Significance
Forests remove about 30% of anthropogenic CO2 emissions through photosynthesis and return almost 40% of incident precipitation back to the atmosphere via transpiration. The trade-off between photosynthesis and transpiration through stomata, the water-use efficiency (WUE), is an important driver of plant evolution and ecosystem functioning, and has profound effects on climate. Using stable carbon and oxygen isotope ratios in tree rings, we found that WUE has increased by a magnitude consistent with estimates from atmospheric measurements and model predictions. Enhanced photosynthesis was widespread, while reductions in stomatal conductance were modest and restricted to moisture-limited forests. This result points to smaller reductions in transpiration in response to increasing atmospheric CO2, with important implications for forest–climate interactions, which remain to be explored.
Keywords: tree rings, stable isotopes, water-use efficiency, AmeriFlux, CO2 fertilization
Abstract
Multiple lines of evidence suggest that plant water-use efficiency (WUE)—the ratio of carbon assimilation to water loss—has increased in recent decades. Although rising atmospheric CO2 has been proposed as the principal cause, the underlying physiological mechanisms are still being debated, and implications for the global water cycle remain uncertain. Here, we addressed this gap using 30-y tree ring records of carbon and oxygen isotope measurements and basal area increment from 12 species in 8 North American mature temperate forests. Our goal was to separate the contributions of enhanced photosynthesis and reduced stomatal conductance to WUE trends and to assess consistency between multiple commonly used methods for estimating WUE. Our results show that tree ring-derived estimates of increases in WUE are consistent with estimates from atmospheric measurements and predictions based on an optimal balancing of carbon gains and water costs, but are lower than those based on ecosystem-scale flux observations. Although both physiological mechanisms contributed to rising WUE, enhanced photosynthesis was widespread, while reductions in stomatal conductance were modest and restricted to species that experienced moisture limitations. This finding challenges the hypothesis that rising WUE in forests is primarily the result of widespread, CO2-induced reductions in stomatal conductance.
Plants assimilate carbon dioxide (CO2) that moves through stomatal openings in foliage. This leads to unavoidable losses of water via transpiration and results in costs associated with accessing water, maintaining the transpiration stream, and repairing damage caused by drought (1–3). The trade-off between photosynthesis (A) and transpiration, reflected by water-use efficiency (WUE), is at the core of ecosystem functioning, underlying global-scale vegetation–climate interactions and the terrestrial water cycle.
Methods for estimating WUE differ in scale (individual leaf, plant, ecosystem) and in whether the abiotic influence of atmospheric evaporative demand is considered. Leaf WUE is defined as the ratio of A and transpiration. Transpiration is the product of stomatal conductance, gs, and the difference in intercellular and atmospheric water-vapor pressure divided by the total atmospheric pressure, which is often presented as vapor pressure deficit (VPD) (4). Multiplying WUE by VPD yields the intrinsic WUE (iWUE = A/gs), which is not sensitive to increased transpiration driven by abiotic changes in VPD, and thus is more closely coupled with plant ecophysiological function (4). The iWUE can be derived independently from the carbon isotope composition (δ13C) of plant material and that of atmospheric CO2, whose difference reflects discrimination against 13C (Δ13C) occurring during diffusion of CO2 through stomata and assimilation by photosynthesis. Intrinsic WUE is directly linked to the ratio of intercellular (ci) to atmospheric (ca) CO2 (ci/ca) (4, 5). Ecosystem WUE is calculated as the ratio between gross primary production (GPP) and evapotranspiration (ET), both derived from eddy covariance (EC) flux measurements. An ecosystem-scale analog of iWUE, called the inherent WUE (WUEei), can be readily calculated as WUEei = GPP × VPD/ET (6). Others (7) have calculated the underlying WUE (uWUE = WUEei/√VPD = (GPP × √VPD)/ET), which incorporates information about stomatal closure at high VPD (typically assumed to be a function of 1/√VPD), and thus should be more closely coupled to changes in A.
Both global-scale atmospheric δ13C values (8) and tree ring δ13C chronologies (9–11) have revealed a trend of increasing iWUE in recent decades and suggest that ci has increased in proportion with ca, leading to a near-constant ci/ca ratio. This is indicative of an active plant response to increasing ca, involving proportional changes in A and gs (12). In contrast, ecosystem-scale EC data from 21 temperate forests suggest an even larger increase in WUEei and that ci has remained constant (6). This result implies that gs decreases strongly in response to rising ca, and implementing this physiological response in a land surface model leads to larger reduction in ET and increase in runoff than large-scale observations show (13). Resolving these discrepancies in quantitative changes in WUE and explaining the underlying mechanisms is key to understanding and projecting vegetation responses and changes in the terrestrial water cycle under future ca and climatic conditions.
Here, we used tree-ring α-cellulose δ13C and δ18O to document 30-y trends in iWUE, ci, and gs measured for 12 tree species (6 conifers and 6 broadleaves) at 8 forests within the AmeriFlux network, including 5 mesic and 3 xeric sites (Methods and SI Appendix, Fig. S1 and Table S1). We estimated changes in iWUE from δ13C and used δ18O to estimate the 18O enrichment in leaf water above the source water, Δ18OLW. The latter reflects variability in transpiration and gs only (14), as opposed to δ13C, which reflects changes in both A and gs. We assessed trends in radial growth as a surrogate of photosynthetic carbon gain by computing the basal area increment (BAI) from tree-ring widths at all sites. Finally, we compared tree-ring isotope estimates of WUE (iWUE) with those obtained from EC data (WUEei) and with predictions of iWUE changes derived from a model that predicts an optimal ci/ca ratio, balancing carbon gain and water costs as a function of changing environmental conditions (2, 15).
