Abstract
Background
This study aimed to investigate the clinical significance of postoperative serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) in patients who required hip replacement surgery for traumatic fractured neck of femur.
Material/Methods
A retrospective study included 180 patients who had hip replacement surgery for traumatic fractured neck of femur and a control group of 100 patients. Differences between the two groups were compared for serum levels of IL-1β, IL-17, and TNF-α, and the Harris Hip Score (HHS) (maximum 100 points) using Pearson’s correlation.
Results
Serum levels of IL-1β, IL-17, and TNF-α in the control group were significantly lower than those in the study group (P<0.05). According to the HHS, there were 53 patients in the excellent group, 65 patients in the good group, 43 patients in the fair group and 19 patients in the poor group. Postoperative indicator analysis showed significant differences in IL-1β, IL-17, and TNF-α levels between the four groups (P<0.05). Clinical indicators increased from the excellent group to the poor group, with significant differences between the four groups (P<0.05). Postoperative levels of IL-1β, IL-17, and TNF-α were significantly decreased (P<0.05). Pearson’s correlation analysis showed a significant correlation with the clinical indicators (P<0.05).
Conclusions
In patients with hip replacement surgery for traumatic fractured neck of femur, measurement of postoperative serum levels of IL-1β, IL-17, and TNF-α were shown to be potential prognostic indicators.
MeSH Keywords: Interleukin-1beta; Receptors, Interleukin-17; Tumor Necrosis Factor-alpha
Background
Worldwide, with the increasing aging population, there has been increasing attention to the quality of life, safety, and health in the elderly [1]. Recently, the rapid development of the economy has improved population living standards, including increased ownership and use of motor vehicles. The incidence of road traffic accidents has risen significantly, with an increased prevalence of bone injury and fracture caused by trauma [2]. Femoral neck fracture is common in clinical practice and can present as complete or partial fracture. The incidence of fracture of the femoral neck in the elderly is significantly greater than that in young people, and the elderly are prone to fracture during falls and from torsion associated with lack of mobility [3,4]. Following femoral neck fracture, patients have limited mobility and may not be able to walk, and this can severely impact the activities of daily life [5]. Currently, the primary approach to the treatment of traumatic femoral neck fracture is artificial hip joint replacement, but although surgery can result in functional improvement, the postoperative prognosis varies between patients. Currently, no prognostic indicator can predict postoperative outcome in patients following hip replacement surgery for traumatic fractured neck of the femur [6].
Tumor necrosis factor-α (TNF-α) is a functional cytokine with a wide range of biological effects in humans. Studies have shown that TNF-α has a dual regulatory role in the repair process of fractures that can inhibit new bone formation and stimulate bone resorption [7]. Interleukin-1β (IL-1β) and interleukin-17 (IL-17) are members of the interleukin family. IL-1β is a pro-inflammatory cytokine, and when tissue is damaged or local edema occurs, levels of circulating IL-1β rise rapidly [8]. Previous studies have shown that IL-1β levels increase rapidly after bone fracture [9]. IL-17 is a pro-inflammatory cytokine secreted by Th17 cells and is associated with the production of other inflammatory cytokines, including IL-6, IL-8, and tumor necrosis factor-α (TNF-α) and is involved in the recruitment of inflammatory cells, including macrophages and neutrophils [10]. A recent study has shown that IL-17 plays an important role in bone repair, but its role as a prognostic biomarker for bone healing following surgery in patients with bone fracture remains unclear [11].
Therefore, this study aimed to investigate the clinical significance of serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) in patients who required hip replacement surgery for traumatic fracture of the neck of the femur.
Material and Methods
Patient data
This study was approved by the Medical Ethics Committee of Gansu Provincial Hospital West Campus. A retrospective study included 180 patients with orthopedic trauma and fractured neck of the femur who underwent surgery at our hospital from January 2016 to June 2017.
