Significance
Tumor necrosis factor (TNF) is a cytokine that induces signaling via two receptors, TNFR1 and TNFR2. TNF signaling via TNFR1 contributes to development and maintenance of neuropathic pain. Here, we show that TNFR2 is essential for recovery from neuropathic pain across sexes. Treatment of male and female neuropathic mice with a TNFR2 agonist resulted in long-lasting recovery from neuropathic pain. We identified Tregs as the cellular mediator of the analgesic effect of TNFR2. Indeed, TNFR2 agonist administration alleviated peripheral and central inflammation and promoted neuroprotection in a Treg-dependent manner, indicating that TNFR2-dependent modulation of immunity is neuroprotective. We therefore argue that TNFR2 agonists might be a class of nonopioid drugs that can promote long-lasting pain recovery in males and females.
Keywords: TNF, TNFR2, tmTNF, neuropathic pain, Tregs
Abstract
Tumor necrosis factor receptor 2 (TNFR2) is a transmembrane receptor that is linked to immune modulation and tissue regeneration. Here, we show that TNFR2 essentially promotes long-term pain resolution independently of sex. Genetic deletion of TNFR2 resulted in impaired neuronal regeneration and chronic nonresolving pain after chronic constriction injury (CCI). Further, pharmacological activation of TNFR2 using the TNFR2 agonist EHD2-sc-mTNFR2 in mice with chronic neuropathic pain promoted long-lasting pain recovery. TNFR2 agonist treatment reduced neuronal injury, alleviated peripheral and central inflammation, and promoted repolarization of central nervous system (CNS)-infiltrating myeloid cells into an antiinflammatory/reparative phenotype. Depletion of regulatory T cells (Tregs) delayed spontaneous pain recovery and abolished the therapeutic effect of EHD2-sc-mTNFR2. This study therefore reveals a function of TNFR2 in neuropathic pain recovery and demonstrates that both TNFR2 signaling and Tregs are essential for pain recovery after CCI. Therefore, therapeutic strategies based on the concept of enhancing TNFR2 signaling could be developed into a nonopioid therapy for the treatment of chronic neuropathic pain.
Neuropathic pain affects ∼8% of the general population and is caused by a damage to the somatosensory nervous system (1). It is associated with hypersensitivity to painful (hyperalgesia) and nonpainful (allodynia) stimuli (2) and negatively impacts a person’s level of functioning and quality of life. The resistance to available pain therapies makes chronic neuropathic pain a major unmet medical need (1). Recent findings suggest that the immune system plays a role in mediating neuropathic pain (3).
Tumor necrosis factor (TNF) is a master regulator of the immune system that initiates and orchestrates inflammation. Up-regulated levels of TNF have been shown at the site of injury in animal models of peripheral nerve injury and in neuropathic humans (4), and endoneurial application of TNF was shown to induce pain symptoms in the absence of an injury (5). Interestingly, the two TNF receptors (TNFR), TNFR1 and TNFR2, induce diverse and sometimes opposing biological responses. Whereas TNFR1 signaling promotes inflammation and tissue degeneration, TNFR2 contributes to immune regulation as well as tissue homeostasis and regeneration (6, 7). We and others have shown that TNFR1 mediates mechanical allodynia and thermal hyperalgesia and plays a role for both development of neuropathic pain, as well as maintenance of pain (8, 9). Our recent data indicate sex-specific differences in TNFR1-dependent pain pathways and show that TNFR1 expression is essential for pain development in male mice, but not in female mice. Accordingly, pharmaceutical blocking of TNFR1 signaling was only therapeutic in males, but not in female animals (10), while the same drug was therapeutic in females for motor disease (11, 12). In contrast to the general role of TNFR1 for pain development and maintenance, TNFR2 seems to be more important for the early phase of inflammation and neuropathic pain development (13, 14). However, TNFR2 has a well-documented role in tissue regeneration and immune modulation (6, 7, 15). The immunomodulatory properties of TNFR2 are mainly mediated by the expansion and stabilization of regulatory T cells (Tregs) (16, 17), a highly specialized subpopulation of T cells that function to suppress immune responses. Previous data indicate that enrichment or depletion of Tregs at the time of injury influence the development of neuropathic pain in models of peripheral nerve injury (18, 19), indicating a regulatory role of Tregs during the initiation of pain.
It has been suggested that the mechanisms of development of chronic pain states differed between sexes, implying a potential need for different strategies to address chronic pain in men and women (20). We therefore investigated the role of TNFR2 for pain recovery across sexes. We determined that expression of TNFR2 is essential for pain recovery and that systemic administration of a TNFR2 agonist is therapeutic for peripheral nerve injury-induced neuropathic pain via Tregs independently of sex.
Results
Recovery from Mechanical Allodynia Is Dependent on Presence of Tregs.