Results
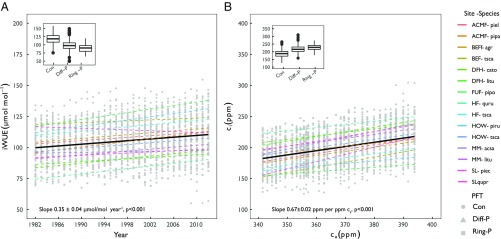
Tree-ring δ13C-derived iWUE significantly increased over the last 30 y, with a slope for the combined data set of 0.35 ± 0.04 μmol⋅mol−1⋅y−1 (P < 0.001) (Fig. 1A). Conifers showed higher iWUE values than broadleaf species (SI Appendix, Table S2), and within broadleaf species, ring porous species had lower iWUE than diffuse porous species (Fig. 1A). The trends in iWUE were positively associated with ca, temperature, and VPD, and negatively associated with precipitation and soil moisture. One-half of the variance in the linear mixed-effects model, however, was explained by the variation in iWUE among species, and individual trees within each species (SI Appendix, Table S2). At the species level, iWUE increased through time for most species, except Pinus echinata at SL where iWUE decreased, and Acer saccharum at MM and Tsuga canadensis at BEF where no trend was detected (SI Appendix, Fig. S2 and Table S3). Relative changes in iWUE (2012 relative to 1982) ranged between −11.5% and 26.9% with an average of 13.8% (±8.6%) across all sites and species (SI Appendix, Fig. S3A).
Fig. 1.
Changes in intrinsic water-use efficiency and intercellular CO2. Trend in intrinsic water-use efficiency (iWUE) (A) and in the intercellular CO2 (ci) in relation to changes in atmospheric CO2 (ca) (B) across the 12 species (n = 5 replicates per species) at 8 AmeriFlux sites in the United States. Insets show changes in iWUE and ci for the different species grouped by plant functional type (PFT): coniferous (Con), diffuse porous (Diff-P), and ring porous (Ring-P) broadleaf species. The full name of each species is provided in SI Appendix, Table S1.
We observed a consistent increase in the relationship between ci and ca (slope, 0.67 ± 0.022 ppm⋅ppm−1; P < 0.001; Fig. 1B and SI Appendix, Fig. S4 and Table S4) and in the ci/ca ratio over time across all 12 species, with conifers showing lower ci/ca ratios than broadleaf species (SI Appendix, Fig. S5 and Table S5). Similarly, carbon isotope discrimination (Δ13Cc) increased with increasing ca by 0.008 ± 0.002‰ ppm−1 of ca (SI Appendix, Fig. S6 and Table S6), with higher sensitivity for conifers (slope, 0.009 ± 0.002 ppm−1 of ca) compared with ring porous broadleaves (slope, 0.002 ± 0.003‰ ppm−1 of ca).
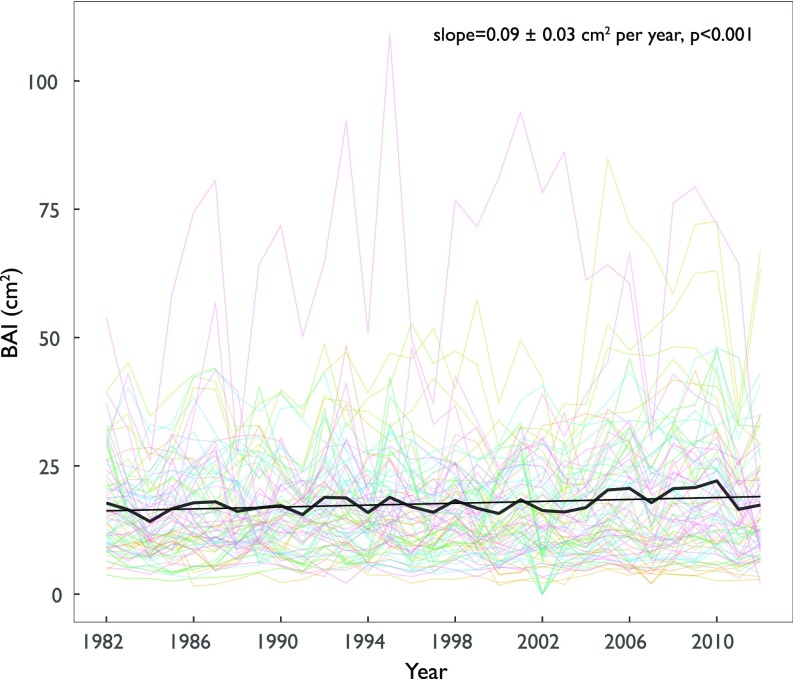
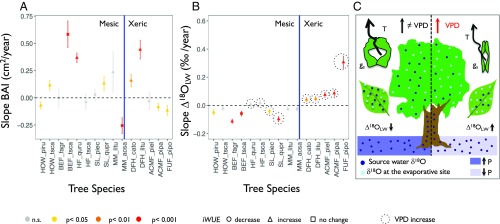
The trends in BAI and Δ18OLW were used to constrain the contribution of A and gs, respectively, in driving the observed changes in iWUE. On average, BAI increased over the last 30 y, with a slope for the combined dataset of 0.09 ± 0.03 cm2⋅y−1 (P < 0.001) (Fig. 2), although the trend was not consistent over site–species combinations (Fig. 3A and SI Appendix, Figs. S7 and S8). Directionality of changes in Δ18OLW obtained from measured tree-ring δ18O (Methods and SI Appendix, Supplementary Text and Fig. S9) revealed that the mechanisms responsible for increasing iWUE were influenced by differences across sites in moisture conditions (SI Appendix, Figs. S10–S12 and Table S1). For mesic sites, we observed no changes or a reduction in Δ18OLW (Fig. 3B), indicative of constant or increasing gs (Fig. 3C). BAI, however, increased or remained constant for the majority of the species, with the exception of Picea rubens and Acer saccharum, where it decreased (Fig. 3A). In contrast, at xeric sites, the Δ18OLW showed a significant upward trend indicative of reduction in gs (Fig. 3 B and C), which, however, negatively impacted tree growth only in the case of conifers (Fig. 3A).
