Abstract
Background
Adequate microcirculatory perfusion is essential for the provision of oxygen to the liver following transplantation. Data from the Oxygen Persufflation in Liver Transplantation (OPAL) study (ISRCTN00167887) were analyzed from liver transplants performed at a single center to determine the role of factors affecting the hepatic microcirculation and early allograft dysfunction (EAD).
Material/Methods
Retrospective data from 116 patients from the Oxygen Persufflation as Adjunction in Liver Transplantation (OPAL) study who underwent liver transplantation at a single center were analyzed. Oxygen saturation of hemoglobin (SO2), relative capillary hemoglobin concentration (rHb), relative tissue blood flow (rBF) using laser Doppler flow measurements, and the Oxygen-to-See (O2C) spectrometry were measured and with post-transplant allograft function were analyzed using univariate and multivariate logistic regression statistics.
Results
Livers donors had a median donor risk index of 1.8. Most liver transplant recipients were men (60.3%), with a median age of 54 years (IQR, 23–68 years). Mean post-transplant 3-month survival was 90.5%. The EAD rate was 22.4%, the median SO2 was 78% (IQR, 29.5–95.8%), the median rHb was 55.6 AU (IQR, 16.8–74.8 AU), and the median rBF was 110.1 AU (IQR, 35.8–406.8 AU). Multivariate logistic regression analysis showed that tissue SO2 (p=0.01), body mass index (BMI) of the transplant recipient (p=0.002), serum alanine transaminase (ALT) of the donor (p=0.02), and portal blood flow (p=0.01) were predictive factors for EAD.
Conclusions
Non-invasive investigations of the liver microcirculation and hemoglobin oxygenation were shown to be predictive factors for EAD following liver transplantation.
MeSH Keywords: Perfusion Imaging, Primary Graft Dysfunction, Risk Assessment
Background
In liver transplantation, the allograft is reperfused in the recipient and is known to result in ischemia-reperfusion injury, which is dependent on preservation injury and the recipient status. The resulting cell edema, release of free oxygen radicals, and their interaction with macromolecules lead to an inflammatory reaction and endothelial impairment [1–6]. Ultimately, reduced early allograft function can occur in liver allografts. Disturbances in the liver microcirculation are of major importance during the early reperfusion period following liver transplantation. The microcirculation provides the required oxygen and nutrient supply, as well as clearance of metabolic waste products from the parenchyma, which are vital to early allograft function.
Therefore, early and reliable methods of assessment of graft function after liver transplantation are required to identify patients who are at increased risk of early allograft dysfunction (EAD). In 2019, Olthoff and colleagues defined EAD based on one or more laboratory findings that included a bilirubin level ≥10 mg/dL on day 7, an international normalized ratio (INR) ≥1.6 on day 7, and a serum alanine or aspartate aminotransferase >2000 IU/L within the first 7 days after liver transplantation [7]. The incidence of EAD was found to be 23%, and the predictive validity of the EAD showed that donor age and the MELD score were risk factors [7].
In this study, data were analyzed from the recently reported findings from the Oxygen Persufflation in Liver Transplantation (OPAL) study (ISRCTN00167887) of 116 liver transplants performed at a single center [8,9]. The aim of this study was to determine the role of factors affecting the hepatic microcirculation that may be predictive for EAD following liver transplantation.
Material and Methods
Study population
Data from the Oxygen Persufflation in Liver Transplantation (OPAL) study (ISRCTN00167887), a single center randomized controlled trial that included 116 patients who underwent liver transplantation were retrospectively analyzed for this study [8,9]. The data were used to determine the factors underlying the development of early allograft dysfunction (EAD) regarding hepatic microvascular perfusion without including the main results of the OPAL study, which have been recently published [9].
This study was approved by the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Data in the OPAL study were collected prospectively during the randomized controlled trial and analyzed retrospectively [9]. In accordance with German law, all allografts were procured from brain-dead organ donors. Inclusion criteria for the OPAL study were men and women >18 years of age, scheduled for first liver transplantation and with an available graft who were willing to participate in the study and who provided written informed consent [8,9]. Inclusion criteria for the donor liver were organs allocated by the ‘rescue offer’ allocation protocol from Eurotransplant, or donors ≥55 years of age. Exclusion criteria were recipients listed as high urgent (HU) cases, simultaneous participation in other clinical trials, and patients who were positive for human immunodeficiency virus (HIV) infection [8].
