/silicon “cycle” on early Earth would likely have involved ultramafic rock and therefore increased magnesium values rather than the low magnesium carbonate forming today (Power et al., 2013).
Several studies consider the chemical products of autotrophic and heterotrophic microbial metabolic processes (Castanier et al., 2000; Bennett et al., 2001; Sánchez-Román et al., 2008; Power et al., 2011; Pace et al., 2016) in addition to the chemical requirements for the formation of carbonates and other authigenic minerals (e.g., Sánchez-Román et al., 2014; Ruff and Farmer, 2016). Most of these studies, which are based on field observations and validated by laboratory experiments, explore the role of microbes in mineral formation. Indeed, results suggest that the range of inorganic changes in the conditions alone is insufficient to induce mineral precipitation. Consequently, these studies suggest that biological processes must play a major role in mineral precipitation. Carbonate minerals could possibly comprise a biosignature when taken in the geologic and atmospheric context.
5.1.6.2. Organic molecules
5.1.6.2.1. Nucleic acids
Almost all known terrestrial life uses DNA as its genetic material, except for some RNA viruses. Of course, it is arguable whether viruses are living in the same sense that other organisms are alive (Lai and Cavanagh, 1997). It is possible that extraterrestrial biology could use an alternative information storage molecule (Pinheiro et al., 2012; Cleaves et al., 2015), and whether evolutionary processes would universally result in the same biochemistry is unknown. It may be reasonable to begin searching for living systems that we are familiar with, and detection of unambiguously extraterrestrial nucleic acids could provide a strong biosignature.
Detection of nucleic acid biomarkers in extraterrestrial environments, for example, Mars, could indicate whether a signal came from organisms with common ancestries to those on Earth (Mojarro et al., 2017; Pontefract et al., 2017). The abundance of nucleic acid building blocks produced in the cosmos may have pushed all life to use nucleic acids as genetic materials (Callahan et al., 2011); thus, using nucleic acids as extraterrestrial biomarkers may provide an unambiguous biosignature. Still, there are multiple known ways that the environment can make the compounds that comprise nucleic acids.
5.1.6.2.2. Amino acids and peptides
Peptides are another major class of biopolymer present in all extant life on Earth. These are polymers of a limited set of 20 common amino acids, and importantly, in terrestrial life, amino acids are exclusively l-enantiomers (except glycine, which is achiral). How life evolved to use specifically this set of amino acids or enantiomers is unknown (Blackmond, 2010; Ilardo et al., 2015). Although extant life only uses 20 proteinogenic amino acids, many of which have been found in extraterrestrial samples (Kvenvolden et al., 1970), extraterrestrial examples of proteinogenic amino acids occur alongside many other types of nonproteinogenic amino acids (Cronin and Pizzarello, 1997; Ambrogelly et al., 2007). Nonproteinogenic amino acids are, by definition, not found in proteins although some are found in natural products (Walsh et al., 2013). The observation of proteinogenic amino acids by itself does not constitute a biosignature, as abiotic processes can also form these molecules (Miller, 1953; Mullen and Sutherland, 2007; Aubrey et al., 2009; Higgs and Pudritz, 2009), and even peptides are not necessarily biosignatures, as they can also be formed abiotically (Leman et al., 2004; Danger et al., 2012; Kitadai et al., 2017). However, the exclusive detection of extraterrestrial homochiral peptides of significant length is not probabilistically favorable and may be an unambiguous biosignature (Orgel, 1998). This raises questions of whether homochiral peptides might be produced abiotically from a racemic pool of abiotically produced amino acids (Mathew et al., 2004; Córdova et al., 2005; Meierhenrich et al., 2005).
Still, recent studies have shown that functional (Mohamed et al., 2017) d-peptides can be produced biotically (Katoh et al., 2017). Perhaps large quantities of either homochiral peptides, or even the coexistence of both types of homochiral peptides (but not mixed chirality peptides), observed beyond the Earth would be indicative of extraterrestrial life. Unfortunately, biopolymers tend to degrade over time, and the structural information that may allow them to serve as biosignatures can be lost over relatively short time periods.
5.1.6.2.3. Distribution of molecules
When the isotopic or structural information of a molecule is not sufficiently diagnostic of its origin, the relative concentration of the molecule in the environment compared with other chemically related molecules may instead be used as a biosignature. Due to thermodynamic and kinetic constraints on the rate of formation of molecules during abiotic synthesis, a continuous spectrum of molecules, enriched in kinetically allowable low-molecular-weight compounds, is expected. For example, hydrocarbons synthesized via FTT processes are characterized by an exponential decrease in abundance with increasing number of carbon atoms (Sherwood Lollar et al., 2002). This is in stark contrast to biological systems where metabolism results in the synthesis of only a specific subset of compounds.
Through enzymatic catalysis, organisms can rapidly synthesize compounds, even those that require a high energy of formation, because of the evolutionary benefits imparted by their synthesis (Dorn et al., 2011). The principle that biological metabolism uses a discontinuous subset of biochemicals is hypothesized to be universal to all forms of life (McKay, 2004; Davies et al., 2009). On Earth, uneven distribution patterns suggestive of biological origins are particularly evident in larger organic molecules where biosynthesis uses two-carbon building blocks, for example, in the case of enriching fatty acids of even carbon number in the environment (Botta et al., 2008).
5.1.6.2.4. Allotropes
Some elements and minerals can exist in more than one kinetically stable form in the same physical state (solid, liquid, or gas). For elements, these forms are known as allotropes. Examples of allotropes include diamond, fullerene, and graphite for carbon, molecular oxygen (O2) and ozone (O3) for oxygen, and the numerous types known for sulfur, including various cyclo and catena allotropes. Since one allotrope may be more kinetically favored by a biological synthesis mechanism over an abiotic mechanism, the relative abundances of these may serve as a biomarker.
5.1.6.2.5. Cells/compartments
Common chemical constituents characterize terrestrial life. The interactions of these constituents define living systems and, for biochemical reactions to occur over appropriate timescales, the concentration of biomolecules must be relatively high (Matsuura et al., 2012; Sunami et al., 2016). This is generally achieved through the formation of cellular and subcellular compartments. Life uses a range of compartmentalization techniques, from the membraneless stress and p granules and nucleoli (Montgomery, 1898; Brangwynne et al., 2015) to a range of membrane-bounded organelles and cells themselves. The organization of cells is not uniform. For bacteria and archaea, nuclei are absent. Most eurkaryotes have a single nucleus, some, for example, Bryopsis plumosa (Kim et al., 2001) and Caulerpa prolifera (Kaplan and Hagemann, 1991) are multinucleate giant cells.
The different possibilities for the emergence of membrane-based compartmentalization have led to a significant research effort to build prebiotically plausible synthetic cell analogues that are capable of mimicking certain aspects of extant life (Szostak et al., 2001; Kurihara et al., 2011; Kuruma, 2015; Trantidou et al., 2017). Analogs demonstrating metabolism, growth, replication, division, and evolution have been devised in the laboratory. These research efforts not only describe plausible options for the earliest forms of life on Earth but also lead to questions of how life can be defined in general terms and pose questions about the kind of compartments and their components that could be considered unambiguous extraterrestrial biosignatures.
5.1.6.3. Mineral biosignatures (and abiosignatures)
Relatively little focus has been applied to using minerals as biomarkers. Mineral speciation and mineral morphology are two phenomena that might be used as biosignatures to reveal an extant or fossil biosphere.
5.1.6.3.1. Mineral species as biosignatures
Earth boasts >5300 approved named mineral species, each with a unique chemical composition and crystal structure (rruff.info/ima). About 1500 of these diverse minerals can be unambiguously shown to originate through nonbiological, igneous, or metamorphic processes. In addition, hundreds of alteration minerals formed by hydration reactions, or species formed through evaporation of saline solutions, may occur on nonliving worlds (Hazen et al., 2008; Hazen and Ferry, 2010; Hazen, 2013). As noted earlier, all purported biosignatures must be evaluated in the environmental context, and mineral species are no exception. Consequently, the occurrence of these species alone cannot be used to claim a biological origin.
In contrast, two-thirds of known mineral species on Earth arise directly or indirectly through biological alteration of the near-surface environment. Most abundant among these are minerals formed through the oxidative alteration of other minerals, notably thousands of oxidized minerals contain multivalent elements sensitive to oxidation/reduction, including transition metals (e.g., Cu, Fe, Mn, Ni) and metalloids (e.g., As, Sb), and nonmetals (e.g., C, S). Some minerals, such as the microbial precipitate hazenite [KNaMg2(PO4)2·14H2O] (Yang et al., 2011), arise exclusively through biological activity. In addition, over 60 organic minerals, including oxalates, hydrocarbons, derivatives of guano, urinary tract minerals (e.g., struvite, NH4MgPO4·6H2O) (Sánchez-Román et al., 2007), and one geoporphyrin (abelsonite; NiC31H32N4), are unambiguously the by-products of biological activity (Hazen et al., 2013).
Can mineral species that appear to be unambiguously biological on Earth occur through purely physical and/or chemical processes on other planets and moons? There are a few thousand minerals that arise from oxidative weathering—presumably the consequence of oxygenic photosynthesis on Earth, but that might form abiotically on more oxidized worlds. Similarly, hydrocarbon minerals on Earth are usually associated with coal and other carbon-rich deposits assumed to arise from geologically modified or decayed biomass. Hydrocarbon minerals likely arise from purely physical and chemical processes on Titan (Cornet et al., 2015). Except for a few distinctive organic minerals derived from complex biomolecules (Table 1), it is not yet possible to point to a single mineral or suite of mineral species that provide unambiguous evidence for an extant or fossil biosphere on another planet or moon. Thus, by themselves and without other biosignatures from organic molecules, isotopic and elemental compositions, such as mineral assemblages, are only “permissive” evidence.
Table 1.
Organic Mineral Species Unambiguously Derived from Biomolecules
| Mineral | Formula | Biological source |
|---|---|---|
| Abelsonite | NiC31H32N4 | Chlorophyll-derived porphyrin |
| Guanine | C5H3(NH2)N4O | DNA/RNA |
| Oxammite | (NH4)2(C2O4)H2O | Derived from guano |
| Tinnunculite | C5H4N4O3·2H2O | Uric acid dihydrate; guano |
| Urea | CO(NH2)2 | The principal component of urine |
| Uricite | C5H4N4O3 | Uric acid; metabolic breakdown of purines |
5.1.6.3.2. Mineral morphologies as biosignatures
The most familiar and convincing mineral biosignatures are morphological in character. Biomineralized shells, teeth, and bones composed of carbonate, silica, or phosphate minerals retain obvious evidence of biological function. Stromatolites, burrows, and other trace fossils, coprolites, and other macroscopic fossils also provide convincing morphological biosignatures preserved in mineralized structures. Microscopic fossils such as diatom frustules, amebic tests, radiolarian skeletons, plant biominerals (phytoliths), and more also are distinctively biological. Many of these mineral morphologies are also treated as objects below.
In a few cases, the morphology of individual crystal grains may point to an unambiguous biological origin, such as microbially precipitated minerals that display morphologies not otherwise likely to occur. Uraninite (UO2) is an interesting mineral example whose morphology appears to have changed through deep time as a consequence of biology (Hazen et al., 2009). In Archean rocks before the Great Oxidation Event (GOE), uraninite is typically coarse grained, occurring abiotically in both igneous formations and as stream-eroded grains in sediments (Rasmussen and Buick, 1999). However, more recent formations display concentrations of nanouraninite by strains of Geobacter, Desulfovibrio, and Shewanella, which may couple acetate oxidation to the reduction of aqueous uranyl cations, UO22+ to nanouraninite (Lovely et al., 1991; Spear et al., 2000; Fayek et al., 2005; Long, 2008; Sharp et al., 2008). Although nanouraninite is still ambiguous as a biosignature, the role of microbes in modifying mineral morphology represents an important opportunity in future biomarker research.
5.1.6.4. Chemical disequilibrium
The cycle of reactive oxygen species (ROS) provides an example of linked abiotic and biotic processes that may help to distinguish biotic from abiotic conditions (Fig. 7). ROS are produced in the environment in the forms of hydrogen peroxide (H2O2), hydroxyl radical, and superoxide, among others. The abiotic ROS cycle produces chemical disequilibria in redox states of some transition elements, notably Fe and Mn (e.g., Doane, 2017).
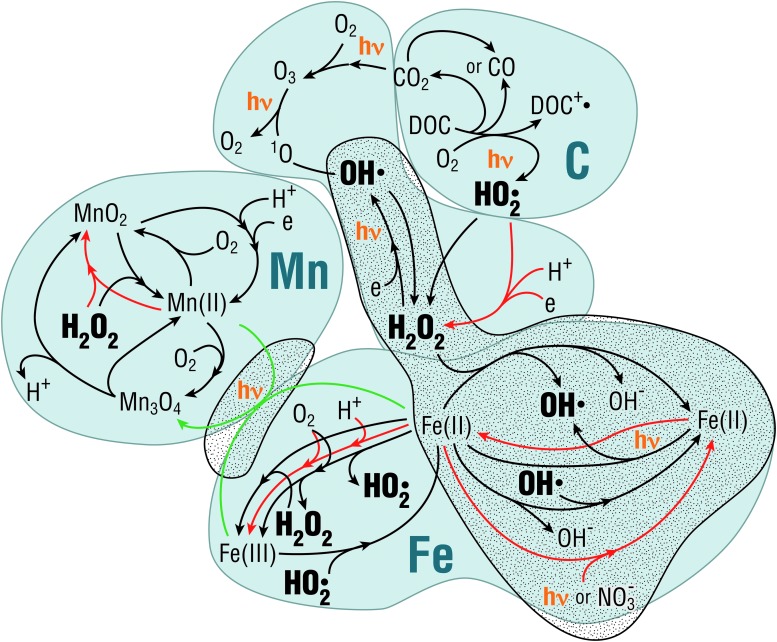
FIG. 7.
Linked biotic and abiotic processes illustrate formation and degradation of reactive oxygen species in aquatic systems, including photochemical processes. Three systems are used to illustrate the processes: carbon, which is the dominant mechanism in most aquatic systems, manganese, and iron (cf. Wilson et al., 2000; Duesterberg et al., 2008; Doane, 2017). Black arrows are abiotic reactions. Red arrows are biotic reactions. Green arrows are inferred reactions. The stippled area shows reactions that occur in the absence of oxygen. Dots by chemical compounds indicate an unpaired electron, that is, free radicals. Image N.W.H.
In most modern surface waters, the main abiotic pathway for ROS production involves photoreactive dissolved organic matter. Yet, reactions involving multivalent elements are likely the most relevant for early Earth and planetary systems (Ramesh et al., 2016; Doane, 2017 and references therein). ROS production could have been much higher on early Earth before oxygenation of the atmosphere because of the absence of an ozone layer (and thus a high flux of intermediate wavelength ultraviolet radiation [UVB: 280–315 nm]) and the higher concentrations of reduced multivalent elements in near-surface environments. These reactions cycle elements abiotically through redox states, leading to accumulation of ROS depending on the rates of formation and degradation.