All patients were diagnosed with a traumatic femoral neck fracture. Patients who underwent hip replacement surgery were in the study group, which included 100 men and 80 women, with an age range of 22–68 years and mean age of 50.41±10.33 years. The study inclusion criteria were age >18 years, patients who required hip replacement surgery for traumatic fracture of the femoral neck who were without malignancy, and without contraindications to surgery, without immune disease, and patients with adequate clinical data. Patients were included if they or their families were able to understand and sign informed consent to participate in the study.
Study exclusion criteria were patients with congenital defects, autism, cognitive or hearing impairment, patients and their families who could not adhere to treatment and follow-up, and patients who lacked clinical data. Also, 100 healthy volunteers were included in the control group, including 62 men and 38 women, with an age range of 20–70 years and mean age of 48.39±9.58 years.
Serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) measured by enzyme-linked immunoassay (ELISA)
The enzyme-linked immunoassay (ELISA) kits used were obtained from Qiyi Biological Technology, Shanghai and included a kit for tumor necrosis factor-α (TNF-α) (QY-H10038), interleukin-1β (IL-1β) (99008), and interleukin-17 (IL-17) (R10-79). ELISA was performed according to the manufacturer’s instructions. Briefly, serum samples were added to 50 μL of standard solutions in the wells, and 50 μL of distilled water and 50 μL of primary antibodies were added to the control well. The remaining wells included 40 μL of samples and 10 μL of biotin-labeled secondary antibodies were incubated at 37°C for 30 min. The plates were washed five times for 30 seconds, and 50 μL of the enzyme-labeled solution was added to each well, and the plate was sealed and incubated at 37°C for 60 min. After washing five times, and dried, horseradish peroxidase (HRP) at 100 μL/well was added, and the plate was sealed and incubated at 37°C for 15 min in the dark. The chromogen, 3,3′, 5,5;-tetramethylbenzidine (TMB) at 100 μL per well was added and incubated at room temperature for 20 min in the dark. Finally, a clearing solution of 50 μL per well was added, and the maximum absorption wavelength of 450 nm was detected within 15 min using a microplate region. The experiments were performed in triplicate.
Outcome measurements
The main outcome measurements were the differences in serum levels of IL-1β, IL-17, and TNF-α between the study group and the control group. The Harris Hip Score (HHS) and serum IL-1β, IL-17 and TNF-α levels were measured at one week after surgery. The maximum HHS was 100 and was divided into four categories, including the excellent group with an HHS of ≥90, the good group with an HHS of 80–89, the fair group with a HHS of 70–79, and the poor group with an HHS <70. A higher HHS indicated better patient recovery after surgery.
The secondary outcome measurements were the relationships between indicators of the study group, which were compared using Pearson’s correlation analysis.
Statistical analysis
Data were analyzed using SPSS version 20.0 software (Guangzhou Pomine Info. Tech. Co., Ltd., China), and the data was drawn by using GraphPad Prism version 7 (Shanghai Beka Communication Equipment Co., Ltd., China). Data were expressed as the mean ± standard deviation (SD) and as the percentage (%). The chi-squared (χ2) test was used. Comparison between groups was performed by independent sample t-test. Comparison before and after the treatment in the groups was performed by the paired t-test. Multiple group analysis was performed by analysis of variance (ANOVA) and expressed as the F-value. Paired comparisons were performed by post hoc analysis and the least significant difference (LSD) t-test. The relationships between indicators were analyzed by Pearson’s correlation. A P-value <0.05 was considered to be statistically significant.
Results
Comparison of the clinical and demographic data of the control group and study group
Comparison of the clinical and demographic data between the control group and the study group showed that there was a significant difference in the white blood cell (WBC) count and blood platelet (PLT) count between the two groups (P<0.05), and there was no significant difference in the other indicators (P>0.05) (Table 1).
Table 1.