Definition of the organ rescue allocation
To rescue a donor liver after refusal by more than three liver transplant centers for potential recipients in the Model of End-Stage Liver Disease (MELD)-based allocation system the allocation mechanism was switched by the allocation organization (Eurotransplant). These organs were offered locally for transplant centers with an appropriate recipient or to the first center to accept them, using the multiple-refusal/competitive rescue offer procedure. Organ rescue offers were also conducted in cases with non-stable donors, prolonged cold ischemic times, or for other logistical problems during retrieval.
Definition of early allograft dysfunction (EAD)
Early allograft dysfunction (EAD) was defined according to the current guidelines [7], as a bilirubin ≥10 mg/dL on postoperative day 7 and/or an International Normalized Ratio (INR) ≥1.6 on postoperative day 7 and/or serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >2000 IU/L within the first 7 days [7]. Each case was classified as EAD or non-EAD. Currently, EAD represents the best-validated and clinically relevant parameter for early liver allograft assessment after liver transplantation [7].
Surgical procedures and immunosuppression
Donor operations were performed by specially trained local teams and followed the standards of the local protocols for organ procurement within the different Eurotransplant regions. Standard surgical transplant techniques were used for liver transplantation. The standard technique for liver transplantation included complete caval replacement and anastomosis of the portal vein, hepatic artery, and bile duct using end-to-end anastomoses. According to the study protocol of the OPAL study [8,9], donor organs were randomized into two groups [8,9]. Half of the organs were treated by the routine procedures of static cold storage, and the other half were treated by retrograde oxygen persufflation for at least 90 minutes, as previously described [8,9]. All patients were treated in the Intensive Care Unit (ICU) after liver transplantation, and perioperative care was similar in both groups as well as the use of immunosuppression [8,9]. Intravenous corticosteroids (1,000 mg methylprednisolone) were used intraoperatively [8,9]. Postoperatively, standard immunosuppression consisted of calcineurin-inhibitors in combination with corticosteroids and mycophenolate mofetil, following standard protocols [8,9].
Measurements of hepatic microcirculation and liver perfusion
One hour after allograft reperfusion, non-invasive measurements of hepatic microcirculation were performed. A probe was placed at four predefined locations at the surface of the liver and measurements were made of oxygen saturation of hemoglobin (SO2), relative capillary hemoglobin concentration (rHb), and relative tissue blood flow (rBF) by a combination of laser Doppler flow measurements and Oxygen-to-See (O2C) spectrometry (LEA Medizintechnik GmbH, Giesen, Germany). Of the four measurements taken for each parameter, the mean and interquartile range (IQR) were calculated.
SO2 was the parameter used to measure local tissue hypoxia. The capillary venous oxygen saturation reflects the balance between oxygen delivery and consumption, with 75% of the blood volume distributed to the venous capillary system. Saturation of hemoglobin was given as a percentage. The rHb is an indicator of the amount of hemoglobin in the liver parenchyma. Therefore, the filling of the microvessels is dependent on capillary density, capillary recruitment, and venous filling and was represented in arbitrary units (AU). This parameter represented venous congestion.
The measurement of the rBF demonstrated the volume flow in relative units and indicated ischemia or hyperemia. Relative tissue blood flow was given in arbitrary units (AU).
Measurement of hemodynamic parameters
One hour after reperfusion, measurements of portal venous and arterial inflow were conducted by Doppler ultrasound. At the same time, global hemodynamic parameters also included the cardiac index and central venous pressure, which were measured during routine anesthesia.