The formation and degradation rates of ROS depend on intrinsic rate constants, temperature, pressure, and the concentrations of reactants. All ROS are damaging to life, which has developed mechanisms to detoxify H2O2 and superoxides although not able to detoxify hydroxyl radicals. Microbes rapidly degrade H2O2 and superoxide (Wilson et al., 2000), limiting the maximum concentration achievable in surface waters. In the absence of microbes, it is possible that H2O2, and superoxide concentrations (in the presence of O2) would continue to increase until the rate of the degradation equals the rate of formation, which could lead to very high concentrations of environmental H2O2 and superoxide. Thus, high photochemically achievable concentrations of ROS could mean that there is no life, while lower but constant maxima could mean that life is present. Such a subtle distinction would require a highly nuanced interpretation. For example, Meadows et al. (2018) compare abiotic mechanisms of O2 production on exoplanets with the early emergence of photosynthesis as a source of O2 on Earth. The environmental conditions comprising planetary atmospheres and stellar spectra, under several models of such conditions, could produce biosignature false positives.
Krissansen-Totton et al. (2018) argue that the presence of disequilibria, specifically in carbon-bearing gases, CO2 and CH4, is a potential biosignature that can be detected in planetary atmospheres. Indeed, these two species of carbon would not be expected to occur together, and both can be detected remotely. They point out, though, that photochemical processes, among others, must be considered, particularly with respect to photodissociation of methane. The abiotic, and potentially biotic, rates of the formation and degradation reactions are key for allowing the concentrations of either carbon dioxide or methane to build up. Photochemical processes, particularly in the presence of suitable catalysts (e.g., Habisreutinger et al., 2013), have the potential to put environmental pressure on planetary systems and maintain disequilibria.
5.2. Objects
The term ‘objects’ describes physical features produced by life, such as fossils, microbialites (in particular stromatolites), and biotextures as well as some physical features that are inferred to be related to life products (visible or invisible), such as concretions. Life is embedded in its environmental context and spatial scales are important for interpreting biogenicity. Characteristics from the macroscale down to the submicron scale need to be accounted for as much as possible although typically not all present in a given specimen, feature, or material type. Identifiable morphologies of potentially habitable environments at larger scales are visible with current instrumentation on Mars (e.g., orbiters); however, most are still ambiguous without the ability to evaluate features at smaller scales. Multiple methods examining features at multiple scales are needed to adequately characterize samples or systems to increase the level of certainty that a record of life is preserved. Spatial distributions of potentially biogenic features must also be characterized to understand context and move toward more certainty when identifying biosignatures.
Objects can be produced by organisms over a variety of scales, from macroscopic stromatolites (e.g., Awramik and Buchheim, 2015; Suosaari et al., 2016) down to submicron biominerals, such as microbially generated magnetite (e.g., Stolz, 1993; Stal, 2012). Extreme environments are often thought of as the most likely sites for the origins of life on both Earth and Mars. However, it is important to remember that organisms adapt to their environment during evolution, and the origins of life may occur under milder conditions (Cleaves and Chalmers, 2004).
5.2.1. Objects as biosignatures
Object biomarkers can have varying degrees of ambiguity with respect to their biogenicity. For example, bones are unambiguous biosignatures at the macroscale, because they can only be formed by vertebrates. However, macroscale textures in sedimentary rocks are potentially more ambiguous, and can form either biotically, as in the case of MISS (Noffke et al., 2008) and stromatolitic lamination (Lee et al., 2000), or by abiological physical processes, such as fluid flow or turbulence (McLoughlin et al., 2008; Bower, 2011; Menon et al., 2016), and abiological chemical processes, such as the abiotic precipitation of calcium carbonate (Pope and Grotzinger, 2000; McLoughlin et al., 2008). To further complicate matters, the formation of stromatolitic lamination can involve a combination of abiotic and biotic processes (e.g., Riding, 2008; Suosaari et al., 2016; Tosti and Riding, 2017). Continued investigation of the abiotic and biotic factors that can lead to stromatolite formation is necessary to discriminate their variable contributions (Awramik and Grey, 2005).
At smaller scales, morphological microbial body fossils can provide strong fossil evidence (Levett et al., 2016) or be ambiguous, since many of the same mineral species involved with fossilization also occur in abiotic systems, and even mineral morphologies can mimic microbial ones in ancient rocks (e.g., Bower et al., 2015, 2016; Crosby and Bailey, 2018). In addition, many of these body fossils have different morphological identification features at a variety of scales, which when observed collectively reduce the uncertainty of interpretation. The consensus is that multiple lines of evidence that combine chemistry, morphology, geologic context, and other features at different scales are required to determine biogenicity with confidence. Herein, we describe objects that can potentially be used to infer a record of biological activity on a variety of scales (from the large scale down to the small scale), followed by a brief discussion of biosignature preservation.
5.2.1.1. Kilometer-scale context
Individual objects as biosignatures do not occur at large regional scales, except for some stromatolites. Massive stromatolite beds, a few to several meters thick, can be traced over ∼1000 km in the Mesoproterozoic Atar Group, Mauritania (Bertrand-Sarfati and Moussine-Pouchkine, 1988).
One of the first requirements in the search for past records of life is recognizing environments capable of high bioproductivity, long-duration habitability, and high preservation potential (Hays et al., 2017). This is a critical first step in the search for a recognizable biosignature, although the topic of habitable environments is too large to be covered in detail here.
To recognize km-scale environments conducive to habitability and preservation of biosignatures, we need to be able to interpret the past or present habitability of environments. For example, indicators of such environments include clays (i.e., hydrated aluminosilicates with layered crystal structures) and carbonate lithologies combined with diagnostic large-scale morphologies such as layered rocks or deltas from orbital data in the context of Mars. Especially, attractive environments include exhumed and exposed sedimentary units, exposed subsurface deposits, spring environments, and regions where mafic rocks and sediments are layered over sulfate-rich sedimentary rocks (as sulfates record both the presence of water and contain sulfur—a potentially good energy source).
Subsurface environments are good candidates for having hosted continuous and long-lived potentially habitable environments—particularly the subsurface of Mars, and the liquid interiors of Europa and Enceladus presumably overlying rocky centers. The subsurface of a rocky planet such as the Earth often contains redox gradients at a variety of spatial scales, which could provide energy for chemolithotrophs on Mars (Boston et al., 1992). In addition, these redox gradients and the potential for chemolithoautotrophy would likely involve reactions that create rapid mineral precipitation (e.g., the creation of iron-bearing, sulfate, or carbonate diagenetic cements) that increases the probability for preservation of life.
Examples of exposed subsurface environments on Mars include Margaritifer Terra where chaotic terrain is hypothesized to have resulted from expulsion of subsurface fluid (e.g., Carr, 1979; Thomas et al., 2017). In addition, raised ridges that are resistant to erosion relative to the surrounding rock have been interpreted as possible examples of subsurface mineralization that has preferentially cemented these fractures, rendering them harder than the rest of the unit (Thomas et al., 2017).
The environments of springs on ancient Earth and Mars have similarly high probabilities for both production and preservation (Hays et al., 2017). Springs may not have the longevity of some other environments but may present ephemeral refugia (on geologic timescales) for life to survive inhospitable conditions, such as the Late Heavy Bombardment on Earth and increasingly inhospitable surface conditions during the Hesperian period on Mars. Rapid mineral precipitation can entomb microbes in these environments and preserve biogenic features over geologic timescales (e.g., Potter-McIntyre et al., 2017).
A specific example of mafic deposits over layered sulfates (such as N.E. Syrtis on Mars) (Ehlmann and Mustard, 2012) may represent habitats that have excellent production and preservation potential. On Earth, analog research on mafic intrusions into sulfate-rich sedimentary rocks shows that these environments are promising astrobiological targets (Foster et al., 2010). These are subsurface environments that would be locally sterilized during mafic emplacement. However, the fluid accompanying the mafics would mobilize sulfur and other bioavailable elements to supply the environment with fresh reactants for metabolism. Some degree of sterilization could create an ecological niche for organisms and an environment rich with nutrients, increasing the chances for high biological production and high preservation due to rapid mineral precipitation.
The potential for kilometer-scale contexts described above can support more insightful interpretations of meter-scale and smaller objects than analyzing those objects alone. In the absence of such bridging information, isolated objects may be more ambiguous and subject to multiple interpretations. The more subtle the smaller object or feature, the more the kilometer-scale view of the environment can contribute critical information.
5.2.1.2. Meter-scale objects
Meter-scale biosignatures can include microbialites (stromatolites), microbially induced sedimentary structures (aka MISS) on extensive bedding plane surfaces, and concretions that are visible to the naked eye and are often spatially distributed from the meter to the hundreds of meters scale (e.g., Awramik, 1992; Noffke et al., 2008; Potter et al., 2011; Chan et al., 2012; Fralick and Riding, 2015; Potter-McIntyre et al., 2017). Some macroscale features represent the collective physical remains of structured microbial communities (Awramik, 1992), where diagnostic wavy laminar, domical, clotted, conical, branching, and stratiform morphologies would not form in such finely laminated deposit were it not for the microbial influences. Indeed, in Archean rocks, the macrostructures associated with microstructures (e.g., stromatolitic lamination and MISS) can be extensive and compelling as a morphological biosignature (Noffke et al., 2008; Noffke and Awramik, 2013). Stromatolites and certain types of microbial mats are further discussed in a later section on patterns.
Trace fossils (ichnofossils) record the activities of a variety of different types of organisms and are visible at the submeter scale. Common terrestrial trace fossils include footprints, burrows, root traces, and imprint textures. For example, charophytes (freshwater algae) can leave characteristic imprints in rock that consist of 1 mm by 5–10 mm shallow (<0.5 mm) vugs (Potter-McIntyre et al., 2014). Some evidence suggests that syneresis cracks are biological in origin and can also be considered microbial trace fossils (Harazim et al., 2013; Mariotti et al., 2014). Other microbial trace fossils also occur at micron scales in the form of mineral precipitation patterns or microborings (Staudigel et al., 2015; Nikitczuk et al., 2016).
Taken collectively, these textures can provide clues about paleoenvironments and the types of communities that inhabited them. However, physicochemical changes to sedimentary deposits over geologic time can also result in the formation of similar abiotic textures in ancient rocks (e.g., Grosch and McLoughlin, 2015; Davies et al., 2016). For example, mudcracks can resemble burrows in a cross-sectional view, or clotted paleosol development can resemble burrow textures. Larger organisms and collections of organisms (biofilms, microbial mats) may be preserved but may be significantly reduced in size due to compaction during diagenesis (Bower et al., 2017) and dewatering. Consistency in parameters such as texture, size, and orientation can be useful in differentiating biotic from abiotic signals but examining these features at a variety of scales is still necessary to deduce biogenicity in ancient rocks.
5.2.1.3. Centimeter-scale objects
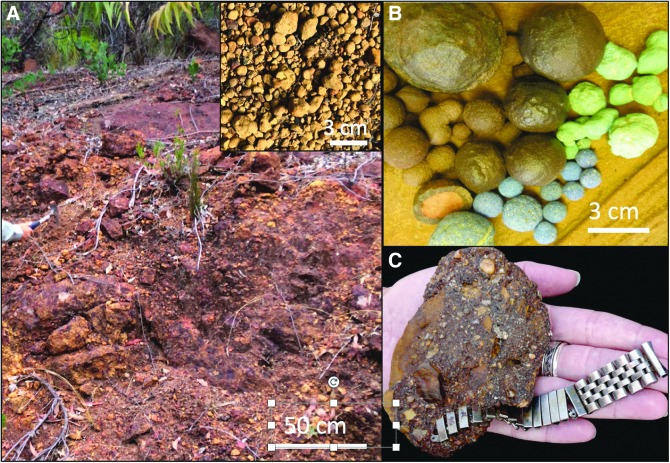
Obvious, identifiable centimeter-scale fossils need little explanation of their biogenicity because we have a great deal of contextual knowledge about life on Earth, but large putative fossil materials on other planets may be much more difficult to interpret or even notice. Carrying this idea further, a range of other cm-scale objects could contain biosignatures similar with microbialites but perhaps even on finer microscopic scales. This range could include nonskeletal carbonate grains such as coated grains, including oncoids, ooids, pisolites, and others (Flügel, 2010). Iron oxide nodules of Earth's near-surface critical zone can exhibit a suite of varieties from those with clear biosignatures (e.g., hematite and goethite paleosol mineralization that preserve fruiting bodies, fungi, organic matter, and bacteria; Anand and Verrall, 2011) (Fig. 8A), to others that are ambiguous at best (Fig. 8B, C).
FIG. 8.
Iron oxide mineral precipitates have various biomediated to ambiguous origins. (A) Bauxitic paleosols of the Yilgarn Craton, Western Australia, show deep weathered zones, heavily influenced by plants to microbes and bacteria. Upper right inset shows loose paleosol pisolite (pisoliths) with various microbial forms (Anand and Verrall, 2011). (B) Concretions of goethite (brown), malachite (green), and azurite (blue) mineralogies from Utah are more ambiguous in their origins, and lack any fossil nuclei. (C) Iron oxide concretions around human-made objects (e.g., metal watch band from the Chesapeake Bay) suggest rapid, biomediated cementation on the orders of years. Images (A, B) M.A.C.; image (C) S. Godfrey, supplied by R.M.H.
Concretions (Fig. 8B) are diagenetic, cemented mineral masses that comprise another example of centimeter-scale objects (although concretions can vary from meter- to millimeter-scales) with or without clear relationships to identifiable fossils (e.g., Raiswell et al., 2000; Mozley and Davis, 2005; Potter et al., 2011). Concretions without any obvious fossil nuclei are often assumed to be products of physical cementation, although it may just be a problem of recognizing subtle biosignatures.
If all of Earth's surface to subsurface waters has harbored microbes throughout most of the rock record, then biomediation must be considered possible for any authigenic mineral, regardless of whether there is a visible fossil nucleus or not. Furthermore, it is evident that cementation and even recrystallization can happen quickly if the conditions are right on timescales of several years (Fig. 8C) (e.g., Coleman, 1993; Melim and Spilde, 2011). Thus, unresolved outstanding issues about concretions include our ability to distinguish the biotic and abiotic processes involved in their formation, and the possibility that biomineralization can occur by microbial alteration of the pore fluid chemistry to a thermodynamically favorable environment that triggers precipitation.
Concretions are of great interest in planetary exploration, as “blueberries” believed to be concretions have already been found at multiple places on Mars (e.g., Chan et al., 2004, 2005; Squyres et al., 2004; Grotzinger et al., 2005; Calvin et al., 2008). Concretions are evidence of groundwater involved in cementation, and thus, if Earth examples preserve biosignatures, there is a similar possibility that such signatures could be found on other planetary bodies such as Mars.
5.2.1.4. Micron-scale biosignatures
Microbial body fossils can persist over billions of years on Earth, for example, in units such as the 1.88 Ga Gunflint Chert (e.g., Barghoorn and Tyler, 1965; Schopf et al., 2002). For rocks older than ∼2 Ga, there is morphologic evidence at macroscale (see section 5.2.1.2) and microscale, which, combined with petrographic data and chemical signatures (e.g., stable isotope data, mineral phases, kerogen), allows for unambiguous interpretation of these as biogenic features (e.g., Schopf et al., 2002, 2007, 2018). Unfortunately, in most rocks of this age, these signatures are often ambiguous due to geologic processes that over time alter and obscure much of the original fabrics, such as original minerals or cellular remains, at the microscale. Pore spaces within rocks can preserve body fossils (Lanier, 1989) or other evidence of biotic interactions, but they can also be filled in with abiotic carbon-rich fluids (Bower et al., 2016).
