Significance
The use of insecticides in agriculture is one of the suggested causes of the decline in insect populations. Neonicotinoids are among the most widely used insecticides. However, they have important negative side effects, especially for pollinators and other beneficial insects feeding on floral nectar and pollen. We identified an exposure route: Neonicotinoids reach and kill beneficial insects when they feed on the most abundant carbohydrate source for insects in agroecosystems, honeydew. Honeydew is the excretion product of phloem-feeding hemipteran insects such as aphids, mealybugs, whiteflies, or psyllids. This route of exposure is likely to affect a much wider range of beneficial insects and crops than contaminated nectar. Therefore, it should be included in future environmental risk assessments of neonicotinoids.
Keywords: environmental risk assessment, thiamethoxam, honeydew, pollinators, biological control agents
Abstract
Pest control in agriculture is mainly based on the application of insecticides, which may impact nontarget beneficial organisms leading to undesirable ecological effects. Neonicotinoids are among the most widely used insecticides. However, they have important negative side effects, especially for pollinators and other beneficial insects feeding on nectar. Here, we identify a more accessible exposure route: Neonicotinoids reach and kill beneficial insects that feed on the most abundant carbohydrate source for insects in agroecosystems, honeydew. Honeydew is the excretion product of phloem-feeding hemipteran insects such as aphids, mealybugs, whiteflies, and psyllids. We allowed parasitic wasps and pollinating hoverflies to feed on honeydew from hemipterans feeding on trees treated with thiamethoxam or imidacloprid, the most commonly used neonicotinoids. LC-MS/MS analyses demonstrated that both neonicotinoids were present in honeydew. Honeydew with thiamethoxam was highly toxic to both species of beneficial insects, and honeydew with imidacloprid was moderately toxic to hoverflies. Collectively, our data provide strong evidence for honeydew as a route of insecticide exposure that may cause acute or chronic deleterious effects on nontarget organisms. This route should be considered in future environmental risk assessments of neonicotinoid applications.
Growing evidence of important declines in insect populations has caused great concern because of the valuable ecosystem services that insects provide, such as pollination, biological control, nutrient cycling, and providing food sources to higher trophic levels in the food web (1–7). Some of the suggested causes for the decline in insect populations are the loss of their natural habitat, climate change, and the widespread use of insecticides (1–4, 7). Insecticide applications usually result in rapid mortality of the target herbivore species. However, insecticides can also affect beneficial insects directly, as well as indirectly through the food chain (8, 9). Neonicotinoids are among the most widely used and toxic insecticides, accounting for more than 20% of the world´s insecticide market (10). In 2012, they were used in important crops such as citrus, cotton, oilseed rape, soybean, ornamentals, fruits, greenhouse vegetables, potato, rice, sunflower seed, or maize (11). In that year, imidacloprid and thiamethoxam accounted for the largest share of authorized insecticide use in Europe, with 30 and 25%, respectively (11). In Europe, 70% of the neonicotinoid treatments were sprays, whereas less than 20% were seed treatments, and the rest were other application methods such as drip irrigation (11). In 2014, 33% of the 239,000 ha dedicated to citrus production in California (USA) (12, 13) was treated with soil or foliar applications of imidacloprid and this insecticide remained in trees for more than 1 y (14). These neonicotinoid-treated trees can be infested by various species of phloem-feeding insects that survive the treatment and excrete honeydew (15, 16).
In contrast to previous generations of insecticides, neonicotinoids act systemically throughout the plant. Their use is questioned because of the impact on beneficial insects, mainly bees (1, 17). One of the best-known routes of exposure of beneficial insects to neonicotinoids is through contaminated floral nectar and pollen (9, 17). Neonicotinoids reach these plant-derived food sources at concentrations ranging from 0.7 to 39 µg/kg (14, 17, 18). Many insects are exposed to neonicotinoids when they feed on nectar and pollen during the flowering period of crops. However, floral nectar and pollen are scarce and limited to only the brief flowering period in many agroecosystems (19, 20).
Honeydew is the most important source of carbohydrates in many ecosystems, especially in agricultural fields (19–22). Honeydew is the sugar-rich excretion of phloem-feeding insects such as aphids, whiteflies, mealybugs, coccids, and psyllids that feed on crops, weeds, or the surrounding vegetation. This rich and ubiquitous food source is exploited by many beneficial insects, including bees, ants, parasitic wasps, and predators (19, 22), increasing their fitness by feeding on honeydew (19, 20, 22–24). For instance, a great number of ant species, which protect honeydew producers, feed on honeydew and would not survive without it (22). Similarly, more than 50% of the naturally occurring parasitic wasps collected in wheat fields and citrus orchards had recently fed on honeydew (25, 26). Most of these parasitic wasps would die in less than 2 d without feeding on honeydew (20). Bees, as well as other pollinators, also feed on honeydew when nectar is scarce (27, 28).
Because honeydew is produced by insects that feed on phloem, it can contain plant secondary metabolites that are excreted by these phloem feeders (29). Since neonicotinoids are transported through the phloem, honeydew may be an important source of these insecticides in the environment. This, however, has remained unexplored. Here, we investigated whether honeydew excreted by phloem-feeding insects contains neonicotinoid residues that can affect insects feeding on it. The presence of insecticide in honeydew would elucidate a route of insecticide exposure to the many organisms that feed on honeydew. To this aim, the hoverfly Sphaerophoria rueppellii, which is a pollinator in the adult stage and a predator in the juvenile stage, and the hymenopteran parasitic wasp Anagyrus pseudococci were fed ad libitum with honeydew excreted by Planococcus citri settled on 1-y-old citrus trees. Infested trees were treated with the neonicotinoids thiamethoxam (trade name Actara 25WG) and imidacloprid (trade name Confidor 20LS) under 2 potential scenarios. To test the most common mode of application, insecticides were applied via the soil at the recommended concentrations. In a second scenario, insecticides were applied as a foliar spray at 50% of the recommended concentrations to test the effects when low doses of neonicotinoids reach honeydew producers. This second scenario represents exposure through 1) insecticide drift to untreated plots, 2) partial exposure to insecticide when a spray does not reach all parts of the plant due to incorrect insecticide application or unfavorable climatic conditions, or 3) when neonicotinoids remain in the plant for long periods at lower concentrations (14, 30). Neonicotinoids can remain in plants for several months (31, 32) and even for more than 1 to 3 y after the application in perennial crops such as citrus (14, 33). During this long period, hemipterans can feed on plants and excrete honeydew contaminated with neonicotinoids at different concentrations that may cause lethal and sublethal effects on beneficial insects. Moreover, a recent study has demonstrated that neonicotinoids are present in lower than recommended rates in 93% of organic soils and crops, that had not been treated with neonicotinoids for the last 10 y (34). The presence and concentration of imidacloprid and thiamethoxam in the honeydew samples were further analyzed for both soil- and foliar-treated trees using liquid chromatography–mass spectrometry (LC-MS/MS).
