Significance
Different intensity levels of aggression surround our daily lives, terminating in occasional individuals with violence. What nervous system mechanisms exist that set limits on such widely differing expressions of aggression? Is sex one of them? A generalization associated with aggression is that males fight at higher intensity levels than females. In a Drosophila melanogaster aggression model, it was found that males fight at higher intensity levels than females, and that hierarchical relationships are established only in male fights. However, our screen of a Drosophila library of Gal4 drivers yielded a single line that triggered exceptionally high levels of aggression selectively in female flies. Here the roots of that hyperaggression are traced to a very small set of female brain-specific neurons.
Keywords: aggression, female aggression, doublesex, cholinergic, Drosophila melanogaster
Abstract
In the Drosophila model of aggression, males and females fight in same-sex pairings, but a wide disparity exists in the levels of aggression displayed by the 2 sexes. A screen of Drosophila Flylight Gal4 lines by driving expression of the gene coding for the temperature sensitive dTRPA1 channel, yielded a single line (GMR26E01-Gal4) displaying greatly enhanced aggression when thermoactivated. Targeted neurons were widely distributed throughout male and female nervous systems, but the enhanced aggression was seen only in females. No effects were seen on female mating behavior, general arousal, or male aggression. We quantified the enhancement by measuring fight patterns characteristic of female and male aggression and confirmed that the effect was female-specific. To reduce the numbers of neurons involved, we used an intersectional approach with our library of enhancer trap flp-recombinase lines. Several crosses reduced the populations of labeled neurons, but only 1 cross yielded a large reduction while maintaining the phenotype. Of particular interest was a small group (2 to 4 pairs) of neurons in the approximate position of the pC1 cluster important in governing male and female social behavior. Female brains have approximately 20 doublesex (dsx)-expressing neurons within pC1 clusters. Using dsxFLP instead of 357FLP for the intersectional studies, we found that the same 2 to 4 pairs of neurons likely were identified with both. These neurons were cholinergic and showed no immunostaining for other transmitter compounds. Blocking the activation of these neurons blocked the enhancement of aggression.
Aggression, an innate behavior seen across vertebrate and invertebrate species, is usually displayed by both sexes in competition for resources and in the selection of desired mates (1, 2). In most species, female fights are at lower intensity levels than male fights; however, exceptions to this generalization exist, such as maternal aggression in defense of the young in mammalian species (3, 4). In humans, abnormal expression of this behavior is often associated with psychiatric disorders, such as major depressive disorder, anxiety disorders, postpartum psychosis, and posttraumatic stress disorder (PTSD). The incidence of these disorders is significantly higher in women than in men (5). PTSD is commonly associated with men as a result of the trauma of war, but women actually have a higher risk than men of PTSD following a traumatic experience (6). Finally, unbridled violence released within groups of animals, including humans, is a serious problem in society.
The biological basis for aggression remains poorly understood in any species, but several valuable animal models have been developed that are beginning to yield important information about the nervous system roots of this essential behavior. Among these models is a fruit fly (Drosophila melanogaster) system. Aggression in Drosophila was first reported more than 100 y ago by Sturtevant (7), and these studies were followed up relatively slowly over many decades with field and laboratory studies (1, 8–11). A particularly elegant study by Hoffman in 1987 (12) quantified aggressive behavior between male fruit flies in a laboratory setting. Field work was carried out with females (13–15), but relatively little laboratory work was performed until a detailed study characterized and quantified male and female aggression in dyadic pairings between flies (16). These studies demonstrated that some behavioral patterns seen in the pairings during fights were female-specific (high posture fencing, head butts, shoves, elevated wings, and charging), some were male-specific (boxing, lunging, extended wing threats), and some were seen in both males and females (fencing). Male fights usually ended with the establishment of hierarchical relationships in which winners denied access to resources by losers. In contrast, female fights commonly ended with sharing of resources. Genes of the sex determination hierarchy (fruitless [fru] and transformer [tra]) were found to be key elements in establishing whether flies fought using male or female patterns of aggression (17). Switching the patterns of aggression used in the nervous system without changing the sex of other body regions (possible because sex is cell-specific in flies) leads to confusion in behavioral interactions between the flies (18). After such switches, males will attack females displaying male patterns of aggression in social interactions.
In males, important effects on the intensity of aggression have been reported by altering the expression of amines (serotonin and octopamine; refs. 19 and 20) and peptides (Tachykinins and Neuropeptide F; refs. 21 and 22); however, relatively little work has been done on the neurochemical or neurohormonal regulation of aggression in females. An exception may be the recent demonstration that during copulation, the transfer of sperm along with sex peptides from males to females (23) alters aggression levels in females. In mammals, another neurochemical influence on female aggression may be via activation of neurons expressing the Esr1+ form of the estrogen receptor (24).
In the studies presented here, we initiate efforts to unravel the circuitry concerned with the expression of high levels of aggression in female fruit flies. We focus on whether female aggression results from activation of a different circuitry than male aggression, whether the same neurohormones and neurotransmitters are involved, and on whether female and male aggression results from differential activation of similar circuitries in male and female flies.
To begin these studies, we screened (25) a portion of the FlyLight collection of Gal4 driver lines that had been generated at the Janelia Research Campus (26, 27). By activation of neurons targeted by a variety of these Gal4 driver lines using a thermal-sensitive cation channel (dTrpA1; ref. 28), we found a single line (GMR26E01-Gal4, here abbreviated as R26E01) that triggers unusually high levels of aggression in female flies. Upon heat activation, these females fought at such high levels of intensity that it required high-speed video examination of the resulting behavior to decode the patterns involved. The videos showed a greatly increased use of only female patterns of aggression in these encounters; no similar increases in aggression were seen in male flies. Further behavioral examination of the R26E01 > TrpA1 females showed that these flies were not generally hyperactive, and that there were no obvious effects on courtship behavior. When paired against wild-type females, heat-activated R26E01 > TrpA1 flies attacked first. In addition, in contrast to what is seen in normal female fights, these bouts often ended in the formation of hierarchical relationships.
Immunocytochemical studies found this Gal4 driver widely expressed in both female and male brains. Therefore, we used an intersectional genetic approach with a library of enhancer trap FLP recombinase (etFLP) lines to attempt to reduce the population of neurons involved. In several cases in which a cluster of well-known doublesex (dsx)-expressing neurons were targeted, the elevated female aggression phenotype was found. Neurons expressing the sex determination hierarchy transcription factors fru and dsx have been found colocalized in varying combinations in brain pC1, pC2, and pC3 clusters of neurons (29, 30). The pC1 cluster contains ∼50 dsx immunopositive neurons in male hemibrains and ∼10 dsx immunopositive neurons in female hemibrains (29). Individual neurons within this cluster have been reported to be involved in courtship in male and female flies (31–33), in aggression in males (33), and in behavioral switching between courtship and aggression in males (33).