Clinical factors for outcome analysis
Standard characteristics of donors and recipients and the impact on the outcome were analyzed that included donor age, gender, body mass index (BMI), cause of death (cerebrovascular accident, hypoxia, trauma, others), cold ischemia time, length of stay in the Intensive Care Unit (ICU), and biopsy-proven macrovesicular and microvesicular steatosis. The use of preservation solution was also documented, including histidine-tryptophan-ketoglutarate (HTK), and the University of Wisconsin organ preservation solution. The organ donor laboratory values included measurement of serum aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl-transferase (γGT), bilirubin, the international normalized ratio (INR), creatinine, sodium, and the donor risk index (DRI) (for Caucasian donors). The organ recipient data included patient age, gender, body mass index (BMI), the Model for End-Stage Liver Disease (MELD) score, duration of surgery, the warm ischemia time, tissue oxygen saturation (SO2), relative capillary hemoglobin concentration (rHb), relative tissue blood flow (rBF), arterial and portal perfusion.
Statistical analysis
Data were shown as the mean and standard error of the mean, or the median and interquartile range (IQR). Graft and patient survival were analyzed using Kaplan-Meier curves and data were compared using the log-rank test. All parameters of hepatic microperfusion were correlated with global hemodynamic status of the recipient. Clinical parameters and measurements of hepatic microcirculation were correlated with the development of EAD following liver transplantation by univariate and multivariate logistic regression analysis. Parameters with p<0.1 in univariate analysis were introduced in the multivariate model. The multivariate model was performed using stepwise selection. A p-value <0.05 was considered statistically significant. JMP version 10.0.0 (SAS Institute Inc., Cary, NC, USA) and SPSS version 24 (IBM, Armonk, NY, USA) were used for statistical analysis.
Results
Donors, recipients, and perioperative characteristics
There were 116 patients included in the present study. The mean age of organ donors was 63 years (±1.26 years). Half (50%) of the donors were male. Donor treatment in the Intensive Care Unit (ICU) lasted for a median of 3.0 days (IQR, 1–19 days) before organ procurement. Organ preservation in the cold was undertaken for 452±13.4 minutes. During implantation of the allograft, a warm ischemic time of 30±0.6 minutes was documented. The Donor Risk Index (DRI) was used for risk assessment and was calculated with a mean of 1.8±0.3.
The majority of recipients (60.3%) in this analysis were male with a mean age of 53.2±0.8 years. A mean laboratory Model for End-Stage Liver Disease (MELD) score before liver transplantation was 14.6±0.6, which represented the severity of liver disease. Indications (or a combination of indications) for liver transplantation included cirrhosis related to alcoholic cirrhosis (31.9%), viral hepatitis (26.7%), hepatocellular carcinoma (HCC) (26.7%), non-alcoholic steatohepatitis (NASH) (6.9%), and other causes (25.9%). The mean duration of the surgical procedure was 267±6.1 minutes. Further details are shown in Table 1.
Table 1.
Donor, recipient and procedural characteristics in 116 patients who underwent liver transplantation.
| Donor | ||
|---|---|---|
| Age (years) | 63.2±1.26 | |
| Gender (Male/Female) | 50%/50% | |
| BMI (kg/m2) | 26.9±0.47 | |
| ICU-stay before donation (days) | 3 (1–19) | |
| CP resuscitation (yes/no) | 32/84 (27.6%/72.4%) | |
| Vasopressor Therapy (yes/no)* | 97/1 (98.9%/1.02%) | |
| Sodium (mmol/l) | 148±0.8 | |
| Creatinine (μmol/l) | 104.3±7.3 | |
| AST (U/l) | 83.1±9.9 | |
| ALT (U/l) | 63.1±10.3 | |
| gGT (U/l) | 81.1±8.3 | |
| Bilirubin (μmol/l) | 11.1±0.8 | |
| Preservation solution (HTK/UW) | 105/11 (90.5%/9.5%) | |
| Microvesicular Steatosis | 40% (0–95%) | |
| Macrovesicular Steatosis | 5% (0–50%) | |
| Cold Ischemic Time | 452±13.4 | |
| Donor Risk Index | 1.8±0.3 | |
| Recipient | ||
| Age (years) | 53.2±0.8 | |
| Gender (Male/Female) | 60.3%/39.7% | |
| BMI (kg/m2) | 26.9±0.5 | |
| labMELD before LT | 14.6±0.6 | |
| Diagnosis (multiple entries possible) | HCV/HCB | 31 (26.7%) |
| HCC | 31 (26.7%) | |
| Alcohol | 39 (33.6%) | |
| NASH | 8 (6.9%) | |
| Others | 29 (25%) | |
| Warm ischemic time (minutes) | 30±0.6 | |
| Time for surgery (minutes) | 267±6.1 | |
Data given as the mean and standard error of the mean (SEM), and the median and range.