Results and Discussion
Toxicity of Honeydew for Hoverflies.
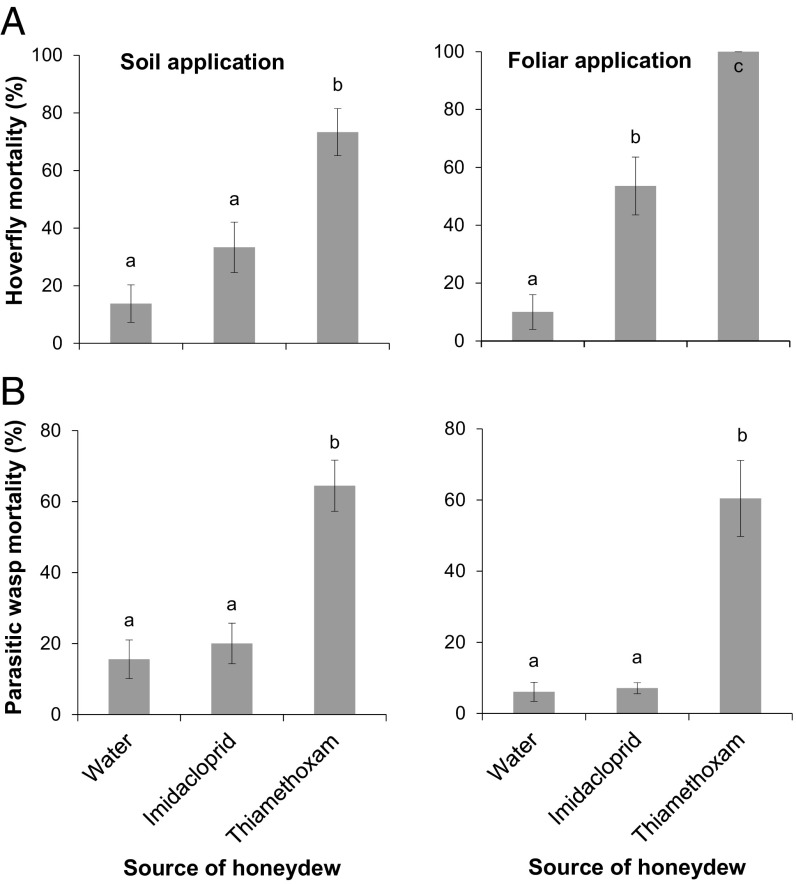
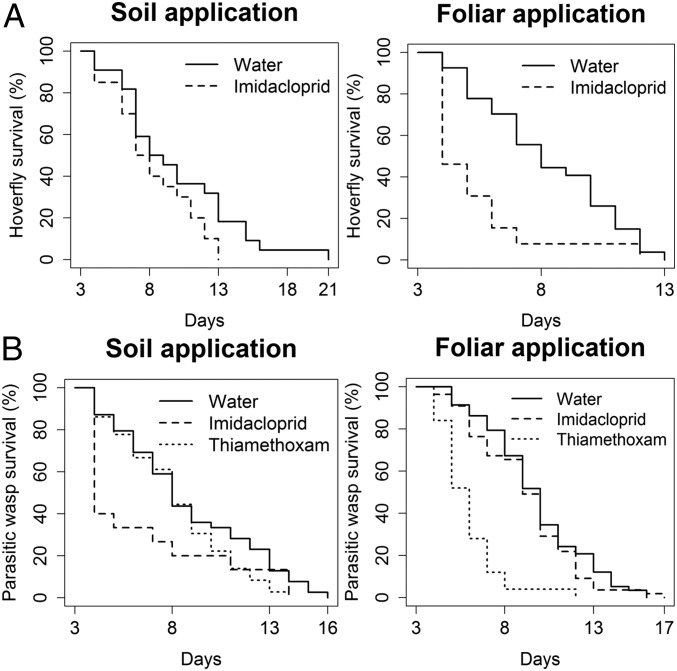
In soil-treated trees, 73.3 ± 8.3% of the hoverflies died within 3 d of feeding on honeydew excreted by mealybugs feeding on thiamethoxam-treated trees, 33.3 ± 8.8% of the hoverflies died in the imidacloprid treatment, and 13.8 ± 6.5% in the control treatment (Generalized Linear Model based on binomial distribution, χ286 = 23.86, P < 0.0001) (Fig. 1 A, Left) (Movie S1). The corrected mortality was 69.1% for the hoverflies fed on honeydew excreted by mealybugs feeding on thiamethoxam-treated trees. The longevity of the surviving hoverflies was assessed daily when they had continuous access to honeydew of the different treatments. After these 3 d, longevity of hoverflies fed on control honeydew (9.9 ± 0.9 d) or honeydew from mealybugs fed on imidacloprid-treated trees (8.3 ± 0.7 d) was similar (Cox’s Proportional Hazards: χ239 = 2.97, P = 0.085) (Fig. 2 A, Left).
Fig. 1.
Mortality of beneficial insects fed on honeydew contaminated with neonicotinoid insecticides. Mortality (mean ± SE) of (A) the pollinating hoverfly S. rueppellii and (B) the parasitic wasp A. pseudococci fed on honeydew of P. citri feeding on water-treated trees or on honeydew of P. citri feeding on soil- (Left) or foliar-treated trees (Right) with the neonicotinoid insecticides imidacloprid or thiamethoxam. Mortality was assessed after feeding on honeydew during 72 h. Columns sharing the same letter are not significantly different from each other (Bonferroni test, P < 0.05).
Fig. 2.
Survival of beneficial insects fed on honeydew contaminated with neonicotinoid insecticides. Survival curves estimated by Kaplan–Meier of (A) the pollinating hoverfly S. rueppellii, and (B), the parasitic wasp A. pseudococci fed on honeydew of P. citri feeding on water-treated trees or on honeydew of P. citri feeding on soil- (Left) or foliar-treated trees (Right) with the neonicotinoid insecticides imidacloprid or thiamethoxam.