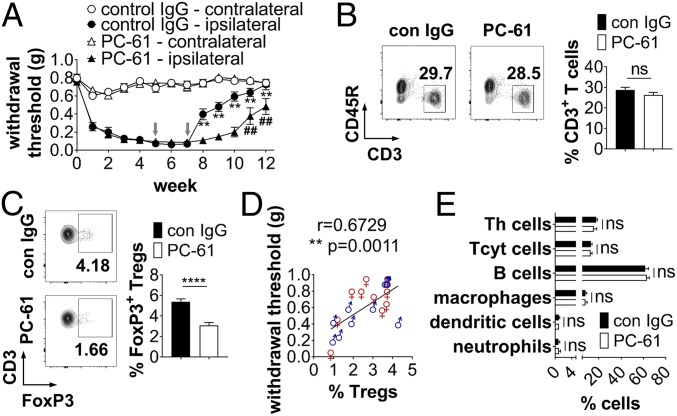
Previous studies indicated that depletion of Tregs increases severity of mechanical allodynia (19) and that expansion of Tregs attenuates development of mechanical hypersensitivity (18) in male animals. Due to the different pathways underlying pain development in males and females, we analyzed the role of Tregs in spontaneous pain recovery in both sexes. First, we confirmed that male and female (SI Appendix, Fig. S1) WT mice start recovering from chronic constriction injury (CCI)-mediated mechanical allodynia 8 wk after peripheral nerve injury. Then, we depleted Tregs in injured male and female mice during the recovery phase starting 5 wk postinjury (wpi) using the anti-CD25 antibody PC-61, which depletes more than 75% of Tregs through immune-dependent mechanisms and is widely used to interrogate Treg function (21). In male and female mice, depletion of Tregs with PC-61 delayed recovery for ∼3 wk compared to control IgG-treated mice (Fig. 1A), with the effect of Treg depletion being stronger in males than in females (SI Appendix, Fig. S2). Whereas the total percentage of splenic CD3+ T cells was not altered (Fig. 1B), we observed a significant reduction of FoxP3+ Tregs 12 wpi (Fig. 1C). Further, the levels of Tregs were correlated with the recovery of both male and female mice from both treatment groups (r = 0.6729, **P = 0.0011, Fig. 1D and SI Appendix, Fig. S2C), indicating that Tregs are important for resolution of mechanical allodynia in males and females. No changes in the percentage of other immune cell subsets were observed between control IgG and PC-61–treated animals (Fig. 1E).
Fig. 1.
Treg depletion delays recovery after CCI. (A) WT mice underwent CCI at week 0. Mechanical allodynia was determined using the Von Frey test over a period of 12 wk. Five and seven weeks after injury, mice were injected with control IgG (con IgG) or PC-61 (500 µg per i.p. injection, gray arrows) to deplete Tregs (n = 10 per group). **P < 0.01 con IgG vs. PC-61 ipsilateral paw, ##P < 0.01 wk 6–10 vs. week 5 PC-61 ipsilateral paw. (B–E) Percentage of splenic CD3+ T cells (B) and CD25+FoxP3+ Tregs (C) (population: CD3+ T cells) was quantified by flow cytometry 12 wpi. Shown are scatterplots from representative experiments. Values in each quadrant are the percentage of positive cells. Quantification shows the mean ± SEM. (D) Correlation of the percentage of splenic Tregs with the withdrawal threshold at 12 wpi (n = 10 per sex). (E) Quantification of the percentage of different splenic immune cell subsets of CCI animals 12 wpi (n = 10 each group). ****P < 0.0001; ns, not significant.
TNFR2 Is Essential for Recovery from Mechanical Allodynia.
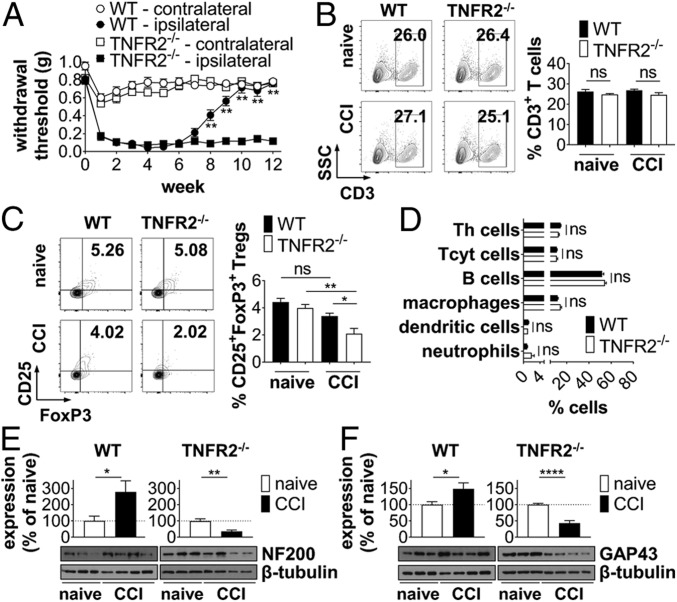
Due to the well-documented role of TNFR2 in neuronal recovery (7) and Treg function (22), we next compared pain recovery of WT and TNFR2−/− mice after CCI. Interestingly, male and female TNFR2−/− animals developed chronic nonresolving pain and did not recover from mechanical allodynia (Fig. 2A and SI Appendix, Fig. S3). Whereas no changes in the percentage of total splenic CD3+ T cells were observed (Fig. 2B), the percentage of CD25+FoxP3+ Tregs was significantly decreased in injured TNFR2−/− mice, compared to injured WT and noninjured TNFR2−/− mice (Fig. 2C). No differences in the percentages of other immune cells were observed between injured WT and TNFR2−/− mice (Fig. 2D). Further, no significant changes were observed in the percentage of all analyzed immune cell subsets between naïve noninjured WT and TNFR2−/− mice (Fig. 2 B and C and SI Appendix, Fig. S3). Interestingly, whereas levels of neurofilament 200 (NF200), a marker for large myelinated A-β fibers, were increased in WT mice, TNFR2−/− mice showed chronic loss of NF200 12 wpi in the spinal cord (Fig. 2E). Similarly, whereas levels of Growth Associated Protein 43 (GAP43), a protein indicative of neuronal repair, were increased in WT mice, TNFR2−/− mice show decreased levels of GAP43 (Fig. 2F), suggesting impaired neurorepair in TNFR2−/− mice following CCI. Levels of CD68 (SI Appendix, Fig. S5A), a protein highly expressed by macrophages, were up-regulated in both WT and TNFR2−/− mice. Similar, expression of calcitonin gene-related peptide (CGRP) was up-regulated after injury in both groups (SI Appendix, Fig. S5B). CGRP is a neuropeptide that is primarily released from sensory neurons and is up-regulated after nerve damage and neuroinflammation (23). Up-regulated CGRP levels have been implicated in pain pathways (24). These data indicate that the expression level of CD68 and CGRP is not influenced by endogenous TNFR2 signaling (SI Appendix, Fig. S5C). Baseline expression levels of all analyzed proteins in naïve WT and TNFR2−/− mice were similar (SI Appendix, Fig. S5D).
Fig. 2.