Fig. 2.
Changes in basal area increment (BAI). Trend in BAI across the 12 species (n = 5 replicates per species) at 8 AmeriFlux sites in the United States.
Fig. 3.
Changes in BAI and Δ18OLW, and physiological link between Δ18OLW and gs. Slopes (±SE) of the temporal changes in BAI (A) and Δ18OLW (B) for the investigated species. Slopes significantly different from zero (SI Appendix, Table S7) are denoted with different colors, according to the P values. The black circle denotes sites where VPD increased. The vertical blue line separates mesic from xeric sites. C summarizes the physiological link between the Δ18OLW and changes in transpiration (T) and gs. The arrows indicate when the slope of Δ18OLW was either greater than or less than zero.
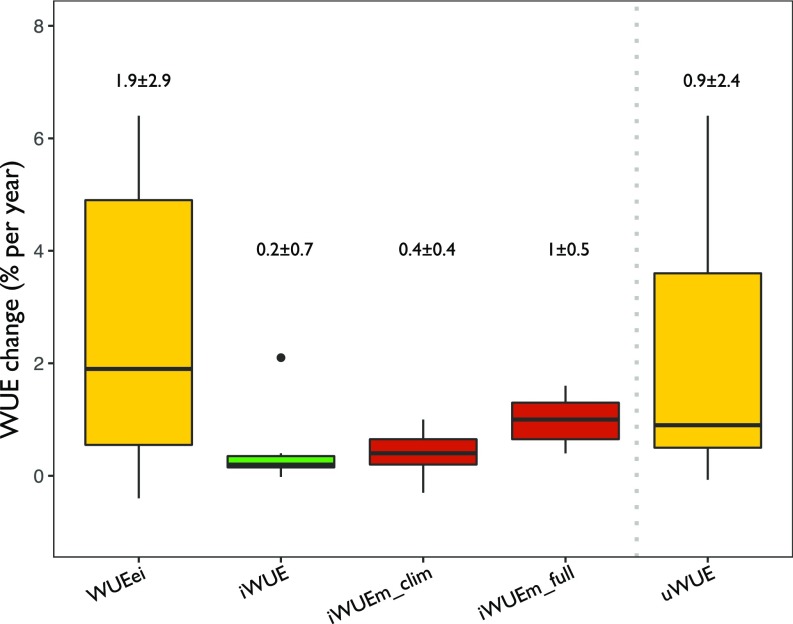
For the period of 1992–2012 (concurrent with EC measurements), tree-ring and model-derived estimates of iWUE agreed well, but EC-derived WUEei had steeper temporal trends. We observed a median Sen’s slope of 0.2 (±0.7)% y−1 for the tree-ring derived iWUE, 0.4 (±0.4)% y−1 for model prediction including only climate, and 1.0 (±0.5)% y−1 including climate and CO2 interactions. In contrast, WUEei from EC showed a significantly higher slope (P < 0.05) and higher variability among sites than the other 2 approaches, with a median Sen’s slope that was 9.5 times higher (1.9 ± 2.7% y−1) than that obtained for iWUE (Fig. 4). Nonetheless, the EC-derived WUE trend fell within the range of model predictions, which included both climate and CO2 effects (Fig. 4).
Fig. 4.
Comparing tree-ring δ13C and EC-based estimates of WUE and prediction from the carbon–water optimality model. Distribution of Sen’s slopes of year-by-year percentage changes in WUE (percentage per year) relative to the first year flux data were available at each site and obtained by considering 3 different methods: ecosystem fluxes (WUEei and uWUE), tree-ring δ13C (iWUE), and prediction from the carbon–water optimality model (16). In this latter case, we show predictions when only climate (iWUEm_Clim) and both climate and CO2 effects were included (iWUEm_full). Numbers above each boxplot indicate the median ± SD.
Discussion
Tree-ring isotope chronologies presented in this study fill the knowledge gap for underrepresented species (SI Appendix, Fig. S1 and ref. 16) in the highly productive forests of the Eastern United States. Changes in iWUE observed in our study are similar in magnitude to those reported for other temperate forests in the northern hemisphere (9–11) (SI Appendix, Fig. S3) and to those derived from the global estimate of a 20% increase over the 20th century inferred from atmospheric δ13C-CO2 observations (8). An increase in iWUE has been mostly associated with a proportional adjustment of A and gs to the increase in ca, which leads to an increase in ci (but at a slower rate than ca), and a constant ci/ca ratio (12). Our results show that, for every ppm of ca over the last 30 y, ci increased by 0.67 ± 0.022 ppm, consistent with rates reported for a large scale study in Europe (11), however inconsistent with EC observations (6) showing a constant ci for the 1992–2010 period (SI Appendix, Fig. S13). Moreover, we also found that the increase in iWUE was not achieved—at least not for all of the species—by a proportional adjustment of A and gs (12). Instead, it reflects a dynamic leaf gas exchange response to ca and climate (17), with increase in A, and small changes in gs modulated by differences in moisture conditions among sites.
