Significance
S-nitrosylation, the addition of a nitric oxide (NO) moiety to a reactive protein cysteine (Cys) thiol to form an S-nitrosothiol (SNO), is emerging as a pivotal redox-based, posttranslational modification (PTM) during plant immune function. However, the Cys target sites of NO bioactivity and the associated consequences on cellular signaling are not well defined. Our findings suggest that S-nitrosylation of small ubiquitin-like modifier (SUMO)-conjugating enzyme 1 (SCE1) at Cys139 controls SUMOylation, a protein-based PTM that negatively regulates plant immunity through conjugation of SUMO1/2. This Cys is evolutionary conserved and specifically S-nitrosylated in the human homolog, UBC9, implying that this mechanism might be conserved across phylogenetic kingdoms.
Keywords: nitric oxide, S-nitrosylation, immunity
Abstract
SUMOylation, the covalent attachment of the small ubiquitin-like modifier (SUMO) to target proteins, is emerging as a key modulator of eukaryotic immune function. In plants, a SUMO1/2-dependent process has been proposed to control the deployment of host defense responses. The molecular mechanism underpinning this activity remains to be determined, however. Here we show that increasing nitric oxide levels following pathogen recognition promote S-nitrosylation of the Arabidopsis SUMO E2 enzyme, SCE1, at Cys139. The SUMO-conjugating activities of both SCE1 and its human homolog, UBC9, were inhibited following this modification. Accordingly, mutation of Cys139 resulted in increased levels of SUMO1/2 conjugates, disabled immune responses, and enhanced pathogen susceptibility. Our findings imply that S-nitrosylation of SCE1 at Cys139 enables NO bioactivity to drive immune activation by relieving SUMO1/2-mediated suppression. The control of global SUMOylation is thought to occur predominantly at the level of each substrate via complex local machineries. Our findings uncover a parallel and complementary mechanism by suggesting that total SUMO conjugation may also be regulated directly by SNO formation at SCE1 Cys139. This Cys is evolutionary conserved and specifically S-nitrosylated in UBC9, implying that this immune-related regulatory process might be conserved across phylogenetic kingdoms.
The production of nitric oxide (NO) is a conspicuous feature of immune responses in complex eukaryotes (1, 2). In this context, S-nitrosylation, the addition of an NO moiety to a protein cysteine (Cys) thiol to form an S-nitrosothiol (SNO), is thought to be a major route to regulate protein function (3–5). In combination with reactive oxygen intermediates, NO regulates the hypersensitive response (3, 6), a programmed execution of plant cells at sites of attempted infection (7), and the expression of a suite of immune-related genes (8–10). However, the underpinning molecular mechanisms are not well understood.
The small ubiquitin-like modifier (SUMO) is present in all eukaryotes and is essential for viability (11, 12). SUMO is conjugated to target proteins via a pathway analogous to ubiquitylation, involving E1 and E2 enzymes as well as E3 ligases. The SUMO activating enzyme (E1) is a heterodimeric complex that forms a high-energy thioester bond with the C-terminal carboxyl group of SUMO. Next, SUMO is transferred to the SUMO conjugating enzyme (E2), which catalyzes the conjugation of SUMO to its targets. SUMO ligases (E3) enhance the efficiency of conjugation and may contribute to target specificity but are not required for SUMO conjugation in vitro (13).
SUMOylation has been implicated in plant immunity by virtue of its function in hypersensitive response control (14) and signaling integral to salicylic acid (SA), a major immune activator (15–17). Either loss of function of the SUMO E3 ligase, SAP and Miz 1 (SIZ1), or knockdown of SUMO1 and SUMO2, the 2 major stress-responsive SUMO isoforms, results in constitutive SA-dependent gene expression and increased pathogen resistance (16, 17). Furthermore, immune phenotypes of siz1 mutants are dependent on the immune receptor SNC1 (18). Collectively, these studies suggest that in the absence of pathogen challenge, global SUMOylation mediated by SUMO1/2 negatively regulates plant immunity.
Despite the central role of SUMOylation in both plant and animal cell biology, there is currently little insight into the regulatory processes underpinning this posttranslational modification. Here we show that following pathogen recognition, the Arabidopsis SUMO E2 enzyme, SUMO-conjugating enzyme 1 (SCE1), is S-nitrosylated at a highly conserved Cys, Cys139. We show that this site-specific modification inhibits the SUMO-conjugating activity of both SCE1 and its human homolog UBC9, suggesting that this might constitute an evolutionary conserved mechanism of regulating levels of SUMO conjugates in cells. Furthermore, expression of mutant SCE1(C139S) in Arabidopsis results in elevated levels of SUMOylated proteins after pathogen infection, compromised immune gene activation, and increased disease susceptibility. Thus, these data suggest that after immune activation, increasing NO levels are in part transduced into immune responses by inhibiting global conjugation of SUMO1/2 through S-nitrosylation of SCE1.
Results
S-Nitrosylation of SUMO E2 Enzymes Inhibits SUMOylation In Vitro.