In foliar-treated trees, all hoverflies died within 3 d of feeding on honeydew excreted by mealybugs feeding on thiamethoxam-treated trees, 53.5 ± 10% of the hoverflies died in the imidacloprid treatment, and only 10 ± 6% in the control treatment (GLM based on quasibinomial distribution, F 2, 87 = 46.22, P < 0.0001) (Fig. 1 A, Right). The corrected mortality was 100 and 48.4% for the hoverflies fed on honeydew excreted by mealybugs feeding on thiamethoxam and imidacloprid-treated trees, respectively. After these 3 d, hoverflies that fed on honeydew excreted by mealybugs feeding on trees treated with imidacloprid (8.4 ± 0.7 d) lived significantly shorter than those fed on honeydew produced on control trees (11.3 ± 0.6 d) (Cox’s Proportional Hazards: χ21 = 7.68, P = 0.0056) (Fig. 2 A, Right). The different translocation routes of the 2 insecticides in the plant might explain the differential toxicity of honeydew excreted by mealybugs feeding on trees treated with thiamethoxam or imidacloprid. Thiamethoxam is a phloem-transported insecticide whereas imidacloprid is translocated mostly via xylem (35, 36). Therefore, phloem feeders such as P. citri are more likely to excrete thiamethoxam in their honeydew.
Toxicity of Honeydew for Parasitic Wasps.
In soil-treated trees, 64.4 ± 7.2% of the parasitic wasps died within 3 d of feeding on honeydew excreted by mealybugs that fed on trees treated with thiamethoxam, whereas 20 ± 5.7% died in the imidacloprid treatment. Mortality in the control was 15.6 ± 5.5% (GLM, based on binomial distribution, χ2137 = 31.87, P < 0.0001) (Fig. 1 B, Left). The corrected mortality was 59% the parasitic wasps fed on honeydew excreted by mealybugs feeding on thiamethoxam-treated trees. The longevity of the surviving parasitic wasps was assessed daily while they had continuous access to honeydew of the different treatments. After these 3 d, longevity of parasitic wasps fed on honeydew from control trees (8.1 ± 0.5 d) or honeydew from mealybugs fed on imidacloprid (8.8 ± 0.6 d) or thiamethoxam-treated trees (6.33 ± 0.95 d) was similar (Cox’s Proportional Hazards: χ287 = 4.48, P = 0.11) (Fig. 2 B, Left).
In foliar-treated trees, 60.1 ± 10.7% of the parasitic wasps died within 3 d of feeding on honeydew excreted by mealybugs that fed on trees treated with thiamethoxam, whereas only 7.1 ± 1.5% died in the imidacloprid treatment. Mortality in the control was 6.1 ± 2.7% (GLM, based on quasibinomial distribution, F2, 27 = 23.98, P < 0.0001) (Fig. 1 B, Right). The corrected mortality was 57.4% the parasitic wasps fed on honeydew excreted by mealybugs feeding on thiamethoxam-treated trees. After these 3 d, parasitic wasps that fed on honeydew excreted by mealybugs feeding on thiamethoxam-treated trees lived significantly shorter (7.8 ± 0.5 d) than those fed on control honeydew (12.1 ± 0.4 d) or on honeydew of mealybugs that had fed on imidacloprid-treated trees (11.4 ± 0.4 d) (Cox’s Proportional Hazards: χ22 = 43.06, P < 0.0001) (Fig. 2 B, Right). Longevity of parasitic wasps fed on control honeydew or honeydew from mealybugs fed on imidacloprid-treated trees was similar (Fig. 2B). Both neonicotinoids resulted in higher mortality in the hoverfly than in the parasitic wasp. This may be due to a greater feeding rate and/or a lower detoxification capacity of the hoverfly. For example, bumblebees are more susceptible than honey bees to ingested neonicotinoids because their feeding rate is greater (37). In our study, we also observed qualitatively that the hoverflies ingested more honeydew than the parasitic wasps.
Detection of Neonicotinoids in Honeydew.
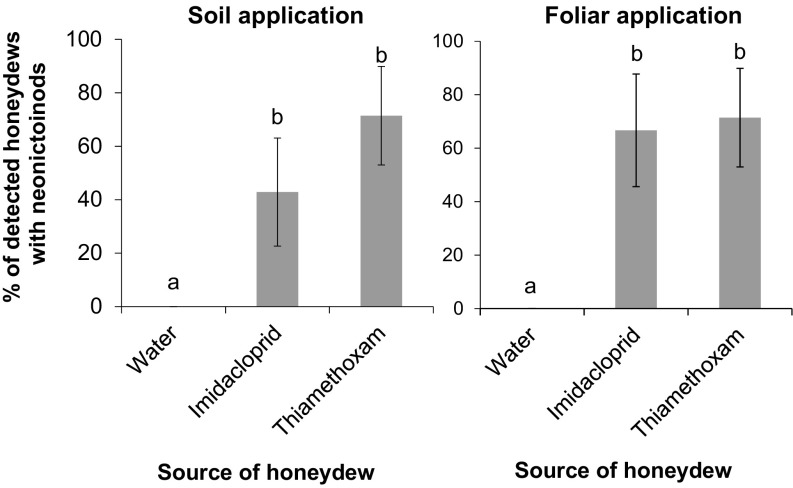
The presence and concentration of imidacloprid and thiamethoxam in the honeydew samples were further analyzed for both soil- and foliar-treated trees using LC-MS/MS (SI Appendix, Figs. S1–S3). In soil-treated trees, thiamethoxam was detected in mealybug-produced honeydew from 71.4 ± 18.4% of the trees sampled throughout the 5 d that the experiment lasted (Fig. 3 and SI Appendix, Table S4). These samples contained 18.3 ± 7.6 ng of thiamethoxam/mL of honeydew (ppb). Imidacloprid was detected in mealybug-produced honeydew from 42.9 ± 20.2% of the trees sampled throughout the 5 d of the experiment. These samples contained 15.6 ± 1.4 ng of imidacloprid/mL of honeydew (ppb). Neither thiamethoxam nor imidacloprid was detected in honeydew produced by mealybugs feeding on water-treated trees (Fisher’s exact test, P = 0.031). In foliar-treated trees, thiamethoxam was detected in mealybug-produced honeydew from 66.7 ± 21.1% of the trees sampled throughout the 5 d that the experiment lasted (Fig. 3 and SI Appendix, Table S5). Imidacloprid was detected in mealybug-produced honeydew from 71.4 ± 18.4% of the trees sampled throughout the 5 d of the experiment (Fig. 3 and SI Appendix, Table S5). These samples contained 68.1 ± 11.6 ng of imidacloprid/mL of honeydew (ppb). As in the previous experiment, neither thiamethoxam nor imidacloprid were detected in honeydew samples collected from control trees (Fisher’s exact test, P = 0.023).