Archean cherts, especially, exhibit both biotic and abiotic features that can be morphologically and chemically similar at the micron scale (Bower et al., 2016). Chemical gradients that record fluid/rock/biota interactions within diagenetic cements can be observed in thin section and can provide useful information, and this remains to be further developed as a tool for interpreting biogenicity (Potter-McIntyre et al., 2014).
Biotic and abiotic jarosite [KFe(SO4)2(OH)6] is indistinguishable at micrometer to submicrometer scales. A similar conclusion regarding scale was reached following experiments in which biomediated and abiotic mineral precipitates of Ca-sulfates were compared: compositional differences were apparent only at the submicron scale (Bower et al., 2015). This is also true for mineral habits: mineral examples created in the laboratory via biotic and abiotic processes often cannot be differentiated unless examined at the submicron scale. It is imperative to consider scale context when searching for biosignatures, and some biosignatures may need to be examined over multiple scales for unambiguous interpretation.
5.2.2. Preservation potential of objects as biosignatures
Inorganic processes that affect sediments following deposition are broadly referred to as “diagenesis.” The field of “taphonomy” (Efremov, 1940) studies how the biological remains and/or the by-products of organisms are transformed and preserved as they pass from the biosphere to the lithosphere (Cadée, 1991; Behrensmeyer et al., 2000; Allison and Bottjer, 2011). Both diagenesis and taphonomy affect the overall preservational potential of biosignatures.
There are positive factors that favor preservation. (1) Rapid and early diagenetic cementation in detrital systems that lowers sediment permeability, which along with anoxia, can greatly reduce rates of organic matter degradation and can essentially “freeze” biogenic features and help preserve them. (2) In chemical sedimentary systems, preservation is enhanced by rapid entombment in finely crystalline chemical precipitates, particularly where primary mineral phases are chemically stable and resist dissolution and aqueous weathering (Farmer, 1999b). On Earth, these favorable lithotypes include cherts and phosphorites, along with less stable carbonates and shales, which are the most common host rocks for the fossil record on Earth. (3) Selective biases such as mineralized skeletons (Dart, 1949; Lawrence, 1968; Olson, 1980; Seilacher, 1992) or large organisms and collections of organisms (biofilms, microbial mats) can also have higher preservation potential than individual microbes (Bower et al., 2017; Hays et al., 2017).
The potential for a preserved record of extraterrestrial microbial life on other planets in our Solar System, such as Mars (e.g., Farmer 1995; Farmer and Des Marais, 1999; Ruff and Farmer, 2016), has been fueled by studies of microbial biosignature preservation in a variety of modern and ancient terrestrial analog environments (e.g., Konhauser et al., 2001; Schopf et al., 2012). It is also important to understand the different environments and controls that exist and operate on planets. For example, a 3.5 Ga rock on Mars will not have undergone the extensive metamorphism of a similar age rock on Earth nor will it have been in contact with diagenetic fluids for billions of years. Even young Earth rocks (several millions of years) often show evidence of superimposed multiple precipitation/dissolution events and mobilization of diagenetic minerals (e.g., Potter et al., 2011).
Based on extensive studies on Earth, it is apparent that biomineralization and preservation of biosignatures are dependent on natural context. A holistic approach of the entire environment—from the kilometer down to the submicron scale—needs to be examined, as opposed to just the “parts.” Another helpful approach could be to develop probabilistic models to reduce uncertainty in biosignature confirmation (e.g., Bayesian statistics approach of Walker et al., 2018). These models can be used together with more traditional physical data to build a more robust approach to biosignature identification to deal with degrees of certainty.
Not all fossils will likely be pristine or unambiguous, and this can be quantified by careful measurement of the number of individual organismal fossils, number of traits or characteristics, sizes, and population densities of organisms. This is already done in the paleontology community where face recognition-type algorithms have been used to automatically identify trilobites (e.g., Wei, 1994; Cope et al., 2012).
5.3. Patterns
Here, we define a biopattern as a spatial and/or temporal organization of any of the substances and objects produced directly or indirectly by the processes of life discussed above, and a biosignature in its true sense. One of the earliest interpretations of biopatterns as biosignatures dates to Xenophanes (c. 570 BC–c. 475 BC), who observed structures in rocks inland from the ocean that resembled marine shells and fish and hence fossils (biosignatures) and concluded that an ocean (containing bivalves and fish) once occupied the inland region (Burnet, 1930; McKirahan, 1994). Of course, modern examination of fossils and their morphology and possibly chemical life traces are central to paleontology, biology, and geology and offer a record of evolution and the history of life. However, we now understand that there is a vast array of potential biopatterns ranging in scale from nanoscale biochemical patterns to multiple kilometer-scale brushlands and forest tree growth patterns. Such patterns do not rely on a particular chemistry or morphology, but only that patterns of some sort can be recognizable, analyzable, and ultimately tied to specific biological processes (Fig. 9A–C).
FIG. 9.
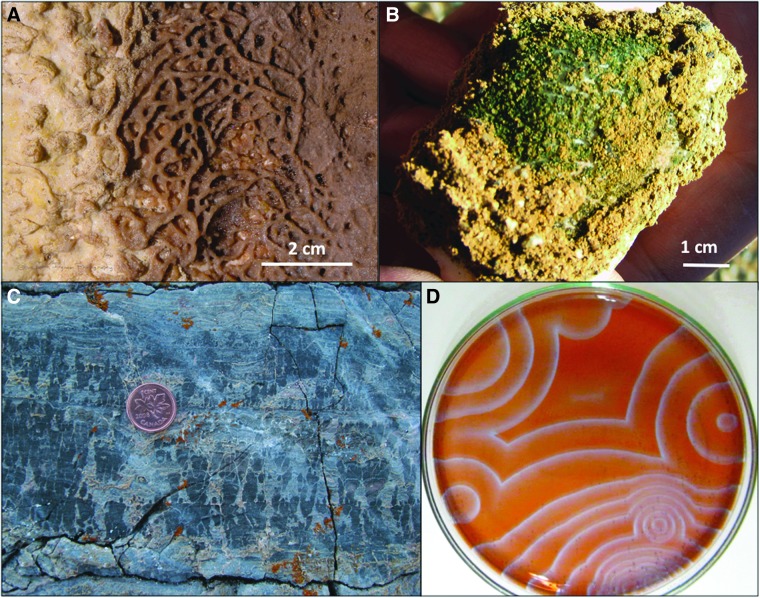
Mineral patterns can be biomediated (A–C) or a result of abiotic chemical reactions (D). (A) A modern lithification front of biovermiculation, Cueva de Villa Luz, Mexico. View ∼8 cm across. Image: K. Ingham. (B) The underside of a hypolithic rock shows highly miniaturized biovermiculation patterns of cyanobacteria (genus Chroococcidiopsis) that live along the soil interface, Strzelecki Desert, Australia. Image: P.J.B. (C) Centimeter-size columnar-branching and multifurcate dolomitic stromatolites show millimeter-thick lamination patterns from the Paleoproterozoic McLeary Formation of the Belcher Supergroup, Canada. Image: D.P. (D) In an established abiotic B-Z reaction, chemical oscillation rings create life-like patterns. In this example, the red color arises from the redox indicator ferroin (commercial 25 mM phenanthroline ferrous sulfate) used in the experiment, and blue-gray lines represent redox fronts extending radially outward from oxidation spots in the geometric centers. Glass dish diameter 100 mm. B-Z, Belousov–Zhabotinsky. Image: D.P.
Abiotic reactions can create life-like patterns (Fig. 9D), but when clear biologically controlled patterns exist and abiotic mimics adequately ruled out, then they may be strong candidates for consideration as universal biosignatures (Schubert et al., 2017). In this work, we focus only on physical manifestations of visually identifiable spatial patterns.
Physical patterns can vary widely in characteristics but are typically sinuous, curving, or spatially arranged due to the physics that govern biological growth (Meron et al., 2004). Biopatterns can be influenced by environmental factors such as ultraviolet light, as is done in industrial biopatterning for biomedical applications from tissue engineering to fundamental cell studies (Whitesides et al., 2001). Biopatterns can also be influenced by the presence or absence of sunlight. The shape of stony corals (hexacorals) changes as a function of water depth, which consequently affects light penetration. For example, branching in Porites sillimaniani decreases with depth (see Kaandorp and Kübler, 2001).
Biopatterns can include concretions (Suga and Nakahara, 2012; Yoshida et al., 2015), layering in stromatolites (Semikhatov et al., 1979; Awramik, 1992), and biovermiculations in caves and deserts (Thiéry et al., 1995; Klausmeier, 1999; HilleRisLambers et al., 2001; Schubert et al., 2017). Biopatterns are not just passive responses of biological systems. For example, increasing density of soil crust patterns is correlated with diversity, metabolic activity, and capacity to restructure the soil (Mogul et al., 2017).
Biovermiculations are worm-like or hieroglyphic-like patterns often occurring in biological mats or thin films formed by communities of microbes. Most commonly, biovermiculations (bioverms) occur in caves (Fig. 9A) or ancient ruins but can be found in desert soil crusts, hypersaline creek algae growth, and even in modern walls and buildings. Of particular interest in the study of early life and life in extreme environments is that microbial communities can grow under hypolithic rocks (Fig. 9B), providing a small “greenhouse”-like environment where biology can be protected in an otherwise uninhabitable or deadly environment.
The patterns can be traced back to the early studies in morphogenesis (e.g., of the coloration patterns on animals) (Turing, 1952). In resource-constrained environments, biological systems form patterns that may serve to optimize their return on the effort to acquire needed resources (Schubert et al., 2017). These patterns persist over time, partly because cave environments are not perturbed by surface weather and only rarely affected by events such as flooding or animal activities. Thus, microbial activities result in ongoing mineralization of patterns that can provide evidence of life even when microbial activities may have long ceased.
Biopatterns can be preserved across geological timescales, but challenges to preservation (Hays et al., 2017) exist, most notably the living structures must be covered rapidly or self-mineralize (e.g., Boston et al., 2001). DNA and protein sequence information typically undergo rapid degradation over very short geological timescales, with the exact amount of time dependent on environmental factors such as temperature, humidity, and the encasing matrix. However, diagenesis can preserve biochemicals such as amino acids from structures such as eggshell and bone (Bada et al., 1999), allowing inference of the original shape of soft-body parts.
In addition, characteristic laminated structures of stromatolites (Fig. 9C) are easily recognized over 3.5 billion years of the geologic record (Hofmann et al., 1999; Allwood et al., 2006), although many ancient putative examples still engender heated debate. Currently, a debate exists over the putative stromatolites from the 3.7 Ga Isua supercrustals (Nutman et al., 2016; Allwood et al., 2018). Stromatolites are still forming today in many different environments, including normal salinity marine, hypersaline marine, streams, lakes (both freshwater and saline), and thermal springs. Stromatolite structures are a primitive, fundamental outcome of life adapted to living in shallow photic environments, and their morphological preservation is rationalized to have been more favorable before the advent of organisms that graze on them. Biopatterns in caves commonly also lithify even as they grow, providing a mechanism for their long-term preservation (Boston et al., 2009).
Biopatterns in surface environments must be entombed and permineralized like traditional fossils or encased in various salts and other evaporites for preservation. Even with all these challenges, on a planet where biology has spread globally and existed for billions of years (e.g., Earth and potentially early Mars), it is reasonable that a large number of biopatterns would be preserved and could thus be interpretable as biosignatures.
5.3.1. Biopatterns in stromatolites
Stromatolites and other microbialites constitute an important group of biosignatures that present biopatterns (Fig. 9C). The term microbialite (Burne and Moore, 1987) encompasses various types of organosedimentary deposits that bind and trap sediment, including stromatolites (laminated), thrombolites (clotted), dendrolites (composed of cm-size shrubs), and leiolites (which are structureless). These four microbial types have biopatterns, but establishing the role, if any, of biology in forming the structures or patterns has been contentious. Stromatolites have been at the forefront of this debate (Grotzinger and Knoll, 1999; Awramik and Grey, 2005; McLoughlin et al., 2008; Allwood, 2016).
Numerous criteria have been developed to increase the level of confidence that a stromatolite is biogenic (Awramik and Grey, 2005). Given these, biopatterns occur at three different observational levels: macrostructure, mesostructure, and microstructure. Macrostructure refers to the overall shape. Common shapes include millimeter- to decimeter-size distinctive cones, domes, and columns. Some shapes are difficult to attribute to nonbiologic processes, specifically when their geologic context is considered (e.g., in subaqueously deposited sedimentary rocks, primarily carbonates). Mesoscale is intermediate between macro- and microstructure and refers to the internal structure visible to the unaided eye and serves as the scale for identifying the four types of microbialites. The defining characteristic of a stromatolite is lamination. A common biopattern is alternating, thinner dark laminae with thicker light laminae (at the millimeter or less scale), with laminae across the structure having variable thicknesses (non-isopachous). Microscale structure is studied with the aid of a microscope. Biopatterns include the growth position of microbial fossils, the arrangement of sediment grains, and the sharpness of the boundaries between dark and light laminae.
Microorganisms living in chemical sedimentary accreting systems influence the form of precipitation indirectly by providing surfaces for mineral nucleation. These influences and inherited forms are difficult to distinguish at the nanoscale but are often seen clearly at larger spatial scales. Visible laminae such as those used to define true stromatolites (vs. abiotic examples such as the lamina inside a geode) are usually visualized and studied at spatial scales much coarser than unit cells, although the cells provide the basic building blocks.
In biofilms, organic forms are mostly visualized at the micron scale, where they provide templates for mineral precipitates that nucleate on them and eventually entomb whole organisms. The form of a captured organism may retain important aspects of the external shape of cells during accretion. However, only the basic aspects of the inherited form are usually preserved, and, once the organism is fully entombed, it no longer controls the inherited form, because the geometry of unit cell accretion is controlled inorganically at a fine scale.
Although stromatolites form only rarely in surface environments today (McNamara and Awramik, 1992), they are relatively common in the fossil record, particularly in the Proterozoic (Peters et al., 2017). Stromatolites are readily seen by the naked eye and criteria have been developed to establish their biogenicity. In many caves, stromatolite-like laminated structures are very common and formed microbially but not in response to light direction as they are not photosynthetic but heterotrophic or chemolithotrophic (Melim et al., 2009). They usually are pendant structures that grow in pools and fossilize very well, but living examples are known (Melim et al., 2015).
Computational modeling offers a novel way to distinguish biological from abiological patterns. If the “ruleset” of the generating system (e.g., the environmental factors and resulting organism behaviors that lead to the pattern) can be identified, it can then be compared with known biological and abiological systems. Differential equation-based models (von Hardenberg et al., 2001; Meron et al., 2004) and cellular automata (Dunkerley, 1997; Schubert et al., 2017) are equivalent ways to model biological systems in silico (Strader et al., 2011). Determining mathematical and statistical rules that govern biological growth processes and their interactions with mineralization processes is nontrivial but important for many problems. Consequently, various techniques have been used, including machine learning (Richards et al., 1990; Campbell et al., 2004; Placzek, 2014; Gurikov et al., 2016), coevolution (Juille and Pollack, 1998), and histogram-based methods (Schubert et al., 2017).