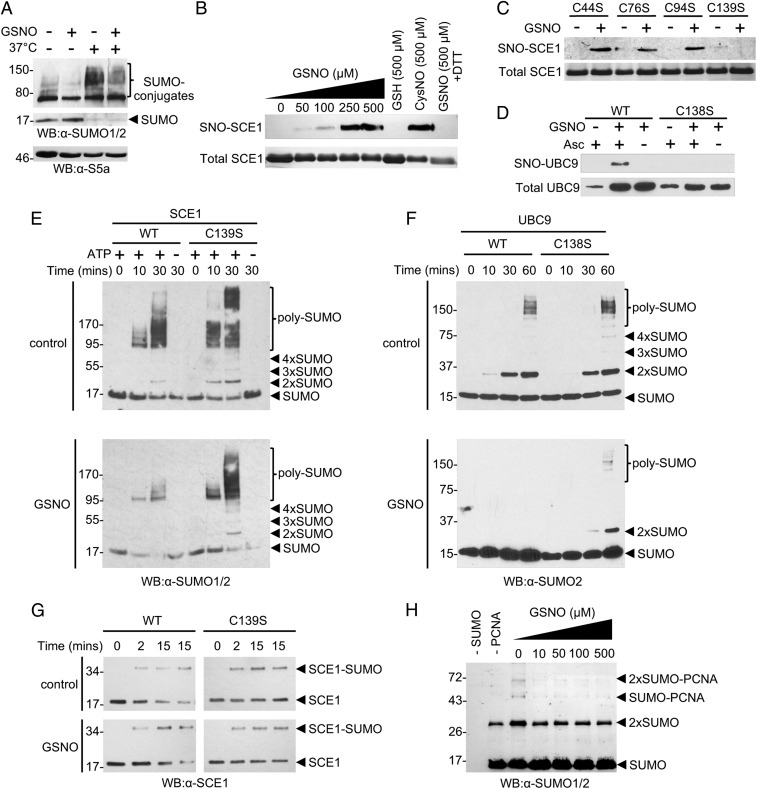
To determine whether NO might help sculpt the plant immune response by regulating SUMOylation, protoplasts were isolated from wild-type (WT) Arabidopsis plants and used to monitor the potential impact of the natural NO donor, S-nitrosoglutathione (GSNO), on this modification following heat shock. This is a well-established method to increase SUMO2/3 or SUMO1/2 conjugates in human or plant cells, respectively (19, 20). This SUMOylation response was also observed in protoplasts after exposure to 37 °C for 15 min, with a concurrent decrease in levels of free SUMO (Fig. 1A). Pretreatment of protoplasts with 1 mM GSNO inhibited this response and also reduced levels of SUMO conjugates under resting conditions, suggesting that SUMOylation is inhibited by GSNO.
Fig. 1.
S-nitrosylation of SUMO E2 enzymes inhibits SUMOylation in vitro. (A) WT protoplasts were pretreated with or without 1 mM GSNO, followed by a 15-min incubation at either 22 °C or 37 °C. Protein extracts were analyzed by Western blot analysis against SUMO1/2 and S5a (loading control). (B) Purified recombinant SCE1 was subjected to the stated treatments before the BST. Total SCE1 was detected by staining with Coomassie blue, while SNO-SCE1 was detected by Western blot analysis against biotin. (C) Each Cys mutant form of SCE1 was incubated with either 100 μM GSH or GSNO as in B. (D) Purified recombinant UBC9 was incubated with or without 500 μM GSNO before the BST. Biotinylated protein (SNO-UBC9) was enriched by streptavidin pull-down, followed by detection by Western blot analysis against His-tag. The omission of ascorbate (−Asc) served as a negative control for the BST. (E) The stated proteins were incubated with or without 500 μM GSNO for 20 min, then added to the in vitro SUMOylation reactions, which were incubated at 30 °C for the stated times. SUMO1 species were detected by Western blot analysis against SUMO1/2. (F) Proteins were incubated as in E, then added to the in vitro SUMOylation reactions and incubated at 37 °C for the stated times, followed by Western blot analysis against SUMO2. (G) Proteins were incubated as in E, then added to a reaction mix containing the E1 heterodimer and SUMO1, followed by the addition of ATP and incubation at 30 °C for the stated times. The SCE1-SUMO1 thioester was observed by Western blot analysis against SCE1. (H) SCE1 was pretreated with the stated concentrations of GSNO before being added to the reaction mixtures. SUMOylated forms of PCNA were visualized by Western blot analysis against SUMO1/2.
Since NO can modulate protein activity by S-nitrosylation, we hypothesized that components of the SUMOylation machinery might be targeted by this posttranslational modification. The sole Arabidopsis SUMO E2 conjugating enzyme, SCE1, has 4 Cys residues, including its active site Cys94, that could be potential targets of S-nitrosylation. To test this, we used the biotin-switch technique (BST), which specifically replaces protein SNOs with a biotin label (21). Purified recombinant SCE1 was efficiently S-nitrosylated by GSNO in a concentration-dependent manner in vitro. Furthermore, another natural NO donor, CysNO, also S-nitrosylated SCE1 (Fig. 1B). Informatively, this modification could be reversed by DTT, consistent with S-nitrosylation of SCE1 on a given Cys thiol. Mutagenesis of the Cys residues within SCE1 established that only mutation of Cys139 prevented S-nitrosylation by GSNO and subsequent detection by BST (Fig. 1B). This finding was further confirmed by mass spectrometry (SI Appendix, Fig. S1).