In an attempt to further restrict the numbers of neurons involved, we used a dsxFLP line in combination with UAS > stop > dTrpA1myc; R26E01-Gal4 flies and ended up finally with a dsx+ immunostaining group of 2 to 4 pairs of neurons within female brain pC1 clusters. No similar immunostained neurons were found in male brains. In the adult female brain, these neurons displayed extensive bilateral arbors of presynaptic endings in the anterior protocerebrum through the posterior of the brain to the inferior slope. Immunostaining for neurotransmitters or their biosynthetic enzymes demonstrated that members of this group of neurons stained positively for choline acetyltransferase (cholinergic neurons), stained weakly for GABA (GABAergic neurons), and did not stain for tyrosine hydroxylase (dopaminergic and octopamine neurons), tryptophan hydroxylase (serotonin neurons), tyrosine decarboxylase 2 (octopamine neurons), or furin (for peptidergic neurons). Activation of this group of neurons partially duplicated the elevated selective female aggression phenotype, while blocking their activity had no effect. Taken together, our results support the suggestion that activation of a small group of pC1 dsx neurons causes Drosophila females to fight at a high level of aggression using female patterns of aggression.
Results
Activation of R26E01 Neurons with TrpA1 Selectively Enhances Hyperaggression in Female Flies.
Drosophila females exhibit aggression when competing for resources, such as food (SI Appendix and Movie S1). However, when screening lines from the Drosophila Fly Light collection of Gal4 driver lines in aggression assays, we found a driver line (R26E01-Gal4) that, when combined with the UAS-dTrpA1 line and activated by temperature, yielded what appeared to be unusually high and continuous levels of aggression in female flies. Moreover, no food was present to stimulate competition (SI Appendix and Movie S2; compare Movies S2 to S4 with Movie S1). At 20 °C, the dTrpA1 channel is closed, and at 29 °C, the cation channel is activated, permitting conductance to increase and depolarization to occur (28). In the behavioral screen, we reared flies at 20 °C to minimize dTrpA1 channel openings and fought the flies at 29 °C to increase channel opening and thereby activate the targeted neurons. Following heat activation, almost continuous high-intensity interactions were observed between the R26E01 > TrpA1 females. During these fights, both flies stood in elevated postures with their wings up and legs in almost constant motion. No similar effects were seen in pairings of males of this genotype at 20 °C or 29 °C (SI Appendix and Movie S6) or in R26E01 > TrpA1 females paired at the lower temperature of 20 °C. However, the interactions observed were sufficiently complex that it was difficult to determine whether the flies were using mixed female and male patterns of aggression (e.g., for females, head butts [including shoves] and charges with elevated wings; for males, lunges and tussling) (16), or even whether behavioral patterns seen during courtship and aggression might be intermingled. To reveal what was happening during the periods of activation, we recorded fights using a high-speed camera at 400 fps and analyzed the interactions in terms of the use of now-recognizable behavioral patterns (SI Appendix and Movie S5). The results showed that the thermally activated R26E01 > TrpA1 female flies used only female patterns of aggression during their encounters.
Analysis of Fighting Behavior in R26E01 > TrpA1 Females.
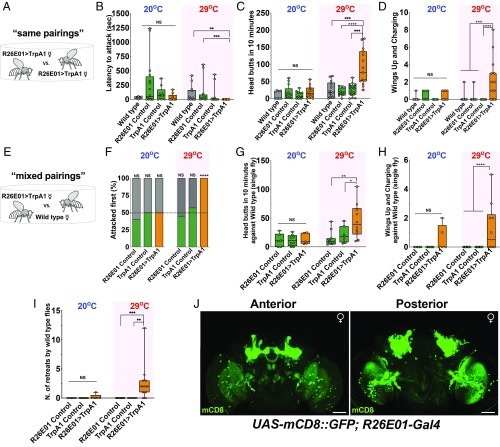
We compared the latency to attack and 2 behavioral patterns characteristic of female aggression—head butting (including shoves that are difficult to distinguish in the top views used in these studies for scoring) and wings up/charging (16)—in fights between pairs of females. The experimental flies expressed two transgenes (R26E01-Gal4 driver and UAS-dTrpA1 as its effector), while the controls were parent lines carrying single transgenes or Canton-S females. In experiments carried out with same genotype pairings (“same pairings”, R26E01 > TrpA1 vs. R26E01 > TrpA1), no important differences were seen in any of the parameters measured at 20 °C (Fig. 1 A–D), but at 29 °C, the experimental flies showed large increases in the measured fight patterns (Fig. 1 C and D). In mixed genotype pairing fights (“mixed pairings”; R26E01 > TrpA1 vs. control Canton-S females; Fig. 1 E–H) similar results were seen, except possibly in the “wings-up/charging” pattern, where at 20 °C the experimental flies also showed a small but nonsignificant increase in the times this pattern was observed. Flies expressing both transgenes (R26E01 > TrpA1) showed a faster latency to initiate a first attack at 29 °C but only compared with 1 of the 2 controls and wild type (Fig. 1B). However, in all cases at 29 °C, the experimental flies initiated the first attack against Canton S females (Fig. 1F). R26E01 > TrpA1 flies also appeared to form hierarchical relationships, in which single flies remained on the food cup after chasing their opponents from the food surface (SI Appendix and Movie S4). Canton-S female flies retreated more often from the food cup when fighting against thermally activated R26E01 > TrpA1 females compared with the retreats observed in fights with the transgenic controls (Fig. 1I). The enhanced aggression was not due to a generalized increase in activity of the experimental flies at the elevated temperature as measured in the locomotor assay of midline crosses at 20 °C and 29 °C (SI Appendix, Fig. S1A). “High-posture fencing” (a somewhat rare pattern of female aggression) was also seen with temperature activation, but this pattern was not scored because it was difficult to separate from others due to the almost constant movement of the flies (SI Appendix and Movie S3). We also found that R26E01 > TrpA1 females spent a considerably larger fraction of the time in close interaction with their opponents, whether alive or headless (SI Appendix, Fig. S1B).
Fig. 1.