Data missing for 18 donors.
Patient and graft outcome
Graft survival after 1 month and 1 year were 95.7% and 79.9%, respectively. Patient survival after 1 month and 1 year were 95.7% and 80.7%, respectively. Postoperatively, temporary hemodialysis was initiated in 13 recipients (11.2%) for a median of 6 days (IQR, 1–44 days). The median stay in the Intensive Care Unit (ICU) and hospital stay for the recipients was 3 days (IQR, 1–45 days) and 19 days (IQR, 1–114) days, respectively.
Two out of 116 patients (1.7%) were retransplanted for primary non-function (PNF) of the liver transplant, and 26 (22.4%) of the 116 recipients developed early allograft dysfunction (EAD). The most frequent criterion leading to the diagnosis of EAD was elevated transaminases within the first 7 postoperative days. Graft survival after 30 days in patients with EAD was 92.3% compared with 97.8% in non-EAD patients (p=0.02). The 12-month graft survival rates in EAD patients and non-EAD patients were 76% and 89.5%, respectively (p=0.02). Patients with EAD showed a 30-day survival of 92.3% compared to 96.7% in those without EAD (p=0.14). The 12-month survival for EAD and non-EAD patients was 72.9%, and 83%, respectively (p=0.14) (Figure 1).
Figure 1.
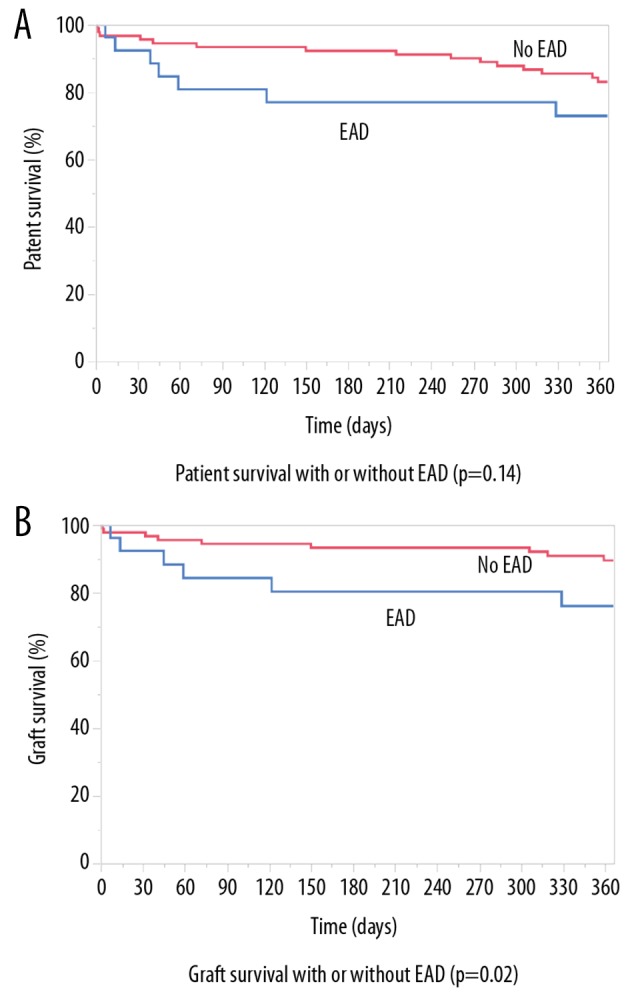
Patient and liver graft survival with and without early allograft dysfunction (EAD). (A) Patient survival with or without early allograft dysfunction (EAD) (p=0.14). (B) Graft survival with or without EAD (p=0.02).