TNFR2−/− mice have chronic nonresolving pain. (A) WT and TNFR2−/− mice underwent CCI at week 0. Mechanical allodynia was determined using the Von Frey test over a period of 12 wk (mean ± SEM, WT: n = 12, TNFR2−/−: n = 20) **P < 0.01 WT vs. TNFR2−/− ipsilateral paw. (B–D) Percentage of splenic CD3+ T cells (B) and CD25+FoxP3+ Tregs (C) (population: CD3+ T cells) and and other immune cell subsets (D) was quantified by flow cytometry 12 wpi. Shown are scatterplots from representative animals. Values in each quadrant are the percentage of positive cells. Quantification shows the mean ± SEM, WT: n = 8, TNFR2−/−: n = 10. Expression of NF200 (E) and GAP43 (F) in the spinal cord of naïve and injured WT and TNFR2−/− mice was quantified by Western blot analysis 12 wpi. Shown are representative blots and the mean ± SEM of optical density of each protein normalized to β-tubulin, percentage of protein expression of naïve mice (n = 6 naïve, n = 8 CCI each group). *P < 0.05; **P < 0.01; ****P < 0.0001; ns, not significant.
TNFR2 Agonism Is Therapeutic for Neuropathic Pain.
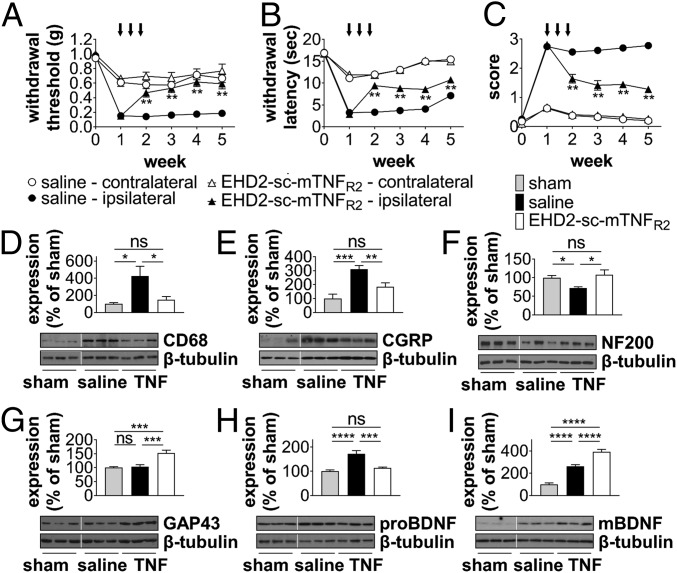
TNFR2 was shown to be expressed on Tregs, activated T cells, myeloid cells, and other immune cells (25). We here confirmed that Tregs and myeloid cells expressed the highest levels of TNFR2. TNFR2 expression was increased on almost all analyzed immune cell subsets, besides peripheral neutrophils and spinal cord macrophages after CCI (SI Appendix, Figs. S6 and S7). We next investigated whether exogenous activation of TNFR2 is therapeutic in the CCI model. To selectively activate TNFR2, we used the mouse TNFR2 agonist EHD2-sc-mTNFR2 (26, 27). Treatment with EHD2-sc-mTNFR2 was started 1 wpi (day 7) and repeated twice at day 10 and 13. Male and female mice had significantly recovered from mechanical allodynia 1 wk after treatment start and although treatment was stopped after the third injection, mice did not relapse (Fig. 3A and SI Appendix, Fig. S8A). In addition to its therapeutic effect on tactile allodynia, TNFR2 agonism was also therapeutic on thermal hyperalgesia (Fig. 3B and SI Appendix, Fig. S8B) and cold allodynia (Fig. 3C and SI Appendix, Fig. S8C), demonstrating a general role of TNFR2 for neuropathic pain recovery. Confirming that the therapeutic effect observed after TNFR2 agonist treatment in WT mice is through on-target effects by activating TNFR2, TNFR2−/− mice did not respond to EHD2-sc-mTNFR2 treatment (SI Appendix, Fig. S9). TNFR2 agonist treatment resulted in reduced microglia/macrophage activity, as seen by reduced up-regulation of CD68 expression in the spinal cord (Fig. 3D) and a reduction of the injury-mediated CGRP increase (Fig. 3E). In line with our data suggesting that TNFR2−/− mice show chronic neurodegeneration and impaired recovery after CCI (Fig. 2 E and F), we observed increased NF200 (Fig. 3F) and GAP43 (Fig. 3G) levels. We further detected that TNFR2 agonist treatment reduced expression of the precursor of brain-derived neurotrophic factor (preBDNF) (Fig. 3H), which promotes neuronal cell death and retraction of growth cones (28). In contrast, increased levels of the mature form of BDNF (mBDNF), a neurotrophin that promotes neuronal survival and regeneration, were found in animals treated with EHD2-sc-mTNFR2 (Fig. 3H).
Fig. 3.
TNFR2 agonism promotes recovery from neuropathic pain. WT mice underwent CCI or sham injury at week 0. Mechanical allodynia (A), thermal hyperalgesia (B), and cold allodynia (C) were determined over a period of 5 wpi. On days 7, 10, and 13, mice were treated with saline or EHD2-sc-mTNFR2 (10 mg/kg) via i.p. injection (black arrows, mechanical allodynia: n = 19 each group, thermal hyperalgesia/cold allodynia: n = 12 each group) **P < 0.01 saline vs. EHD2-sc-mTNFR2 ipsilateral paw. (D–I) Expression of CD68, CGRP, NF200, GAP43, proBDNF, and mBDNF in the spinal cord was quantified by Western blot analysis 5 wpi. Shown are representative blots and the mean ± SEM of optical density of each protein normalized to β-tubulin, percentage of protein expression of sham (sham: n = 6, saline/EHD2-sc-mTNFR2 [TNF]: n = 8). *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
TNFR2 Agonism Reduces Central and Peripheral Inflammation.