Structural modeling of SCE1 based on its human homolog UBC9 revealed that Cys139 is likely solvent-exposed and thus accessible for modification (SI Appendix, Fig. S2A). This contrasts with Cys44 and Cys76, which are located in the interior of the protein structure with their side chains orientated inward, and Cys94, which sits within the active site cleft. Importantly, the Cys corresponding to Arabidopsis Cys139 is highly conserved in various higher eukaryotes (SI Appendix, Fig. S2B), suggesting a possible functional role. To test this, the corresponding residue (Cys138) in human UBC9 was mutated, and the protein was subjected to BST analysis. Similar to SCE1, UBC9 was specifically S-nitrosylated at Cys138 (Fig. 1D).
After identifying Cys139 of SCE1 and Cys138 of Ubc9 as sites of S-nitrosylation in vitro, we sought to uncover the effect of these modifications on enzymatic activity. By reconstituting the Arabidopsis SUMO machinery in vitro, the formation of poly-SUMO1 chains served as a readout of E2 activity and revealed that both WT and C139S forms of SCE1 are equally capable of rapidly forming SUMO1 chains (Fig. 1E). Therefore, it appears that mutation of Cys139 does not affect enzyme activity in vitro. However, pretreatment of WT SCE1 with GSNO inhibited its SUMO-conjugating activity (Fig. 1E). Importantly, this effect was not observed with GSNO pretreatment of the C139S protein, suggesting that specific modification of Cys139 inhibits SCE1 activity (Fig. 1E). We also confirmed that SUMO chain formation was inhibited by pretreating SCE1 with another NO donor, CysNO (SI Appendix, Fig. S2C).
Next, we examined whether S-nitrosylation of UBC9 affected its SUMO-conjugating activity by monitoring the in vitro formation of poly-SUMO2 chains using the reconstituted human SUMO machinery (22). Similar to SCE1, only GSNO pretreatment of WT, but not C138S UBC9, inhibited SUMO conjugating activity (Fig. 1F).
SUMO first forms a thioester with the E1 heterodimer before it is transferred to the active site of the E2, where it also establishes a thioester linkage. Subsequently, SUMO is conjugated to its target substrate, forming an isopeptide bond. Thus, inhibition of SCE1 by S-nitrosylation of Cys139 can occur at either SUMO-SCE1 thioester formation or the transfer of SUMO from SCE1 to the given target. To discriminate between these 2 alternative possibilities, we performed in vitro SCE1-SUMO1 thioester formation assays. Both WT and C139S SCE1 proteins were equally capable of forming thioester bonds with SUMO1, and this reaction was unaffected by pretreatment with GSNO (Fig. 1G). This suggests that the inhibition of poly-SUMO1 chain formation by GSNO observed in Fig. 1E did not result from inhibition of SCE1-SUMO1 thioester formation.
We next determined whether SNO formation at Cys139 might interfere with SUMO1 transfer to a given target. To explore this, we performed in vitro SUMOylation reactions using Saccharomyces cerevisiae proliferating cell nuclear antigen (ScPCNA), a model substrate (23). Similar to poly-SUMO chain formation, the SUMOylation of ScPCNA was inhibited by GSNO pretreatment of SCE1 (Fig. 1H). Collectively, these data suggest that mutation of SCE1 Cys139 does not affect the activity of this enzyme and significantly, S-nitrosylation of this redox-active residue blunts SCE1 function by inhibiting the ability of SCE1 to transfer SUMO to its substrates, rather than interfering with SCE1-SUMO thioester formation.
S-Nitrosylation of SCE1 Cys139 Inhibits SUMOylation In Vivo.
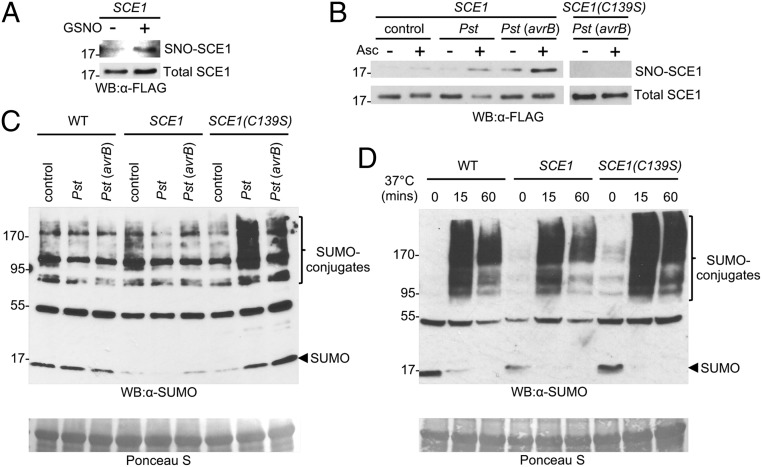
We next explored whether SCE1 is subjected to S-nitrosylation in vivo by generating transgenic plants expressing either FLAG epitope-tagged WT or C139S SCE1. Comparable expression between these lines was confirmed (SI Appendix, Fig. S3 A and B) and SUMO1/2 coimmunoprecipitated with both FLAG-SCE1 and FLAG-C139S at the expected SCE1-SUMO thioester molecular weight, suggesting that these proteins are active in vivo (SI Appendix, Fig. S3C). We tested whether SCE1 expressed in plants can be S-nitrosylated by GSNO by subjecting protein extracts to BST analysis, followed by isolation of S-nitrosylated proteins and their subsequent analysis by Western blotting using an anti-FLAG antibody, to detect the possible presence of FLAG-SCE1 among these protein SNOs. Very little SNO-SCE1 was detected under basal conditions, but preincubating the extracts with 1 mM GSNO resulted in S-nitrosylation of SCE1 (Fig. 2A) suggesting that FLAG-SCE1 expressed in vivo can be S-nitrosylated.