Measurements of the hepatic microcirculation
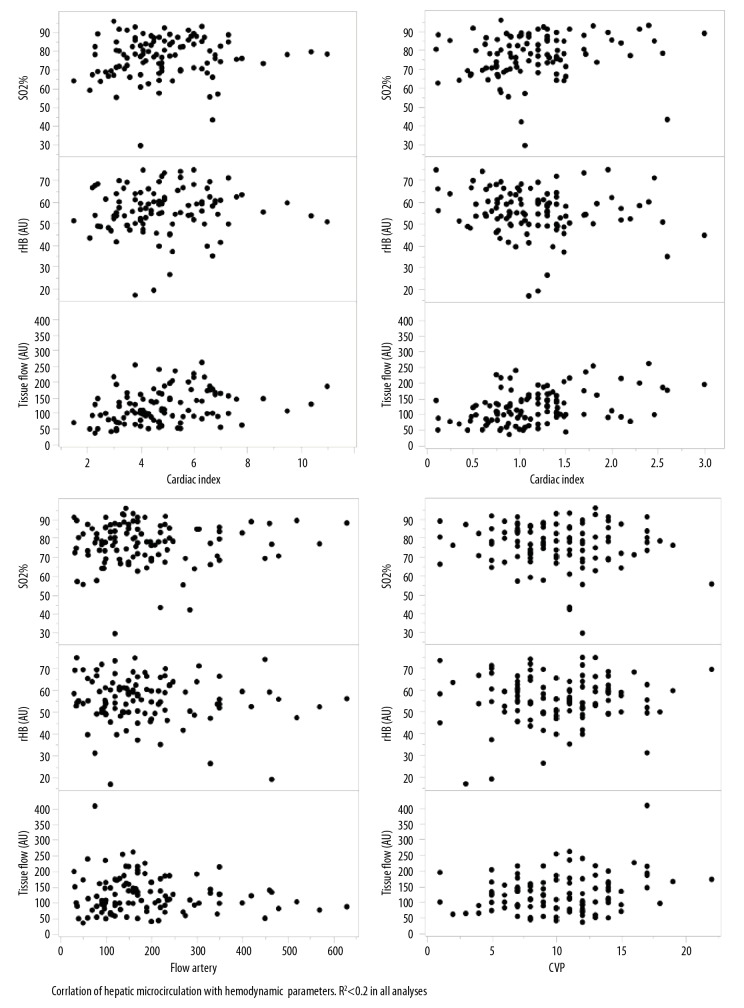
After reperfusion, oxygen saturation of hemoglobin showed a median of 78% (IQR, 70.1–85.2%). The median relative capillary hemoglobin concentration was 55.6 AU (IQR, 49.8–62.2 AU). The median relative tissue blood flow was 110.1 AU (IQR, 84.8–160.3 AU). In terms of global perfusion characteristics measured at the same point as the microcirculation, the flow in the hepatic artery and the portal vein showed median values of 160 ml/min (IQR, 30–630 ml/min) and 1.1 l/min (IQR, 0.1–3.3 l/min), respectively. Also, the median cardiac index was 4.7 (IQR, 1.5–11), and the median central venous pressure was 10 mm H2O (IQR, 1–22 mmH2O). The parameters of the microcirculation were correlated with the global perfusion characteristics to exclude the effects of the hepatic microperfusion on the global hemodynamic state of the recipient (Figure 2). There was a significant correlation (p<0.001) between the rBF and the portal flow and the cardiac index, an R2 was <0.2 in all analyses.
Figure 2.
Correlation between hepatic microcirculation and hemodynamic parameters. R2 <0.2 in all analyses.
The association between hepatic perfusion and EAD
Univariate analysis showed that there was a significant association between functional parameters of the hepatic microcirculation and the development of EAD. The numerical data for the groups of patients with and without EAD are shown in Table 2. The most significant association with the development of EAD was portal blood flow (p=0.008). The microcirculatory perfusion parameters of SO2 and rHb did not reach statistical significance (p=0.07, respectively). Relative capillary blood flow (rBFmean) (p=0.31) and hepatic artery flow (p=0.81) were not significantly associated with the development of EAD.
Table 2.