General clinical and demographic characteristics of the study group and the control group.
| Characteristic | Study group group (n=180) n (%) | Control group (n=100) n (%) | t/χ2 | P-value | |
|---|---|---|---|---|---|
| Gender | Male | 100 (55.56) | 62 (62.00) | 1.095 | 0.295 |
| Female | 80 (44.44) | 38 (38.00) | |||
| Age (years) | >50 | 65 (36.11) | 27 (27.00) | 2.419 | 0.120 |
| ≤50 | 115 (63.89) | 73 (73.00) | |||
| Hypertension | Yes | 43 (23.89) | 20 (20.00) | 0.558 | 0.455 |
| No | 137 (76.11) | 80 (80.00) | |||
| Diabetes | Yes | 53 (29.44) | 26 (26.00) | 0.377 | 0.539 |
| No | 127 (70.56) | 74 (74.00) | |||
| Smoking | Yes | 110 (61.11) | 68 (68.00) | 1.317 | 0.251 |
| No | 70 (38.89) | 32 (32.00) | |||
| Alcohol history | Yes | 32 (17.78) | 12 (12.00) | 1.620 | 0.203 |
| No | 148 (82.22) | 88 (88.00) | |||
| Education | >Junior middle school | 120 (66.67) | 58 (58.00) | 2.085 | 0.149 |
| <Junior middle school | 60 (33.33) | 42 (42.00) | |||
| BMI (kg/m2) | 22.84±1.58 | 22.57±1.66 | 1.345 | 0.180 | |
| WBC (109/l) | 4.69±1.84 | 11.25±1.69 | 29.416 | 0.000 | |
| Hb (g/l) | 135.35±15.48 | 136.24±13.88 | 0.478 | 0.633 | |
| PLT (109/l) | 193.84±52.19 | 238.34±56.88 | 6.619 | 0.000 |
BMI – body mass index; WBC – white blood cell; Hb – hemoglobin; PLT – platelet.
Serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) measured by enzyme-linked immunoassay (ELISA) at one week after surgery
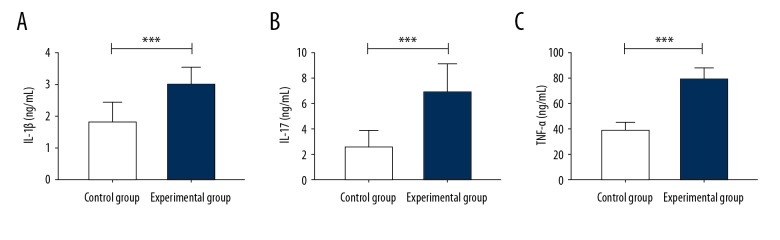
Comparison of postoperative levels of serum IL-1β, IL-17, and TNF-α between the two groups showed that the levels of serum IL-1β, IL-17 and TNF-α in the control group were significantly lower than those in the study group (P<0.05) (Table 2, Figure 1).
Table 2.
Serum levels of levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α).
| Group | Study (n=100) | Control (n=180) | t-value | P-value |
|---|---|---|---|---|
| IL-1β (ng/ml) | 1.82±0.65 | 3.02±0.58 | 15.903 | 0.000 |
| IL-17 (pg/ml) | 2.54±1.36 | 6.88±2.33 | 21.961 | 0.000 |
| TNF-α (pg/ml) | 38.54±6.84 | 78.92±8.79 | 55.454 | 0.000 |
IL-1β – interleukin-1β; IL-17 – interleukin-17; TNF-α – tumor necrosis factor-α.
Figure 1.
Serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) measured by enzyme-linked immunoassay (ELISA). (A) Serum levels of IL-1β level in the control group are significantly lower than in the study group. (B) ELISA shows that serum IL-17 levels in the control group are significantly lower than that in the study group. (C) ELISA shows that serum TNF-α levels in the control group are significantly lower than in the study group. *** P<0.001. IL-1β – interleukin-1β; IL-17 – interleukin-17; TNF-α – tumor necrosis factor-α.