Fig. 2.
S-nitrosylation of SCE1 Cys139 inhibits SUMOylation in vivo. (A) Protein extracts from 35S::FLAG-SCE1 plants were subjected to the BST with or without preincubation with 1 mM GSNO. Biotinylated proteins were enriched by streptavidin affinity pull-down, followed by Western blot analysis against FLAG. (B) Protein extracts from controls or plants inoculated with 107 cfu/mL Pst DC3000 or Pst DC3000 (avrB) (6 hpi) were subjected to the BST, and both SNO-SCE1 and total SCE1 were detected by Western blot against FLAG. (C) Plants were inoculated as in B, and leaf tissue was collected at 6 hpi. Protein extracts were then analyzed by Western blot analysis against SUMO1/2. Ponceau S staining of the large subunit of Rubisco indicates equal loading. (D) Liquid-grown seedlings of the stated lines were exposed to 37 °C for the indicated times and analyzed as in C.
Global SNO levels are increased in Arabidopsis on pathogen recognition (24, 25), so we performed the BST on plants challenged with either the virulent bacterial pathogen Pseudomonas syringae pv. tomato (Pst) DC3000 or an avirulent strain expressing AvrB, recognized by the RPM1 resistance protein in WT Col-0 plants (26). Low levels of SNO-SCE1 were detected in noninoculated plants (Fig. 2B), while SNO-SCE1 levels were increased at 6 h postinoculation (hpi) with either Pst DC3000 or especially Pst DC3000 (avrB), suggesting that S-nitrosylation of SCE1 is enhanced in response to a pathogen challenge (Fig. 2B). Importantly, SCE1(C139S) was not S-nitrosylated in response to attempted pathogen ingress (Fig. 2B), implying that Cys139 is also the site of SNO formation in vivo. Furthermore, total SNO levels increased significantly at 6 hpi following challenge with same Pst DC3000 strains (SI Appendix, Fig. S4A). Collectively, these data suggest that after pathogen recognition, global SNO levels are increased, promoting S-nitrosylation of SCE1 at Cys139.
Since SCE1(C139S) is insensitive to S-nitrosylation and this modification was shown to inhibit SUMO-conjugating activity in vitro, we next examined the impact of SCE1(C139S) expression on global SUMOylation levels in either the absence or presence of a pathogen challenge. Consistent with a previous report (16), Pst DC3000 inoculation had no observable effect on global SUMOylation in leaves of WT plants, with similar results observed for SCE1-expressing plants (Fig. 2C). Strikingly, in the SCE1(C139S) line, SUMO conjugate levels were increased after challenge with either Pst DC3000 or Pst DC3000 (avrB) (Fig. 2C). In a similar fashion, SCE1(C139S) plants also exhibited increased and prolonged SUMOylation following heat shock, which also results in rapid NO synthesis (27) (Fig. 2D). Thus, S-nitrosylation of Cys139 following engagement of the pathogen-triggered nitrosative burst may be required to suppress SCE1 activity and, by extension, SUMOylation during plant immune function.
Cys139 of SCE1 Is Required for Immunity and Stress-Induced Gene Expression.
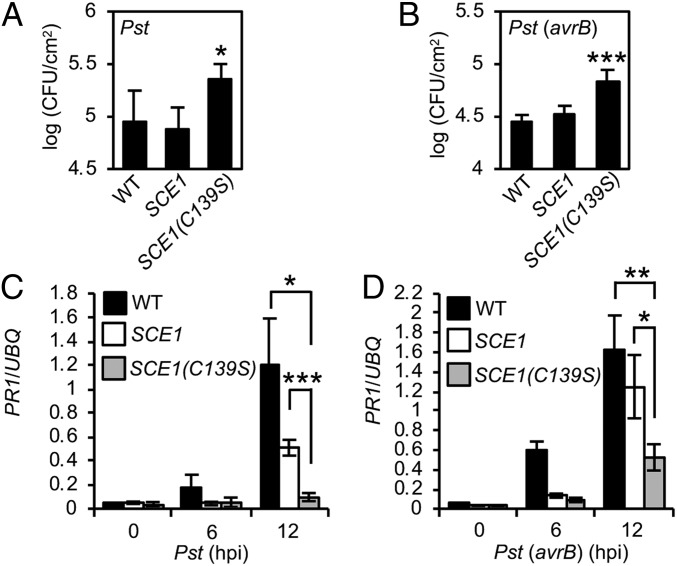
After establishing that S-nitrosylation of SCE1 at Cys139 is driven by attempted pathogen infection, we next explored the biological consequences of this redox-based modification on plant disease resistance. Bacterial growth assays revealed that compared with WT and SCE1-expressing plants, which showed similar levels of pathogen growth, SCE1(C139S) plants were more susceptible to infection by Pst DC3000 (Fig. 3A). Similarly, the SCE1(C139S) line exhibited increased growth of Pst DC3000 (avrB) (Fig. 3B). Additional independent transgenic lines showed similar results (SI Appendix, Fig. S4 B and C). However, the difference in bacterial titer between Pst DC300 and Pst DC3000 (avrB) was similar between SCE1- and SCE1(C139S)-expressing plants, that suggesting RPM1-mediated disease resistance might not be impacted. In aggregate, these data imply that SNO formation at Cys139 of SCE1 is required for full basal disease resistance.