One method currently being explored that directly observes pattern formation involves taking time-lapse images of slowly growing biological communities that develop recognizable patterns such as biovermiculations or mat structures. By comparing two successive pictures, one can see how they have changed spatiotemporally, which in turn specifies the rules to be used in the modeling. Time series comparison is straightforward and thus the basis of most techniques. In contrast, only one high-resolution picture is needed using histogram techniques (Schubert et al., 2017), because only spatial comparisons are done, making it a particularly promising method for examining rock strata or other potentially biological patterns.
In the histogram method (Fig. 10), any region of interest in a biopattern image is first selected and converted to two colors (e.g., Red = R and Green = G) based on clustering or user experience. Separate histograms are then made around each R or G pixel, indexed by the number of neighbors that are green in a preselected region around the points, designating these as histogram R and histogram G. The number of neighbors corresponds to the density of green pixels in that region, which roughly corresponds to the amount of biological competition for some limiting factor. The histograms are then compared with each other bin by bin, where either histogram R or G will have a higher frequency, that is, the density of red or green pixels at a given distance from a red or green pixel is determined by the ruleset, which becomes evident in the histogram. Such a comparison is shown in Fig. 10, in which red corresponds to abiological material and green corresponds to biological material.
FIG. 10.
Histogram comparison used to determine a ruleset for a biopattern. Neighbors are the number of cells with biology within a preselected range, called the radius. Frequency is the number of cells (either abiological or biological) in the entire image that have the number of neighbors specified by the horizontal axis. The image is not guaranteed to have all possible configurations of neighbors, so there are sometimes no data available. These patterns specify the underlying rules. A, abiological; B, biological; ND, no data; OA, only abiological; OB, only biological. Image: K.E.S.
The histogram method has been validated using single states of a known cellular automaton, and the predicted ruleset compared with an actual ruleset. The result for one such system is shown in Fig. 11, where the ruleset was estimated at each iteration of the cellular automaton only using the histograms from that iteration.
FIG. 11.
Histogram comparison of the number of neighbors for biological and abiological “cells” at each iteration of a modeled cellular automaton versus a ruleset determined from an actual biological system. The vertical axis is iterations (essentially time). Each row is a histogram of the cellular automaton state, where color indicates the relative amount of biological versus abiological “cells.” Gray indicates no data, green indicates more biological “cells” at that number of neighbors, while pink indicates more abiological. Dark green indicates only biological cells having that number of neighbors and dark pink indicates only abiological cells having that number of neighbors. Image: K.E.S.
The actual rule boundaries are shown by the light blue lines and the rules are listed at the top. Gray indicates regions lacking data (i.e., where no cell had that minimum number of neighbors). Dark green indicates that only histogram G (biological) had a nonzero value for that density/neighbor count, while dark pink indicates only histogram R (abiological) had a nonzero value. Light green indicates that histogram B had a higher frequency than histogram A at that density, and light pink indicates that histogram A had a higher frequency than histogram B at that density. It is readily apparent that boundaries between the histogram regions outlined above correspond very closely to the actual rules for almost all iterations after an initial start-up. Although the spatial pattern of pixels changes continuously over time according to the ruleset, the relative frequencies stay constant, because these are defined by the rules. Assuming that a pattern encountered was well established, the histogram technique should give a good estimate of the rules, which can then be used to putatively distinguish biological from abiological patterns.
While more work needs to be done to develop these histogram techniques, biopatterns offer an exciting potential biomarker that could be detected by using machine-learning techniques. Machine-learning schemes that can use such cellular automata logic and can build up a basis of experience over the course of training can function much like a well-seasoned field scientist but with the added benefit of immediate quantitative rationales for making a particular biotic/abiotic call on a specific pattern. The application of such automated decision calls on patterns can be applied to future robotic missions to planetary targets of astrobiological interest to provide immediacy of remote decision-making without continuous direct Earth control.
5.3.1.1. Abiotic chemically induced patterns
Many geological processes are driven by cyclic processes, including seasons, tides, and day/night cycles. In turn, these large-scale physical processes can induce cyclic behaviors in biological and geochemical systems. Indeed, stromatolite laminations have been attributed in some cases to seasonal variation and day/night cycles among other forcing factors (Seong-Joo et al., 2000). There are also a variety of regular banding patterns observable in many mineral types, for example, the banding observed in minerals such as malachite [Cu2CO3(OH)2]. A possible explanation for some of these regular mineralogical patterns has been attributed to Liesegang phenomena, which are the result of reaction/diffusion-type physicochemical systems (Hartman et al., 1934). A variety of such systems have been studied in the laboratory, and collectively the ability of both natural biological and abiological phenomena to produce intriguingly complex patterns is well known (Ball, 1999) (Fig. 9D).
The potential importance of reaction diffusion systems in biosignature detection is that there may be a variety of phenomena that can produce seemingly highly ordered systems, which may not be easily attributable to or distinguishable from biological processes. Besides those already identified, there may be many that remain to be discovered and that may operate over much longer timescales.
5.3.2. Isotopic patterns
Physical, chemical, and biological processes can all produce stable isotope fractionation. Fractionation of isotopes among states, phases, and biological organic matter is driven by differences in the energy stored in bonds. Thus, patterns of isotopic abundances between substances can reflect these differences, but their combination with contextual data substantially strengthens the veracity of isotopic patterns as biosignatures. Two major elemental systems are described below.
5.3.2.1. Carbon isotopes
One example of a biological isotope fractionation process is the fixation of inorganic carbon by autotrophs. In these pathways, CO2 containing the lighter isotope of carbon, 12C, is energetically more easily manipulated than CO2 containing 13C, resulting in enrichment in 12C in the resulting fixed organic carbon. The utility of stable isotope fractionation patterns as a biosignature depends on the isotopic composition of both the putative biological material and its starting material. However, some abiotic chemical processes produce isotopic fractionations similar with biological ones and biogenicity is often hotly debated on both sides, for example, see the disagreement over interpretation of iron isotopes in the ancient rock record in Guildbaud et al. (2011) and Czaja et al. (2012). Environmental context supports the observed fractionation as abiotic if mineralogical and geochemical processes alone can explain the observed signatures. Alternatively, the presence of complex organic compounds and metabolic products might suggest biological element cycling with isotopic fractionation. Patterns of isotopic abundances, among individual types or classes of biomolecules, can be very compelling as biosignatures (e.g., Hayes, 2001).
5.3.2.2. Sulfate isotope signatures
Transition from abiotic to biotic: In early Earth history before the rise of abundant atmospheric O2, abiotic photochemistry of S-species derived from volcanic outgassing was a primary driver for cycling surface sulfur. These gas-phase photochemical reactions produce mass-independent sulfur isotope fractionation that can be detected in sulfides older than 2.4 Ga. There is evidence for microbially mediated sulfur and sulfate reduction in the Paleoarchean (Shen et al., 2001; Philippot et al., 2007; Wacey et al., 2010). As atmospheric O2 levels increased during the early Paleoproterozoic due to the rise of oxygenic photosynthesis, evaporitic sulfates accumulated in the sedimentary rock record, along with isotopic evidence for further biologically mediated sulfate reduction beginning around 2.7–2.5 Ga (Canfield and Raiswell, 1999).
The effects of biology on S cycling are also intertwined with other direct and indirect biological processes. Most notably, the rise of biogenic atmospheric O2 significantly altered the photochemical processes that affect S exhaled by volcanism, and this has left a strong isotopic fractionation signal in the geological record (Farquhar et al., 2000). By the time of the GOE, biology and oxidative weathering, both of which exhibit mass-dependent fractionation, had come to dominate S cycling.
Uncertainties persist when isotopes are invoked as a single line of evidence for a biosignature, especially for samples from the early Archean (3.7–3.8 Ga). For example, δ13C values of graphite in a highly metamorphosed sedimentary rock are not a reliable indicator of biogenicity because there are nonbiological processes that can produce graphite having δ13C values that are more negative, relative to coexisting carbonates, similar to the pattern that is observed for organic products of biological metabolism (e.g., Ray, 2009). Hence, the δ13C of graphite should be regarded as a consistent but insufficient criterion for biogenicity. This criterion partially explains continuing controversies about the earliest Archean biosignatures. However, in the case of younger (3.47 Ga) less metamorphosed rocks with carbon (particulate kerogen), isotopic biosignatures are less controversial (Schopf et al., 2018).
6. Summary and Recommendations
The concept of “biosignatures” encompasses a suite of continuous phenomena with end members of life versus non-life for different features and parameters, many of which can act synergistically or antithetically with each other producing a great deal of complexity. All interpretations are further complicated by the fact that there are many overlaps between biotic and abiotic signals in the rock record when studies focus on individual signatures or even just a few signatures taken together. There is the additional complication that biotic versus abiotic features may appear very similar or even identical under some circumstances and may require several lines of evidence to determine their true origins. The field of astrobiology and the search for extraterrestrial life are heavily biased by our singular terrestrial perspective. Current knowledge of biosignatures is fundamentally based on three classifications of expressions of life: substances, objects, and patterns, but there may be additional dimensions that require consideration. Given these daunting challenges for biosignature identification, we discuss three approaches for future research in astrobiology.
6.1. Scales and context
There is a fundamental need to understand biosignatures in their geologic context and across multiple spatial and temporal scales. It is important to look at the whole picture, not just the individual part or sum of parts of a system. Spatial scaling and the distribution relationships can determine the mappable extent of biosignatures. There is a tendency in science to focus examination of biosignatures at one particular scale, particularly when searching for an analogue to a specific terrestrial example. However, the complexity of biosignatures requires an integrative approach encompassing tools and methodologies as well as the context for each scale and type of data. Both classical and newer analytical methods will need to be used, as well as approaches that can span traditional research boundaries.
Astrobiology as a discipline needs more analog studies at multiple nested scales, with clear context for each example. Spatial and temporal scales (both modern and ancient) are also important. For the best understanding of how biosignatures form and are preserved, we must account for macroscale characteristics all the way down to sub-microscale ones, with lateral as well as vertical distribution and characterization.
Future studies should clearly establish the context and conduct examinations across multiple scales, wherever possible. Potential biosignatures can be ambiguous yet intellectually seductive, and thus, many independent lines of evidence and tools are required to keep us honest in our inquiries (Boston et al., 2001). Quantification of biosignature metrics whenever at all possible can help establish detection confidence and allow for more confident assignment of “weightings” or ranked prioritizations that can be used in the search for life.
6.2. Community-accepted standards
How can one determine whether a planetary phenomenon is the result of life? A set of community-accepted standards of abiological versus biological characteristics is needed. There is likely little left in Earth's near-surface and subsurface environment that has not been altered in one way or another by biological processes, and thus, we may need to rely heavily on controlled abiotic experiments to provide “clean” determinations. A set of standards to be used globally across multiple instrument platforms would be helpful.
Simulation of abiotic conditions in controlled experiments is one way to attempt to define the abiotic “end member.” Samples containing known biosignatures can be experimentally altered and aged (e.g., by placing them under temperature and pressure conditions simulating diagenesis). It remains unknown whether such experiments can be scaled directly to natural systems. For example, it is notoriously difficult to reproduce the processes of petroleum and coal formation in the laboratory; these processes appear to require long periods of heating at relatively low temperatures. It is not always possible to substitute higher intensities of temperature or pressure for time, and there may be many unknown feedbacks occurring in natural systems or even understood feedbacks that cannot be produced in the laboratory.
More community-accepted standards of abiosignatures or abiotic features would be tremendously helpful, just as analytical standards can be critical to provide consistency in measurements across multiple instrument platforms. Such standards are still being developed and debated by scientists. The development and use of mathematical models and statistical methods to examine probabilities for accurately identifying biotic processes over abiotic ones may have application at multiple scales. Such mathematical models and patterns could also be used together with traditional, physical data for a more robust approach to biosignature identification.
6.3. Data management
The power of cybertechnology offers opportunities at every stage and level of the study of astrobiology, the search for biosignatures, and the attempts to understand the origins of life. A universal data management system could handle appropriate curation and cross-calibration of standards. More open data and sample sharing would facilitate interdisciplinary and international collaboration and the use of data analytics to identify the best pathways forward.
Astrobiology research can be high risk because there are so many overlapping variables to consider, because there is often such lack of consensus as to what constitutes a biosignature, and because it requires so many different disciplinary approaches to effectively answer the outstanding questions. However, more interdisciplinary collaboration among scientists studying astrobiology, and support for open data sharing and data management systems, can be effective in bridging communication and overcoming disciplinary barriers, transforming the science and the potential for new discoveries across multiple temporal and spatial scales (e.g., Park Boush et al., 2017). Although the infrastructure to accomplish the goals that we set forth here may require considerable investment of effort and funding, it could provide multiple benefits for the astrobiology community, including access to user-friendly tools for data mining of existing data sets that would also be engaging to citizen scientists, students, and educators.
Coupled with the way science is conducted, there are additional challenges with biosignatures that are physical objects, namely their curation, an area that seems to have fallen out of favor in modern times but which is critical to our knowledge base. Importantly, such physical collections must be collated with all their associated information (e.g., metadata). A data management system for biosignature and abiosignature samples would further facilitate interdisciplinary collaboration in that working on and thinking about a common body of samples could bring great intellectual power to bear on the challenges articulated here. Other paleobiology databases (e.g., paleobiodb.org) and stratigraphic databases (e.g., macrostrat.org) are helping geoscientists sort out important temporal and spatial relationships in searchable, aggregator platforms.
Data management systems would facilitate new exploration by using “big data,” especially for a field such as astrobiology that relies on interdisciplinary data. Complex patterns and relationships could be discoverable by leveraging the cyber infrastructure and technology currently available but underutilized. The network of relationships between minerals and biology (Hazen et al., 2008) is an example of a major discovery facilitated by computational collaborations.
An integrated GIS (Geographic Information System) framework of databases that can be interrogated with a search engine, or having multiple layers of science information, including spatial locations, is a long way off. However, the vision of how it can benefit the community has to start now, even though this is a long-term investment.
In the cosmic perspective, terrestrial bias still makes it difficult to fully understand what an abiotic, habitable planet would look like. Yet at the same time, we are at an exciting cusp, poised to move forward with an enthusiastic and interdisciplinary community of scientists and an expanding toolbox of methods to uncover more details about both biosignatures and abiosignatures. An integration of studies across scales and contexts, using quantitative methods involving agreed-upon standards, and making use of cyberinfrastructure, will collectively guide future explorations for the origins of Earth life and the potential existence of biosignatures in extraterrestrial examples.