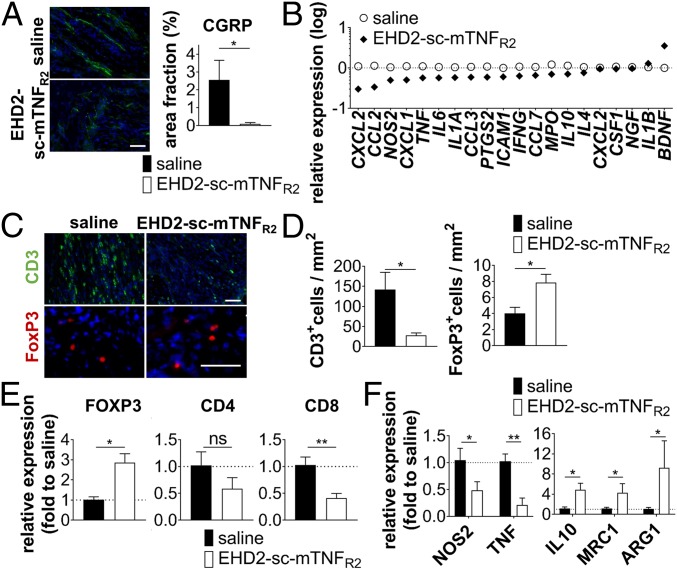
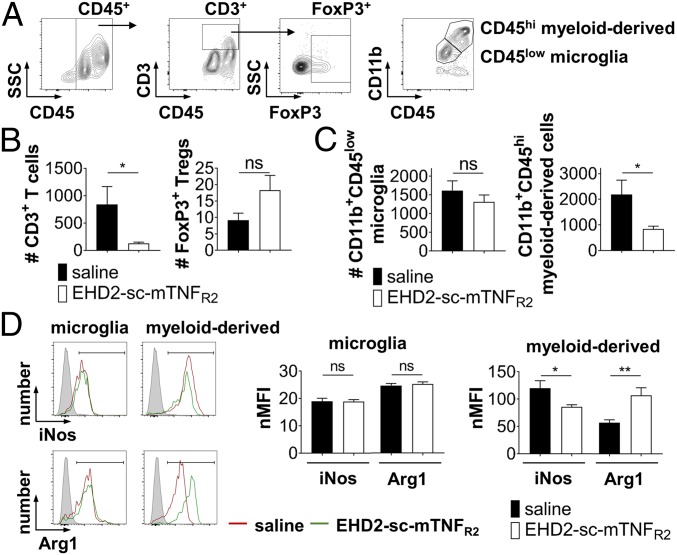
In addition to down-regulating CGRP expression in the spinal cord, EHD2-sc-TNFR2 dramatically decreased the number of CGRP+ neurons in the sciatic nerve (Fig. 4A), indicating decreased neuronal injury and pain signaling in the peripheral nervous system. One of the first clinical signs of peripheral nerve injury is infiltration of immune cells into the peripheral nerve. Concurrently with the reduced pain sensitivity of EHD2-sc-mTNFR2-treated mice, the expression of the great majority of inflammatory markers in the sciatic nerve was down-regulated in TNFR2 agonist-treated mice, whereas expression of BDNF was up-regulated after TNFR2 agonist treatment (Fig. 4B). Compared to saline treatment, we observed reduced infiltration of CD3+ T cells (Fig. 4 C and D), but increased infiltration of FoxP3+ Tregs (Fig. 4 C and D) into the peripheral nerve. Similarly, FOXP3 expression was increased in the spinal cord (Fig. 4E) of mice treated with EHD2-sc-mTNFR2 after injury, whereas CD4 and CD8A gene expression levels in the spinal cord were decreased in EHD2-sc-mTNFR2–treated mice (Fig. 4E). Further, we observed that compared to saline administration, mice treated with EHD2-sc-mTNFR2 showed reduced expression of TNF and NOS2 and increased expression of IL10, MRC1, and ARG1 (Fig. 4F). We therefore next investigated immune cell infiltration into the spinal cord by flow cytometry. Indeed, we confirmed that a reduced number of T effector cells infiltrated the spinal cord after TNFR2 agonist treatment (Fig. 5 A and B). Although we detected higher numbers of FoxP3+ Tregs in the spinal cord after EHD2-sc-mTNFR2 administration, these changes were not significant (Fig. 5 A and B). Further, TNFR2 agonist treatment reduced the infiltration of infiltrating myeloid-derived cells into the spinal cord but did not impact the number of microglia (Fig. 5 A and C). Interestingly, whereas TNFR2 agonist treatment did not impact microglia polarization, expression of inducible nitric oxide synthase (iNos) was decreased and expression of arginase 1 (Arg1) was increased after EHD2-sc-mTNFR2 administration in myeloid-derived cells (Fig. 5D).
Fig. 4.
TNFR2 agonism promotes antiinflammatory responses in the sciatic nerve and spinal cord. (A) CGRP levels were quantified by immunofluorescence analysis 2 wpi. Shown are representative pictures and quantification of CGRP signal (mean ± SEM, n = 4 per group). (B) Expression of different genes in the sciatic nerve was quantified by quantitative real-time PCR. Data are shown as log (fold expression to naive) (mean ± SEM, saline: n = 4, EHD2-sc-mTNFR2: n = 6). (C and D) Infiltration of CD3+ and FOXP3+ T cells into the sciatic nerve was quantified by immunofluorescence analysis 2 wpi (mean ± SEM, n = 4 per group). (E and F) Gene expression of FOXP3, CD4, CD8A, NOS2, TNF, IL10, MRC1, and ARG1 in the spinal cord 2 wpi was quantified by quantitative real-time PCR (mean ± SEM, saline n = 4, EHD2-sc-mTNFR2 n = 6). *P < 0.05; **P < 0.01; ns, not significant. (Scale bars, 50 µm.)
Fig. 5.