Univariate logistic regression analysis of hepatic perfusion parameters for the development of early allograft dysfunction (EAD).
| EAD n=26 | No EAD n=90 | p-Wert | |
|---|---|---|---|
| SO2mean (%) | 80.1±6.8 | 75.8±12.2 | 0.07 |
| rHBmean (AU) | 58.7±8.7 | 54.6±10.9 | 0.07 |
| Flowmean (AU) | 114.7±42.5 | 127.8±62.5 | 0.31 |
| FlowHA (ml/min) | 184.9±118.8 | 191.9±124.3 | 0.81 |
| FlowPV (l/min) | 0.92±0.5 | 1.25±0.6 | 0.008 |
Data given as the mean and standard error of the mean (SEM), and the median and range.
The association between clinical parameters and the development of EAD
Univariate logistic regression analysis identified few factors associated with the development of EAD with p<0.1, which included the body mass index (BMI) of the transplant recipient (p=0.053), the last donor serum ALT (p=0.09), and the organ cold ischemic time (p=0.07). These factors and the perfusion parameters were included in a multivariate logistic regression model for the development of EAD. The results are shown in Table 3. Independent associations with the development of EAD were the recipient BMI, the last donor ALT, the SO2 measurements, and the portal blood flow.
Table 3.
Multivariate regression analysis of hepatic perfusion parameters for the development of early allograft dysfunction (EAD).
| Odds ratio | 95% confidence intervall | p-Value | |
|---|---|---|---|
| Recipient BMI | 0.85 | 0.76–0.95 | 0.002 |
| Donor ALT | 0.995 | 0.991–0.999 | 0.02 |
| SO2 | 0.93 | 0.87–0.99 | 0.01 |
| FlowPV | 4.2 | 1.27–14.2 | 0.009 |
Data given as the mean and standard error of the mean (SEM), and the median and range.
Discussion
In this study, data were analyzed from the recently reported findings from the Oxygen Persufflation in Liver Transplantation (OPAL) study (ISRCTN00167887) of 116 liver transplants performed at a single center [8,9]. This study aimed to determine the role of factors affecting the hepatic microcirculation that may be predictive for early allograft dysfunction (EAD) following liver transplantation. In terms of hepatic microperfusion, this study was the first to evaluate the range of values for oxygen saturation of hemoglobin (SO2), relative capillary hemoglobin concentration (rHb), and relative tissue blood flow (rBF) in clinical liver transplantation. Also, multivariate analysis identified hepatic oxygen saturation of hemoglobin (SO2) as an independent predictor for the development of EAD. Other factors, independently associated with the development of EAD, were the body mass index (BMI) of the recipient, the last donor serum alanine transaminase (ALT) laboratory value, and the portal blood flow after reperfusion.
Hepatic microperfusion is believed to be key for successful early allograft function after transplantation, to provide sufficient oxygen and nutrient supply to the liver transplant, as well as clearance of metabolic waste products from the parenchyma, which are prerequisites for any organ function. The measurement of SO2, rHb, and rBF can provide insights into the microcirculatory environment of the transplanted organ. The SO2 measures the oxygen saturation of hemoglobin after delivery of oxygen to the parenchyma, as most blood volume in the microcirculation is found in the venous system.
Therefore, the balance between oxygen delivery and consumption can be assessed in the liver transplant as the rHb indicates the amount of hemoglobin in the liver parenchyma from the microvessels, and increased filling can identify venous congestion. Also, the rBF demonstrates the volume of blood flow and ischemia or hyperemia in relative units. The interaction between these variables identifies several reasons for tissue ischemia, including hypoxia caused by venous congestion (rHb), reduced blood flow (rBF), and reduced oxygen saturation. Hypoxia due to ischemia reduces blood flow so that oxygen extraction increases and the oxygen saturation is decreased. Relative ischemia based on increased metabolism is characterized by increased oxygen extraction, reduced oxygen saturation, and often increased blood flow. In the present study, EAD showed a specific pattern of hepatic microcirculatory disturbance when compared with liver transplants without EAD.
An increase in SO2 indicates less oxygen consumption in organs, with suboptimal function after reperfusion. In the present study, this finding was observed in the setting of increased capillary filling, suggesting venous congestion and organ edema. Cell swelling and edema are characteristics of severe ischemia-reperfusion injury [9–11], resulting from preservation injury. A significant reduction of the hepatic portal inflow further undermined the setting of increased portal resistance due to cell swelling, narrowing of the sinusoids and ultimately resulting in disturbances of organ perfusion. Multivariate analysis in this study identified SO2 and portal blood flow as independent predictors for the development of EAD.