Hyperaggression in female Drosophila. (A) Schematic of assay in which a R26E01 > TrpA1 female is paired with another female of the same genotype in a fighting chamber at 20 °C or 29 °C. (B) Shorter latencies to attack displayed during thermogenetic activation of R26E01 > TrpA1-positive neurons in same genotype group pairings of female flies (Kruskal–Wallis, H = 20.7, P = 0.0001; n = 10 to 15 flies) compared with control R26E01Gal4/+ (***P = 0.0002) and wild-type (**P = 0.0036) females. Dunn’s multiple-comparison post hoc test determined these values. (C) An increase in the total number of head butts during thermogenetic activation of R26E01 neurons (Kruskal–Wallis, H = 24.84, P < 0.0001; n = 13 to 17 pairs). R26E01 > TrpA1 females used more head butts compared with R26E01/+ (****P < 0.0001), TrpA1/+ (***P = 0.0006), and wild-type pairs (***P = 0.0007). Dunn’s multiple-comparison post hoc tests determined these values. (D) Differences were found in the number of times wings were elevated during fights (Kruskal–Wallis, H = 26.06, P < 0.0001; n = 14 to 19 pairs). R26E01 > TrpA1 activated females elevated their wings more often compared with control R26E01/+ (***P = 0.0003) and TrpA1/+ (****P < 0.0001) and wild-type (***P = 0.0008) females. Dunn’s multiple-comparison post hoc tests determined these values. (E) Schematic of the assay in which a R26E01 > TrpA1 female was paired with a wild-type female in a fighting chamber at 20 °C or 29 °C. (F) R26E01 > TrpA1 (orange bar) female flies initiated attacks 100% of the time against wild-type Canton-S (gray bars), compared with 45% for R26E01/+ (green bar) and 57% for TrpA1/+ (green bar) flies (χ2 test, χ2 = 76.05; ****P < 0.0001). (G) Differences in the number of head butts between groups observed at 29 °C (Kruskal–Wallis, H = 10, P = 0.0067; n = 7 to 11 flies). R26E01 > TrpA1 flies displayed more head butts against their wild-type opponents compared with R26E01/+ (**P = 0.0030) and TrpA1/+ (*P = 0.0193) flies. Dunn’s multiple-comparison post hoc tests determined these values. (H) Differences were also seen in the number of wings up and charging (Kruskal–Wallis, H = 25.0, P < 0.0001; n = 7 to 11 flies). R26E01 > TrpA1 flies displayed more wings up and charging compared with R26E01/+ (****P = 0.0010) and TrpA1/+ (****P = 0.0010) flies. Dunn’s multiple-comparison post hoc tests determined these values. (I) Canton-S female flies retreated more times from the food cup (Kruskal–Wallis, H = 18.39, P < 0.0001; n = 10 to 11 flies) when fighting with R26E01 > TrpA1 female flies than when fighting with control genotype R26E01/+ (***P = 0.0004) and TrpA1/+ (**P = 0.0015) flies. Dunn’s multiple-comparison post hoc tests determined these values. (J) Confocal image of female brain of UAS-mCD8::GFP/+;R26E01-Gal4/+ flies immunostained with anti-GFP antibody (green). Z-stack projection is shown. (Scale bars: 50 µm.) Data in B–D, F, and I: center line, median; boxes, first and third quartiles; whiskers, range. Circles represent individual values. NS, not significant at P > 0.05. Aggressive behaviors were measured over a 40-min period.
To estimate the population of neurons involved in the phenotype in these initial studies, the R26E01-Gal4 driver was used to drive green fluorescent protein (GFP) expression. Immunocytochemical examination showed GFP to be widely distributed in the brain, with extensive labeling of neurons in the mushroom bodies, suboesophageal zone (SEZ), lateral accessory lobe (LAL), fan-shaped body, central complex, and antennal lobes (Fig. 1J). We also observed expression in the ventral nervous system (see Fig. 3C). When tsh-Gal80 was expressed along with R26E01 > TrpA1 to limit expression in the ventral nerve cord, similar behavioral results as seen with R26E01 > TrpA1 were obtained. This suggests that expression in the brain alone is sufficient to enhance female-female aggression.
Fig. 3.
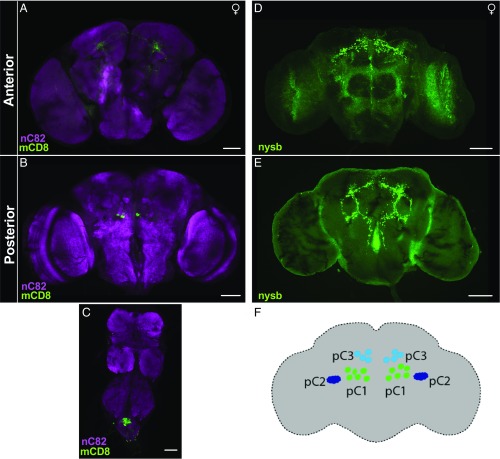
Sexually dimorphic pC1 dsx neurons. (A and B) Confocal Z-stack images of UAS > stop > mCD8::GFP; R26E01-Gal4/dsxFLP anterior (A) and posterior (B) female adult brain with bilateral GFP expression in dsx neurons, as visualized with anti-mCD8 (membrane-bound GFP) (green) and neuropil counterstained with nc82 (magenta). The number of neurons varied from 2 to 4 pairs of neurons bilaterally. (C) Some neurons were also expressed in the abdominal ganglion in females. (D and E) Their presynaptic expression in the anterior (D) and posterior (E) regions of the brain, expressing UAS > stop > synaptotagmin (presynaptic marker) tagged with GFP (green). (F) Schematic of pC1–3 neuronal clusters found in the posterior region of the female brain (29–31, 33, 36, 37).
To confirm that the effects observed in these initial observations were specific to aggression in females, we searched for courtship differences between experimental and control females with Canton-S males (SI Appendix, Fig. S2) and effects on male-male aggression between experimental and control males (SI Appendix, Fig. S3). In the courtship studies, we measured latency to court, courtship vigor index (CVI), latency to copulate, and copulation duration. In all parameters measured, no differences were seen in courtship behavior at 20 °C and 29 °C (SI Appendix, Fig. S2). This is consistent with the observations in the high-speed videos of our initial studies, in which we observed no behavioral patterns related to courtship (SI Appendix and Movie S5). For male aggression, we scored the latency to attack and the numbers of lunges and wing threats seen at 20 °C and 29 °C. No differences in aggression were seen between control and experimental males at either temperature (SI Appendix, Fig. S3 and Movie S6).
Intersectional Approach to Reduce the Population of Neurons Involved with Female Hyperaggression.
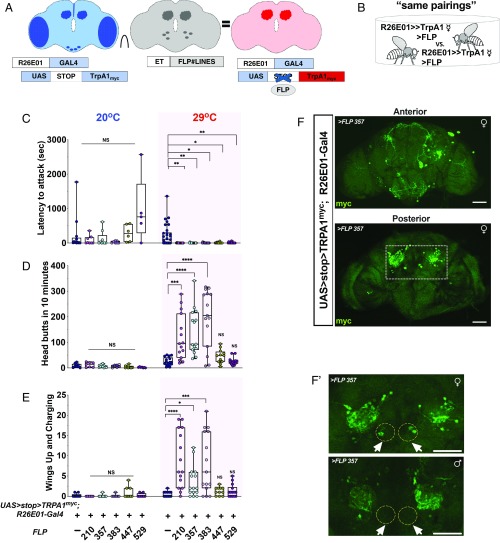
To reduce the populations of neurons involved in generating the elevated female aggression phenotype, we used an intersectional approach previously developed in our laboratory (34) (Fig. 2A). In this approach, a library of et-FLP lines was generated, all of which expressed a FLP recombinase in different populations of central nervous system neurons. The experimental flies for these experiments were females constructed in the following manner: females carrying UAS > stop > dTrpA1myc; R26E01-Gal4 transgenes were crossed individually to males from a selection of 113 of our collection of et-FLP lines. The progeny of these crosses contained a subset of neurons expressing Gal4 under control of a first regulatory element, its target effector, and the flp recombinase, synthesized under control of a second regulatory element. The flp recombinase excises the stop cassette, allowing expression of the heat-inducible dTrpA1 channel tagged with myc only in neurons capable of utilizing both regulatory elements. The myc tag allows us to identify targeted neurons using an antibody to the Myc-derived polypeptide (35).