Fig. 3.
Honeydew contaminated by neonicotinoid insecticides. Percentage (mean ± SE) of soil-treated trees (Left) or foliar-treated trees (Right) with P. citri honeydew contaminated by neonicotinoids. Neonicotinoids were detected using LC-MS/MS. Columns with different letters are significantly different from each other (Fisher´s exact test, P < 0.05; number of trees per treatment = 6 to 7).
Our results demonstrate that honeydew is a route of exposure to neonicotinoids for beneficial insects. Honeydew contaminated with neonicotinoids may be present in numerous ecosystems. These insecticides are used worldwide in many crops that, concurrently, are infested by honeydew producers. Moreover, these insecticides even occur in 93% of organic soils and crops, that had not been treated with neonicotinoids for the last 10 y (34). Our study focused on citrus trees. As mentioned above, citrus is not the only crop in which neonicotinoids are routinely applied. For instance, in 2011, 79 to 100% of corn and 34 to 44% of soybean seeds were treated with neonicotinoids in the United States (35.1 and 32.5 million ha, respectively). These crops are infested by phloem-feeding insects that continuously excrete honeydew when they are resistant/tolerant to neonicotinoids or when neonicotinoid concentration in the plant decreases and they can feed and develop at these lower concentrations (13, 38).
The high accessibility of honeydew excreted by numerous phloem-feeding insect species throughout the year suggests that contaminated honeydew represents a highly toxic carbohydrate source for beneficial arthropods (19, 20, 22). For example, predators (21), ants (22), pollinators such as honey bees, solitary bees, bumblebees (19, 22, 28) and even vertebrates like birds (39) have been observed feeding on honeydew. Unavoidably, insecticides applied to control insect pests may have repercussions on organisms at different trophic levels. Insecticides taken up by lower trophic levels, i.e., herbivores, can cascade up to higher trophic levels of a food web. In addition to the direct pathway of contamination through nectar, honeydew readily drops from colonies and hence there is further potential for nontarget soil-dwelling organisms to be affected via this route.
Conclusion
Due to the negative effects of neonicotinoids on nontarget organisms, especially honey bees, the European Commission has recently banned the use of imidacloprid, thiamethoxam, and clothianidin in open agroecosystems in the member states after a risk assessment report of the European Food Safety Authority (11). As with the previous assessments, exposure of beneficial insects to the substances was assessed via 3 routes: residues in bee pollen and nectar; dust drift during the sowing/application of the treated seeds; and water consumption. These decisions, however, did not consider that honeydew, which is more abundant than nectar, could be an important additional route of insecticide exposure for beneficial insects, including pollinators. This route of exposure is likely to affect a much wider range of beneficial insects than contaminated nectar and, thus, should be included in future environmental risk assessments.
Materials and Methods
Insects and Experimental Conditions.
The phloem-feeding herbivorous insect P. citri was obtained from the State Insectary of Valencia (Almassora, Spain), where it was reared on potato sprouts and transported to the Instituto Valenciano de Investigaciones Agrarias (IVIA) (Moncada, Spain) as crawlers (first nymphal instar). The parasitic wasp Anagyrus pseudococci and the predator-pollinator S. rueppellii were obtained from the commercial companies Koppert Biological Systems S.L and Biobest Biological Systems, respectively. Pupae of both species were introduced into wooden and glass rearing boxes (51 × 51 × 41 cm) with holes in the wall that were covered with mesh. Rearing boxes were kept in the laboratory at room temperature until adults emerged. Unfed newly emerged parasitic wasps and hoverflies were collected daily between 9:00 and 11:00 AM and used in the experiments. All experiments were carried out in different climatic chambers for each insect at 25 ± 2 °C, 75 ± 10% RH, and a photoperiod of 14:10 h (L:D).
We selected hoverflies and parasitic wasps of honeydew-producing insects because it is known that they feed on honeydew in the field and also use honeydew as cues to locate their hosts (25–27, 40–43). Therefore, they are extensively in contact with honeydew in the field. Moreover, we selected a hoverfly because hoverflies represent one of the most important groups of pollinators (44); some genera of hoverflies are also predators during their larval stage (45); and, finally, they are highly sensitive to insecticides and their populations are in decline (2, 46). A parasitic wasp was selected because these wasps represent one of the main groups of beneficial insects in agriculture (45, 47, 48). One of the most important examples of biological control in the world is based on Anagyrus parasitoids (49–51).
Plant Infestation and Insecticide Application.
Twenty-seven and 45 potted clementine trees cv. Clementina de Nules grafted on “Macrophyla” (Citrus sinensis × Poncirus trifoliata) were reared and infested for the foliar and soil insecticide applications, respectively. Trees were 2 y old and ∼1 m high and they were maintained in a greenhouse at IVIA. The environmental conditions were 22 ± 5 °C, 70 ± 20% RH, and natural photoperiod (January-April). Clementine trees were watered 3 times per week and were fertilized once per week with Sofertirrig fertilizer (18-18-18 N-P-K). They were infested with P. citri crawlers on February 28, 2018, for the foliar insecticide application and January 22, 2017, for the soil insecticide application. To infest them, 1.5-mL centrifuge tubes half-filled with P. citri crawlers were placed on the crown of each plant.
The neonicotinoids used in this research were thiamethoxam [Thiamethoxam (25%), Actara 25 WG, Syngenta] and imidacloprid [Imidacloprid (20%), Confidor 20 LS, Bayer]. Two potential scenarios were tested. First scenario: Insecticides were applied via the soil at the recommended concentrations to test the most common mode of application (16, 52–54). For this, we applied each insecticide solution or distilled water (control treatment) to 15 clementine plants per treatment directly on the soil on March 23, 2018. Neonicotinoids were applied onto the soil at the dose recommended by the producer. A concentration of 0.3 g of active ingredient of thiamethoxam/1 L of distilled water or 0.75 mL of imidacloprid/1 L of distilled water was applied on 15 different plants per treatment. Untreated controls were watered using only distilled water. We used different 0.5-L glass jars for each treatment to water plants.