Acknowledgments
This article is an outgrowth of an international workshop sponsored by ELSI (Earth-Life Science Institute) EON (ELSI Origins Network) held at the Tokyo Institute of Technology, Japan. The EON is supported by a grant from the John Templeton Foundation. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation. The authors thank the group of participants in the workshop who contributed to the development of the ideas and concepts presented here. In addition to the listed authors, they gratefully acknowledge the workshop participation of N. Aubert-Kato, A. Baccouche, N. Bapat, Y. Fuji, C. Giri, S. Ida, J. Kirschvink, I. Mamajanov, A. Panikar, M. Sharma, Y. Ueno, S. Zang, and M. Voytek. The authors gratefully acknowledge two anonymous reviewers who provided helpful comments and suggestions to improve this article.
Abbreviations Used
- ELSI
Earth-Life Science Institute
- EON
ELSI Origins Network
- FTT
Fischer–Tropsch Type
- GIS
Geographic Information System
- GOE
Great Oxidation Event
- LNRE
Large Number of Rare Events
- MISS
microbially induced sedimentary structures
- ROS
reactive oxygen species
- SEM
scanning electron microscopy
Author Disclosure Statement
No competing financial interests exist.
Associate Editor: Lewis Dartnell
References
- Ader M., Thomazo C., Sansjofre P., Busigny V., Papineau D., Laffont R., Cartigny P., and Halverson G.P. (2016) Interpretation of the nitrogen isotopic composition of Precambrian sedimentary rocks: assumptions and perspectives. Chem Geol 429:93–110 [Google Scholar]
- Allison P.A. and Bottjer D.J. (2011) Taphonomy: process and bias through time, 1. In Topics in Geobiology 32, edited by P.A. Allison and D.J. Bottjer, Springer, New York, 600 pp [Google Scholar]
- Allwood A.C. (2016) Geology: evidence of life in Earth's oldest rocks. Nature 537:500–501 [DOI] [PubMed] [Google Scholar]
- Allwood A.C., Walter M.R., Kamber B.S., Marshall C.P., and Burch I.W. (2006) Stromatolite reef from the early Archaean era of Australia. Nature 441:714–718 [DOI] [PubMed] [Google Scholar]
- Allwood A.C., Rosing M.T., Flannery D.T., Hurowitz J.A., and Heirwegh C.M. (2018) Reassessing evidence of life in 3,700-million-year-old rocks of Greenland. Nature 563:241–244 [DOI] [PubMed] [Google Scholar]
- Ambrogelly A., Palioura S., and Söll D. (2007) Natural expansion of the genetic code. Nat Chem Biol 3:29–35 [DOI] [PubMed] [Google Scholar]
- Amend J.P. and Shock E.L. (1998) Energetics of amino acid synthesis in hydrothermal ecosystems. Science 281:1659–1662 [DOI] [PubMed] [Google Scholar]
- Anand R.R. and Verrall M. (2011) Biological origin of minerals in pisoliths in the Darling Range of Western Australia. Aust J Earth Sci 58:823–833 [Google Scholar]
- Aubrey A.D., Cleaves H.J., and Bada J.L. (2009) The role of submarine hydrothermal systems in the synthesis of amino acids. Orig Life Evol Biosph 39:91–108 [DOI] [PubMed] [Google Scholar]
- Awramik S.M. (1992) The history and significance of stromatolites. In Early Organic Evolution: Implications for Mineral and Energy Resources, edited by Schidlowski M., Golubic S., Kimberley M.M., McKirdy D.M. and Trudinger P.A., Springer-Verlag, Berlin, pp 435–449 [Google Scholar]
- Awramik S.M. and Buchheim H.P. (2015) Giant stromatolites of the Eocene Green River Formation (Colorado, USA). Geology 43:691–694 [Google Scholar]
- Awramik S.M. and Grey K. (2005) Stromatolites: biogenicity, biosignatures, and bioconfusion. In Astrobiology and Planetary Missions, edited by Richard B. Hoover, Gilbert V. Levin, Alexei Y. Rozanov, and G. Randall Gladstone, Proceedings of SPIE, Bellingham, WA (USA), 5906: pp. 59060P-1-9 [Google Scholar]
- Bada J.L., Wang X.S., and Hamilton H. (1999) Preservation of key biomolecules in the fossil record: current knowledge and future challenges. Philos Trans R Soc B Biol Sci 354:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P. (1999) The Self-Made Tapestry: Pattern Formation in Nature, Oxford University Press, New York [Google Scholar]
- Barghoorn E.S. and Tyler S.A. (1965) Microorganisms from the Gunflint chert. Science 147:563–577 [DOI] [PubMed] [Google Scholar]
- Behrensmeyer A.K., Kidwell S.M., and Gastaldo R.A. (2000) Taphonomy and paleobiology. In Deep Time: Paleobiology's Perspective, edited by Erwin D.H. and Wing S.L., The Paleontological Society, Lawrence, KS, pp 103–147 [Google Scholar]
- Bennett P.C., Rogers J.R., Choi W.J., and Hiebert F.K. (2001) Silicates, silicate weathering, and microbial ecology. Geomicrobiol J 18:3–19 [Google Scholar]
- Berelson W.M., Corsetti F.A., Pepe-Ranney C., Hammond D.E., Beaumont W., and Spear J.R. (2011) Hot spring siliceous stromatolites from Yellowstone National Park: assessing growth rate and laminae formation. Geobiology 9:411–424 [DOI] [PubMed] [Google Scholar]
- Bernard S. and Papineau D. (2014) Graphitic carbons and biosignatures. Elements 10:435–440 [Google Scholar]
- Berner R.A. (1968) Calcium carbonate concretions formed by the decomposition of organic matter. Science 159:195–197 [DOI] [PubMed] [Google Scholar]
- Bernstein M.P., Mattioda A.L., Sandford S.A., and Hudgins D.M. (2005) Laboratory infrared spectra of polycyclic aromatic nitrogen heterocycles: quinoline and phenanthridine in solid argon and H2O. Astrophys J 626:909–918 [Google Scholar]
- Bertrand-Sarfati J. and Moussine-Pouchkine A. (1988) Is cratonic sedimentation consistent with available models? An example from the Upper Proterozoic of the West African craton. Sediment Geol 58:255–276 [Google Scholar]
- Blackmond D.G. (2010) The origin of biological homochirality. Cold Spring Harb Perspect Biol 2:a002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston P.J., Ivanov M.V., and McKay C.P. (1992) On the possibility of chemosynthetic ecosystems in subsurface habitats on Mars. Icarus 95:300–308 [DOI] [PubMed] [Google Scholar]
- Boston P.J., Spilde M.N., Northup D.E., Melim L.A., Soroka D.S., Kleina L.G., Lavoie K.H., Hose L.D., Mallory L.M., Dahm C.N., Crossey L.J., and Schelble R.T. (2001) Cave biosignature suites: microbes, minerals and Mars. Astrobiology 1:25–55 [DOI] [PubMed] [Google Scholar]
- Boston P.J., Spilde M.N., Northup D.E., Curry M.C., Melim L.A., and Rosales-Lagarde L. (2009) Microorganisms as speleogenetic agents: geochemical diversity but geomicrobial unity [Special Paper 1:51–57]. In Hypogene Speleogenesis and Karst Hydrology of Artesian Basins, edited by A.B. Klimchouk and D.C. Ford, Ukrainian Institute of Speleology and Karstology, Simferopol, p 280 [Google Scholar]
- Botta O., Bada J.L., Gomez-Elvira J., Javaux E., Selsis F., and Summons R. (2008) Strategies of life detection: summary and outlook. Space Sci Rev 135:371–380 [Google Scholar]
- Böttcher T. (2018) From molecules to life: quantifying the complexity of chemical and biological systems in the Universe. J Mol Evol 86:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower D.M. (2011) Micro Raman spectroscopic investigations of laminae associated mineral assemblages in 2.9 Ga sandstones of the Pongola Supergroup, South Africa. J Raman Spectrosc 42:1626–1633 [Google Scholar]
- Bower D.M., Hummer D.R., Kyono A., and Steele A. (2015) The co-evolution of Fe-, Ti-oxides and other microbially induced mineral precipitates in sandy sediments: understanding the role of cyanobacteria in weathering and early diagenesis. J Sediment Geol 85:1213–1227 [Google Scholar]
- Bower D.M., Steele A., Fries M.D., Green O.D., and Lindsay J.F. (2016) Raman imaging spectroscopy of a putative microfossil from the ∼3.46 Ga Apex Chert: insights from quartz crystal orientation. Astrobiology 16:169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower D.M., Hummer D.R., and Steele A. (2017) An experimental look at the taphonomy of cyanobacterial mats in siliciclastic sediments. Palaios 32:1–14 [Google Scholar]
- Brangwynne C.P., Tompa P., and Pappu R.V. (2015) Polymer physics of intracellular phase transitions. Nat Phys 11:899 [Google Scholar]
- Burne R.V. and Moore L.S. (1987) Microbialites: organosedimentary deposits of benthic microbial communities. Palaios 2:241–254 [Google Scholar]
- Burnet J. (1930) Early Greek Philosophy, A. and C. Black, Ltd., London, 375 p [Google Scholar]
- Cadée G.C. (1991) The history of taphonomy. In The Processes of Fossilization, edited by Donovan S.K., Columbia University Press, New York, pp 3–21 [Google Scholar]
- Cady S.L. and Noffke N. (2009) Geobiology: evidence for early life on Earth and the search for life on other planets. GSA Today 19:4–10 [Google Scholar]
- Cady S.L., Farmer J.D., Grotzinger J.P., Schopf W., and Steele A. (2003) Morphological biosignatures and the search for life on Mars. Astrobiology 3:351–368 [DOI] [PubMed] [Google Scholar]
- Callahan M.P., Smith K.E., Cleaves H.J., Ruzicka J., Stern J.C., Glavin D.P., House C.H., and Dworkin J.P. (2011) Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc Natl Acad Sci U S A 108:13995–13998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W.M., Shoffner J.D., Johnson J.R., Knoll A.H., Pocock J.M., Squyres S.W., Weitz C.M., Arvidson R.E., Bell J.F., Christensen P.R., and de Souza P.A. (2008) Hematite spherules at Meridiani: results from MI, Mini-TES, and Pancam. J Geophys Res Planets 113: E12S37, doi: 10.1029/2007JE003048 [DOI] [Google Scholar]
- Campbell A., Pham B., and Tian Y.-C. (2004) Mining ecological data with cellular automata. In International Conference on Cellular Automata, Springer Berlin Heidelberg, Berlin, Heidelberg, pp 474–483 [Google Scholar]
- Canfield D.E. and Raiswell R. (1999) The evolution of the sulfur cycle. Am J Sci 299:697–723 [Google Scholar]
- Carr M.H. (1979) Formation of Martian flood features by release of water from confined aquifers. J Geophys Res 84:2995–3007 [Google Scholar]
- Castanier S., Métayer-Levrel G.L., and Perthuisot J.P. (2000) Bacterial roles in the precipitation of carbonate minerals. In Microbial Sediments, edited by Riding R.E.and S.M. Awramik, Springer, Berlin, Heidelberg, pp 32–39 [Google Scholar]
- Catling D.C., Krissansen-Totton J., Kiang N.Y., Crisp D., Robinson T.D., DasSarma S., Rushby A.J., Del Genio A., Bains W., and Domagal-Goldman S. (2018) Exoplanet biosignatures: a framework for their assessment. Astrobiology 18:709–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.A., Beitler B., Parry W.T, Ormö J., and Komatsu G. (2004) A possible terrestrial analogue for hematite concretions on Mars. Nature 429:731–734 [DOI] [PubMed] [Google Scholar]
- Chan M.A., Beitler Bowen B., Parry W.T., Ormö J., and Komatsu G. (2005) Red rock and red planet diagenesis: comparisons of Earth and Mars concretions. GSA Today 15:4–10 [Google Scholar]
- Chan M.A., Potter S.L., Bowen B.B., Petersen E.U., Parry W.T., Bowman J.R., Barge L., and Seiler W. (2012) Characteristics of terrestrial ferric oxide concretions and implications for Mars. In Sedimentary Geology of Mars, edited by Grotzinger J.and R. Milliken, Society for Sedimentary Geology, Tulsa, Oklahoma, Special SEPM Publication No. 102, pp 253–270 [Google Scholar]
- Chivian D., Brodie E.L., Alm E.J., Culley D.E., Dehal P.S., DeSantis T.Z., Gihring T.M., Lapidus A., Lin L.H., Lowry S.R., Moser D.P., Richardson P.M., Southam G., Wanger G., Pratt L.M., Andersen G.L., Hazen T.C., Brockman F.J., Arkin A.P., and Onstott T.C. (2008) Environmental genomics reveals a single-species ecosystem deep within earth. Science 322:275–278 [DOI] [PubMed] [Google Scholar]
- Cleaves H.J. (2012) Prebiotic chemistry: what we know, what we don't. Evol Educ Outreach 5:342 [Google Scholar]
- Cleaves H.J. and Chalmers J.H. (2004) Extremophiles may be irrelevant to the origin of life. Astrobiology 4:1–9 [DOI] [PubMed] [Google Scholar]
- Cleaves H.J., Meringer M., and Goodwin J. (2015) 227 Views of RNA: is RNA unique in its chemical isomer space? Astrobiology 15:538–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M.L. (1993) Microbial processes: controls on the shape and composition of carbonate concretions. Mar Geol 113:127–140 [Google Scholar]
- Colman D.R., Poudel S., Stamps B.W., Boyd E.S., and Spear J.R. (2017) The deep hot biosphere: twenty-five years of retrospection. Proc Natl Acad Sci U S A 114:6895–6903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope J.S., Corney D., Clark J.Y., Remagnino P., and Wilkin P. (2012) Plant species identification using digital morphometrics: a review. Expert Syst Appl 39:7562–7573 [Google Scholar]
- Córdova A., Engqvist M., Ibrahem I., Casas J., and Sundén H. (2005) Plausible origins of homochirality in the amino acid catalyzed neogenesis of carbohydrates. Chem Commun (Camb) 15:2047–2049 [DOI] [PubMed] [Google Scholar]
- Cornet T., Cordier D., Bahers T.L., Bourgeois O., Fleurant C., Mouélic S.L., and Altobelli N. (2015) Dissolution on Titan and on Earth: toward the age of Titan's karstic landscapes. J Geophys Res Planets 120:1044–1074 [Google Scholar]
- Cronin J.R. and Pizzarello S. (1997) Enantiomeric excesses in meteoritic amino acids. Science 275:951–955 [DOI] [PubMed] [Google Scholar]
- Crosby C.H. and Bailey J.V. (2018) Experimental precipitation of apatite pseudofossils resembling fossil embryos. Geobiology 16:80–87 [DOI] [PubMed] [Google Scholar]
- Czaja A.D., Johnson C.M., Yamaguchi K.E., and Beard B.L. (2012) Comment on Abiotic pyrite formation produces a large Fe isotope fractionation. Science 335:538. [DOI] [PubMed] [Google Scholar]
- Danger G., Plasson R., and Pascal R. (2012) Pathways for the formation and evolution of peptides in prebiotic environments. Chem Soc Rev 41:5416–5429 [DOI] [PubMed] [Google Scholar]
- Dart R.A. (1949) The predatory implemental technique of the australopithecines. Am J Phys Anthropol 7:1–16 [DOI] [PubMed] [Google Scholar]