The increase in ci across all of the species could partially indicate a CO2 fertilization effect on A. This is consistent with the dependence of A on ci as described by the model for C3 plant photosynthesis (18). Moreover, results from free-air CO2 experiments (FACEs) (19) and independent global measurements (8) based on atmospheric δ13C-CO2 supported an increase in A with rising ca. Lower ci values observed for conifers compared with broadleaves could be related to either differences in gs and mesophyll conductance (20) or in foliar nitrogen concentration (SI Appendix, Fig. S14), the latter being an important determinant of photosynthetic capacity (19). Nevertheless, enhanced A and iWUE in response to ca contribute to maintaining or increasing tree growth for the majority of the species.
Exposing trees to almost 700 ppm of ca increased iWUE (FACEs, SI Appendix, Fig. S3B) by enhancing A but also reducing gs. However, this latter result was not consistent across all of the experiments and studied species (19, 21), and still “the mechanism by which the stomata sense [CO2], and where in the leaf [CO2] is sensed, is unclear” (22). If a reduction in gs under increasing ca occurs across all of the investigated species, this should be reflected in a reduction in leaf transpiration, which in turn leads to less dilution of the enriched (in the heavy isotope 18O) water at the evaporative site by the unenriched water coming from the soil via xylem, thus increasing the Δ18OLW (14, 23) (Fig. 3C). However, Δ18OLW trends show differences among species, which are mostly related to differences among sites in moisture conditions. For all of the species in mesic forests, gs remained constant or increased over the last 30 y (as indicated by the reduction in Δ18OLW), even when VPD increased (at HF and SL). This finding suggests that favorable soil moisture conditions can reduce the value of water lost so that gs remains high and that, for species that do not routinely experience water stress, increasing A to maintain and/or increase tree growth is a more beneficial response to rising ca than conserving water. A reduction in gs (as inferred by the significant increase in Δ18OLW) was observed only for species experiencing more xeric condition since the 1990s and an increase in VPD. Interestingly, in this latter case, we only observed a reduction in BAI for conifers, while tree growth for broadleaf species increased. Conifers in general show a more sensitive response of gs and A to VPD than broadleaf species, likely due to a narrower hydraulic safety margin (24, 25). The difference between mesic and xeric sites for changes in gs was not captured by the carbon–water optimality model, as it predicted a reduction of gs at all sites (SI Appendix, Fig. S15), while observations show reduction only at the xeric sites. Other factors could have affected gs in the studied species, such as reduced sensitivity of gs to the increase in ca in older trees (26), and structural changes of plant hydraulic architecture allowing the water to move through the soil–atmosphere continuum (27). It is often overlooked when working at the ecosystem scale, but known since pioneering studies from Francis Darwin (28), that stomata aperture is ultimately a mechanical process associated with changes in turgor and osmotic pressure in the guard and the epidermal cells (29, 30), supported by the efficiency of the hydraulic system in transporting water (3, 31). Indeed, results from a FACE experiment at one of our sites (DFH) showed that the reduction in gs was not a direct response to ca, but to the whole-plant hydraulic conductance (27). Changes in leaf area index (LAI) can also play a role, although none of the sites in our study showed evidence of temporal trends in LAI (SI Appendix, Fig. S16).
Both tree and ecosystem WUE increased over the investigated years, although with different magnitudes. Our tree-ring results, on one hand, suggest a more modest increase in iWUE compared with a previous EC-based synthesis (6) and the EC analyses in this study. Some of the explanations for the discrepancies between the two approaches could be related to the different timescales they represent (32, 33), or to uncertainties in estimating canopy ET (i.e., nontranspirational water fluxes and/or contribution from understory vegetation ET) (34) and GPP and respiration from ecosystem CO2 fluxes. Two recent studies showed that current methods used for partitioning CO2 fluxes do not account for the light-dependent reduction in respiration, leading to an overestimation of GPP (35, 36), and consequently WUE. Our results based on Δ18OLW, on the other hand, do not support a general reduction of gs in recent decades, except when low moisture limits forest productivity. This finding calls into question the notion that rising ca reduces gs and hence transpiration, at least for mesic ecosystems in the northeastern United States, where, indeed, no significant changes in ET were observed (SI Appendix, Fig. S17). FACE experiments reported reduced transpiration (as assessed through sapflow measurements) under increasing ca (37), but no significant changes in sap flux were also observed (38, 39). This points to smaller reductions in transpiration in response to climate change than previously thought, with important implications for forest–climate interactions (40), which will require additional research to resolve. Moreover, the assumption that different tree species follow the same physiological strategy in response to increasing ca, regardless of the moisture conditions they experience, and that this strategy remains static over time, is probably too simplistic. Whether it is A and/or gs driving increasing WUE depends on a dynamic coordination of functional traits (1–3) and their adjustment to environmental changes. Implementing such a dynamic response challenges the ability of large-scale vegetation models to predict how gs will continue to optimize water loss relative to carbon gain under future CO2 emission scenarios.