Second scenario: Insecticides were applied as a foliar spray at 50% of the recommended concentrations to test the effects when low doses of neonicotinoids reach honeydew producers. For this, we applied each insecticide or distilled water (control treatment) in separate chambers to 9 clementine plants per treatment on April 19, 2019. Plants were temporarily removed from the greenhouse to prevent spray drift and cross-contamination of treatments. Neonicotinoids were applied onto the foliage at half the dose recommended by the producer. A concentration of 0.1 g of thiamethoxam/1 L of distilled water and a concentration of 0.15 mL of imidacloprid/1 L of distilled water were applied on 9 different plants per treatment. Untreated controls were sprayed using only distilled water. We used 2-L manual sprayers and a separate sprayer was used for each insecticide and the control. Insecticides were sprayed until run-off (200 mL). One hour after spraying, we returned the trees to their previous positions in the greenhouse.
Honeydew Collection.
For soil application, we collected honeydew daily from March 24 (+1 d after treatment, DAT) to March 29, 2018 (+5 DAT) by placing Parafilm squares of 10 cm × 10 cm below the plant for 24 h. The collected honeydew for each treatment were labeled and stored at −20 °C in Petri dishes until they were used (22, 23). Honeydew was labeled with information on treatment, tree number, and day of collection. The same procedure was carried out for the foliar application experiment from April 20 to 25, 2019.
Amount of Honeydew Produced by the Mealybugs and Provided to the Hoverflies and Parasitic Wasps.
For the soil application experiment, the amount of honeydew produced by P. citri and the honeydew provided to the beneficial insects, the hoverfly S. rueppellii and the parasitic wasp A. pseudococci, was estimated. The amount of honeydew produced by P. citri per treatment and per day (1, 3, 5, and 10 DAT) in each tree was assessed by counting, under a stereo microscope, the total number of small (less than 150 µm Ø), medium (between 150 and 300 µm Ø), and large (more than 300 µm Ø) honeydew droplets on 3 squares of 1 cm2 each, for 3 randomly collected 25-cm2 Parafilm pieces from the same tree and day. The volume of each categorized droplet was estimated as , where r is the radius of the droplet. Subsequently, we estimated the total volume of honeydew for each 1-cm2 section by summing up the volume of all counted droplets (SI Appendix, Table S1).
To ensure that all insects received honeydew ad libitum in the toxicity assay, the amount of honeydew provided was estimated. The mean volume of honeydew per cm2 of Parafilm in each treatment was multiplied by the area of Parafilm provided per day (SI Appendix, Table S2). The corresponding honeydew-containing Parafilm sections were placed in the Petri dish or glass vials together with wet cotton wool. For all experiments, honeydew was renewed daily to avoid crystallization (22).
Toxicity of Honeydew Excreted by Mealybugs Feeding on Trees Treated with Neonicotinoids as Assessed for Hoverflies and Parasitic Wasps.
We fed the hoverfly S. rueppellii and the parasitic wasp A. pseudococci with honeydew excreted by P. citri feeding on trees that had been treated with thiamethoxam, imidacloprid, or distilled water (control). For the hoverfly S. rueppellii, we confined 30 newly emerged and unfed adults individually in 5.3-cm-diameter Petri dishes with 3-cm-diameter holes covered with muslin mesh to allow ventilation (54–56). For the parasitic wasp A. pseudococci, on the soil application, between 45 and 50 parasitic wasps per treatment were used and placed individually in glass vials 3 cm high and of 0.8 cm diameter covered with wet cotton wool. Instead, for the foliar application, groups of 10 newly emerged and unfed females per Petri dish were used. Ten replicates (each containing 10 new parasitic wasps) per treatment were carried out (100 individuals per treatment).
For the soil and foliar application experiments, Parafilm pieces with honeydew of each treatment were defrosted, observed under the stereo microscope to check for the presence of honeydew, and cut into pieces of different sizes to provide honeydew ad libitum (∼4 cm2 for the Petri dishes and 0.5 cm2 for the glass vials). Petri dishes or glass vials containing the different beneficial insects were kept in the climatic chambers during 72 h and afterward mortality was assessed. Feeding beneficial insects with contaminated honeydew in a no-choice situation represents the most common scenario under field conditions because agriculture is based on large-scale uniformly treated monocultures where floral nectar is scarce, and is limited to only the brief flowering period in flowering crops (57–60).
Effects of Honeydew Excreted by Mealybugs Feeding on Trees Treated with Neonicotinoids on Hoverfly and Parasitic Wasp Longevity.
After 72 h, surviving hoverflies and parasitic wasps of each replicate were placed individually into new containers to study potential sublethal effects on longevity. The surviving hoverflies were kept in the same Petri dishes used previously for the toxicity study. For the soil application experiment, we analyzed a total of 22 hoverflies fed on honeydew from mealybugs feeding on untreated trees, and 20 individuals fed on honeydew from mealybugs feeding on trees treated with imidacloprid. For the foliar application, we analyzed a total of 27 hoverflies fed on honeydew from mealybugs feeding on untreated trees, and 13 fed on honeydew from mealybugs feeding on trees treated with imidacloprid. This experiment was not carried out for thiamethoxam because most individuals had died during the previous experiment.
For the parasitic wasp A. pseudococci in the soil application experiment, parasitic wasps were kept in the same glass vials used for the toxicity assay. We analyzed a total of 36 parasitic wasps fed on honeydew from mealybugs feeding on trees treated with distilled water, 39 on honeydew from mealybugs feeding on trees treated with imidacloprid, and 15 with thiamethoxam. For the foliar application experiment, between 1 and 7 surviving females per replicate were placed individually into glass vials (subreplicates). Each surviving female was used as replicate because there were no significant differences between replicates (females coming from the same Petri dish) in any treatment: survivorship of parasitic wasp females fed on honeydew excreted by mealybugs feeding on trees treated with water (χ29 = 10.1, P = 0.34); mealybugs feeding on trees treated with imidacloprid (χ29 = 13.53, P = 0.16) or thiamethoxam (χ27 = 9.96, P = 0.19) (number of individuals per replicate in SI Appendix, Table S3). Therefore, we analyzed a total of 58 parasitic wasps fed on honeydew from mealybugs feeding on trees treated with distilled water only, 55 on honeydew from mealybugs feeding on trees treated with imidacloprid, and 25 with thiamethoxam
Diets were provided ad libitum daily for each treatment and experiment on both beneficial insects. We checked survival daily until all adults had died. Glass vials and Petri dishes were kept in the climate chambers until all hoverflies and parasitic wasps had died.