The Harris Hip Score (HHS) and IL-1β, IL-17 and TNF-α level at one week after surgery
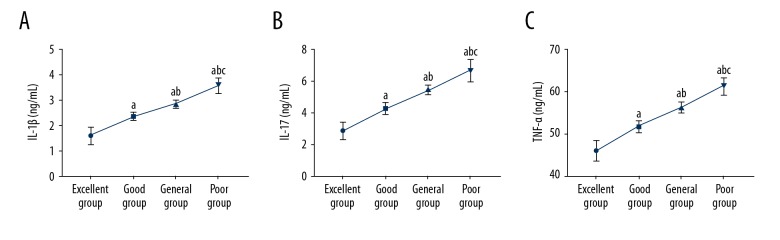
The Harris Hip Score (HHS) identified 53 patients in the excellent group, 65 patients in the good group, 43 patients in the fair group, and 19 patients in the poor group. Analysis of indicators at one week after surgery showed significant differences in IL-1β, IL-17, and TNF-α levels between the four groups (P<0.05). Indicators increased from the excellent group to the poor group, with significant differences between the four groups (P<0.05) (Table 3, Figure 2).
Table 3.
Relationship between the Harris Hip Score (HHS) and serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) at one week after surgery.
| Group | Excellent (n=53) | Good (n=65) | Fair (n=43) | Poor (n=19) | F-value | P-value |
|---|---|---|---|---|---|---|
| IL-1β (ng/ml) | 1.60±0.34 | 2.37±0.16a | 2.87±0.15ab | 3.59±0.30abc | 404.165 | 0.000 |
| IL-17 (pg/ml) | 2.82±0.54 | 4.28±0.38a | 5.46±0.29ab | 6.66±0.69abc | 428.604 | 0.000 |
| TNF-α (pg/ml) | 45.87±2.33 | 51.81±1.48a | 56.25±1.26ab | 61.24±2.15abc | 449.996 | 0.000 |
IL-1β – interleukin-1β; IL-17 – interleukin-17; TNF-α – tumor necrosis factor-α.
Compared with the excellent group (P<0.05).
Compared with the good group (P<0.05).
Compared with the fair group (P<0.05).
The Harris Hip Score (HHS) was out of 100 and was divided into four categories, including the excellent group with an HHS of ≥90, the good group with an HHS of 80–89, the fair group with a HHS of 70–79, and the poor group with a HHS <70.
Figure 2.
Relationship between the Harris Hip Score (HHS) and serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) at one week after surgery. (A) The Harris Hip Score (HHS) significantly decreased with the increased serum levels of IL-1β. (B) The HHS significantly decreased with the increased serum levels of IL-17. (C) The HHS significantly decreased with the increased serum levels of TNF-α. a Compared with the excellent group (P<0.05). b Compared with the good group (P<0.05). c Compared with the fair group (P<0.05). IL-1β – interleukin-1β; IL-17 – interleukin-17; TNF-α – tumor necrosis factor-α.
Comparison of the IL-1β, IL-17, and TNF-α levels before and after surgery in the study group
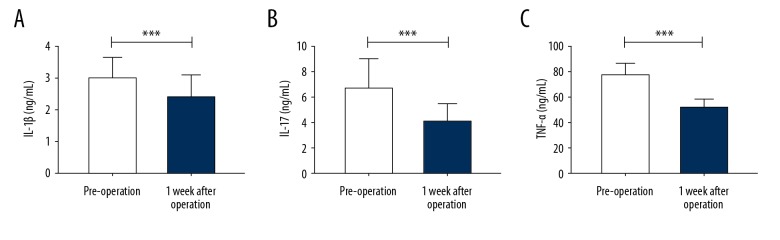
Comparison of the serum levels of IL-1β, IL-17, and TNF-α before and after surgery showed that the levels were significantly reduced after surgery (P<0.05) (Table 4, Figure 3).
Table 4.
Change in serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) before and after surgery.
| Group | Before surgery (n=180) | One week after surgery (n=180) | t-value | P-value |
|---|---|---|---|---|
| IL-1β (ng/ml) | 3.02±0.57 | 2.43±0.67 | 8.288 | 0.000 |
| IL-17 (pg/ml) | 6.78±2.21 | 4.23±1.36 | 12.621 | 0.000 |
| TNF-α (pg/ml) | 78.26±8.62 | 52.38±5.44 | 33.881 | 0.000 |
IL-1β – interleukin-1β; IL-17 – interleukin-17; TNF-α – tumor necrosis factor-α.