- Dartnell L.R., Page K., Jorge-Villar S.E., Wright G., Munshi T., Scowen I.J., Ward J.M., and Edwards H.G.M. (2012) Destruction of Raman biosignatures by ionising radiation and the implications for life detection on Mars. Anal Bioanal Chem 403:131–144 [DOI] [PubMed] [Google Scholar]
- Davies N.S., Liu A.G., Gibling M.R., and Miller R.F. (2016) Resolving MISS conceptions and misconceptions: a geological approach to sedimentary surface textures generated by microbial and abiotic processes. Earth Sci Rev 154:210–246 [Google Scholar]
- Davies P.C.W., Benner S.A., Cleland C.E., Lineweaver C.H., McKay C.P., and Wolfe-Simon F. (2009) Signatures of a shadow biosphere. Astrobiology 9:241–249 [DOI] [PubMed] [Google Scholar]
- Des Marais D.J. (2003) Biogeochemistry of hypersaline microbial mats illustrates the dynamics of modern microbial ecosystems and the early evolution of the biosphere. Biol Bull 204:160–167 [DOI] [PubMed] [Google Scholar]
- Des Marais D.J., Harwit M.O., Jucks K.W., Kasting J.F, Lin D.N., Lunine J.I., Schneider J., Seager S., Traub W.A., and Woolf N.J. (2002) Remote sensing of planetary properties and biosignatures on extrasolar terrestrial planets. Astrobiology 2:153–181 [DOI] [PubMed] [Google Scholar]
- Des Marais D.J., Nuth J.A., III, Allamandola L.J., Boss A.P., Farmer J.D., Hoehler T.M., Jakosky B.M., Meadows V.S., Pohorille A., and Spormann A.M. (2008) The NASA Astrobiology Roadmap. Astrobiology 8:715–730 [DOI] [PubMed] [Google Scholar]
- Doane T.A. (2017) A survey of photogeochemistry. Geochem Trans 18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd M.S., Papineau D., Grenne T., Slack J.F., Rittner M., Pirajno F., O'Neil J., and Little C.T.S. (2017) Evidence for early life in Earth's oldest hydrothermal vent precipitates. Nature 543:60–64 [DOI] [PubMed] [Google Scholar]
- Dorn E.D., Nealson K.H., and Adami C. (2011) Monomer abundance distribution patterns as a universal biosignature: examples from terrestrial and digital life. J Mol Evol 72:283–295 [DOI] [PubMed] [Google Scholar]
- Duesterberg C.K., Mylon S.E., and Waite T.D. (2008) pH effects on iron-catalyzed oxidation using Fenton's reagent. Environ Sci Technol 42:8522–8527 [DOI] [PubMed] [Google Scholar]
- Dunkerley D.L. (1997) Banded vegetation: development under uniform rainfall from a simple cellular automaton model. Plant Ecol 129:103–111 [Google Scholar]
- Efremov J.A. (1940) Taphonomy: new branch of paleontology. Pan Am Geol 74:81–93 [Google Scholar]
- Ehlmann B.L. and Mustard J.F. (2012) An in situ record of major environmental transitions on early Mars at Northeast Syrtis Major. Geophys Res Lett 39: L11202 (7 pages), doi: 10.1029/2012GL051594 [DOI] [Google Scholar]
- Emmeche C. (1997) Aspects of complexity in life and science. Philosophica 59:41–68 [Google Scholar]
- Exoplanet Science Strategy. (2018) National Academies of Sciences, Engineering, and Medicine, Washington, DC: The National Academies Press. 10.17226/25187 [DOI] [Google Scholar]
- Falkowski P.G. (1997) Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387:272–275 [Google Scholar]
- Farmer J.D. (1995) Mars exopaleontology. Palaios 10:197–198 [PubMed] [Google Scholar]
- Farmer J.D. (1999a) Taphonomic modes in microbial fossilization. In Size Limits of Very Small Microorganisms: Proceedings of a Workshop, Space Studies Board, National Research Council, National Academies Press, Washington, DC, pp 94–102 [PubMed] [Google Scholar]
- Farmer J.D. (1999b) Environmental and mineralogical controls on fossilization: key elements in a strategy for Mars exopaleontology. In Fifth International Conference on Mars, Pasadena, CA [Google Scholar]
- Farmer J.D. and Des Marais D. (1999) Exploring for a record of ancient Martian life. J Geophys Res 104:26977–26995 [DOI] [PubMed] [Google Scholar]
- Farquhar J., Bao H., and Thiemens M. (2000) Atmospheric influence of Earth's earliest sulfur cycle. Science 289:756–758 [DOI] [PubMed] [Google Scholar]
- Fayek M.J., Utsunomiya S., Pfiffner S.M., Anovitz L.M., White D.C., Riciputi L.R., Ewing R.C., and Stadermann F.J. (2005) Nanoscale chemical and isotopic characterization of Geobacter sulfurreducens surfaces and bio-precipitated uranium minerals. Can Mineral 43:1631–1641 [Google Scholar]
- Fernández-Remolar D.C., Preston L.J., Sanchez-Roman M., Izawa M.R., Huang L., Southam G., Banerjee N.R., Osinski G.R., Flemming R., Gómez-Ortíz D., and Ballesteros O.P. (2012) Carbonate precipitation under bulk acidic conditions as a potential biosignature for searching life on Mars. Earth Planet Sci Lett 351:13–26 [Google Scholar]
- Flügel E. (2010) Microfacies of Carbonate Rocks: Analysis, Interpretation, and Application, 2nd ed., Springer Verlag, New York, 984 pp [Google Scholar]
- Foster I.S., King P.L., Hyde B.S., and Southam G. (2010) Characterization of halophiles in natural MgSO4 salts and laboratory enrichment samples: astrobiological implications for Mars. Planet Space Sci 58:599–615 [Google Scholar]
- Fox S. and Strasdeit H. (2017) Inhabited or uninhabited? Pitfalls in the interpretation of possible chemical signatures of extraterrestrial life. Front Microbiol 8:1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fralick P. and Riding R. (2015) Steep Rock Lake: sedimentology and geochemistry of an Archean carbonate platform. Earth Sci Rev 151:132–175 [Google Scholar]
- Grosch E.G. and McLoughlin N. (2014) Reassessing the biogenicity of Earth's oldest trace fossil with implications for biosignatures in the search for early life. Proc Natl Acad Sci U S A 111:8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch E.G. and McLoughlin N. (2015) Questioning the biogenicity of titanite mineral trace fossils in Archean pillow lavas. Proc Natl Acad Sci U S A 112:E3090–E3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzinger J.P. and Knoll A.H. (1999) Stromatolites in Precambrian carbonates: evolutionary mileposts or environmental dipsticks? Annu Rev Earth Planet Sci 27:313–358 [DOI] [PubMed] [Google Scholar]
- Grotzinger J.P., Arvidson R.E., Bell J.F., III Calvin, W., Clark B.C., Fike D.A., Golombek M., Greeley R., Haldemann A., Herkenhoff K.E., Jolliff B.L., Knoll A.H., Malin M., McLennan S.M., Parker T., Soderblom L., Sohl-Dickstein J.N., Squyres S.W., Tosca N.J., and Watters W.A. (2005) Stratigraphy and sedimentology of a dry to wet eolian depositional system, Burns formation, Meridiani Planum, Mars. Earth Planet Sci Lett 240:11–72 [Google Scholar]
- Guildbaud R., Butler I.B., and Ellam R.M. (2011) Abiotic pyrite formation produces a large Fe isotope fractionation. Science 332:1548–1551 [DOI] [PubMed] [Google Scholar]
- Gurikov P., Kolnoochenko A., Golubchikov M., Menshutina N., and Smirnova I. (2016) A synchronous cellular automaton model of mass transport in porous media. Comput Chem Eng 84:446–457 [Google Scholar]
- Habisreutinger S.N., Schmidt-Mende L., and Stolarczyk J.K. (2013) Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew Chem Int Ed Engl 52:7372–7408 [DOI] [PubMed] [Google Scholar]
- Harazim D., Callow R.H., and McIlroy D. (2013) Microbial mats implicated in the generation of intrastratal shrinkage (“synaeresis”) cracks. Sedimentology 6:1621–1638 [Google Scholar]
- Hartman R.J., Kanning E.W., and Klee F.G. (1934) Liesegang phenomenon applied to banded malachite. J Chem Educ 11:346 [Google Scholar]
- Hayes J.M. (2001) Fractionation of carbon and hydrogen isotopes in biosynthetic processes. Rev Mineral Geochem 43:225–277 [Google Scholar]
- Hays L.E., Graham H.V., Des Marais D.J., Hausrath E.M., Horgan B., McCollom T.M., Parenteau M.N., Potter-McIntyre S.L., Williams A.J., and Lynch K.L. (2017) Biosignature preservation and detection in Mars analog environments. Astrobiology 17:363–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen R.M. (2013) Paleomineralogy of the Hadean Eon: a preliminary list. Am J Sci 313:807–843 [Google Scholar]
- Hazen R.M. and Ferry J.M. (2010) Mineral evolution: mineralogy in the fourth dimension. Elements 6:9–12 [Google Scholar]
- Hazen R.M., Griffin P.L., Carothers J.M., and Szostak J.W. (2007) Functional information and the emergence of biocomplexity. Proc Natl Acad Sci U S A 104:8574–8581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen R.M., Papineau D., Bleeker W., Downs R.T., Ferry J.M., McCoy T.L., Sverjensky D.A., and Yang H. (2008) Mineral evolution. Am Mineral 93:1693–1720 [Google Scholar]
- Hazen R.M., Ewing R.C., and Sverjensky D.A. (2009) Evolution of uranium and thorium minerals. Am Mineral 94:1293–1311 [Google Scholar]
- Hazen R.M., Downs R.T., Jones A.P., and Kah L. (2013) The mineralogy and crystal chemistry of carbon. In Carbon in Earth, edited by Hazen R.M., Jones A., and Baross J., Mineralogical Society of America, Washington, DC, pp 7–46 [Google Scholar]
- Hazen R.M., Grew E.S., Downs R.T., Golden J., and Hystad G. (2015) Mineral ecology: chance and necessity in the mineral diversity of terrestrial planets. Can Mineral 53:295–323 [Google Scholar]
- Hennet R.J.-C., Holm N.G., and Engel M.H. (1992) Abiotic synthesis of amino acids under hydrothermal conditions and the origin of life: a perpetual phenomenon? Naturwissenschaften 79:361–365 [DOI] [PubMed] [Google Scholar]
- Higgs P.G. and Pudritz R.E. (2009) A thermodynamic basis for prebiotic amino acid synthesis and the nature of the first genetic code. Astrobiology 9:483–490 [DOI] [PubMed] [Google Scholar]
- HilleRisLambers R., Rietkerk M., van den Bosch F., Prins H.H.T., and de Kroon H. (2001) Vegetation pattern formation in semi-arid grazing systems. Ecology 82:50–62 [Google Scholar]
- Hofmann H.J., Grey K., Hickman A.H., and Thorpe R.I. (1999) Origin of 3.45 Ga coniform stromatolites in Warrawoona Group, Western Australia. Geol Soc Am Bull 111:1256–1262 [Google Scholar]
- Humphrey W., Dalke A., and Schulten K. (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38 [DOI] [PubMed] [Google Scholar]
- Hystad G., Downs R.T., and Hazen R.M. (2015) Mineral species frequency distribution conforms to a large number of rare events model: prediction of Earth's “missing” minerals. Math Geosci 47:647–661 [Google Scholar]
- Ilardo M., Meringer M., Freeland S., Rasulev B., and Cleaves H.J. (2015) Extraordinarily adaptive properties of the genetically encoded amino acids. Sci Rep 5:9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.S., Anslyn E.V., Graham H.V., Mahaffy P.R., and Ellington A.D. (2018) Fingerprinting non-terran biosignatures. Astrobiology 18:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley C. and Douglas T. (2012) Topological biosignatures: large-scale structure of chemical networks from biology and astrochemistry. Astrobiology 12:29–39 [DOI] [PubMed] [Google Scholar]
- Judson O.P. (2017) The energy expansions of evolution. Nat Ecol Evol 1:0138. [DOI] [PubMed] [Google Scholar]
- Juille H. and Pollack J.B. (1998) Coevolving the “ideal” trainer: application to the discovery of cellular automata rules. In Genetic Programming 1998: Proceedings of the Third Annual Conference, University of Wisconsin, Morgan Kaufmann, Ithaca, New York, pp 519–527 [Google Scholar]
- Kaandorp J.A. and Kübler J.E. (2001) The Algorithmic Beauty of Seaweeds, Sponges and Corals, Springer Science and Business Media, Berlin, p. 193 [Google Scholar]
- Kaplan D.R. and Hagemann W. (1991) The relationship of cell and organism in vascular plants. Bioscience 41:693–703 [Google Scholar]
- Kasting J.F. and Catling D. (2003) Evolution of a habitable planet. Annu Rev Astron Astrophys 41:429–463 [Google Scholar]
- Katoh T., Tajima K., and Suga H. (2017) Consecutive elongation of D-amino acids in translation. Cell Chem Biol 24:46–54 [DOI] [PubMed] [Google Scholar]
- Kim G.H., Klotchkova T.A., and Kang Y.M. (2001) Life without a cell membrane: regeneration of protoplasts from disintegrated cells of the marine green alga Bryopsis plumosa. J Cell Sci 114:2009–2014 [DOI] [PubMed] [Google Scholar]
- Kitadai N., Oonishi H., Umemoto K., Usui T., Fukushi K., and Nakashima S. (2017) Glycine polymerization on oxide minerals. Orig Life Evol Biosph 47:123–143 [DOI] [PubMed] [Google Scholar]
- Klausmeier C.A. (1999) Regular and irregular patterns in semiarid vegetation. Science 284:1826–1828 [DOI] [PubMed] [Google Scholar]
- Konhauser K.O., Phoenix V.R., Bottrell S.H., Adams D.G., and Head I.M. (2001) Microbial-silica interactions in modern hot spring sinter: possible analogues for Precambrian siliceous stromatolites. Sedimentology 48:415–435 [Google Scholar]
- Kring D.A. (2000) Impact events and their effect on the origin, evolution, and distribution of life. GSA Today 10:1–7 [Google Scholar]
- Krissansen-Totton J., Olson S., and Catling D.C. (2018) Disequilibrium biosignatures over Earth history and implications for detecting exoplanet life. Sci Adv 4:eaao5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara K., Tamura M., Shohda K.I., Toyota T., Suzuki K., and Sugawara T. (2011) Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat Chem 3:775–781 [DOI] [PubMed] [Google Scholar]
- Kuruma Y. (2015) Creation of simple biochemical systems to study early cellular life. Orig Life Evol Biosph 45:359–360 [DOI] [PubMed] [Google Scholar]
- Kvenvolden K., Lawless J., Pering K., Peterson E., Flores J., Ponnamperuma C., Kaplan I.R., and Moore C. (1970) Evidence for extraterrestrial amino acids and hydrocarbons in Murchison meteorite. Nature 228:923–926 [DOI] [PubMed] [Google Scholar]