Neonicotinoid Detection in Honeydew Samples.
After feeding the beneficial insects, the remaining honeydew for both insecticide applications experiment was used to assess the presence of insecticide. For the soil-treated trees, we analyzed 7 samples from each treatment as follows: control honeydew (excreted by mealybugs feeding on water-treated trees), samples of honeydew excreted by mealybugs feeding on trees treated with imidacloprid, honeydew excreted by mealybugs feeding on trees treated with thiamethoxam (SI Appendix, Table S4). For the foliar-treated trees, we analyzed 8 samples of control honeydew (excreted by mealybugs feeding on water-treated trees) coming from 5 trees and 3 d; 17 samples of honeydew excreted by mealybugs feeding on trees treated with imidacloprid from 7 trees and 5 d and 14 samples of honeydew excreted by mealybugs feeding on trees treated with thiamethoxam from 6 trees and 5 different days (SI Appendix, Table S5). Each sample comprised the remaining honeydew for tree and day. The amount of honeydew per sample was assessed as explained in Amount of Honeydew Produced by Mealybugs and Provided to the Hoverflies and Parasitic Wasps. Then, we extrapolated this value to estimate the total volume of honeydew on the Parafilm (25 cm2).
Chemicals.
High-purity (98 to 99.9%) standards of desired insecticides, namely, imidacloprid, thiamethoxam, and its metabolite clothianidin, were purchased from Sigma-Aldrich. Individual standard solutions were prepared in methanol at a concentration of 1 g·L−1. The working standard solution was prepared by mixing the appropriate amounts of individual standard solutions and diluting with methanol to a final concentration of 0.5 mg·L−1. All solutions were stored in 10 mL glass vials at 4 °C in the dark.
Ammonium formate and methanol (gradient grade for liquid chromatography) were obtained from Sigma-Aldrich and Panreac, respectively. High-purity water was prepared using a Milli-Q water purification system (Millipore). Ten millimolar ammonium formate solutions prepared in both Milli-Q water and methanol were used as mobile phase in LC-MS/MS.
Insecticide extraction from honeydew.
All droplets of honeydew from the same tree and day were dissolved in “Sample Diluent Buffer” (Imidacloprid Enzyme-Linked ImmunoSorbent Assay, Microtiter Plate-kit, Abaraxis. Inc.) in case of foliar-treated trees or in 50% methanol in case of soil-treated trees. One hundred microliters of diluent solution were ejected on top of the Parafilm piece containing the honeydew droplets. The diluent solution and the honeydew droplets were stirred gently with the same pipette to dissolve the honeydew and then draw into Eppendorf tubes. In the case of samples dissolved with Sample Diluent Buffer, these 100 µL were mixed with 100 µL of methanol and injected in the LC-MS/MS. The samples dissolved with 50% methanol were used without further dilution to inject in the LC-MS/MS.
Chemical analysis using LC-MS/MS.
The chromatographic instrument was an HP1200 series LC equipped with an automatic injector, a degasser, a quaternary pump, and a column oven combined with an Agilent 6410 triple quadrupole (QQQ) mass spectrometer with an electrospray ionization (ESI) interface (Agilent Technologies). Data were processed using a MassHunter Workstation Software for qualitative and quantitative analysis (GL Sciences). The chromatographic column was a Luna C18 (15.0 cm × 0.21 cm) with a 3-μm particle size (Phenomenex). The column temperature was kept at 30 °C and the volume injected was 5 μL. An isocratic binary mobile phase consisted of 10 mM ammonium formate: in Milli-Q water and in methanol (50:50, vol/vol) a flow rate of 0.3 mL·min−1 was used.
The ESI ionization source parameters were drying gas (nitrogen) flow of 11 L min−1 at temperature of 300 °C, nebulizer pressure of 15 psi (1034.2 mbar), and capillarity voltage of 4,000 V. The QQQ worked in multiple reaction monitoring with both mass spectrometers at unit resolution and a dwell time of 10 ms and a cell accelerator voltage of 7 eV. The particular conditions to determine each insecticide are specified in SI Appendix, Table S6.
Method validation and quality control.
The linearity of the MS/MS method was established with 6 calibration points, using external standards over a concentration range of 1 to 250 ng·mL−1 (equivalent to 2 to 500 ng·g−1 in the extract). The peak area of target analytes was calculated using MassHunter software (Agilent). Each point was obtained as the mean of 3 independent injections. The data were fit to a linear least-squares regression curve with a 1/× weighting that was not forced through the origin. The calibration curves were y = 359 × −42 for thiamethoxam, y = 129x + 83 for imidacloprid, and y = 132x + 27 for clothianidin. All of them provided an r2 > 0.99.
The sensitivity of the method was estimated by establishing the limits of detection (LODs) and quantification (LOQs) using standard solutions prepared in spiked honey samples that were free of insecticides. The LODs were established as the lowest insecticide concentration whose qualified transition (SRM2) presented a signal-to-noise ratio (S/N) ≥3. They were 0.05, 0.03, and 0.04 ng/mL of extract for thiamethoxam, imidacloprid, and clothianidin, respectively. The LOQs were determined also in pure solvent and in spiked honey as the minimum detectable amount of analyte with S/N ≥ 10 for the quantifier (SRM1) transition. All of the LOQs were verified spiking the samples and analyzing them. They were 0.15, 0.1, and 0.12 ng/mL of extract for thiamethoxam, imidacloprid, and clothianidin, respectively. This level of sensitivity allowed the detection and quantification of very low amount of insecticide in the extracts that might be coming from residual contaminations from previous treatments of the trees used in the experiments. In case of soil-treated trees, we have detected imidacloprid in some of the water-treated trees with levels ranging from <LOQ to 0.5 ng mL−1. Hence, and for the sake of accuracy, we have subtracted 0.5 ng mL−1 to all imidacloprid values in this experiment (SI Appendix, Table S4) (61, 62).
Statistical Analysis.