Figure 3.
Comparison of serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) before and after surgery. (A) The serum level of IL-1β decreased at one week after surgery, and there was a significant difference compared with the serum levels before surgery. (B) The serum level of IL-17 decreased at one week after surgery, and there was a significant difference compared with the serum levels before surgery. (C) The serum level of TNF-α decreased at one week after surgery, and there was a significant difference compared with the serum levels before surgery. *** P <0.001. IL-1β – interleukin-1β; IL-17 – interleukin-17; TNF-α – tumor necrosis factor-α.
Relationship between serum levels of IL-1β, IL-17, and TNF-α
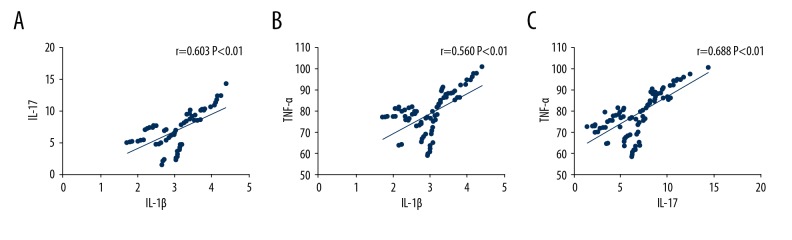
Pearson’s correlation analysis showed a significant correlation between the indicators of serum levels of IL-1β, IL-17, and TNF-α (P<0.05) (Table 5, Figure 4).
Table 5.
Comparisons between serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α).
| Factor | r-value | P-value |
|---|---|---|
| IL-1β vs. IL-17 | 0.603 | 0.000 |
| IL-1β vs. TNF-α | 0.560 | 0.000 |
| IL-17 vs. TNF-α | 0.688 | 0.000 |
IL-1β – interleukin-1β; IL-17 – interleukin-17; TNF-α – tumor necrosis factor-α.
Figure 4.
Comparison of the relationships between interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α). (A) Pearson’s correlation analysis shows a significant correlation between serum levels of IL-1β and IL-17 (r=0.603, P=0.000). (B) Pearson’s correlation analysis shows a significant correlation between serum levels of IL-1β and TNF-α (r=0.560, P=0.000). (C) Pearson’s correlation analysis shows a significant correlation between serum levels of IL-17 and TNF-α (r=0.688, P=0.000). IL-1β – interleukin-1β; IL-17 – interleukin-17; TNF-α – tumor necrosis factor-α.
Discussion
In this study, serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) measured by enzyme-linked immunoassay (ELISA) were significantly reduced in patients after hip replacement for traumatic femoral neck fracture. Using the Harris Hip Score (HHS) for the postoperative outcome, there were significant differences in levels of IL-1β, IL-17, and TNF-α between the excellent group, the good group, the fair group, and the poor group. These findings indicated that IL-1β, IL-17, and TNF-α might be potential prognostic indicators after hip replacement surgery. Correlation analysis showed a positive correlation between serum levels of IL-1β, IL-17, and TNF-α, indicating that there might be a certain synergistic relationship between expression of these cytokines.
Femoral neck fracture is more common in the elderly, due to reduced mobility and an increase in falls, which leads to a significant increase in the incidence of traumatic fractures [12]. The main treatment for femoral neck fracture is surgical hip replacement. Hip replacement surgery is usually successful in replacing the hip joint with an artificial prosthesis [13]. Hip replacement surgery is a routine surgical procedure, and in 2003 in the United States, more than 200,000 people underwent hip replacement surgery [14].