- Lai M.C. and Cavanagh D. (1997) The molecular biology of coronaviruses. Adv Virus Res 48:1–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneuville M., Kameya M., and Cleaves H.J. (2018) Earth without life: a systems model of a global abiotic nitrogen cycle. Astrobiology 18:897–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier W.P. (1989) Interstitial and peloid microfossils from the 2.0 Ga Gunflint Formation: implications for the paleoecology of the Gunflint stromatolites. Precambrian Res 45:291–318 [Google Scholar]
- Lau G.E., Cosmidis J., Grasby S.E., Trivedi C.B., Spear J.R., and Templeton A.S. (2017) Low-temperature formation and stabilization of rare allotropes of cyclooctasulfur (β-S8 and γ-S8) in the presence of organic carbon at a sulfur-rich glacial site in the Canadian High Arctic. Geochim Cosmochim Acta 200:218–231 [Google Scholar]
- Lawrence D.R. (1968) Taphonomy and information losses in fossil communities. Geol Soc Am Bull 79:1315–1330 [Google Scholar]
- Lee S.-J., Browne K.M., and Golubic S. (2000) On stromatolite lamination. In Microbial Sediments, edited by Riding R. and Awramik S.M., Springer, Berlin, pp 16–24 [Google Scholar]
- Leman L., Orgel L., and Ghadiri M.R. (2004) Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306:283–286 [DOI] [PubMed] [Google Scholar]
- Leshin L.A., Mahaffy P.R., Webster C.R., Cabane M., Coll P., Conrad P.G., Archer P.D., Atreya S.K., Brunner A.E., Buch A., and Eigenbrode J.L. (2013) Volatile, isotope, and organic analysis of martian fines with the Mars Curiosity rover. Science 341:1238937. [DOI] [PubMed] [Google Scholar]
- Levett A., Gagen E., Shuster J., Rintoul L., Tobin M., Vongsvivut J., Bambery K., Vasconcelos P., and Southam G. (2016) Evidence of biogeochemical processes in iron duricrust formation. J South Am Earth Sci 71:131–142 [Google Scholar]
- Lindsay J.F. and Brasier M.D. (2002) Did global tectonics drive early biosphere evolution? Carbon isotope record from 2.6 to 1.9 Ga carbonates of Western Australian basins. Precambrian Res 114:1–34 [Google Scholar]
- Long P.E. (2008) Field-scale bioreduction of U(VI) to U(IV) in an alluvial aquifer: evidence for microbially mediated precipitation of uranium under both natural and biostimulated conditions (abstract). Geochim Cosmochim Acta 72:A560 [Google Scholar]
- Lovelock J.E. and Giffin C.E. (1969) Planetary Atmospheres: compositional and other changes associated with the presence of life. Adv Astronaut Sci 25:179–193 [Google Scholar]
- Lovelock J.E. and Margulis L. (1974) Atmospheric homeostasis by and for the biosphere: the Gaia hypothesis. Tellus 26:2–10 [Google Scholar]
- Lovely D.R., Phillips E.J.P., Gorby Y.A., and Landa E.R. (1991) Microbial reduction of uranium. Nature 350:413–416 [Google Scholar]
- Mariotti G., Pruss S., Perron J., and Bosak T. (2014) Microbial shaping of sedimentary wrinkle structures. Nat Geosci 7:736–740 [Google Scholar]
- Mata S.A., Harwood C.L., Corsetti F.A., Stork N.J., Eilers K., Pepe-Ranney C., Berelson W.M., and Spear J.R. (2012) Influences of gas production and filament orientation on stromatolite microfabric. Palaios 27:206–219 [Google Scholar]
- Mathew S.P., Iwamura H., and Blackmond D.G. (2004) Amplification of enantiomeric excess in a proline-mediated reaction. Angew Chem Int Ed Engl 43:3317–3321 [DOI] [PubMed] [Google Scholar]
- Matsuura T., Hosoda K., Kazuta Y., Ichihashi N., Suzuki H., and Yomo T. (2012) Effects of compartment size on the kinetics of intracompartmental multimeric protein synthesis. ACS Synth Biol 1:431–437 [DOI] [PubMed] [Google Scholar]
- McCollom T.M. (2016) Abiotic methane formation during experimental serpentinization of olivine. Proc Natl Acad Sci USA 113:13965–13970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay C.P. (2004) What is life—and how do we search for it in other worlds? PLoS Biol 2:1260–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKirahan V.S. (1994) The Socratic origins of the Cynics and Cyrenaics. In The Socratic Movement, edited by Vander P.A. Waerdt, Cornell University Press, Ithaca, New York, pp 367–391 [Google Scholar]
- McLoughlin N., Wilson L.A., and Brasier M.D. (2008) Growth of synthetic stromatolites and wrinkle structures in the absence of microbes—implications for the early fossil record. Geobiology 6:95–105 [DOI] [PubMed] [Google Scholar]
- McNamara K.J. and Awramik S.M. (1992) Stromatolites: a key to understanding the early evolution of life. Sci Prog 76:345–364 [Google Scholar]
- Meadows V.S., Reinhard C.T., Arney G.N., Parenteau M.N., Schwieterman E.W., Domagal-Goldman S.D., Lincowski A.P., Stapelfeldt K.R., Rauer H., DasSarma S., and Hegde S. (2018) Exoplanet biosignatures: understanding oxygen as a biosignature in the context of its environment. Astrobiology 18:630–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meierhenrich U.J., Nahon L., Alcaraz C., Bredehoft J.H., Hoffmann S.V., Barbier B., and Brack A. (2005) Asymmetric vacuum UV photolysis of the amino acid leucine in the solid state. Angew Chem Int Ed Engl 44:5630–5634 [DOI] [PubMed] [Google Scholar]
- Melim L.A. and Spilde M.N. (2011) Rapid growth and recrystallization of cave pearls in an underground limestone mine. J Sediment Res 81:775–786 [Google Scholar]
- Melim L.A., Liesheidt R., Northup D.E., Spilde M.N., Boston P., and Queen J.M. (2009) A biosignature suite from cave pool precipitates, Cottonwood Cave, New Mexico. Astrobiology 9:907–917 [DOI] [PubMed] [Google Scholar]
- Melim L.A., Northup D.E., Spilde M.N., and Boston P.J. (2015) Update: living Reticulated Filaments from Herbstlabyrinth-Adventhöhle Cave System, Germany. J Cave Karst Stud 77:87–90 [Google Scholar]
- Menon L.R., McIlroy D., Liu A.G., and Brasier M.D. (2016) The dynamic influence of microbial mats on sediments: fluid escape and pseudofossil formation in the Ediacaran Longmyndian Supergroup, UK. J Geol Soc 173:177–185 [Google Scholar]
- Meron E., Gilad E., von Hardenberg J., Shachak M., and Zarmi Y. (2004) Vegetation patterns along a rainfall gradient. Chaos Solitons Fractals 19:367–376 [Google Scholar]
- Miller S.L. (1953) A production of amino acids under possible primitive Earth conditions. Science 117:528–529 [DOI] [PubMed] [Google Scholar]
- Mogul R., Vaishampayan P., Bashir M., McKay C.P., Schubert K., Bornaccorsi R., Gomez E., Tharayil S., Payton G., Capra J., Andaya J., Bacon L., Bargoma E., Black D., Boos K., Brant M., Chabot M., Chau D., Cisneros J., Chu G., Curnutt J., DiMizio J., Engelbrecht C., Gott C., Harnoto R., Hovanesian R., Johnson S., Lavergne B., Martinez G., Mans P., Morales E., Oei A., Peplow G., Piaget R., Ponce N., Renteria E., Rodriguez V., Rodriguez J., Santander M., Sarmiento K., Scheppelmann A., Schroter G., Sexton D., Stephenson J., Symer K., Russo-Tait T., Weigel B., and Wilhelm M.B. (2017) Microbial community and biochemical dynamics of biological soil crusts across a gradient of surface coverage in the Central Mojave Desert. Front Microbiol 8:1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.F., Brezden A., Mohammad H., Chmielewski J., and Seleem M.N. (2017) A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci Rep 7:6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojarro A., Ruvkun G., Zuber M.T., and Carr C.E. (2017) Nucleic acid extraction from synthetic mars analog soils for in situ life detection. Astrobiology 17:747–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery T.S.H. (1898) Comparative cytological studies, with especial regard to the morphology of the nucleolus. J Morphol 15:265–582 [Google Scholar]
- Morrison S.M., Liu C., Eleish A., Prabhu A., Li C., Ralph J., Downs R.T., Golden J.J., Fox P., Hummer D.R., Meyer M.B., and Hazen R.M. (2017) Network analysis of mineralogical systems. Am Mineral 102:1588–1596 [Google Scholar]
- Mozley P. and Davis J.M. (2005) Internal structure and mode of growth of elongate calcite concretions; evidence for small-scale, microbially induced, chemical heterogeneity in groundwater. Geol Soc Am Bull 117:1400–1412 [Google Scholar]
- Mullen L.B. and Sutherland J.D. (2007) Simultaneous nucleotide activation and synthesis of amino acid amides by a potentially prebiotic multi-component reaction. Angew Chem Int Ed Engl 46:8063–8066 [DOI] [PubMed] [Google Scholar]
- Nielsen L.P. (1992) Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol Lett 86:357–362 [Google Scholar]
- Nikitczuk M.P.C., Schmidt M.E., and Flemming R.L. (2016) Candidate microbial ichnofossils in continental basaltic tuffs of central Oregon, USA: expanding the record of endolithic microborings. Geol Soc Am Bull 128:1270–1285 [Google Scholar]
- Noffke N. and Awramik S.M. (2013) Stromatolites and MISS—differences between relatives. GSA Today 23:4–9 [Google Scholar]
- Noffke N., Gerdes G., Klenke T., and Krumbein W.E. (1996) Microbially induced sedimentary structures—examples from modern sediments of siliciclastic tidal flats. Zentralbl Geol Paläontol Teil I 1:307–316 [Google Scholar]
- Noffke N., Beukes N., Bower D.M., Hazen R.M., and Swift D.J.P. (2008) An actualistic perspective into Archean worlds—(cyano-)bacterially induced sedimentary structures in the siliciclastic Nhlazatse Section, 2.9 Ga Pongola Supergroup, South Africa. Geobiology 6:5–20 [DOI] [PubMed] [Google Scholar]
- Nutman A.P., Bennett V.C., Friend C.R., Van Kranendonk M.J., and Chivas A.R. (2016) Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 537:535–538 [DOI] [PubMed] [Google Scholar]
- Olson E.C. (1980) Taphonomy: its history and role in community evolution. In Fossils in the Making: Taphonomy and Paleoecology, edited by Behrensmeyer A.K. and Hill A.P., University of Chicago Press, Chicago, IL, pp 5–19 [Google Scholar]
- Orgel L.E. (1998) Polymerization on the rocks: theoretical introduction. Orig Life Evol Biosph 28:227–234 [DOI] [PubMed] [Google Scholar]
- Pace A., Bourillot R., Bouton A., Vennin E., Galaup S., Bundeleva I., Patrier P., Dupraz C., Thomazo C., Sansjofre P., Yokoyama Y., Franceschi M., Anguy Y., Pigot L., Virgone A., and Visscher P. (2016) Microbial and diagenetic steps leading to the mineralisation of Great Salt Lake microbialites. Sci Rep 6:31495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. (2001) The universal nature of biochemistry. Proc Natl Acad Sci U S A 98:805–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papineau D., De Gregorio B.T., Fearn S., Kilcoyne D., Purohit R., and Fogel M.L. (2016) Nanoscale petrographic and geochemical insights on the origin of Paleoproterozoic stromatolitic phosphorites from Aravalli, India. Geobiology 14:3–32 [DOI] [PubMed] [Google Scholar]
- Papineau D., She Z., and Dodd M.S. (2017) Chemically-oscillating reactions during the diagenetic oxidation of organic matter and in the formation of granules in late Paleoproterozoic chert from Lake Superior. Chem Geol 47:33–54 [Google Scholar]
- Park Boush L., Lehnert K., Myrbo A., Noren A., Peters S., Singer B., and Williams J. (2017) What's your delta? EarthRates-A New NSF funded research coordination network for linking scales across the sedimentary crust. Sediment Rec 15:4–8 [Google Scholar]
- Parker E.T., Cleaves H.J., Bada J.L., and Fernández F.M. (2016) Quantitation of α-hydroxy acids in complex prebiotic mixtures via liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 30:2043–2051 [DOI] [PubMed] [Google Scholar]
- Pavlov A.A., Vasilyev G., Ostryakov V.M., Pavlov A.K., and Mahaffy P. (2012) Degradation of the organic molecules in the shallow subsurface of Mars due to irradiation by cosmic rays. Geophys Res Lett 39:5 [Google Scholar]
- Pepe-Ranney C., Berelson W.M., Corsetti F.A., Treants M., and Spear J.R. (2012) Cyanobacterial construction of hot spring siliceous stromatolites in Yellowstone National Park. Environ Microbiol 14:1182–1197 [DOI] [PubMed] [Google Scholar]
- Peters S.E., Husson J.M., and Wilcots J. (2017) The rise and fall of stromatolites in shallow marine waters. Geology 45:487–490 [Google Scholar]
- Philippot P., van Zuilen M.A., Lepot K., Thomazo C., Farquhar J., and Van Kranendonk M.J. (2007) Early Archaean micro-organisms preferred elemental sulfur, not sulfate. Science 317:1534–1537 [DOI] [PubMed] [Google Scholar]
- Pinheiro V.B., Taylor A.I., Cozens C., Abramov M., Renders M., Zhang S., Chaput J.C., Wengel J., Peak-Chew S.-Y., McLaughlin S.H., Herdewijn P., and Holliger P. (2012) Synthetic genetic polymers capable of heredity and evolution. Science 336:341–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek B. (2014) Neighborhood selection and rules identification for cellular automata: a rough sets approach. In International Conference on Parallel Processing and Applied Mathematics, Springer, Berlin, Heidelberg, pp 721–730 [Google Scholar]
- Pontefract A., Zhu T.F., Walker V.K., Hepburn H., Lui C., Zuber M.T., Ruvkun G., and Carr C.E. (2017) Microbial diversity in a hypersaline sulfate lake: a terrestrial analog of ancient Mars. Front Microbiol 8:1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M.C. and Grotzinger J.P. (2000) Controls on fabric development and morphology of tufas and stromatolites, Uppermost Pethei Group (1.8 Ga), Great Slave Lake, Northwest Canada. In Carbonate Sedimentation and Diagenesis in the Evolving Precambrian World, edited by Grotzinger J.P.and N.P. James, Society for Sedimentary Geology, Tulsa, Oklahoma, Special SEPM Publication No. 67, pp 103–121 [Google Scholar]
- Potter S.L., Chan M.A., Petersen E.U., Dyar M.D., and Sklute E. (2011) Characterization of Navajo Sandstone concretions; Mars comparison and criteria for distinguishing diagenetic origins. Earth Planet Sci Lett 301:444–456 [Google Scholar]
- Potter-McIntyre S.L., Chan M.A., and McPherson B.J. (2014) Concretion rormation in volcaniclastic host rocks: evaluating the role of organics, mineralogy, and geochemistry in early diagenesis. J Sediment Res 84:875–892 [Google Scholar]