TNFR2 agonism reduces infiltration of inflammatory immune cells into the spinal cord. Immune cells were isolated form the lumbar spinal cord of CCI mice 2 wpi and analyzed by flow cytometry. (A) Shown is the representative gating strategy. Number of infiltrating CD3+ T cells and CD3+FoxP3+ Tregs (B) and CD11b+CD45low microglia and infiltrating CD11b+CD45hi myeloid cells (C) was quantified by flow cytometry. (D) Expression of iNos and Arg1 in microglia and infiltrating myeloid cells was quantified by flow cytometry. Shown are representative histograms and the quantification of the (isotype) normalized median fluorescence intensity (nMFI) of the iNos and Arg1 signal (mean ± SEM, n = 10 per group) *P < 0.05; **P < 0.01; ns, not significant.
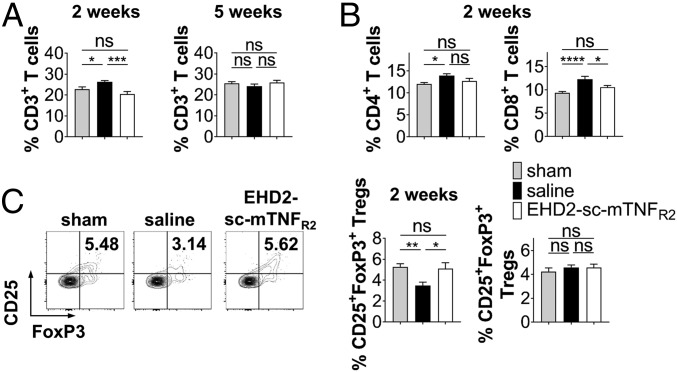
Quantification of splenic immune cells showed that percentage of T cell subsets was significantly elevated 2 wpi. EHD2-sc-mTNFR2–treated mice did not show an increase in the percentage of CD3+, CD4+, or CD8+ T cells (Fig. 6 A and B), indicating that selective activation of TNFR2 abolished injury-mediated immune cell expansion. No changes in the percentage of CD3+, CD4+, or CD8+ T cells were detected 5 wpi (Fig. 6A and SI Appendix, Fig. S10A). Interestingly, we observed a significant reduction in the percentage of splenic CD25+FoxP3+ Tregs 2 wpi in the saline-treated group compared to sham injured mice. However, no differences in the percentage of Tregs was observed in EHD2-sc-mTNFR2–treated mice (Fig. 6C). This is in line with previous observations that TNFR2 agonism increases Treg proliferation (27, 29). However, we cannot exclude that other mechanisms contribute to the increased Treg fractions, such as increased stability/survival or altered biodistribution. No differences between the groups were observed 5 wpi (Fig. 6C). Further, EHD2-sc-mTNFR2 administration prevented injury-induced reduction in the percentage of splenic CD11b+CD115−Ly6G+ neutrophils and CD68+ monocytes/macrophages and increased the percentage of CD11c+MHCII+ dendritic cells 2 wpi. Comparable to T lymphocytes, no changes in the percentages of neutrophils, macrophages, and dendritic cells were observed 5 wpi. Percentages of CD45R+ B cells were not changed 2 and 5 wpi (SI Appendix, Fig. S10). Since both Tregs and TNFR2 on central nervous system (CNS)-resident cells, such as microglia, have been shown to reduce neuroinflammation and to promote neuroprotection (15, 30, 31), we investigated whether EHD2-sc-mTNFR2 was transported into the spinal cord. Indeed, we were able to detect EHD2-sc-mTNFR2 after 2 and 5 wk in the spinal cord (SI Appendix, Fig. S11).
Fig. 6.
TNFR2 agonism promotes antiinflammatory responses in the periphery. Percentage of splenic CD3+ T cells (A), CD4+ and CD8+ T cells (B), and CD25+FoxP3+ Tregs (C) (population: CD3+ T cells) was quantified by flow cytometry. Shown are scatterplots from representative experiments. Values in each quadrant are the percentage of positive cells. Quantification shows the mean ± SEM (2 wk: n = 16 each group, 5 wk: sham/EHD2-sc-mTNFR2: n = 16 and saline: n = 18). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant.
TNFR2 Agonism Promotes Pain Recovery via Tregs.
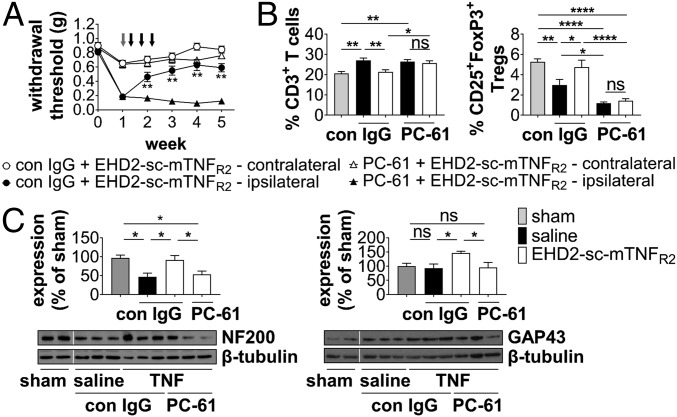
We next investigated whether the therapeutic effect of EHD2-sc-mTNFR2 is mediated via Tregs. Beginning 7 d after injury, male and female mice were first administered a control IgG or PC-61 for Treg depletion and then on days 9, 12, and 15, mice were treated with EHD2-sc-mTNFR2. Whereas mice treated with control IgG showed TNFR2 agonist-dependent recovery, mice that received the combination of PC-61 and EHD2-sc-mTNFR2 did not recover from mechanical allodynia (Fig. 7A and SI Appendix, Fig. S12). While EHD2-sc-mTNFR2 treatment reduced the injury-mediated increase in splenic CD3+, CD4+, and CD8+ T cells in control IgG-treated mice 2 wpi, no significant reduction was observed in mice treated with PC-61 and EHD2-sc-mTNFR2 (Fig. 7B and SI Appendix, Fig. S13), indicating that EHD2-sc-mTNFR2–mediated Treg activity is responsible for suppression of T cell expansion. As expected, Treg levels were significantly decreased in PC-61–treated mice 2 and 5 wpi (Fig. 7B and SI Appendix, Fig. S13). In contrast, PC-61-induced depletion of Tregs did not abolish the increase in splenic CD11b+CD115−Ly6G+ neutrophils, CD68+ monocytes/macrophages, and CD11c+MHCII+ dendritic cells percentages observed after treatment with EHD2-sc-mTNFR2 (SI Appendix, Fig. S13), suggesting that these effects do not contribute to the therapeutic effect of EHD2-sc-mTNFR2 in the CCI model. Besides a reduction in the percentage of Tregs (Fig. 7B), no major changes were observed 5 wpi (SI Appendix, Fig. S15). Finally, depletion of Tregs abolished the neuroprotective effects of EHD2-sc-mTNFR2 treatment, i.e., animals that received the combination of PC-61 and EHD2-sc-mTNFR2 did show a decrease in NF200 levels and no increase in GAP43 levels compared to EHD2-sc-mTNFR2 treatment alone (Fig. 7C).