Fig. 3.
Cys139 of SCE1 is required for resistance to Pst DC3000. (A and B) Plants were inoculated with 105 cfu/mL Pst DC3000 (A) or Pst DC3000 (avrB) (B), and leaf discs were assayed for bacterial growth at 3 dpi. Data points represent mean ± SD (n = 6 biological replicates), with asterisks indicating significant difference from WT (P < 0.05, Student’s t test). (C) Plants were inoculated with 106 cfu/mL Pst DC3000, and leaf tissue was harvested at the stated times. The expression of PR1 was analyzed by qPCR and normalized against the constitutively expressed UBQ5. Data points represent mean ± SD (n = 3) of 3 independent biological samples, and asterisks represent significant differences between the indicated samples (*P < 0.05, ***P < 0.0001, Student’s t test). (D) Plants were inoculated with 106 cfu/mL Pst DC3000 (avrB), and PR1 expression was analyzed as in C.
To uncover the molecular basis of these observations, the expression of the SA marker gene, PR1, was monitored after Pst DC3000 inoculation. As expected, PR1 expression was induced in WT plants and, more pertinently, in SCE1 plants at 12 hpi (Fig. 3C). In contrast, PR1 expression was compromised in SCE1(C139S) plants (Fig. 3C). Furthermore, Pst DC3000 (avrB)-induced PR1 expression was also reduced at 12 hpi in SCE1(C139S) plants (Fig. 3D).
Since pathogen-induced gene expression was compromised in SCE1(C139S) plants that also displayed higher levels of SUMO conjugation, we tested whether similar links between SUMO conjugate levels and transcriptional responses might exist in response to heat shock. It is well established that heat stress induces NO levels in plants (28), so we monitored expression of the heat-stress marker gene HsfA3 in seedlings after exposure to 37 °C for 1 h. HsfA3 expression was induced to similar levels in WT and SCE1 plants but was not induced in SCE1(C139S) plants (SI Appendix, Fig. S4D) suggesting that signaling through C139 of SCE1 is also required for optimal transcriptional responses to heat stress.
S-Nitrosylation of SCE1 Impacts Plant Immunity.
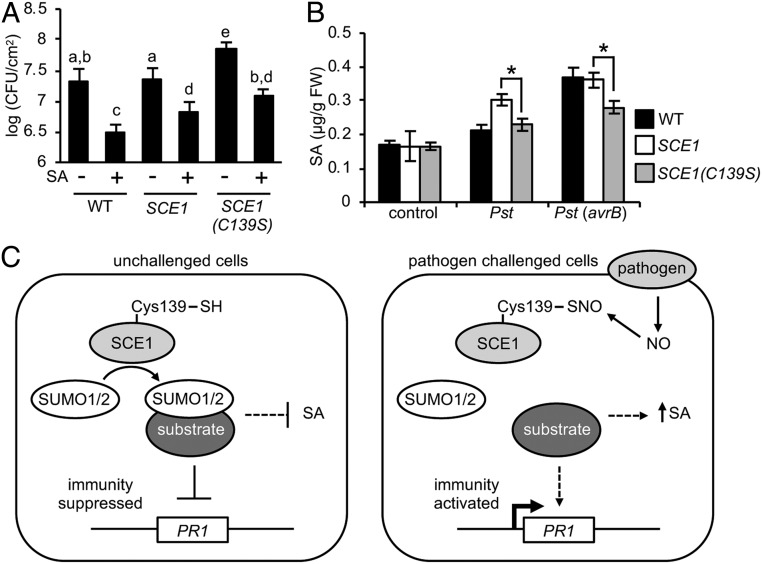
We next tested SA-induced immunity in SCE1 and SCE1(C139S) plants. As expected, pretreatment of WT plants with SA resulted in dramatically less growth of Pst DC3000 compared with mock-treated lines (Fig. 4A). In agreement with previous experiments, mock-treated SCE1(C139S) plants showed significantly higher levels of Pst DC3000 growth compared with mock-treated WT and SCE1 plants; however, SA treatment of both SCE1 and SCE1(C139S) plants reduced the titer of Pst DC3000 to similar levels (Fig. 4A).