Fig. 2.
Female aggression is driven by a subset of neurons. (A) Intersectional strategy used to isolate neurons in the female brain. et-FLP was used in combination with the R26E01-Gal4 driver and UAS > stop > TrpRPA1myc. (B) Schematic of assay in which a female FLP#∩R26E01 was paired with another female of the same genotype in a fighting chamber at 20 °C or 29 °C. (C) Fighting latencies (Kruskal–Wallis, H = 27.05, P = 0.0007; n = 15 to 20 flies) were significantly shorter for 210FLP∩R26E01 (**P = 0.0042), 357FLP∩R26E01 (**P = 0.0060), 383FLP∩R26E01 (*P = 0.0147), 447FLP∩R26E01 (**P = 0.0159), and 529FLP∩R26E01 (**P = 0.0094) flies compared with controls. Dunn’s multiple-comparison post hoc tests determined these values. (D) Number of head butts in the 10 min after the initial head butt (Kruskal–Wallis, H = 61.15, P < 0.0001; n = 12 to 17 pairs) during thermogenetic activation of R26E01-Gal4 intersected with FLP#∩R26E01-positive neurons. 210FLP∩R26E01 (***P = 0.0007), 357FLP∩R26E01 (****P < 0.0001), and 383FLP∩R26E01 (****P < 0.0001) pairings showed more head butting compared with control pairings. Dunn’s multiple-comparison post hoc tests determined these values. (E) An increase in the number of times flies displayed wings up and charging during the 10-min fight (Kruskal–Wallis, H = 39.34, P < 0.0001, n = 11 to 17 pairs). Fight pairings 210FLP∩R26E01 (****P < 0.0001), 357FLP∩R26E01 (*P = 0.0261), and 383FLP∩R26E01 (***P = 0.0002) held up their wings during charging more often compared with control pairings. Dunn’s multiple-comparison post hoc tests determined these values. (F) Confocal Z-stack images of intersectional expression of TrpPA1myc in female brains of R26E01-Gal4 with 357FLP∩R26E01. (F′) A male brain and female brain immunostained with anti-myc antibody (green). (Scale bars: 50 µm.) Data in C to E: center line, median; boxes, first and third quartiles; whiskers, range. Circles represent individual values. NS, not significant at P > 0.05. Aggressive behaviors were measured over a 40-min period.
The design of the experiment, as above, was to first fight same pairings (of same genotypes) of flies (Fig. 2) and then fight mixed pairings (of different genotypes) where the opponents were Canton-S females. In our initial screen of et-FLP lines, we used females generated from the crosses and examined their behavior at 29 °C, searching for individuals demonstrating enhanced aggression. Of the 113 et-FLP lines screened in this way, 5 showed enhanced female aggression. Next, detailed examinations of the behavior were carried out in same pairings of the flies from the 5 lines that showed high levels of aggression in the screen: 210FLP∩R26E01, 357FLP∩R26E01, 383FLP∩R26E01, 447FLP∩R26E01, and 529FLP∩R26E01. At 20 °C, all experimental female lines showed a latency to attack that was no different from that seen in controls, with the possible exception of slightly (but nonsignificantly) longer latencies to attack in 2 of the lines (447FLP and 529FLP). These small differences disappeared at 29 °C; all 5 lines showed faster attack latencies than controls (Fig. 2C). Three of these (210FLP∩R26E01, 357FLP∩R26E01, and 383FLP∩R26E01) also showed increased head butting (Fig. 2D) and wings up/charging (Fig. 2E) at 29 °C. Moreover, when we paired these 3 et-FLP lines with control Canton-S females in mixed pairings, the experimental flies almost always attacked first at 29 °C (SI Appendix, Fig. S4B), as was seen with the broader Gal4 line (Fig. 1F).
We next compared FLP#∩R26E01 experimental flies with their Canton-S opponents, scoring each separately to demonstrate that only FLP#∩R26E01 flies (experimentals) responded to temperature elevation with enhanced aggression, and that when enhanced aggression is directed at control flies, it does not trigger enhanced aggression in the controls (SI Appendix, Fig. S4 C and D). While large increases in head butting were seen in the mixed pairings at 29 °C with 210FLP∩R26E01, 357FLP∩R26E01, and 383FLP∩R26E01 (SI Appendix, Fig. S4C), much smaller effects were seen in wings up/charging (SI Appendix, Fig. S4D). This difference compared with the high level of wings up/charging responses shown in Fig. 2 might be related to opponent responses; in Fig. 2E (same pairings), the opponent would likely respond with high-level aggression, while in SI Appendix, Fig. S4C, the control opponent would likely mainly retreat from its highly aggressive intersected opponent.
Sexually Dimorphic Neurons Required for Female Hyperaggression.
We next sought to determine which groups of neurons are involved in inducing the hyperaggression phenotype. For this purpose, we examined the expression patterns of neuronal staining of the 3 positive FLP lines using an anti-Myc antibody. With 210FLP∩R26E01 and 383FLP∩R26E01, broad expression of TrpA1Myc was observed that partially overlapped with much of the original total R26E01-Gal4 population (SI Appendix, Fig. S5 A and B); however, with 357FLP∩R26E01, a smaller number of neurons were immunostained (Fig. 2F′). This line showed tagged neurons in the SEZ, LAL, protocerebrum, and calyx of the mushroom body, along with a small number of neurons in the posterior medial region (Fig. 2F). Male brains restricted with 357FLP∩R26E01 showed a similar staining pattern except for a small cluster of neurons not immunostained (circles in Fig. 2F′). Closer examination revealed that a similar small group of neurons was immunostained in all female brains from the 210FLP∩R26E01, 357FLP∩R26E01, and 383FLP∩R26E01 crosses that showed behavioral phenotypes (arrows in Fig. 2F and SI Appendix, Fig. S5 A and B), suggesting that this group of sexually dimorphic neurons might be responsible for the aggression phenotype. The sexually dimorphic staining pattern led us to ask whether these neurons are within the cluster of dsx- expressing neurons that had already been implicated in male and female courtship and in male aggression by other investigators (29–33).
pC1 Neurons Colocalize with dsx and Are Cholinergic.