Fig. 7.
TNFR2 agonism alleviates allodynia via Tregs. (A) WT mice underwent CCI or sham injury at week 0. Allodynia was determined using the Von Frey test over a period of 5 wk. Mice were treated with control IgG (con IgG) or PC-61 (500 µg each, i.p.) after 7 d (gray arrow). On day 9, 12, and 15 mice received i.p. injections with saline or EHD2-sc-mTNFR2 (10 mg/kg, black arrows) (n = 16 each group), **P < 0.01 con IgG + EHD-sc-mTNFR2 vs. PC-61 + EHD2-sc-mTNFR2 ipsilateral paw. (B) Percentage of splenic CD3+ T cells and CD25+FoxP3+ Tregs (population: CD3+ T cells) 2 wpi was quantified by flow cytometry (n = 8–14 mice each group ± SEM). (C) Expression of NF200a and GAP43 in the spinal cord was quantified by Western blot analysis 5 wpi. Shown are representative blots and the mean ± SEM of optical density of each protein normalized to β-tubulin, percentage of protein expression of sham (sham: n = 6, saline/EHD2-sc-mTNFR2[TNF]/PC-61: n = 6). *P < 0.05; **P < 0.01; ****P < 0.0001; ns, not significant.
Discussion
We recently showed that TNFR1 signaling plays an essential role for pain development in males, but not females. Accordingly, pharmacological inhibition of TNFR1 was only therapeutic in males but had no effect on females (10). Here, we demonstrated that pharmacological activation of TNFR2 induces long-lasting recovery from neuropathic pain across sexes, indicating that the analgesic effect of TNFR2 works via a sex-independent pathway. We further provide genetic evidence showing that pain recovery after CCI is abolished in male and female TNFR2−/− mice, uncovering a role of TNFR2 for pain recovery. We further show that exogenous TNFR2 activation by EHD-sc-mTNFR2 loses its therapeutic activity when Tregs are depleted before treatment start. The important role of Tregs for functional recovery after CCI is supported by further data showing that pharmacological depletion of Tregs in the chronic phase of the disease delays recovery. This study therefore provides a basis for a better understanding of the role of Tregs for functional recovery of neuropathic pain and suggests that TNFR2 agonists could be developed into a novel nonopioid therapy for the treatment of neuropathic pain.
It is well documented, that TNF acts as a neuromodulator to increase inflammation and neuropathic pain after injury (32). Endoneurial TNF application into the nervous system results in behavioral and electrophysiological changes associated with induction of thermal hyperalgesia and mechanical allodynia, whereas antagonism of TNF has the opposite effect (33, 34). Further, there is a correlation between TNF expression levels and development of neuropathic pain (33), suggesting an important role of TNF in the initiation of pain. We and others have shown that TNFR1 signaling promotes mechanical allodynia (8) and thermal hyperalgesia (9). In contrast, the role of TNFR2 for pain is less well characterized. Using a model of inflammatory pain, it was shown that after intraplantar injection of complete Freund's adjuvant, heat hyperalgesia was decreased in the early phase in TNFR2−/− mice but reduced in both early and later phases in TNFR1−/− mice (14). While these studies demonstrate that TNFR1 plays a role for both development of neuropathic pain as well as maintenance of pain, they also suggest that TNFR2 is important for the early phase of pain development.
In contrast, we here show that TNFR2 plays an essential role for the resolution of pain via immune-dependent mechanisms. Peripheral immune cells were shown to play a pivotal role for the induction and maintenance of neuropathic pain. Whereas cytokines and neutrophils have been found to play an important role during the early stages of acute pain, T lymphocytes appear to play a central role in chronic neuropathic pain (35). In addition, T cells were shown to play a role in pain resolution as well. In particular, chemotherapy-induced mechanical allodynia was significantly prolonged in T cell-deficient mice (36), while adoptive transfer of T cells via intrathecal injection relieved tactile allodynia after chemotherapy (37). Further, after peripheral nerve injury, microglial cells become activated and change their phenotype to produce inflammatory cytokines and chemokines that facilitate recruitment of leukocytes into the brain (38). Inhibition of microglia activation was shown to alleviate mechanical allodynia and hyperalgesia following spinal nerve ligation (39), and a role for microglia in long-term maintenance of allodynia was postulated (40). Interestingly, recent studies advance the hypothesis that there is a difference between the sexes in the initiation and maintenance of neuroinflammation and development of neuropathic pain. In particular, there appears to be a greater involvement of microglia in males, whereas in females neuroinflammation appears to be primarily driven by adaptive immune cells (41). Our data demonstrate that TNFR2 signaling promotes pain recovery in both males and females, indicating that different pain pathways may be targeted by TNFR2 agonism. We show that exogenous TNFR2 activation reduced expansion of T effector cells in the periphery and infiltration into the nerve and spinal cord. We further observed increased Treg levels in the periphery and the spinal cord after TNFR2 agonist administration. Tregs were previously shown to infiltrate the nerve and spinal cord and to interfere with development of neuropathic pain after peripheral nerve injury (18, 19). We here show that Tregs play a crucial role for pain recovery in peripheral nerve injury-mediated neuropathic pain. Next to immunosuppression, Tregs contribute to tissue regeneration in the periphery and the CNS (42) and may solely account for the therapeutic effect observed after TNFR2 agonist treatment. However, TNFR2 is also expressed on other cells, such as activated T cells, myeloid cells or microglia and TNFR2 signaling in those cells was shown to alleviate neuroinflammation (31, 43, 44). We therefore cannot exclude that TNFR2 signaling in non-Treg cells contributes to its therapeutic effect. Next to reduced immune cell infiltration, we observed a repolarization of CNS-infiltrating myeloid cells into an antiinflammatory phenotype after TNFR2 agonist treatment. In particular, antiinflammatory/reparative macrophages have been indicated to play a role for repair processes after nerve injury (45).