Fig. 4.
S-nitrosylation of SCE1 impacts plant immunity .(A) Plants were sprayed with either 0.5 mM SA or H2O and inoculated with 105 cfu/mL Pst DC3000 at 24 h after spraying. Leaf discs were then assayed for bacterial growth at 3 dpi. Data points represent mean ± SD (n = 6 biological replicates), with letters indicating significant differences between samples (α = 0.05, Tukey–Kramer ANOVA). (B) Plants were inoculated with 106 cfu/mL Pst DC3000 or Pst DC3000 (avrB), and SA levels were measured after 24 h. Data points represent mean ± SD (n = 3 biological replicates), with asterisks representing significant differences between the indicated samples (P < 0.01, Student’s t test). (C) In pathogen-unchallenged plant cells, SCE1-dependent conjugation of SUMO1/2 contributes to the repression of PR1 gene expression in part by limiting SA levels. In pathogen-challenged cells, increasing NO levels associated with attempted pathogen infection promote the S-nitrosylation of SCE1 at Cys139. This inhibits the SUMO-conjugating activity of SCE1 and reduces global SUMOylation, which in turn allows accumulation of SA, relieves repression of PR1 gene expression, and contributes to the activation of immunity.
To confirm that exogenous SA treatment rescued immunity in SCE1(C139S) plants, we monitored SA-induced PR1 expression. SA-induced PR1 expression reached similar levels in WT, SCE1, and SCE1(C139S) plants (SI Appendix, Fig. S5). Therefore, to establish whether the disease-susceptible phenotype of SCE1(C139S) plants is attributed to reduced endogenous SA accumulation, we performed a challenge with Pst DC3000 to induce accumulation of this metabolite and subsequently determined its concentration. SA accumulated to a lesser extent in SCE1(C139S) plants compared with SCE1 plants in response to both Pst DC3000 and Pst DC3000 (avrB) (Fig. 4B). Collectively, these data suggest that S-nitrosylation of SCE1 at C139 is required for maximal SA accumulation and associated disease resistance. Thus, our findings support a model in which attempted pathogen infection promotes increasing levels of NO, leading to S-nitrosylation of SCE1 at Cys139. This serves to limit SUMO1/2 conjugation by SCE1, enabling both the accumulation of SA and maximal activation of SA-dependent defense gene expression (Fig. 4C).
Discussion
SUMO conjugation has been implicated in a plethora of regulatory systems across eukaryotes, including human disease pathways (29). The control of global SUMOylation integral to cellular signaling is currently thought to occur predominantly at the level of each protein target via local regulatory mechanisms, rather than by direct modulation of the core SUMOylation machinery by PTMs (30). However, acetylation or SUMOylation of UBC9 is thought to enable discrimination between individual target substrates (31, 32). Conversely, the redox-active small molecule hydrogen peroxide has been shown to reduce total SUMOylation by driving the formation of disulfide bonds between SUMO E1 and E2 enzymes (33). These modifications have not yet been linked to cellular signaling, however. Our findings suggest that changes in global SUMOylation that underpin plant immune function may result from direct regulation of the SUMOylation apparatus by SNO formation at Cys139 of SCE1. This means of controlling SUMO conjugation may also serve to limit SUMOylation of proteins involved in heat-stress signaling and thus may be a widespread means of transcriptional regulation in general. Significantly, this Cys is evolutionary conserved and specifically S-nitrosylated in the corresponding human enzyme, UBC9, modulating its activity. Therefore, this mechanism might be conserved between plants and animals, thereby providing a potential target for either future agrochemical or pharmaceutical intervention, respectively.
The effect of S-nitrosylation on enzymatic activity can typically be directly mediated through modification of active site Cys residues (4). However, our findings suggest that Cys139 is the sole S-nitrosylation site of SCE1 both in vitro and in vivo. In a mutational study of S. cerevisiae Ubc9, residues close to this area were shown to be important for Smt3p-Smt3p conjugate formation (34). Similar to S-nitrosylation of SCE1 at Cys139, these same mutations did not have any effect on Ubc9-Smt3p thioester formation. The fact that S-nitrosylation of SCE1 at Cys139 does not affect SUMO thioester formation suggests that it does not interfere with binding to the E1 complex. This is not surprising, since a well-defined region of the Ubc9 N terminal has been identified as the binding site for E1:E2 noncovalent interactions (34–37), and Cys139 is located at a distant site near the C terminus. Although there are currently no structures available for components of the Arabidopsis SUMOylation machinery, data from the structure of human Ubc9 in complex with the SUMO substrate RanGAP1 reveal that residues close to Cys138 on the same α-helix are important for interaction with RanGAP1 (38). Mutation of a conserved tyrosine to phenylalanine (Y134F) dramatically reduced the ability of Ubc9 to conjugate SUMO to RanGAP1, suggesting that this residue plays an important role. This tyrosine is conserved in SCE1 (Tyr135), and its side chain is predicted to occupy a similar position in Ubc9. Therefore, a possible mechanism for S-nitrosylation of Cys139 to inhibit SUMOylation is through interference with interactions between Tyr135 and substrate proteins.