Methods
Sites and Environmental Parameters.
Eight forested sites within the AmeriFlux network were selected to represent major temperate forest types for the United States and to span a range of biological properties (32, 41) and climate conditions (SI Appendix, Fig. S1 and Table S1). Mesic and xeric sites were identified based on the long-term changes in precipitation (P), i.e., 1991–2012 compared with 1901–1960 average. Wetter conditions were reported since the 1990s for the sites in the Northeast and the Midwest, whereas precipitation decreased in the case of the three sites in the Southeast and Southwest (42). For the long-term temperature and P data, we referred to available data at meteorological stations near the study sites, obtained from the Global Historical Climatology Network. Growing season (May to September) and annual mean for both temperature and P were calculated from monthly values and indicated along the text as Tgrs and Pgrs and Ta and Pa. VPD was calculated from actual vapor pressure and T (43) from CRU TS 3.23, with data extracted at site locations. Finally, we obtained the standardized precipitation–evaporation index (SPEI), relative to August, with 3-mo lag from the global database (http://sac.csic.es/spei/).
Measure of Carbon and Oxygen Isotope Ratios in Tree Rings.
Wood cores were collected from 10 to 15 trees for each of the dominant and codominant tree species at each site and constructed stable isotope chronologies for the last 30 y (1982–2012). All of the wood cores were dated from the bark to the pith. Ring width measurements were carried out with a sliding scale micrometer (Velmex Measuring System) using MeasureJ2X software (VoorTech Consulting). Ring width series were cross-dated from 1960 to 2012 first within each tree, then among trees, and finally between species within a site by using the COFECHA software (44). For stable isotope analyses, only the 5 trees showing the highest correlation with the mean chronology were selected. BAI was calculated for each tree by following the “outside-in” function to convert raw ring-width measurements to BAI based on the diameter of the tree and the width of each ring moving toward the pith. The method assumes a circular growth pattern. A mean BAI value was computed for each tree species at an annual resolution averaged over 2–3 wood cores sampled per tree. For HF, we used BAI used in ref. 45, and updated to 2012. From each of the 5 trees (n = 2 wood cores per tree), each annual ring from 1982 to 2012 was separated and then shredded by using a razor blade. For the two Quercus species only the latewood was separated, as it better reflects the contribution of sugars from the current year’s photosynthetic activity (45, 46). Wood from each ring was subjected to α-cellulose extraction (47, 48), and then homogenized by using an ultrasonic bath. About 0.3 ± 0.1 mg of each ring sample was weighed in tin capsules and converted to CO2 with an elemental analyzer (ECS 4010; Costech Analytical) coupled to a continuous flow isotope ratio mass spectrometer (Delta PlusXP; ThermoFinnigan) to determine δ13C. An additional 0.3 ± 0.1 mg of each sample was weighed in silver capsules, converted to CO with a pyrolysis elemental analyzer (TC/EA; ThermoFinnigan), and analyzed for δ18O with a continuous flow isotope ratio mass spectrometer (Delta PlusXP; ThermoFinnigan). Carbon and oxygen isotope ratios were expressed in per mil (‰) relative to the Vienna PDB and Vienna SMOW international standards, respectively. Isotope analyses were carried out at the Stable Isotope Core Laboratory (Washington State University). The SDs for internal standards were less than 0.2‰ and 0.4‰ for δ13C and δ18O values, respectively, whereas SDs for Sigma-Aldrich α-cellulose (item C8002, lot 031M0133V) were less than 0.2‰ and 0.6‰ for δ13C and δ18O values, respectively. Finally, mean SDs across replicates on given years and assessed for each species were 0.2‰ and 0.3‰ for δ13C and δ18O values, respectively.
Calculation of Tree WUE.
We derived iWUE from δ13C measured in tree ring α-cellulose (49) based on the well-established theory linking leaf ci/ca with isotopic carbon discrimination, Δ13Cc (5):
| [1] |
δ13Cc and δ13Ca are the carbon isotope compositions of tree ring α-cellulose and ca, a is the isotope fractionation during CO2 diffusion through stomata (4.4‰), and b is the isotope fractionation during fixation by Rubisco (27‰). Note that Eq. 1 is the “simple” form of isotopic discrimination that does not include effects due to mesophyll conductance and photorespiration, which were not available for the species here. We derived ci from the following equation:
| [2] |
ca and δ13C were obtained from Mauna Loa records (50) from 1990 to 2012, while from 1990 back to 1982 we used data published in ref. 51. iWUE (in micromoles of CO2 per mole of H2O) was then calculated using the following equation:
| [3] |
where 1.6 is the molar diffusivity ratio of CO2 to H2O (i.e., gCO2 = gH2O/1.6). The iWUE as derived from Eq. 4 is normalized by a constant VPD of 1 mol⋅mol−1. Intrinsic WUE was converted from micromoles of CO2 per mole of H2O at VPD of 1 mol mol−1 to grams of C/kilogram of H2O normalized at VPD of 1 kPa to better compare the leaf and ecosystem WUE. The conversion factor from micromole/mole at VPD of 1 mol mol−1 to grams of C/kilogram of H2O at 1 kPa is 12/18*100/1,000.
Calculation of Δ18Oc and Δ18O in Leaf Water (Δ18OLW).