In an attempt to refine the expression pattern and target these sexually dimorphic neurons more specifically, we intersected R26E01-Gal4 with dsxFLP and used UAS > stop > mCD8::GFP to label membranes of the targeted neurons. These crosses targeted a greatly reduced population of neurons, including a small number found in a similar location as those targeted in the 357FLP∩R26E01 females (compare Fig. 2 F and F′ and Fig. 3 A and B). In the dsxFLP experiments, immunostaining of the GFP-tagged mCD8 showed neuronal processes only in an anterior view (Fig. 3A) of the brain, but in a posterior view (Fig. 3B), 2 to 4 immunopositive cell bodies per hemisphere were found in the vicinity of the known dsx-positive pC1 cluster of neurons (29–31, 33, 36, 37) (Fig. 3F). In the ventral nerve cord, a cluster of immunostained neurons was found in the abdominal ganglion (Fig. 3C). None of the cell bodies that were immunopositive in females were stained for GFP in males (SI Appendix, Fig. S6). This suggests that these neurons are either absent in males or not targeted by the R26E01-Gal4 driver line.
In an attempt to identify axonal endings of the pC1 neurons, we used a synaptobrevin marker tagged with GFP in UAS > stop > nysb::GFP; R26E01-Gal4/dsxFLP female fly brains. Bilateral projections were extensively distributed throughout the protocerebral regions and into superior and inferior posterior slope regions (Fig. 3 D and E), as has been reported previously (29–31, 33, 36).
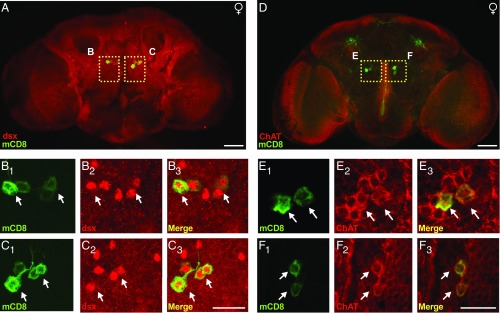
To confirm that the pC1 neurons targeted using dsxFLP expressed dsx, we again used an antibody against GFP to label the targeted neurons and added a second antibody (dsxDBD) that is reportedly specific for the transcription factor (36). As expected, we found that the dsxDBD antibody labeled ∼10 neurons in the pC1 cluster (29, 33). Between 2 and 4 pairs of those neurons on each side of the brain in the area of the pC1 cluster showed colocalization between dsx and GFP (Fig. 4 A–C).
Fig. 4.
pC1 dsx neurons are cholinergic. (A) Immunostaining of GFP in adult female genotype UAS > stop > mCD8::GFP;R26E01-Gal4/dsxFLP counterstained with dsx. (B and C) Higher-magnification (60× objective) of anti-mCD8 (green) (B1 and C1), anti-dsx (red) (B2 and C2), and a merger of B1 and C1 and B2 and C2 (B3 and C3). (D) Immunostaining of cell membrane in the adult female genotype UAS > stop > mCD8::GFP;R26E01-Gal4/dsxFLP counterstained with ChAT. (E and F) Higher-magnification (60× objective) views of anti-mCD8 (green) (E1 and F1), anti-ChAT (red) (E2 and F2), and a merger of E1 and F1 and E2 and F2 (E3 and F3). (Scale bars: 50 µm in A and D; 25 µm in B, C, E, and F.)
Zhou et al. (36) reported that most of the dsx-expressing neurons in the pC1 cluster expressed choline acetyltransferase in their cell bodies and were presumed to be excitatory in function. In addition, the neurons did not express GABA immunostaining. Our results support those findings when the R26E01-Gal4 driver was intersected with 357FLP or dsxFLP. In both cases, the pC1 neurons showed similar immunostaining patterns with the choline acetyltransferase antibody. In all cases, a dark ring of cytoplasmic immunostaining surrounded the putative nuclei of the cells on both sides of the brain (Fig. 4 D–F). Immunostaining with a GABA antibody yielded difficult-to-distinguish light staining of what looked like varicose processes in the immediate vicinity of the pC1 neurons (SI Appendix, Fig. S7). Immunostaining with antibodies to tryptophan hydroxylase, tyrosine decarboxylase 2 (Tdc2), serotonin (5HT), and furin (dFur1) (tested because the original driver was derived from a fragment of the dFurin enhancer region) yielded no positive immunostaining in the vicinity of the pC1 neurons (SI Appendix, Figs. S7 and S8).
Activation of pC1 dsx Neurons Selectively Enhances Female Hyper-Aggression.
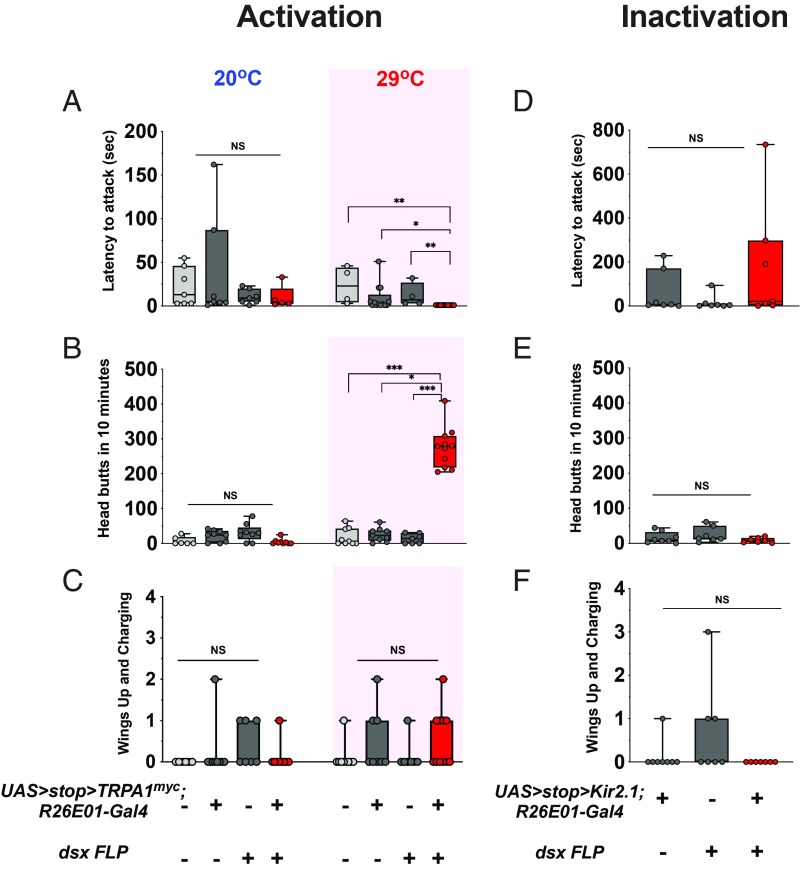
Finally, we felt it was important to determine if the reduced group of dsx neurons could duplicate the phenotype observed with the original R26E01 > TrpA1 and 357FLP∩R26E01 combinations. For these experiments, we used females carrying UAS > stop > dTrpA1myc; R26E01-Gal4 and crossed them to males containing a dsxFLP insertion. The resultant male and female progeny of UAS > stop > dTrpA1myc; R26E01-Gal4/dsxFLP were tested in same sex pairings under our standard male and female aggression assay conditions. In the female pairings, the latency to the first attack was faster than in controls (Fig. 5A). Large increases in head butt numbers were seen at 29 °C, that were comparable to the numbers observed in the original experiments (Fig. 5B, SI Appendix, and Movie S7). Only small effects were seen in the wings up/charging category, however (Fig. 5C). In male aggression assays, no significant enhanced aggression was seen at 29 °C in any of the categories examined (SI Appendix, Fig. S9).