TNF-α is an important pro-inflammatory cytokine that is mainly produced by monocytes and macrophages. Serum levels of TNF-α have been shown to increase in diseases that include malignancy, cardiovascular and cerebrovascular disease, chronic inflammatory disease, and bone fracture [15]. The findings of the present study showed that the serum TNF-α levels in the study group of patients with traumatic femoral neck fracture were significantly increased when compared with the control group, which is a finding that is supported by a previously published study by Ko et al. [16]. Recently, Lim et al. showed that TNF-α promoted angiogenesis in fracture healing in patients with diabetes [17]. Alblowi et al. [18] showed that TNF-α stimulated bone resorption by activating the FOXO1 gene through the receptor activator of nuclear factor kappa-β ligand (RANKL) pathway, indicating that TNF-α plays an important role in fractures and may be a potential prognostic indicator in patients with fracture.
In the present study, serum levels of IL-1β and IL-17 were detected. IL-1 is an inflammatory cytokine secreted by monocytes, endothelial cells, and fibroblasts, mainly in the form of IL-1α and IL-1β, following infection or injury [19]. In the present study, serum levels of IL-1β in the study group were significantly increased when compared with the control group. A previously published study by Lange et al. showed that serum IL-1β levels were significantly increased in the serum of patients with fractures, which is consistent with the findings of the present study [20]. These findings might be explained by the series of pathological changes that follow fracture, including neuroendocrine and metabolic changes, which result in increased serum levels of TNF-α and IL-1β [21]. IL-17, as an early promoter of T cell-induced inflammatory responses, aggravates inflammation by promoting the pro-inflammatory cytokines, IL-1β and TNF-α [22]. In this study, serum IL-17 levels in the control group were significantly lower compared with the study group, supporting a potential prognostic role in patients with fracture. A previously published study showed that IL-17 had a key intermediary role in cellular immune responses in osteogenesis [23], and an in vitro study showed that IL-17 stimulates osteoblast maturation [24], suggesting that IL-17 may play a role in the recovery and prognosis of patients with fracture.
The Harris Hip Score (HHS) is an important clinical indicator used to evaluate patient outcome following artificial hip joint replacement. In this study, the relationships between postoperative IL-1β, IL-17, TNF-α levels, and the HHS were analyzed and showed that the HHS gradually decreased with the increase in serum levels of IL-1β, IL-17 and TNF-α level, indicating an association with postoperative outcome. Detection of serum levels of IL-1β, IL-17, and TNF-α before and after surgery showed that these indicators were significantly reduced after surgery. Correlation analysis of these indicators before showed a positive correlation between these indicators, which might be explained by trauma associated with the stimulation of CD4+ lymphocytes, which induced the secretion of IL-17 by Th17 cells. IL-17 may have interacted with the other factors by inducing an increase in TNF-α and IL-1β levels, and stimulated the cascade of inflammatory mediators to promote increased levels of IL-17.
This study had several limitations. A retrospective clinical study was undertaken that did not investigate the mechanism underlying the association with serum cytokine levels and postoperative outcome. Also, as most of the subjects included in the study were elderly, the influence of undiagnosed or chronic diseases was not excluded and it is unclear whether the findings were applicable to people of all ages. Further studies to investigate the mechanisms associated with the release of inflammatory cytokines, including in vitro studies, and prospective controlled studies with a larger patient study sample size should be performed to validate the findings of this study.
Conclusions
Hip replacement surgery is an effective method of treatment for patients with traumatic femoral neck fracture. The findings from this retrospective study from a single center showed that measurement of postoperative serum levels of interleukin-1β (IL-1β), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) might be potential prognostic indicators of patient outcome.
Footnotes
Source of support: Departmental sources
Conflict of interest
The authors declare no conflict of interest.