To analyze the mortality of the parasitic wasp and the hoverfly after feeding on honeydew for 3 d, we used a generalized linear model with binomial distribution (soil application) or quasibinomial distribution (foliar application) of females after 72 h of feeding on honeydew. The mortality of the parasitic wasps in the foliar insecticide application was calculated as the number of dead parasitic wasps divided by total number of parasitic wasps per Petri dish. In both analyses, honeydew type was the explanatory variable and mortality the dependent variable. A Bonferroni post hoc test using “multcomp” package enabled pairwise comparisons between honeydew treatments. When significant differences between the control and the treated honeydews were found (P < 0.05), mortality was corrected using the Abbott formula. The effect of the honeydew treatments on the parasitic wasp or hoverfly survivorship was represented by Kaplan–Meier survivorship curves and analyzed by a log-rank test using the survival functions of the “Survival” package. The percentage of trees in which neonicotinoids were detected in the collected honeydew was analyzed using a Fisher´s exact test. All tests performed were analyzed using the computer program R (version 3.3.2 for Macintosh).
Supplementary Material
Acknowledgments
We thank Biobest Biological Systems and Koppert Biological Systems for providing the beneficial arthropods used during the bioassays and the State Insectary of Valencia for providing P. citri. We acknowledge Dr. F. L. Wäckers for the recommendation to use the hoverfly in the assays. We also acknowledge P. Bru, J. Catalán, E. Rubio, and P. Cuenca for their valuable help during the experiments; M. Cendoya for helping during the statistical analysis; and Dr. Wim van der Putten for comments on a previous version of the manuscript. This research was partially funded by Instituto Nacional de Investigaciones Agrarias Project RTA2017-00095 and the Conselleria d’Agricultura, Pesca i Alimentació de la Generalitat Valenciana. J.G.-C. was supported by the Spanish Ministry of Economy and Competitiveness, Ramón y Cajal Program (RYC-2013-13834), and M.C.-A. was recipient of INIA Grant CPD2016-0085.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904298116/-/DCSupplemental.
References
- 1.Goulson D., Lye G. C., Darvill B., Decline and conservation of bumble bees. Annu. Rev. Entomol. 53, 191–208 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Hallmann C. A., et al. , More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12, e0185809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potts S. G., et al. , Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Ollerton J., Erenler H., Edwards M., Crockett R., Pollinator declines. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346, 1360–1362 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Thomas J. A., et al. , Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303, 1879–1881 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Dirzo R., et al. , Defaunation in the anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Bayo F., Wyckhuys K. A. G., Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 232, 8–27 (2019). [Google Scholar]
- 8.Desneux N., Decourtye A., Delpuech J.-M., The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Stapel J. O., Cortesero A. M., Lewis W. J., Disruptive sublethal effects of insecticides on biological control: Altered foraging ability and life span of a parasitoid after feeding on extrafloral nectar of cotton treated with systemic insecticides. Biol. Control 17, 243–249 (2000). [Google Scholar]
- 10.Jeschke P., Nauen R., Schindler M., Elbert A., Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59, 2897–2908 (2011). [DOI] [PubMed] [Google Scholar]
- 11.European Food Safety Authority , Evaluation of the data on clothianidin, imidacloprid and thiamethoxam for the updated risk assessment to bees for seed treatments and granules in the EU. EFSA Support Publ 15, 73–83 (2018). [Google Scholar]
- 12.Food Agriculture Organization (FAO) , Statistics yearbook. http://www.fao.org/faostat/en/#data/QC (2013). Accessed 4 January, 2018.
- 13.Douglas M. R., Tooker J. F., Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ. Sci. Technol. 49, 5088–5097 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Byrne F. J., et al. , Determination of exposure levels of honey bees foraging on flowers of mature citrus trees previously treated with imidacloprid. Pest Manag. Sci. 70, 470–482 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Grafton-Cardwell E. E., UC IPM pest management guidelines: Citrus (University of California, DANR/Communications Services, 1996). http://ipm.ucanr.edu/PMG/selectnewpest.citrus.html. Accessed 22 March 2018.
- 16.Grafton-Cardwell E. E., Stelinski L. L., Stansly P. A., Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 58, 413–432 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Whitehorn P. R., O’Connor S., Wackers F. L., Goulson D., Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Kessler S., et al. , Bees prefer foods containing neonicotinoid pesticides. Nature 521, 74–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundgren J. G., Relationships of Natural Enemies and Non-Prey Foods (Springer Science & Business Media, 2009). [Google Scholar]
- 20.Wäckers F. L., van Rijn P. C. J., Heimpel G. E., Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 45, 176–184 (2008). [Google Scholar]
- 21.Hogervorst P. A. M., Wäckers F. L., Carette A. C., Romeis J., The importance of honeydew as food for larvae of Chrysoperla carnea in the presence of aphids. J. Appl. Entomol. 132, 18–25 (2008). [Google Scholar]
- 22.Tena A., Wäckers F. L., Heimpel G. E., Urbaneja A., Pekas A., Parasitoid nutritional ecology in a community context: The importance of honeydew and implications for biological control. Curr. Opin. Insect Sci. 14, 100–104 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Tena A., Llácer E., Urbaneja A., Biological control of a non-honeydew producer mediated by a distinct hierarchy of honeydew quality. Biol. Control 67, 117–122 (2013). [Google Scholar]
- 24.Tena A., Senft M., Desneux N., Dregni J., Heimpel G. E., The influence of aphid-produced honeydew on parasitoid fitness and nutritional state: A comparative study. Basic Appl Ecol 29, 55–68 (2018). [Google Scholar]
- 25.Hogervorst P. A. M., Wäckers F. L., Romeis J., Detecting nutritional state and food source use in field-collected insects that synthesize honeydew oligosaccharides. Funct. Ecol. 21, 936–946 (2007). [Google Scholar]
- 26.Tena A., Pekas A., Wäckers F. L., Urbaneja A., Energy reserves of parasitoids depend on honeydew from non-hosts. Ecol. Entomol. 38, 278–289 (2013). [Google Scholar]