Fig. 5.
pC1 dsx sexually dimorphic neurons are required for induction of female hyperaggressive behavior. (A) dsxFLP∩R26E01 females attacked (Kruskal–Wallis, H = 22.43, P < 0.0001; n = 4 to 11 flies) their opponents sooner compared with wild-type (**P = 0.0011), UAS > stop > TrpRPA1myc/+;R26E01-Gal4/+ (*P = 0.0156), and dsxFLP/+ (**P = 0.0070) control pairings. Dunn’s multiple-comparison post hoc tests determined these values. (B) Increased head butting was observed (Kruskal–Wallis, H = 22.39, P < 0.0001; n = 7 to 11 pairs) in dsxFLP∩R26E01 fight pairings compared with wild-type (***P = 0.0004), UAS > stop > TrpRPA1myc/+;R26E01-Gal4/+ (**P = 0.0060), and dsxFLP/+ (***P = 0.0004) control pairings. Dunn’s multiple-comparison post hoc tests determined these values. (C) No differences were observed in the number of wings up and charging in female fights (n = 7 to 11 pairs). (D–F) Inactivation of dsx neurons does not enhance female aggression. No differences in their latency to attack (D) or in the number of head butts (E) and wings up/charging (F) in dsxFLP∩R26E01 > Kir2.1 pairings compared with control genotype UAS > stop > Kir2.1/+;R26E01/dsxFLP or dsxFLP/+ female fight pairings. Data in A to F: center line, median; boxes, first and third quartiles; whiskers, range. Circles represent individual values. NS, not significant at P > 0.05. Aggressive behaviors were measured over a 40-min period.
To further define the role of the 2 to 4 pairs of dsx neurons identified in the pC1 region of the brain, we asked whether they are required for generation of the higher level patterns of female aggression like head butts, or are mainly concerned with regulating the frequency of usage of such high intensity fight patterns. To examine this, we silenced the cells genetically by expressing the inward rectifier potassium channel Kir2.1 in the target neurons. We used UAS-Kir2.1 rather than UAS-shits to avoid the large temperature shift required (to 30 to 35 °C) to observe the mutant dynamin effect, and to avoid any time lag that might accompany complete blockade of transmitter release with mutant shibire. The female progeny of the Kir flies did display head butts, but in numbers comparable to those observed in control fights (Fig. 5 D–F). Thus, it appears that the dsx neurons identified in these studies are mainly concerned with facilitating a transition to the expression of higher levels of aggression in female flies and not with the establishment of the head butt patterns themselves.
Discussion
The fights of female fruit flies are usually of lower intensity than those of males, and usually end in a sharing of resources (16). As with most generalizations of this type, this is not true in all species examined. That extends to humans as well, where for example, in certain diseases and disorders of the nervous system (5, 6), extremely high levels of female aggression can be seen. It was therefore surprising to find, in a line from the Janelia FlyLight collection, extremely high levels of aggression when the targeted neurons were activated using a thermosensitive dTrpA1 channel. In fact, the movements were so continuous and rapid that we could not, at first, determine whether the activated flies were using male or female patterns of aggression, or even whether courtship behavioral patterns might be mixed in with aggression-related behavioral patterns (SI Appendix and Movie S5). High-speed videos, however, clarified the issue and demonstrated that the R26E01 > TrpA1 flies only were using female patterns of aggression, but at levels higher than any we had seen before (16). Tracking down the expression of the high-level aggression, we found that there was no effect on male aggression or on male or female courtship behavior (the R26E01 > TrpA1 flies were courted normally by wild type males). Surprisingly, we also found that some of the female fights in these experiments resulted in the formation of hierarchical relationships in which the “winners” of these fights were commonly seen in sole possession of the resource.
In our earliest experiments high numbers of neurons were targeted and many of these might have contributed to the high levels of aggression seen. To attempt to reduce this population to a small subset of neurons that still would generate the phenotype, we initiated an intersectional screen with our enhancer trap-flp recombinase library in crosses with flies containing the R26E01-Gal4 driver with UAS > stop > dTrpA1myc, and examining the progeny for high levels of female aggression with heat activation. Several combinations led to progeny that showed the phenotype but only one showed highly restricted numbers of neurons. Among these neurons were a small group (2 to 4 pairs) of neurons from the pC1 cluster of neurons. The pC1, pC2, and pC3 clusters of neurons are a functionally heterogeneous group of neurons-containing fru and/or dsx in varying combinations (29–31, 33, 37–39). It had been reported, however, that within the pC1 and pC3 clusters, specific subgroups of nerve cells were found that showed immunostaining for dsx- alone, and not for fru-, and that these neurons were found only in females (31, 36). Since the phenotype we generated was specific for females, and since females do not produce any of the variety of fru forms that exist, we focused our efforts on dsx as a potential tool to restrict the expression pattern further.
Accordingly, in the next group of experiments we intersected UAS > stop > dTrpA1myc;R26E01-Gal4, with dsxFLP and now found only the 2 to 4 neurons bilaterally expressed in each pC1 cluster that showed immunostaining. In the earliest experiments (prior to the intersectional studies), multiple aspects of female aggression were enhanced (head butts, wings up charging, high posture fencing), but as the population of neurons was restricted further we saw only increases in the numbers of head butts. Those increases were of the same magnitude in the earlier and later studies and both are higher than what would be seen in control females. We have no explanation for the reduction of usage of some of the behavioral patterns other than that with each step of intersection we see fewer pC1 neurons labeled. This might be due to loss of neurons concerned with some of the behavioral patterns seen initially. The 2 to 4 pairs of dsx-expressing cholinergic neurons enable females to go to extremely high levels of aggression displaying high numbers of head butts (mean, 275.3 ± 17.7) during fights. Inactivating these cells by expression of Kir2.1 channels in the neurons does not prevent normal levels of aggression (mean head butts, 8.5 ± 2.6).
Since females generally fight at lower intensity levels than males, it is interesting that activation of a specific subgroup of neurons in females results in extremely high levels of female aggression at intensities comparable to or greater than those generated by male flies. One possible explanation for this finding is that in our routine aggression assays, females are not challenged with conditions that encourage the use of very high levels of aggression. The usual female assay conditions are the same as for males with our standard chambers and a food resource instead of an opposite sex headless fly resource. This might not be a sufficiently strong resource to trigger higher-level aggression in females, especially since a food resource can be readily shared. Another possibility is that, as Rezával et al. (31) have found, latent behavioral patterns exist in female brains (in the Rezával case concerned with male courtship behavior) that are ordinarily suppressed. Perhaps the high-aggression neurons in females also are suppressed except in unique situations that we have not yet duplicated in the laboratory. There may be other possible explanations for this observation, and additional studies are needed to explore this interesting effect.