We calculated the 18O enrichment in tree-ring α-cellulose above the source water, Δ18Oc, according to the following equation (52):
| [4] |
where δ18Oc is the oxygen isotope composition measured in α-cellulose extracted from each ring, while δ18OP is the δ18O of precipitation, which we estimated as described in SI Appendix (SI Appendix, Supplementary Text and Fig. S18). We assume δ18OP to be reflected in the oxygen isotope composition of the soil water (i.e., source water), modified by evaporation (53). We also assume that the tree species at each site had access to water at similar soil depth, so that there are no differences in the δ18O of the source water over the long term. From Δ18Oc, we then estimated Δ18OLW, which is directly linked to transpiration and gs. Notably, less enriched (in 18O) water from the soil and more enriched (in 18O) water at the leaf evaporative sites continuously mix, as a function of transpiration rates and the pathway of water movement through foliar tissues (54, 55) so that lower Δ18OLW results from an increase in transpiration and gs (56). The Δ18Oc can be described by the following equation (57):
| [5] |
where pex is the proportion of oxygen exchanging with local water in the stem during cellulose synthesis, px is the proportional amount of stem water relative to both leaf and stem water in the tissue where cellulose is synthesized, and finally εwc is the isotope fractionation occurring during cellulose synthesis in the stem. Eq. 5 can be rearranged so to derive Δ18OLW as follows:
| [6] |
Generally, a fixed value of 27‰ and 0.4 are considered for εwc and pxpex, respectively. We estimated dampening factors diluting the signal of leaf water δ18O when sugars are translocated to the stem and used for synthesis of cellulose. Specifically, we derived pxpex from Pa and Pgrs (52). Moreover, we also assume a variable εwc, which was derived from Tgrs, based on the equation in ref. 57, showing the dependence of the fractionation factor on temperature.
Flux Data.
We compiled flux and micrometeorological observations from 7 out of the 8 AmeriFlux sites where we sampled wood cores from the dominant species (SI Appendix, Tables S1 and S8). Duke forest was not included because data were not available. Data were acquired from the AmeriFlux data archive, the Fluxnet database, or site PI. In this latter case, gap-filling and partitioning of carbon fluxes were performed by the site PIs. Net carbon fluxes were partitioned into GPP and total ecosystem respiration (Reco) by using the temperature sensitivity of respiration to then extrapolate night to daytime Reco (58). We calculated ET from the latent heat flux. Half-hourly data were aggregated to daily values for the calculation of the growing season (grs) (May to September) GPP (grams of C⋅meter−2⋅grs−1) and ET (kilograms of H2O⋅meter−2⋅grs−1). We only considered GPP and ET obtained during dry canopy conditions (i.e., with no precipitation event) to avoid error introduced from canopy evaporation after precipitation events (6). We calculated inherent (6, 59) and underlying WUE (7) as described in the main text and by using mean of VPD over the grs months.
Estimates of iWUE from the Water–Carbon Optimality Model.
The model predicts an optimal ci/ca ratio that balances carbon gains and water costs and maximizes leaf-level A minus costs. The water–carbon trade-off is governed by the costs arising from the maintenance of carboxylation capacity (Vcmax) and the transpiration stream as a function of gs. The model is based on the Farquhar–von Caemmerer–Berry model for C3 photosynthesis (18), the coordination hypothesis assuming that A operates at the intersection of the light and Rubisco-limited assimilation rates during typical daytime conditions (60), and the least-cost hypothesis assuming an optimal balance of carbon costs as functions of Vcmax and gs (1, 2). Thus, it simulates how photosynthetic parameters (ci, gs, Vcmax, and Jmax) and rates (A, light use efficiency) acclimate to environmental conditions (CO2, T, radiation, VPD, and atmospheric pressure). A complete derivation and description of the model is given in ref. 16. Forcing data used for simulations presented here are annually varying ca, daily mean T, and specific humidity (WATCH-WFDEI) (61), converted to VPD as a function of T and elevation.
Statistical Analyses.
We used R Studio (62) and specific packages for statistical analyses. Linear regressions were employed to explore trends in iWUE, ci, ci/ca, Δ13C, and BAI. Linear mixed models (63) were employed to explain temporal changes in iWUE, ci, ci/ca, and Δ13C as a function of environmental factor and ca (fixed factors). Multiple species (and replicates per species) nested in the sites were included as the random factor. Each of the numerical fixed factors was centered to the mean to remove multicollinearity. An autocorrelation structure of order 1 was included in the model for each site per species combination to allow for the temporal dependency of measurements carried out in subsequent years. Quality of fit was assessed using residual distribution plots, qqnorm plots of standardized residuals against quantiles of standard normals for both individual points and for the random effects. Marginal (only fixed factors) and conditional (fixed plus random factors) proportions of the explained variance (R2m and R2c, respectively) were calculated (64). For the comparison among EC-derived, tree-ring isotope, and predicted WUE, we first calculated the year-by-year percentage changes in WUE relative to the beginning of the EC data record. Then we used the Mann–Kendall Tau nonparametric trend test with Sen’s method to obtain slopes of trend in WUE for the different sites (6). Finally, differences in the slope for WUE among the different methods were assessed by the nonparametric Kruskal–Wallis and post hoc Dunn tests.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Science Foundation (NSF) (Awards 1638688, 1832210, and 1637685) and the National Aeronautics and Space Administration (Award NNX12AK56G). Funding from Marie Sklodowska-Curie Fellowship H2020-MSCA-IF 2015 supported R.G. (Grant 705432) and B.D.S. (Grant 701329), while S.B. was supported by NSF (Award 1229887). R.G. thanks T. Martin, R. Oren, J.-C. Domec, M. Day, L. Lepine, and S. Maxwell for assistance in the field; R. Snyder, L. Buzinski, and C. Madison for assistance in the lab; and Z. Zhou, M. Mencuccini, and M. Cuntz for useful discussion. We thank the AmeriFlux Management Project and Fluxnet for supporting the operation of flux towers. Research at US-Bar is supported by the NSF (Award DEB-1114804), the Northeastern States Research Cooperative, and the US Department of Agriculture Forest Service’s Northern Research Station. We thank the two anonymous reviewers and the editor for positive comments and constructive suggestions on earlier versions of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The dataset, including all tree-ring data, has been deposited with the Environmental Data Initiative (EDI), https://portal.edirepository.org/nis/mapbrowse?scope=edi&identifier=401.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905912116/-/DCSupplemental.