To our knowledge, this is the first study that has demonstrated the relevance of portal blood flow for the development of EAD, as defined by Olthoff et al. [7]. Other studies have shown the clinical relevance of portal inflow in terms of other clinical endpoints, including allograft survival [12], or alternative definitions of EAD [13], and graft injury [14]. A recent study showed a clear correlation between hepatic macroperfusion (portal venous flow, and hepatic arterial flow) with perfusion from the hepatic microcirculation in clinical liver transplantation [15]. This finding is in contrast to the findings of the present study, where a correlation with low statistical significance (R2 <0.2) was observed for rBF and portal flow. Such differences might result from the use of different devices and different measurement parameters of hepatic microperfusion.
Because portal blood flow can be measured non-invasively after reperfusion, this parameter might be routinely assessed in combination with hepatic microcirculation for risk assessment of the transplanted allograft. In support of the findings from the present study, Fechner et al. showed that the intraoperative assessment of the renal microcirculation could predict the development of delayed graft function (DGF) after renal transplantation [16]. An increased rHb and altered SO2 indicated venous congestion, which was believed to be due to organ edema from ischemia-reperfusion injury [16]. Currently, there is some controversy regarding the optimal method of measurement of altered portal venous flow and SO2. Although agents that include prostaglandins, endothelin antagonists, and nitric oxide agonists are believed to preserve microvascular structure and microcirculation in the liver, a potential cytoprotective effect has been shown for the use of prostaglandin after liver transplantation [17]. However, the benefit of these agents has not been shown in a recent meta-analysis of preclinical studies [18]. Therefore, further clinical studies are needed to identify potential drug candidates to prevent EAD based on measurements of hepatic microcirculation.
Currently, the definition and clinical criteria of EAD are being re-evaluated based on objective laboratory parameters that can also address complicated postoperative clinical outcome, and reflects both patient and allograft survival rates [7,19–21]. In the present study, reduced outcomes were found in cases of EAD (Figure 1), but the prediction of the risk of EAD remains a challenge that requires further study [22]. The ultimate goal remains to identify patients who are at risk of EAD as early as possible so that they can be managed clinically to reduce morbidity and mortality following liver transplantation.
Conclusions
This study used data from the Oxygen Persufflation in Liver Transplantation (OPAL) study (ISRCTN00167887), which were analyzed from liver transplants performed at a single center to determine the role of factors affecting the hepatic microcirculation and early allograft dysfunction (EAD). Non-invasive methods of measurement of the microcirculation showed that oxygen saturation of hemoglobin in the liver might be used with clinical factors in the assessment of liver reperfusion quality after transplantation. In the future, routine measurements of the hepatic microcirculation might contribute to the prediction of EAD in patients undergoing liver transplantation.
Acknowledgments
We are indebted to all of our co-workers at the University Hospital Essen, who provided skillful and assiduous care of the patients included in this study. This publication is part of the doctoral thesis of J. Belker.
Footnotes
Source of support: The study includes analysis of data from the randomized controlled single center study, Oxygen Persufflation in Liver Transplantation (OPAL) (ISRCTN00167887), which was supported by the German Research Foundation (DFG MI 470/14/2)
Conflict of interest
None.