After CCI, neurodegenerative changes have been observed in the spinal cord (8). In our experiments, we observed a transient decrease in NF200 levels of the spinal cord after injury, indicating a loss of large myelinated A-β fibers. Importantly, TNFR2−/− mice with chronic nonresolving allodynia showed prolonged NF200 loss, whereas TNFR2 agonist treatment either prevented NF200 loss or promoted NF200 recovery after injury. This is supported by our data that TNFR2−/− mice show reduced spinal GAP43 levels after injury and that TNFR2 agonist treatment increased GAP43 levels in the spinal cord. GAP43 is expressed at high levels in neuronal growth cones and is recognized as a supporting factor for axonal growth and synaptic remodeling (46). The chronic loss of GAP43 in TNFR2−/− mice after CCI and the increased levels of GAP43 after TNFR2 agonist treatment therefore indicate that TNFR2 signaling may promote neuroprotection and/or neuronal remodeling. In support of this potential neurorepair function of TNFR2 agonism, we found decreased levels of proBDNF and increased levels of mBDNF in the spinal cord of EHD2-sc-mTNFR2–treated mice. However, next to its role for neurorepair, BDNF signaling via the tropomyosin receptor kinase B (TrkB) also has a well-documented pronociceptive role (28, 47). Therefore, further studies are necessary to validate the potential neuroreparative role of TNFR2 in the CCI model. Interestingly, the TNFR2 agonist-dependent increase in NF200 and GAP43 levels was abolished when Tregs were depleted prior to EHD2-sc-mTNFR2 treatment. This suggests that Tregs contribute to the observed neuroprotective responses. Indeed, Tregs were shown to promote neuroprotective activities (48). However, it needs to be determined, whether this is a direct effect of Tregs on neurons or indirect via Treg-mediated changes in glial cells or other immune cells.
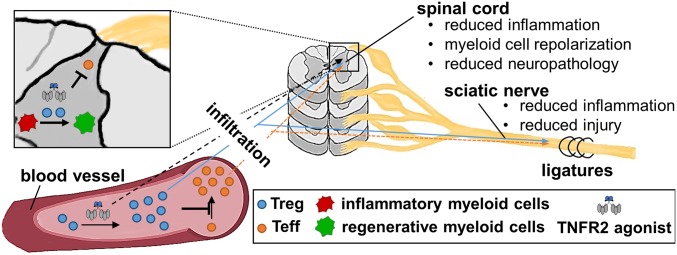
In summary, our data demonstrate that exogenous TNFR2 activation is therapeutic for neuropathic pain via Treg-dependent responses. TNFR2 agonism after CCI results in reduced recruitment of myeloid cells and T cells and increased infiltration of Tregs into the nervous system. Further, CNS-infiltrating myeloid cells switched to an antiinflammatory/reparative phenotype after systemic TNFR2 agonist treatment. These changes ultimately result in reduced neuropathology and long-term recovery from neuropathic pain (Fig. 8). We propose that TNFR2 plays an essential role for pain recovery via Tregs and that Treg therapies, such as TNFR2 agonists, may hold great promise as nonopioid analgesic treatments to reduce neuropathic pain.
Fig. 8.
Exogenous TNFR2 activation reduces peripheral and central inflammation and promotes neuronal recovery via Tregs. Treatment with the TNFR2 agonist EHD2-sc-mTNFR2 leads to increased fraction of Tregs, which suppress injury-induced expansion of peripheral T cells. This results in decreased infiltration of T cells along with enhanced infiltration of Tregs into the sciatic nerve and the dorsal horn of the spinal cord. In the spinal cord, infiltrating Tregs and CNS-infiltrating EHD2-sc-mTNFR2 may further suppress T cell activity, promote polarization of proinflammatory myeloid cells into an antiinflammatory/reperative phenotype, and reduce neuronal injury. These changes ultimately result in alleviation of neuropathic pain.
Materials and Methods
Detailed methods can be found in SI Appendix, SI Materials and Methods. The TNFR2 agonist EHD2-scTNFR2 was described previously (27). Animal care and treatment were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of Drexel University under protocol numbers 20295/20628. Male and female mice were analyzed separately. Since behavioral and biological responses in males and females followed the same trend, figures within the manuscript summarize combined data obtained in male and female mice. Behavioral data are shown separated for males and females in the supplementary data.
Supplementary Material
Acknowledgments
We thank Fan Yang for animal care and Doris Göttsch for technical assistance. R.F. was supported by Deutsche Forschungsgemeinschaft Research Fellowship FI 2138/1-1 and J.R.B. by Drexel’s Drexel Area of Research Excellence and NIH Grant from National Institute of Neurological Disorders and Stroke R01NS051709-14.
Footnotes
Conflict of interest statement: R.F. and J.R.B. are named inventors on patent applications covering the use of TNFR2 agonists for neuropathic pain. R.F., R.E.K., and K.P. are named inventors on patent applications covering the TNFR2 agonists technology.
This article is a PNAS Direct Submission. P.M.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902091116/-/DCSupplemental.