References
- 1.Wright I. J., Reich P. B., Westoby M., Least-cost input mixtures of water and nitrogen for photosynthesis. Am. Nat. 161, 98–111 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Prentice I. C., Dong N., Gleason S. M., Maire V., Wright I. J., Balancing the costs of carbon gain and water transport: Testing a new theoretical framework for plant functional ecology. Ecol. Lett. 17, 82–91 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Sperry J. S., et al. , Predicting stomatal responses to the environment from the optimization of photosynthetic gain and hydraulic cost. Plant Cell Environ. 40, 816–830 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Ehleringer J. R., Hall A. E., Farquhar G. D., “Introduction: Water use in relation to productivity” in Stable Isotopes and Plant Carbon–Water Relations, Ehleringer J. R., Hall A. E., Farquhar G. D., Eds. (Academic Press, New York, 1993), pp. 3–8. [Google Scholar]
- 5.Farquhar G. D., Ehleringer J. R., Hubick K. T., Carbon isotope discrimination and photosynthesis. Ann. Rev. Plant Physiol. Mol. Biol. 40, 503–537 (1989). [Google Scholar]
- 6.Keenan T. F., et al. , Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499, 324–327 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Zhou S., Yu B., Huang Y., Wang G., The effect of vapor pressure deficit on water use efficiency at the subdaily time scale. Geophys. Res. Lett. 41, 5005–5013 (2014). [Google Scholar]
- 8.Keeling R. F., et al. , Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis. Proc. Natl. Acad. Sci. U.S.A. 114, 10361–10366 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peñuelas J., Canadell J. G., Ogaya R., Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 20, 597–608 (2011). [Google Scholar]
- 10.Leonardi S., et al. , Assessing the effects of nitrogen deposition and climate on carbon isotope discrimination and intrinsic water-use efficiency of angiosperm and conifer trees under rising CO2 conditions. Glob. Change Biol. 18, 2925–2944 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Frank D. C., et al. , Water-use efficiency and transpiration across European forests during the Anthropocene. Nat. Clim. Chang. 5, 579–584 (2015). [Google Scholar]
- 12.Saurer M., Siegwolf R. T. W., Schweingruber F. H., Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Change Biol. 10, 2109–2120 (2004). [Google Scholar]
- 13.Knauer J., et al. , The response of ecosystem water-use efficiency to rising atmospheric CO2 concentrations: Sensitivity and large-scale biogeochemical implications. New Phytol. 213, 1654–1666 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Farquhar G. D., Cernusak L. A., Barnes B., Heavy water fractionation during transpiration. Plant Physiol. 143, 11–18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., et al. , Towards a universal model for carbon dioxide uptake by plants. Nat. Plants 3, 734–741 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Dekker S. C., Groenendijk M., Booth B. B. B., Huntingford C., Cox P. M., Spatial and temporal variations in plant water‐use efficiency inferred from tree‐ring, eddy covariance and atmospheric observations. Earth Syst. Dyn. 7, 525–533 (2016). [Google Scholar]
- 17.Voelker S. L., et al. , A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: Evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Glob. Change Biol. 22, 889–902 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Farquhar G. D., von Caemmerer S., Berry J. A., A biochemical model of photosynthetic CO2 assimilation in leaves of C 3 species. Planta 149, 78–90 (1980). [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth E. A., Rogers A., The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Flexas J., Ribas-Carbó M., Diaz-Espejo A., Galmés J., Medrano H., Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 31, 602–621 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Purcell C., et al. , Increasing stomatal conductance in response to rising atmospheric CO2. Ann. Bot. 121, 1137–1149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long S. P., Ainsworth E. A., Rogers A., Ort D. R., Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 55, 591–628 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Cooper L. W., Norby R. J., Atmospheric CO2 enrichment can increase the 18O content of leaf water and cellulose: Paleoclimatic and ecophysiological implications. Clim. Res. 4, 1–11 (1994). [Google Scholar]
- 24.Choat B., et al. , Global convergence in the vulnerability of forests to drought. Nature 491, 752–755 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Carnicer J., Barbeta A., Sperlich D., Coll M., Peñuelas J., Contrasting trait syndromes in angiosperms and conifers are associated with different responses of tree growth to temperature on a large scale. Front. Plant Sci. 4, 409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medlyn B. E., et al. , Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Change Biol. 17, 2134–2144 (2011). [Google Scholar]
- 27.Domec J.-C., Smith D. D., McCulloh K. A., A synthesis of the effects of atmospheric carbon dioxide enrichment on plant hydraulics: Implications for whole-plant water use efficiency and resistance to drought. Plant Cell Environ. 40, 921–937 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Darwin F., Observations on stomata. Proc. R. Soc. Lond. 63, 413–417 (1898). [Google Scholar]