- 27.Vosteen I., Gershenzon J., Kunert G., Hoverfly preference for high honeydew amounts creates enemy-free space for aphids colonizing novel host plants. J. Anim. Ecol. 85, 1286–1297 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Konrad R., Wäckers F. L., Romeis J., Babendreier D., Honeydew feeding in the solitary bee Osmia bicornis as affected by aphid species and nectar availability. J. Insect Physiol. 55, 1158–1166 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Züst T., Agrawal A. A., Population growth and sequestration of plant toxins along a gradient of specialization in four aphid species on the common milkweed Asclepias syriaca. Funct. Ecol. 30, 547–556 (2015). [Google Scholar]
- 30.Rondeau G., et al. , Delayed and time-cumulative toxicity of imidacloprid in bees, ants and termites. Sci. Rep. 4, 5566 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonmatin J. M., et al. , Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 22, 35–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrne F. J., et al. , Evaluation of neonicotinoid, organophosphate and avermectin trunk injections for the management of avocado thrips in California avocado groves. Pest Manag. Sci. 68, 811–817 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Cowles R. S., Montgomery M. E., Cheah C. A. S. J., Activity and residues of imidacloprid applied to soil and tree trunks to control hemlock woolly adelgid (Hemiptera: Adelgidae) in forests. J. Econ. Entomol. 99, 1258–1267 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Humann‐Guilleminot S., et al. , A nation-wide survey of neonicotinoid insecticides in agricultural land with implications for agri-environment schemes. J. Appl. Ecol. 56, 1502–1514 (2019). [Google Scholar]
- 35.Nauen R., Ebbinghaus-Kintscher U., Salgado V. L., Kaussmann M., Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pestic. Biochem. Physiol. 76, 55–69 (2003). [Google Scholar]
- 36.Weichel L., Nauen R., Uptake, translocation and bioavailability of imidacloprid in several hop varieties. Pest Manag. Sci. 60, 440–446 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Cresswell J. E., Robert F. X. L., Florance H., Smirnoff N., Clearance of ingested neonicotinoid pesticide (imidacloprid) in honey bees (Apis mellifera) and bumblebees (Bombus terrestris). Pest Manag. Sci. 70, 332–337 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Guedes R. N. C., Smagghe G., Stark J. D., Desneux N., Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 61, 43–62 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Gaze P. D., Clout M. N., Effects of plantation forestry on birds in New Zealand. J. Appl. Ecol. 21, 795–815 (1984). [Google Scholar]
- 40.Steppuhn A., Wäckers F. L., HPLC sugar analysis reveals the nutritional state and the feeding history of parasitoids. Funct. Ecol. 18, 812–819 (2004). [Google Scholar]
- 41.Lee J. C., Andow D. A., Heimpel G. E., Influence of floral resources on sugar feeding and nutrient dynamics of a parasitoid in the field. Ecol. Entomol. 31, 470–480 (2006). [Google Scholar]
- 42.Calabuig A., et al. , Ants impact the energy reserves of natural enemies through the shared honeydew exploitation. Ecol. Entomol. 40, 687–695 (2015). [Google Scholar]
- 43.Franco J. C., et al. , Kairomonal response of the parasitoid Anagyrus spec. nov. near pseudococci to the sex pheromone of the vine mealybug. Entomol. Exp. Appl. 126, 122–130 (2008). [Google Scholar]
- 44.Rader R., et al. , Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. U.S.A. 113, 146–151 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jervis M., Insects as Natural Enemies : A Practical Perspective (Springer, 2005).
- 46.Sánchez-Bayo F., Environmental science. The trouble with neonicotinoids. Science 346, 806–807 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Wajnberg E., Bernstein C., Van Alphen J., Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications (John Wiley & Sons, 2008). [Google Scholar]
- 48.Heimpel G. E., Mills N. J., Biological Control: Ecology and Applications (Cambridge University Press, 2017). [Google Scholar]
- 49.Herren H. R., Neuenschwander P., Biological control of cassava pests in Africa. Annu. Rev. Entomol. 36, 257–283 (1991). [Google Scholar]
- 50.Zeddies J., Schaab R. P., Neuenschwander P., Herren H. R., Economics of biological control of cassava mealybug in Africa. Agric. Econ. 24, 209–219 (2001). [Google Scholar]
- 51.Wyckhuys K. A., et al. , Biological control of an invasive pest eases pressures on global commodity markets. Environ. Res. Lett. 13, 094005 (2018). [Google Scholar]
- 52.Qureshi J. A., Kostyk B. C., Stansly P. A., Insecticidal suppression of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) vector of huanglongbing pathogens. PLoS One 9, e112331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boina D. R., Bloomquist J. R., Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 71, 808–823 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Cocuzza G. E. M., et al. , A review on Trioza erytreae (African citrus psyllid), now in mainland Europe, and its potential risk as vector of huanglongbing (HLB) in citrus. J. Pest. Sci. 90, 1–17 (2017). [Google Scholar]
- 55.Bell H. A., Kirkbride-Smith A. E., Marris G. C., Edwards J. P., Gatehouse A. M. R., Oral toxicity and impact on fecundity of three insecticidal proteins on the gregarious ectoparasitoid Eulophus pennicornis (Hymenoptera: Eulophidae). Agric. For. Entomol. 6, 215–222 (2004). [Google Scholar]
- 56.Tooming E., et al. , Behavioural effects of the neonicotinoid insecticide thiamethoxam on the predatory insect Platynus assimilis. Ecotoxicology 26, 902–913 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Urbaneja A., et al. , Efficacy of five selected acaricides against Tetranychus urticae (Acari: Tetranychidae) and their side effects on relevant natural enemies occurring in citrus orchards 64, 834–842 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Planes L., et al. , Lethal and sublethal effects of spirotetramat on the mealybug destroyer, Cryptolaemus montrouzieri. J. Pest. Sci. 86, 321–327 (2013). [Google Scholar]
- 59.Saska P., et al. , Treating prey with glyphosate does not alter the demographic parameters and predation of the Harmonia axyridis (Coleoptera: Coccinellidae). J. Econ. Entomol. 110, 392–399 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Gurr G. M., Wratten S. D., Landis D. A., You M., Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 62, 91–109 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Masiá A., Campo J., Vázquez-Roig P., Blasco C., Picó Y., Screening of currently used pesticides in water, sediments and biota of the Guadalquivir River Basin (Spain). J. Hazard. Mater. 263, 95–104 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Calatayud-Vernich P., Calatayud F., Simó E., Suarez-Varela M. M., Picó Y., Influence of pesticide use in fruit orchards during blooming on honeybee mortality in 4 experimental apiaries. Sci. Total Environ. 541, 33–41 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.