References
- 1.Murphy K. L., Bethea J. R., Fischer R., (2017) “Neuropathic pain in multiple sclerosis-current therapeutic intervention and future treatment perspectives” Multiple Sclerosis: Perspectives in Treatment and Pathogenesis, Zagon I. S., McLaughlin P. J., Eds. (Codon Publications, Brisbane, Australia: ). [PubMed] [Google Scholar]
- 2.Jensen T. S., Finnerup N. B., Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 13, 924–935 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Austin P. J., Moalem-Taylor G., The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 229, 26–50 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Lindenlaub T., Sommer C., Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 105, 593–602 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Wagner R., Myers R. R., Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport 7, 2897–2901 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Fischer R., Kontermann R., Maier O., Targeting sTNF/TNFR1 signaling as a new therapeutic strategy. Antibodies 4, 48–70 (2015). [Google Scholar]
- 7.Probert L., TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience 302, 2–22 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Dellarole A., et al. , Neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav. Immun. 41, 65–81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer C., Schmidt C., George A., Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp. Neurol. 151, 138–142 (1998). [DOI] [PubMed] [Google Scholar]
- 10.del Rivero T., Fischer R., Yang F., Swanson K. A., Bethea J. R., Tumor necrosis factor receptor 1 inhibition is therapeutic for neuropathic pain in males but not in females. Pain 160, 922–931 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Novrup H. G., et al. , Central but not systemic administration of XPro1595 is therapeutic following moderate spinal cord injury in mice. J. Neuroinflammation 11, 159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brambilla R., et al. , Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain 134, 2736–2754 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantin C. E., et al. , Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J. Neurosci. 28, 5072–5081 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., et al. , TNF-α contributes to spinal cord synaptic plasticity and inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2. Pain 152, 419–427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y., et al. , Essential protective role of tumor necrosis factor receptor 2 in neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 113, 12304–12309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X., Bäumel M., Männel D. N., Howard O. M., Oppenheim J. J., Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J. Immunol. 179, 154–161 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Chen X., et al. , TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J. Immunol. 190, 1076–1084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin P. J., Kim C. F., Perera C. J., Moalem-Taylor G., Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 153, 1916–1931 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Lees J. G., Duffy S. S., Perera C. J., Moalem-Taylor G., Depletion of Foxp3+ regulatory T cells increases severity of mechanical allodynia and significantly alters systemic cytokine levels following peripheral nerve injury. Cytokine 71, 207–214 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Sorge R. E., Totsch S. K., Sex differences in pain. J. Neurosci. Res. 95, 1271–1281 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Setiady Y. Y., Coccia J. A., Park P. U., In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur. J. Immunol. 40, 780–786 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Yang S., Wang J., Brand D. D., Zheng S. G., Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front. Immunol. 9, 784 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell F. A., King R., Smillie S. J., Kodji X., Brain S. D., Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 94, 1099–1142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyengar S., Ossipov M. H., Johnson K. W., The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 158, 543–559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta A. K., Gracias D. T., Croft M., TNF activity and T cells. Cytokine 101, 14–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer R., et al. , Novel strategies to mimic transmembrane tumor necrosis factor-dependent activation of tumor necrosis factor receptor 2. Sci. Rep. 7, 6607 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer R., et al. , Selective activation of tumor necrosis factor receptor 2 induces anti-inflammatory responses and alleviates experimental arthritis. Arthritis Rheumatol. 70, 722–735 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Hempstead B. L., Brain-derived neurotrophic factor: Three ligands, many actions. Trans. Am. Clin. Climatol. Assoc. 126, 9–19 (2015). [PMC free article] [PubMed] [Google Scholar]
- 29.Chopra M., et al. , Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. J. Exp. Med. 213, 1881–1900 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dombrowski Y., et al. , Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 20, 674–680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao H., et al. , Opposing functions of microglial and macrophagic TNFR2 in the pathogenesis of experimental autoimmune encephalomyelitis. Cell Rep. 18, 198–212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung L., Cahill C. M., TNF-alpha and neuropathic pain–A review. J. Neuroinflammation 7, 27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schäfers M., Svensson C. I., Sommer C., Sorkin L. S., Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 23, 2517–2521 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer C., et al. , Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 913, 86–89 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Coraggio V., et al. , Neuroimmune-driven neuropathic pain establishment: A focus on gender differences. Int. J. Mol. Sci. 19, E281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krukowski K., et al. , CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J. Neurosci. 36, 11074–11083 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X. J., et al. , Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 24, 1374–1377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou H., Lapointe B. M., Clark S. R., Zbytnuik L., Kubes P., A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J. Immunol. 177, 8103–8110 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Raghavendra V., Tanga F., DeLeo J. A., Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 306, 624–630 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Tawfik V. L., Nutile-McMenemy N., Lacroix-Fralish M. L., Deleo J. A., Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav. Immun. 21, 238–246 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Sorge R. E., et al. , Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081–1083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma A., Rudra D., Emerging functions of regulatory T cells in tissue homeostasis. Front. Immunol. 9, 883 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller P. G., Bonn M. B., McKarns S. C., Transmembrane TNF-TNFR2 impairs Th17 differentiation by promoting Il2 expression. J. Immunol. 195, 2633–2647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye L. L., Wei X. S., Zhang M., Niu Y. R., Zhou Q., The significance of tumor necrosis factor receptor type II in CD8+ regulatory T cells and CD8+ effector T cells. Front. Immunol. 9, 583 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu P., et al. , Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 14, 1335–1342 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holahan M. R., A shift from a pivotal to supporting role for the growth-associated protein (GAP-43) in the coordination of axonal structural and functional plasticity. Front. Cell. Neurosci. 11, 266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siniscalco D., Giordano C., Rossi F., Maione S., de Novellis V., Role of neurotrophins in neuropathic pain. Curr. Neuropharmacol. 9, 523–529 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gendelman H. E., Appel S. H., Neuroprotective activities of regulatory T cells. Trends Mol. Med. 17, 687–688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.