References
- 1.McCann P. Urban futures, population ageing and demographic decline. Cambridge Journal of Regions Economy and Society. 2017;10:543–57. [Google Scholar]
- 2.Rubin G, Peleg K, Givon A, Rozen N. Upper extremity open fractures in hospitalized road traffic accident patients: Adult versus pediatric cases. J Orthop Surg Res. 2017;12:157. doi: 10.1186/s13018-017-0657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozsurekci C, Arik G, Halil MG. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin Nutrition. 2015;35:1053–58. doi: 10.1016/j.clnu.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Guan L, Wang M, Zhao B. The feature of fracture in elderly patients in Beijing Jishuitan Hospital from 2009 to 2016. Chin J Emerg Med. 2017;26:860–64. [Google Scholar]
- 5.Davenport SJ, Arnold M, Hua C, et al. Physical activity levels during acute inpatient admission after hip fracture are very low. Physiother Res Int. 2015;20:174–81. doi: 10.1002/pri.1616. [DOI] [PubMed] [Google Scholar]
- 6.Park I, Koehle M, Deveza LR. Total hip replacement surgical guide tool. 9,408,618. US Patent. 2016
- 7.Bilal O, Giulia B, Pierre M. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol. 2014;5:48. doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L, Lars DH, Song X, et al. [Effect of moxibustion on IL-1β and IL-2 in rat models of rheumatoid arthritis]. Journal of Acupuncture and Tuina Science. 2010;8:149–53. [in Chinese] [Google Scholar]
- 9.Loi F, Córdova LA, Pajarinen J, et al. L Inflammation, fracture and bone repair. Bone. 2016;86:119–30. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaffen S. IL-17 receptor composition. Nat Rev Immunol. 2015;16:4. doi: 10.1038/nri.2015.2. [DOI] [PubMed] [Google Scholar]
- 11.Ono T, Okamoto K, Nakashima T, et al. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun. 2016;7:10928. doi: 10.1038/ncomms10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polinder-Bos HA, Emmelot-Vonk MH, Gansevoort RT, et al. High fall incidence and fracture rate in elderly dialysis patients. Netherlands J Med. 2014;72:509–15. [PubMed] [Google Scholar]
- 13.Shang S, Zheng Y, Xiao Z. Application of Orem self-care mode on hip function recovery of elderly patients with artificial hip replacement. Modern Clinical Nursing. 2016;15:19–22. [Google Scholar]
- 14.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–85. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 15.Long H, Xia Y, Liu D, et al. Clinical value of TNF-αin serum of patients with colorectal cancer. Int J Lab Med. 2017;38:1319–21. [Google Scholar]
- 16.Ko FC, Rubenstein WJ, Lee EJ, et al. TNF-α and sTNF-RII are associated with pain following hip fracture surgery in older adults. Pain Medicine. 2017;19:169–77. doi: 10.1093/pm/pnx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim JC, Ko KI, Mattos M, et al. TNFα contributes to diabetes impaired angiogenesis in fracture healing. Bone. 2017;99:26. doi: 10.1016/j.bone.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alblowi J, Kayal RA, Siqueira M, et al. High levels of tumor necrosis factor-α contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175:1574–85. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revu S, Wu J, Henkel M, et al. IL-23 and IL-1β drive human Th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Rep. 2018;22:2642. doi: 10.1016/j.celrep.2018.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange J, Sapozhnikova A, Lu C, et al. Action of IL-1β during fracture healing. J Orthopaedic Res. 2010;28:778. doi: 10.1002/jor.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amanvermez R, Gunay M, Piskin A, et al. TNF-α, IL-1β, and oxidative stress during fracture healing with or without ankaferd. Bratisl Lek Listy. 2013;114:621–24. doi: 10.4149/bll_2013_132. [DOI] [PubMed] [Google Scholar]
- 22.Simone VD, Franzè E, Ronchetti G, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-κB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493–503. doi: 10.1038/onc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abusrer SA. The role of interleukin-17 and RANKL in the regulation of bone destruction in arthritis. J Polit Econ Econ Hist. 2004;52:31–39. [Google Scholar]
- 24.Wang Y, Kim J, Chan A, et al. A two-phase regulation of bone regeneration: IL-17F mediates osteoblastogenesis via C/EBP-β in vitro. Bone. 2018;116:47–57. doi: 10.1016/j.bone.2018.07.007. [DOI] [PubMed] [Google Scholar]