The means by which SUMOylation by SUMO1/2 regulates plant immunity are now beginning to emerge. Loss of SIZ1 function results in elevated SA levels, constitutive activation of PR gene expression, and increased resistance to Pst DC3000 (16). These phenotypes were reverted to WT by expression of the bacterial salicylate hydroxylase NahG, which degrades SA, suggesting that the phenotypes of siz1 mutant plants are due to the elevated levels of SA. More recently, evidence has emerged that SIZ1-mediated SUMOylation of TPR1 inhibits its transcriptional repressor activity (39). Since TPR1 functions together with SNC1 to activate plant immunity (40), it appears that the TPR1/SNC1 complex is a central node of SUMO-mediated immune signaling in plants (18, 39, 41). Interestingly, studies also suggest that SUMO1/2 conjugation regulated by SUMO proteases might have a positive role in SA signaling. Mutation of the SUMO proteases OVERLY TOLERANT TO SALT1 and -2 (OTS1/2) has been shown to increase SA levels in plants, leading to constitutive activation of SA signaling pathways (42). Similarly, the SUMO protease EARLY IN SHORT DAYS 4 (ESD4) has also been shown to accumulate SA (43). Therefore, SUMOylation by SUMO1/2 appears to have both positive and negative effects on SA-mediated immunity. Indeed, SUMOylation can both positively and negatively regulate immune responses in animals depending on the substrate proteins affected (44). Therefore, the substrates that are SUMOylated at a given time under certain cellular conditions can have diverse effects on signaling at the organism level.
To further elucidate the complex roles of SUMOylation in plant immunity, proteins that are SUMOylated after immune activation must be identified and the effect of their modification studied. Interestingly, the central regulator of SA-mediated immunity, the transcription coactivator NONEXPRESSOR OF PATHOGENESIS RELATED 1 (NPR1) has recently been identified as a SUMO substrate (15). Modification of NPR1 by SUMO3 appears to be regulate its stability and binding to cognate transcription factors promoting immunity. In Arabidopsis, SUMO3 is expressed at lower levels and appears to be conjugated to far fewer proteins compared with SUMO1/2 (17, 20). Furthermore, patterns of SUMO3 conjugation do not appear to be affected by various cellular stresses that increase conjugation of SUMO1/2 (20). However, SUMO3 expression is strongly induced by SA, suggesting a key role in SA-mediated immunity (17). Thus, SUMO3 conjugation is also a key regulatory mechanism in plant immunity. Nonetheless, the fact that suppression by SUMO1/2 conjugation appears to be required to prevent the induction of immune function (17) suggests that defense responses are constitutively primed and ready for rapid deployment. Thus, our proposed model may constitute a molecular mechanism by which the nitrosative burst associated with attempted pathogen ingress is perceived and translated into immune activation.
Materials and Methods
Detailed descriptions of materials and methods used in this study, including plant growth conditions, pathogen inoculations, protoplast isolation, protein analyses, gene expression analyses, and statistics, are provided in SI Appendix, Materials and Methods. All primers used are listed in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
We thank Ron Hay and Ellis Jaffray (University of Dundee) for providing the human proteins and constructs used in this study. This work was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) studentship to M.J.S. (BB/F017073/1), a Pakistan Higher Education Commission scholarship to S.I.M., a Royal Society Research Fellowship (UF090321) and research grant to S.H.S. (Rg110495), and BBSRC research grant BB/DO11809/1 to G.J.L.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900052116/-/DCSupplemental.
References
- 1.Delledonne M., Xia Y., Dixon R. A., Lamb C., Nitric oxide functions as a signal in plant disease resistance. Nature 394, 585–588 (1998). [DOI] [PubMed] [Google Scholar]
- 2.MacMicking J. D., et al. , Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 94, 5243–5248 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun B. W., et al. , S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 478, 264–268 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Hess D. T., Matsumoto A., Kim S. O., Marshall H. E., Stamler J. S., Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 6, 150–166 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Scheler C., Durner J., Astier J., Nitric oxide and reactive oxygen species in plant biotic interactions. Curr. Opin. Plant Biol. 16, 534–539 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Chen J., Vandelle E., Bellin D., Delledonne M., Detection and function of nitric oxide during the hypersensitive response in Arabidopsis thaliana: Where there’s a will, there’s a way. Nitric Oxide 43, 81–88 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Coll N. S., Epple P., Dangl J. L., Programmed cell death in the plant immune system. Cell Death Differ. 18, 1247–1256 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durner J., Wendehenne D., Klessig D. F., Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. U.S.A. 95, 10328–10333 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zago E., et al. , Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol. 141, 404–411 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui B., et al. , S-nitrosylation of the zinc finger protein SRG1 regulates plant immunity. Nat. Commun. 9, 4226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay R. T., SUMO: A history of modification. Mol. Cell 18, 1–12 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Saracco S. A., Miller M. J., Kurepa J., Vierstra R. D., Genetic analysis of SUMOylation in Arabidopsis: Conjugation of SUMO1 and SUMO2 to nuclear proteins is essential. Plant Physiol. 145, 119–134 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desterro J. M., Rodriguez M. S., Kemp G. D., Hay R. T., Identification of the enzyme required for activation of the small ubiquitin-like protein SUMO-1. J. Biol. Chem. 274, 10618–10624 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Hanania U., Furman-Matarasso N., Ron M., Avni A., Isolation of a novel SUMO protein from tomato that suppresses EIX-induced cell death. Plant J. 19, 533–541 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Saleh A., et al. , Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe 18, 169–182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J., et al. , Salicylic acid-mediated innate immunity in Arabidopsis is regulated by SIZ1 SUMO E3 ligase. Plant J. 49, 79–90 (2007). [DOI] [PubMed] [Google Scholar]