References
- 1.Khandoga A, Enders G, Luchting B, et al. Impact of intraischemic temperature on oxidative stress during hepatic reperfusion. Free Radic Biol Med. 2003;35:901–9. doi: 10.1016/s0891-5849(03)00430-1. [DOI] [PubMed] [Google Scholar]
- 2.Toledo-Pereyra LH. Organ preservation for transplantation. 2nd ed. Landes Bioscience; Austin, Texas, USA: [Google Scholar]
- 3.Boncompagni E, Gini E, Ferrigno A, et al. Decreased apoptosis in fatty livers submitted to subnormothermic machine-perfusion respect to cold storage. Eur J Histochem. 2011;55:e40. doi: 10.4081/ejh.2011.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham JA, Guarrera JV. “Resuscitation” of marginal liver allografts for transplantation with machine perfusion technology. J Hepatol. 2014;61:418–31. doi: 10.1016/j.jhep.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–63. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 6.Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: A new paradigm? Transpl Int. 2015;28:690–99. doi: 10.1111/tri.12576. [DOI] [PubMed] [Google Scholar]
- 7.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–49. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 8.Minor T, Pütter C, Gallinat A, et al. Oxygen persufflation as adjunct in liver preservation (OPAL): Study protocol for a randomized controlled trial. Trials. 2011;12:234. doi: 10.1186/1745-6215-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallinat A, Hoyer DP, Sotiropoulos G, et al. Oxygen persufflation in liver transplantation results of a randomized controlled trial. Bioengineering. 2019;6:35. doi: 10.3390/bioengineering6020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duval M, Plin C, Elimadi A, et al. Implication of mitochondrial dysfunction and cell death in cold preservation – warm reperfusion-induced hepatocyte injury. Can J Physiol Pharmacol. 2006;84:547–54. doi: 10.1139/y06-014. [DOI] [PubMed] [Google Scholar]
- 11.McKeown CM, Edwards V, Phillips MJ, et al. Sinusoidal lining cell damage: The critical injury in cold preservation of liver allografts in the rat. Transplantation. 1988;46:178–91. [PubMed] [Google Scholar]
- 12.Spitzer AL, Dick AAS, Bakthavatsalam R, et al. Intraoperative portal vein blood flow predicts allograft and patient survival following liver transplantation. HPB (Oxford) 2010;12:166–73. doi: 10.1111/j.1477-2574.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasavada BB, Chen CL, Zakaria M. Portal flow is the main predictor of early graft dysfunction regardless of the GRWR status in living donor liver transplantation – A retrospective analysis of 134 patients. Int J Surg. 2014;12:177–80. doi: 10.1016/j.ijsu.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Kelly DM, Shiba H, Nakagawa S, et al. Hepatic blood flow plays an important role in ischemia-reperfusion injury. Liver Transpl. 2011;17:1448–56. doi: 10.1002/lt.22424. [DOI] [PubMed] [Google Scholar]
- 15.Pulitano C, Joseph D, Sandroussi C, et al. Postreperfusion microcirculatory derangements after liver transplantation: Relationship to hemodynamics, serum mediators, and outcome. Liver Transpl. 2017;23:527–36. doi: 10.1002/lt.24721. [DOI] [PubMed] [Google Scholar]
- 16.Fechner G, von Pezold J, Luzar O, et al. Modified spectrometry (O2C Device) of intraoperative microperfusion predicts organ function after kidney transplantation: A pilot study. Transplant Proc. 2009;41:3575–79. doi: 10.1016/j.transproceed.2009.06.234. [DOI] [PubMed] [Google Scholar]
- 17.Shin M, Song S-H, Kim J-M, et al. Effectiveness of intraportal prostaglandin E1 administration after liver transplantation. Transplant Proc. 2012;44:500–4. doi: 10.1016/j.transproceed.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 18.Yamanaka K, Houben P, Bruns H, et al. A systematic review of pharmacological treatment options used to reduce ischemia-reperfusion injury in rat liver transplantation. PLoS One. 2015;10:e0122214. doi: 10.1371/journal.pone.0122214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoyer DP, Paul A, Gallinat A, et al. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int. 2015;35:156–63. doi: 10.1111/liv.12443. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Xin EY, Liao B, et al. Development and validation of a nomogram for predicting incidence of early allograft dysfunction following liver transplantation. Transplant Proc. 2017;49:1357–63. doi: 10.1016/j.transproceed.2017.03.083. [DOI] [PubMed] [Google Scholar]
- 21.Friedman BH, Wolf JH, Wang L, et al. Serum cytokine profiles associated with early allograft dysfunction in patients undergoing liver transplantation. Liver Transpl. 2012;18:166–76. doi: 10.1002/lt.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agopian VG, Harlander-Locke MP, Markovic D, et al. Evaluation of early allograft function using the liver graft assessment following transplantation risk score model. JAMA Surg. 2018;153(5):436–44. doi: 10.1001/jamasurg.2017.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]