The pC1, pC2, and pC3 clusters of neurons constitute a fascinating, albeit relatively small, subgroup of neurons within male and female brains of Drosophila. They appear to serve as a fundamental center concerned with the choice, regulation, and expression of most or all of the sex-specific behavioral patterns associated with courtship and aggression in male and female fruit flies. Elaboration of the details of how these neurons interact with each other to generate social behavior might not be far off, once again demonstrating the value of model systems for explaining how animal behavior works, which remains one of the great challenges in biological sciences.
Experimental Procedures
Fly Stocks and Rearing.
Fly lines were maintained on standard cornmeal medium and reared at 25 °C and 50% relative humidity on a fixed 12-h light/dark cycle. Behavioral experiments were performed within the first 3 h after lights on. For the experiments in which TrpA1 was introduced into neurons, the flies were reared at 20 °C. To obtain control genotype females, Gal4 or UAS fly lines were crossed to our cantonized w1118 stock (backcrossed to Canton-S for 6 generations) to generate heterozygous controls. Canton-S flies served as wild-type controls. The following fly lines were obtained from the Bloomington Drosophila Stock Center: R26E01-Gal4 (#60510), UAS-mCD8::GFP (#32186), UAS-dTrpA1 (#26263), UAS > stop > TrpA1myc (#66871) (35), UAS > stop > Kir2.1 (#67686), UAS > stop > myr::GFP(#55810), UAS > stop > mCD8::GFP, and UAS > stop > nsyb::GFP (a gift from Barry Dickson, HHMI Janelia Farm Research Campus) (40). An enhancer trap et-FLP library was generated as described previously (34). dsxFLP (41) was a gift from Stephen Goodwin, Oxford University. Detailed genotypes of flies and sample sizes used for each figure are provided in SI Appendix.
Experimental Setup and Design.
All behavioral assays were performed in a newly developed experimental chamber that involves minimal handling of flies (42). Males and females were reared in social isolation in glass vials containing 1.5 mL of standard fly food from the late pupal stage from eclosion to the time of their use in the behavioral experiments. Flies expressing dTRPA1, their controls, and Canton-S “target” flies were raised at 20 °C and 50% relative humidity. Their pupae were isolated and on eclosion housed singly for 7 to 10 d in a vial containing 1.5 mL of standard fly food medium before being transferred to fight chambers at either 29 °C or 20 °C for the behavioral experiments. All aggression and courtship assays were videotaped and analyzed manually.
Aggression Assays.
The experimental protocol has been described previously (43). In brief, flies were anesthetized with CO2 at 48 h before the behavioral assays, and a dot of acrylic paint was applied on the dorsal thorax to facilitate visual tracking of individual flies. Aggression assays were performed between same-genotype pairs or mixed pairings in which a female experimental fly fought a Canton-S wild-type female opponent. For the mixed pairings assays, 1 fly was marked with acrylic paint for genotype identification. The fight arena contained a food cup with standard fly food and a drop of yeast paste on the surface to serve as an attractive resource to induce fighting behavior. Aggressive patterns were scored during a 10-min period after the first head butt in female-female aggression assays or the first lunge in male-male aggression assays. The latency to attack was defined as the difference in the time between the first encounter (social interaction lasting at least ∼2 s) on the food cup and the occurrence of the first head butt. In the top view of our behavioral chamber, it was difficult to distinguish between the head butt and shove behavioral patterns; thus, our number of head butts probably includes shoves as well, with the majority being head butts. Retreats were defined when the fly left the food cup following head butts from the opponent.
Courtship Assays.
All courtship behaviors, including, singing, tapping, wing extension, licking, chasing, and attempts at copulation, were scored manually. The CVI is defined as the fraction of a 10-min observation period spent by the male in courtship behavior after onset of the behavior. Latency to court is defined as the difference in time between when flies first interact and begin courtship behavior, and copulation latency is defined as the difference in time between the first encounter and the onset of copulation. Copulation duration is defined as the time difference between the start and end of successful copulation. In these experiments, we measured the courtship behavior of Canton-S males and experimental female flies for 10 min.
Locomotion Assays.
Single flies were loaded into individual chambers of a 12-well culture plate (well dimensions, 2.4 cm diameter × 2 cm). No food source was added to the wells. A central dividing line was drawn on the bottom of each well. After a 10-min acclimation period, locomotion was scored as the numbers of midline crossings over the next 5 min.
Immunostaining and Microscopy.
Immunocytochemistry studies were performed as described previously (34) with few modifications. Adult fly brains were dissected in PBS, immediately fixed in 4% formaldehyde for ∼30 min, incubated with primary antibodies for at least 16 h at 4 °C, treated with secondary antibodies for at least 3 h, and mounted in Vectashield H-1000. Tissues were washed 3 times in PBS plus 0.1% Triton-X for ∼20 min at room temperature after fixation and each of the antibody incubations. Fixed tissues were blocked with PBT plus 0.5% BSA and PBT plus 3% normal goat serum for at least 1 h, followed by incubation with primary and secondary antibodies, respectively. The following primary antibodies were used: mouse anti-myc (1:1,000; Abcam, ab32 9E10), rat anti-mCD8a (1:100; Invitrogen; MCD0800), mouse anti-GFP (1:500; Invitrogen, A11120), mouse anti-nc82 (1:20; Developmental Studies Hybridoma Bank [DSHB]), mouse anti-ChaT 4B1 (1:50; DSHB), mouse anti-dsxDBD (1:50; DSHB), rabbit anti-Tdc2 (1:1,000; Abcam, ab128225), rabbit anti-tyrosine hydroxylase (1:500; Novus Biologicals), rabbit anti-5HT (1:1,000; Sigma-Aldrich, S5545), rabbit anti-GABA (1:500; Sigma-Aldrich, A2052), and rabbit anti-dFur1 (1:500; provided by Anton Roebroeck, University of Leuven). The secondary antibodies used included Alexa Fluor 488 anti-mouse (1:300), Alexa Fluor 488 goat anti-rat (1:300), Alexa Fluor 594 goat anti-mouse (1:300), and Alexa Fluor 568 goat anti-rabbit (1:300) (all from Invitrogen). Fluorescence images were acquired with an Olympus FluoView FV1000 confocal microscope and processed with ImageJ imaging software.
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism 8 software. The Shapiro-Wilk test of normality (passed at P > 0.05) was initially used to determine data distribution. If the data did not pass the normality distribution test, nonparametric testing was performed. All data except those for copulation duration were analyzed with nonparametric tests, including the Mann–Whitney U test for between-group comparisons, the Wilcoxon test for comparison within paired samples, and the χ2 tests for evaluating the relationship between 2 populations. For comparisons of 3 or more groups, Kruskal–Wallis one-way ANOVA was performed to determine whether there was a treatment effect. The effect was considered statistically significant at P < 0.05; this allowed us to reject the idea that the difference was due to random sampling. The Kruskal–Wallis test was followed by Dunn's multiple-comparison test to identify the differences. Differences were considered statistically significant at P < 0.05. Outliers were tested with the Grubb test, and true outliers were excluded.