- 17.van den Burg H. A., Kini R. K., Schuurink R. C., Takken F. L., Arabidopsis small ubiquitin-like modifier paralogs have distinct functions in development and defense. Plant Cell 22, 1998–2016 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gou M., et al. , Sumoylation E3 ligase SIZ1 modulates plant immunity partly through the immune receptor gene SNC1 in Arabidopsis. Mol. Plant Microbe Interact. 30, 334–342 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Saitoh H., Hinchey J., Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 275, 6252–6258 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Kurepa J., et al. , The small ubiquitin-like modifier (SUMO) protein modification system in Arabidopsis: Accumulation of SUMO1 and -2 conjugates is increased by stress. J. Biol. Chem. 278, 6862–6872 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Jaffrey S. R., Snyder S. H., The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 2001, pl1 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Tatham M. H., et al. , Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 276, 35368–35374 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Colby T., Matthäi A., Boeckelmann A., Stuible H.-P., SUMO-conjugating and SUMO-deconjugating enzymes from Arabidopsis. Plant Physiol. 142, 318–332 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feechan A., et al. , A central role for S-nitrosothiols in plant disease resistance. Proc. Natl. Acad. Sci. U.S.A. 102, 8054–8059 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maldonado-Alconada A. M., et al. , Proteomic analysis of Arabidopsis protein S-nitrosylation in response to inoculation with Pseudomonas syringae. Acta Physiol. Plant. 33, 1493–1514 (2011). [Google Scholar]
- 26.Grant M. R., et al. , Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269, 843–846 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Xuan Y., Zhou S., Wang L., Cheng Y., Zhao L., Nitric oxide functions as a signal and acts upstream of AtCaM3 in thermotolerance in Arabidopsis seedlings. Plant Physiol. 153, 1895–1906 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parankusam S., Adimulam S. S., Bhatnagar-Mathur P., Sharma K. K., Nitric oxide (NO) in plant heat stress tolerance: Current knowledge and perspectives. Front. Plant Sci. 8, 1582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flotho A., Melchior F., Sumoylation: A regulatory protein modification in health and disease. Annu. Rev. Biochem. 82, 357–385 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Bossis G., Melchior F., SUMO: Regulating the regulator. Cell Div. 1, 13 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh Y. L., et al. , Ubc9 acetylation modulates distinct SUMO target modification and hypoxia response. EMBO J. 32, 791–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knipscheer P., et al. , Ubc9 sumoylation regulates SUMO target discrimination. Mol. Cell 31, 371–382 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Bossis G., Melchior F., Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 21, 349–357 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Bencsath K. P., Podgorski M. S., Pagala V. R., Slaughter C. A., Schulman B. A., Identification of a multifunctional binding site on Ubc9p required for Smt3p conjugation. J. Biol. Chem. 277, 47938–47945 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Tatham M. H., et al. , Role of an N-terminal site of Ubc9 in SUMO-1, -2, and -3 binding and conjugation. Biochemistry 42, 9959–9969 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Knipscheer P., van Dijk W. J., Olsen J. V., Mann M., Sixma T. K., Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J. 26, 2797–2807 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capili A. D., Lima C. D., Structure and analysis of a complex between SUMO and Ubc9 illustrates features of a conserved E2-Ubl interaction. J. Mol. Biol. 369, 608–618 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernier-Villamor V., Sampson D. A., Matunis M. J., Lima C. D., Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108, 345–356 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Niu D., et al. , SIZ1-Mediated SUMOylation of TPR1 suppresses plant immunity in Arabidopsis. Mol. Plant 12, 215–228 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Zhu Z., et al. , Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc. Natl. Acad. Sci. U.S.A. 107, 13960–13965 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammoudi V., et al. , The Arabidopsis SUMO E3 ligase SIZ1 mediates the temperature dependent trade-off between plant immunity and growth. PLoS Genet. 14, e1007157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey M., et al. , Stability of small ubiquitin-like modifier (SUMO) proteases OVERLY TOLERANT TO SALT1 and -2 modulates salicylic acid signalling and SUMO1/2 conjugation in Arabidopsis thaliana. J. Exp. Bot. 67, 353–363 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villajuana-Bonequi M., et al. , Elevated salicylic acid levels conferred by increased expression of ISOCHORISMATE SYNTHASE 1 contribute to hyperaccumulation of SUMO1 conjugates in the Arabidopsis mutant early in short days 4. Plant J. 79, 206–219 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Hannoun Z., Maarifi G., Chelbi-Alix M. K., The implication of SUMO in intrinsic and innate immunity. Cytokine Growth Factor Rev. 29, 3–16 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.