- Potter-McIntyre S.L., Williams J., Phillips-Lander C., and O'Connell L. (2017) Taphonomy of microbial biosignatures in spring deposits: a comparison of modern, Quaternary and Jurassic examples. Astrobiology 17:216–230 [DOI] [PubMed] [Google Scholar]
- Power I.M., Wilson S.A., Small D.P., Dipple G.M., Wan W., and Southam G. (2011) Microbially mediated mineral carbonation: roles of phototrophy and heterotrophy. Environ Sci Technol 45:9061–9068 [DOI] [PubMed] [Google Scholar]
- Power I.M., Harrison A.L., Dipple G.M., Wilson S.A., Kelemen P.B., Hitch M., and Southam G. (2013) Carbon mineralization: from natural analogues to engineered systems. Rev Mineral Geochem 77:305–360 [Google Scholar]
- Prokurowski G., Lilley M.D., Seewald J.S., Fruh-Green G.L., Olson E.J., Lupton J.E., Sylva S., and Kelley D.S. (2008) Abiogenic hydrocarbon production at Lost City hydrothermal field. Science 319:604–607 [DOI] [PubMed] [Google Scholar]
- Raiswell R., Fisher Q.J., Cope J.C.W., and Curtis C.D. (2000) Mudrock-hosted carbonate concretions; a review of growth mechanisms and their influence on chemical and isotopic composition. J Geol Soc London 157:239–251 [Google Scholar]
- Ramesh T., Nayak B., Amirbahman A., Tripp C.P., and Mukhopadhyay S. (2016) Application of ultraviolet light assisted titanium dioxide photocatalysis for food safety: a review. Innov Food Sci Emerg Technol 38:105–115 [Google Scholar]
- Rasmussen B. and Buick R. (1999) Redox state of the Archean atmosphere: evidence from detrital minerals in ca. 3250–2750 Ma sandstones from the Pilbara Craton, Australia. Geology 27:115–118 [Google Scholar]
- Ray J.S. (2009) Carbon isotopic variations in fluid-deposited graphite: evidence for multicomponent Rayleigh isotopic fractionation. Int Geol Rev 51:45–57 [Google Scholar]
- Ribas I., Guinan E.F., Güdel M., and Audard M. (2005) Evolution of the solar activity over time and effects on planetary atmospheres. I. High-energy irradiances (1–1700 A). Astrophys J 622:680–694 [Google Scholar]
- Richards F.C., Meyer T.P., and Packard N.H. (1990) Extracting cellular automaton rules directly from experimental data. Physica D 45:189–202 [Google Scholar]
- Riding R. (2008) Abiogenic, microbial and hybrid authigenic carbonate crusts: components of Precambrian stromatolites. Geol Croat 61:73–103 [Google Scholar]
- Ruff S.W. and Farmer J.D. (2016) Silica deposits on Mars with features resembling hot spring biosignatures at El Tatio in Chile. Nat Commun 7:13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushdi A.I. and Simoneit B.R.T. (2004) Condensation reactions and formation of amides, esters, and nitriles under hydrothermal conditions. Astrobiology 4:211–224 [DOI] [PubMed] [Google Scholar]
- Russell M.J. (2003) The importance of being alkaline. Science 302:580–581 [DOI] [PubMed] [Google Scholar]
- Sagan C., Thompson W.R., Carlson R., Gurnett D., and Hord C. (1993) A search for life on Earth from the Galileo spacecraft. Nature 365:715. [DOI] [PubMed] [Google Scholar]
- Sánchez-Román M., Rivadeneyra M.A., Vasconcelos C., and McKenzie J.A. (2007) Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol Ecol 61:273–284 [DOI] [PubMed] [Google Scholar]
- Sánchez-Román M., Vasconcelos C., Schmid T., Dittrich M., McKenzie J.A., Zenobi R., and Rivadeneyra M.A. (2008) Aerobic microbial dolomite at the nanometer scale: implications for the geologic record. Geology 36:879–882 [Google Scholar]
- Sánchez-Román M., Fernández-Remolar D., Amils R., Sánchez-Navas A., Schmid T., San Martin-Uriz P., Rodriguez N., McKenzie J.A., and Vasconcelos C. (2014) Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Sci Rep 4:4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf J.W. and Kudryavtsev A.B. (2012) Biogenicity of Earth's earliest fossils: a resolution of the controversy. Gondwana Res 22:761–771 [Google Scholar]
- Schopf J.W., Kudryavtsev A.B., Agresti D.G., Wdowiak T.J., and Czaja A.D. (2002) Laser–Raman imagery of Earth's earliest fossils. Nature 416:73–76 [DOI] [PubMed] [Google Scholar]
- Schopf J.W., Kudryavtsev A.B., Czaja A.D., and Tripathi A.B. (2007) Evidence of Archean life: stromatolites and microfossils. Precambrian Res 158:141–155 [Google Scholar]
- Schopf J.W., Farmer J.D., Foster I.S., Kudryavtsev A.B., Gallardo V.A., and Espinoza C. (2012) Gypsum-permineralized microfossils and their relevance to the search for life on Mars. Astrobiology 12:619–633 [DOI] [PubMed] [Google Scholar]
- Schopf J.W., Kitajima K., Spicuzza M.J., Kudryavtsev A.B., and Valley J.W. (2018) SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlate carbon isotope compositions. Proc Natl Acad Sci U S A 115:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K.E., Cai R., Gomez E., and Boston P.J. (2017) Using extremophile behavior to identify biological targets of opportunity. In Proceedings of the 6th International Conference on Space Mission Challenges for Information Technology (SMC-IT), Institute of Electrical & Electronics (IEEE), Madrid, Spain, pp 33–37 [Google Scholar]
- Seilacher A. (1992) Dynamic taphonomy: the process-related view of fossil-lagerstätten. In Conferencias de la Reunión de Tafonomía y Fosilización, edited by S. Fernández- López, Editorial Complutense, Madrid, pp 109–125 [Google Scholar]
- Semikhatov M.A., Gebelein C.D., Cloud P., Awramik S.M., and Benmore W.C. (1979) Stromatolite morphogenesis progress and problems. Can J Earth Sci 16:992–1015 [Google Scholar]
- Seong-Joo L., Browne K.M., and Golubic S. (2000) On stromatolite lamination. In Microbial Sediments, edited by Riding R.and S.M. Awramik, Springer, Berlin, Heidelberg, pp 16–24 [Google Scholar]
- Sharp J.O., Schofield E., Junier P., Veeramani H., Suvorova E., Bargar J.R., and Bernier-Latmani R. (2008) Systematic investigation of the product of microbial U(VI) reduction by different bacteria (abstract). Geochim Cosmochim Acta 72:A852 [Google Scholar]
- Shen Y., Buick R., and Canfield D.E. (2001) Isotopic evidence for microbial sulphate reduction in the early Archaean era. Nature 410:77–81 [DOI] [PubMed] [Google Scholar]
- Sherwood Lollar B., Westgate T., Ward J., Slater G.F., and Lacrampe-Couloume G. (2002) Abiogenic formation of alkanes in the Earth's crust as a minor source for global hydrocarbon reservoirs. Nature 416:522–524 [DOI] [PubMed] [Google Scholar]
- Sigman D., Karsh K., and Casciotti K. (2009) Ocean process tracers: nitrogen isotopes in the ocean. In Encyclopedia of Ocean Sciences, edited by Steele J., Thorpe S., and Turekian K., Academic Press, Cambridge, Massachusetts, pp 4138–4153 [Google Scholar]
- Skirrow R.G., Jaireth S., Huston D.L., Bastrakov E.N., Schofield A., van der Wielen S.E., and Barnicoat A.C. (2009) Uranium mineral systems: processes, exploration criteria and a new deposit framework. Geosci Aust Rec 20:44 [Google Scholar]
- Smith K.C. (2016) Life is hard: countering definitional pessimism concerning the definition of life. Int J Astrobiol 15:277–289 [Google Scholar]
- Spear J.R., Figueroa L.A., and Honeyman B.D. (2000) Modeling reduction of U(VI) under variable sulfate concentrations by sulfate-reducing bacteria. Appl Environ Microbiol 66:3711–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squyres S.W., Grotzinger J.P., Arvidson R.E., Bell J.F., III Calvin, W., Christensen P.R., Clark B.C., Crisp J.A., Farrand W.H., Herkenhoff K.E., Johnson J.R., Klingelhofer G., Knoll A.H., McClennan S.M., McSween H.Y., Morris R.V., Rice J.W., Rieder R., and Soderblom L.A.(2004) In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science 306: 1709–1714 [DOI] [PubMed] [Google Scholar]
- Stal L.J. (2012) Cyanobacterial mats and stromatolites. In: Ecology of Cyanobacteria II: Their Diversity in Space and Time, edited by Whitton B.A., Springer, Netherlands, pp 65–125 [Google Scholar]
- Staudigel H., Furnes H., and DeWit M. (2015) Paleoarchean trace fossils in altered volcanic glass. Proc Natl Acad Sci U S A 112:6892–6897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz J.F. (1993) Magnetosomes. Microbiology 139:1663–1670 [Google Scholar]
- Strader B., Schubert K., Quintana M., Gomez E., Curnutt J., and Boston P. (2011) Software tools and algorithms for biological systems, chapter estimation, modeling, and simulation of patterned growth in extreme environments. In Advances in Experimental Medicine and Biology, edited by H. Arabnia and Q.N. Tran, Springer, NY, 696: pp 157–170 [DOI] [PubMed] [Google Scholar]
- Stüeken E.E., Kipp M.A., Koehler M.C., and Buick R. (2016) The evolution of Earth's biogeochemical nitrogen cycle. Earth Sci Rev 160:220–239 [Google Scholar]
- Suga S. and Nakahara H. (2012) Mechanisms and Phylogeny of Mineralization in Biological Systems: Biomineralization 90 Springer, New York [Google Scholar]
- Summons R.E., Albrecht P., McDonald G., and Moldowan J.M. (2008) Molecular biosignatures. Space Sci Rev 135:133–159 [Google Scholar]
- Sunami T., Ichihashi N., Nishikawa T., Kazuta Y., and Yomo T. (2016) Effect of liposome size on internal RNA replication coupled with replicase translation. ChemBioChem 17:1282–1289 [DOI] [PubMed] [Google Scholar]
- Suosaari E., Reid R., Playford P., Foster J., Stolz J., Casaburi G., Hagan P., Chirayath V., Macintyre I., and Planavsky N. (2016) New multi-scale perspectives on the stromatolites of Shark Bay, Western Australia. Sci Rep 6:20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J.W., Bartel D.P., and Luisi P.L. (2001) Synthesizing life. Nature 409:387–390 [DOI] [PubMed] [Google Scholar]
- Tashiro T., Ishida A., Hori M., Igisu M., Koike M., Méjean P., Takahata N., Sano Y., and Komiya T. (2017) Early trace of life from 3.95 Ga sedimentary rocks in Labrador, Canada. Nature 549:516–518 [DOI] [PubMed] [Google Scholar]
- Thiéry J., d'Herbès J.M., and Valentin C.M. (1995) A model simulating the genesis of banded vegetation patterns in Niger. J Ecol 83:497–507 [Google Scholar]
- Thomas R.J., Potter-McIntyre S.L., and Hynek B.M. (2017) Large-scale fluid-deposited mineralization in Margaritifer Terra, Mars. Geophys Res Lett 44:6579–6588 [Google Scholar]
- Thomazo C. and Papineau D. (2013) The evolution of the nitrogen cycle on the early Earth. Elements 9:345–351 [Google Scholar]
- Tosti F. and Riding R. (2017) Fine-grained agglutinated elongate columnar stromatolites: tieling Formation, ca 1420 Ma, North China. Sedimentology 64:871–902 [Google Scholar]
- Trantidou T., Friddin M., Elani Y., Brooks N.J., Law R.V., Seddon J.M., and Ces O. (2017) Engineering compartmentalized biomimetic micro-and nanocontainers. ACS Nano 11:6549–6565 [DOI] [PubMed] [Google Scholar]
- Turing A.M. (1952) The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biol Sci 237:37–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hardenberg J., Meron E., Shachak M., and Zarmi Y. (2001) Diversity of vegetation patterns and desertification. Phys Rev Lett 87:198101–198114 [DOI] [PubMed] [Google Scholar]
- Wacey D., McLoughlin N., Whitehouse M.J., and Kilburn M.R. (2010) Two co-existing sulfur metabolisms in a ca. 3400 Ma sandstone. Geology 38:1115–1118 [Google Scholar]
- Walker S.I., Bains W., Cronin L., DasSarma S., Danielache S., Domagal-Goldman S., Kacar B., Kiang N.Y., Lenardic A., Reinhard C.T., Moore W., Schwieterman E.W., Shkolnik E.L., and Smith H.B. (2018) Exoplanet biosignatures: future directions. Astrobiology 18:779–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.T., O'Brien R.V., and Khosla C. (2013) Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew Chem Int Ed Engl 52:7098–7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Liu C.Q., Mabeley S.C., Wang F., and Hartmann J. (2016) Coupling of carbon and silicon geochemical cycles in rivers and lakes. Sci Rep 6:35832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K.-Y. (1994) Statistical pattern recognition in paleontology using SIMCA-MACUP. J Paleontol 68:689–703 [Google Scholar]
- Westall F., Foucher F., Bost N., Bertrand M., Loizeau D., Vago J.L., Kminek G., Gaboyer F., Campbell K.A., Bréhéret J.G., Gautret P., and Cockell C.S. (2015) Biosignatures on Mars: what, where, and how?: implications for the search for Martian life. Astrobiology 15:998–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides G.M., Ostuni E., Takayama S., Jiang X.Y., and Ingber D.E. (2001) Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 3:335–373 [DOI] [PubMed] [Google Scholar]
- Wilson C.L., Hinman N.W., and Sheridan R.P. (2000) Hydrogen peroxide formation and decay in iron-rich geothermal waters: the relative roles of abiotic and biotic mechanisms. Photochem Photobiol 71:691–699 [DOI] [PubMed] [Google Scholar]
- Woese C. (1998) The universal ancestor. Proc Natl Acad Sci U S A 95:6854–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyborn L.A.I., Heinrich C.A., and Jaques A.L. (1994) Australian Proterozoic mineal systems: essential ingredients and mappable criteria. AusIMM Publ Series 5/94:109–115 [Google Scholar]
- Yang H., Sun H.J., and Downs R.T. (2011) Hazenite, KNaMg2(PO4)2·14H2O, a new biologically related phosphate mineral, from Mono Lake, California, U.S.A. Am Mineral 96:675–681 [Google Scholar]
- Yoshida H., Ujihara A., Minami M., Asahara Y., Katsuta N., Yamamoto K., Sirono S., Maruyama I., Nishimoto S., and Metcalfe R. (2015) Early post-mortem formation of carbonate concretions around tusk-shells over week-month timescales. Sci Rep 5:14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaikin A.N. and Zhabotinsky A.M. (1970) Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature 225:535–537 [DOI] [PubMed] [Google Scholar]
- Zerkle A. and Mikhail S. (2017) The geobiological nitrogen cycle: from microbes to the mantle. Geobiology 15:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]