Supplementary Material
Acknowledgments
We thank members of the E.A.K. laboratory for many helpful discussions and support for this project. Special thanks to Tally Lambert from the Nikon Imaging Center at Harvard Medical School for generating the high-speed videos. We also thank the Neurobiology Department and the Neurobiology Imaging Facility for consultation and instrument availability that supported this work. This facility is supported in part by the Neural Imaging Center as part of a National Institute of Neurological Disorders and Stroke P30 Core Center grant (NS072030). Special thanks also to Jacqueline Palavicino for figure and panel design. These studies were supported by NIH R35-MIRA grant 1-R35-GM118137-01 (to E.A.K.). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907042116/-/DCSupplemental.
References
- 1.Kravitz E. A., Huber R., Aggression in invertebrates. Curr. Opin. Neurobiol. 13, 736–743 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Lorenz K., On Aggression (Harcourt, New York, ed. 1, 1966). [Google Scholar]
- 3.Lonstein J. S., Gammie S. C., Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci. Biobehav. Rev. 26, 869–888 (2002). [DOI] [PubMed] [Google Scholar]
- 4.St John R. D., Corning P. A., Maternal aggression in mice. Behav. Biol. 9, 635–639 (1973). [DOI] [PubMed] [Google Scholar]
- 5.Gulland A., Women have higher rates of mental disorders than men, NHS survey finds. BMJ 354, i5320 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Ditlevsen D. N., Elklit A., Gender, trauma type, and PTSD prevalence: A re-analysis of 18 Nordic convenience samples. Ann. Gen. Psychiatry 11, 26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturtevant A. H., Experiments on sex recognition and the problem of sexual selection in Drosophilia. J. Anim. Behav. 5, 351–366 (1915). [Google Scholar]
- 8.Baier A., Wittek B., Brembs B., Drosophila as a new model organism for the neurobiology of aggression? J. Exp. Biol. 205, 1233–1240 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Dow M. A., von Schilcher F., Aggression and mating success in Drosophila melanogaster. Nature 254, 511–512 (1975). [DOI] [PubMed] [Google Scholar]
- 10.Jacobs M. E., Influence of light on mating of Drosophila melanogaster. Ecology 41, 182–188 (1960). [Google Scholar]
- 11.Kravitz E. A., Fernandez M. P., Aggression in Drosophila. Behav. Neurosci. 129, 549–563 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann A. A., A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. simulans. Anim. Behav. 35, 807–818 (1987). [Google Scholar]
- 13.Shelly T. E., Defense of oviposition sites by female oriental fruit flies (Diptera: Tephritidae). Fla. Entomol. 82, 339–346 (1999). [Google Scholar]
- 14.Pritchard G., The ecology of a natural population of Queensland fruit fly, Dacus tryoni, II: The distribution of eggs and its relation to behaviour. Aust. J. Zool. 17, 293–311 (1969). [Google Scholar]
- 15.Ueda A., Kidokoro Y., Aggressive behaviours of female Drosophila melanogaster are influenced by their social experience and food resources. Physiol. Entomol. 27, 21–28 (2002). [Google Scholar]
- 16.Nilsen S. P., Chan Y. B., Huber R., Kravitz E. A., Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 101, 12342–12347 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan Y. B., Kravitz E. A., Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 104, 19577–19582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vrontou E., Nilsen S. P., Demir E., Kravitz E. A., Dickson B. J., fruitless regulates aggression and dominance in Drosophila. Nat. Neurosci. 9, 1469–1471 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Zhou C., Rao Y., Rao Y., A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 11, 1059–1067 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Alekseyenko O. V., Lee C., Kravitz E. A., Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One 5, e10806 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahina K., et al. , Tachykinin-expressing neurons control male-specific aggressive arousal in Drosophila. Cell 156, 221–235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dierick H. A., Greenspan R. J., Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 39, 678–682 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Bath E., et al. , Sperm and sex peptide stimulate aggression in female Drosophila. Nat. Ecol. Evol. 1, 0154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashikawa K., et al. , Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat. Neurosci. 20, 1580–1590 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKellar C. E., Wyttenbach R. A., A protocol demonstrating 60 different Drosophila behaviors in one assay. J. Undergrad. Neurosci. Educ. 15, A110–A116 (2017). [PMC free article] [PubMed] [Google Scholar]
- 26.Jenett A., et al. , A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991–1001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer B. D., et al. , Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 105, 9715–9720 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamada F. N., et al. , An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rideout E. J., Dornan A. J., Neville M. C., Eadie S., Goodwin S. F., Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 13, 458–466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand A., et al. , Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics 158, 1569–1595 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rezával C., et al. , Activation of latent courtship circuitry in the brain of Drosophila females induces male-like behaviors. Curr. Biol. 26, 2508–2515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y., Budaye S. S., Mahringer D., Drosophila female-specific brain neuron elicits persistent position- and direction-selective male-like social behaviors. https://www.biorxiv.org/content/10.1101/594960v1 (31 March 2019).
- 33.Koganezawa M., Kimura K., Yamamoto D., The neural circuitry that functions as a switch for courtship versus aggression in Drosophila males. Curr. Biol. 26, 1395–1403 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Alekseyenko O. V., Chan Y. B., Li R., Kravitz E. A., Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 110, 6151–6156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Philipsborn A. C., et al. , Neuronal control of Drosophila courtship song. Neuron 69, 509–522 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Zhou C., Pan Y., Robinett C. C., Meissner G. W., Baker B. S., Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83, 149–163 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Sanders L. E., Arbeitman M. N., Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev. Biol. 320, 378–390 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee G., Hall J. C., A newly uncovered phenotype associated with the fruitless gene of Drosophila melanogaster: Aggression-like head interactions between mutant males. Behav. Genet. 30, 263–275 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Robinett C. C., Vaughan A. G., Knapp J. M., Baker B. S., Sex and the single cell, II: There is a time and place for sex. PLoS Biol. 8, e1000365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J. Y., Kanai M. I., Demir E., Jefferis G. S., Dickson B. J., Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 20, 1602–1614 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Rezával C., Nojima T., Neville M. C., Lin A. C., Goodwin S. F., Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 24, 725–730 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trannoy S., Chowdhury B., Kravitz E. A., Handling alters aggression and “loser” effect formation in Drosophila melanogaster. Learn. Mem. 22, 64–68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S., Lee A. Y., Bowens N. M., Huber R., Kravitz E. A., Fighting fruit flies: A model system for the study of aggression. Proc. Natl. Acad. Sci. U.S.A. 99, 5664–5668 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.