- 29.Buckley T. N., The control of stomata by water balance. New Phytol. 168, 275–292 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Franks P. J., Farquhar G. D., The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 143, 78–87 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mencuccini M., The ecological significance of long-distance water transport: Short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ. 26, 163–182 (2003). [Google Scholar]
- 32.Guerrieri R., Lepine L., Asbjornsen H., Xiao J., Ollinger S. V., Evapotranspiration and water use efficiency in relation to climate and canopy nitrogen in U.S. forests. J. Geophys. Res. Biogeosci. 121, 2610–2629 (2016). [Google Scholar]
- 33.Yi K., et al. , Linking variation in intrinsic water-use efficiency to isohydricity: A comparison at multiple spatiotemporal scales. New Phytol. 221, 195–208 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Knauer J., et al. , Towards physiologically meaningful water-use efficiency estimates from eddy covariance data. Glob. Change Biol. 24, 694–710 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Wehr R., et al. , Seasonality of temperate forest photosynthesis and daytime respiration. Nature 534, 680–683 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Keenan T. F., et al. , Widespread inhibition of daytime ecosystem respiration. Nat. Ecol. Evol. 3, 407–415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cech P. G., Pepin S., Körner C., Elevated CO2 reduces sap flux in mature deciduous forest trees. Oecologia 137, 258–268 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Norby R. J., Zak D. R., Ecological lessons from free-air CO2 enrichment (FACE) experiments. Annu. Rev. Ecol. Evol. Syst. 42, 181–203 (2011). [Google Scholar]
- 39.Tor-ngern P., et al. , Increases in atmospheric CO2 have little influence on transpiration of a temperate forest canopy. New Phytol. 205, 518–525 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Bonan G. B., Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Ollinger S. V., et al. , Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: Functional relations and potential climate feedbacks. Proc. Natl. Acad. Sci. U.S.A. 105, 19336–19341 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melillo J. M., Richmond T., Gary W. Y., Climate Change Impacts in the United States: The Third National Climate Assessment (US Global Change Research Program, 2014). [Google Scholar]
- 43.Allen R. G., Pereira L. S., Raes D., Smith M., Crop evapotranspiration–Guidelines for computing crop water requirements (FAO Irrigation and Drainage Paper 56, Food and Agriculture Organization, Rome, 1998).
- 44.Holmes R. L., Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 43, 69–78 (1983). [Google Scholar]
- 45.Belmecheri S., et al. , Tree-ring δ13C tracks flux tower ecosystem productivity estimates in a NE temperate forest. Environ. Res. Lett. 9, 074011 (2014). [Google Scholar]
- 46.Helle G., Schleser G. H., Beyond CO2‐fixation by Rubisco‐An interpretation of 13C/12C variations in tree rings from novel intra‐seasonal studies on broad‐leaf trees. Plant Cell Environ. 27, 367–380 (2004). [Google Scholar]
- 47.Leavitt S. W., Danzer S. R., Method for batch processing small wood samples to holocellulose for stable-carbon isotope analysis. Anal. Chem. 65, 87–89 (1993). [Google Scholar]
- 48.Sternberg L. d. S. L., “Oxygen and hydrogen isotope ratios in plant cellulose: Mechanisms and applications” in Stable Isotopes in Ecological Research, Rundel P. W., Ehleringer J. R., Nagy K. A., Eds. (Springer, 1989), pp. 124–141. [Google Scholar]
- 49.Guerrieri R., et al. , Stable carbon and oxygen isotopes in tree rings and basal area increment from mature temperate forests within the AmeriFlux network. Environmental Data Initiative. 10.6073/pasta/1b7c06e5dd81341f704888e23b2069ae. Deposited 16 July 2019. [DOI]
- 50.Thoning T. W., Kitzis D. R., and Crotwell A.; NOAA ESRL Global Monitoring Division , Data from “Atmospheric carbon dioxide dry air mole fractions from quasi-continuous measurements at Mauna Loa, Hawaii, Version 08-15.” https://www.esrl.noaa.gov/gmd/ccgg/trends/data.html. Accessed 15 May 2015.
- 51.McCarroll D., Loader N. J., Stable isotopes in tree rings. Quat. Sci. Rev. 23, 771–801 (2004). [Google Scholar]
- 52.Cheesman A. W., Cernusak L. A., Infidelity in the outback: Climate signal recorded in Δ18O of leaf but not branch cellulose of eucalypts across an Australian aridity gradient. Tree Physiol. 37, 554–564 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Dawson T. E., Mambelli S., Plamboeck A. H., Templer P. H., Tu K. P., Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 33, 507–559 (2002). [Google Scholar]
- 54.Barbour M. M., Roden J. S., Farquhar G. D., Ehleringer J. R., Expressing leaf water and cellulose oxygen isotope ratios as enrichment above source water reveals evidence of a Péclet effect. Oecologia 138, 426–435 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Farquhar G. D., Lloyd J., “Carbon and oxygen isotope effects in the exchange of carbon dioxide between plants and the atmosphere” in Stable Isotope and Plant Carbon–Water Relations, Ehleringer J. R., Hall A. E., Farquhar G. D., Eds. (Academic Press, New York, 1993), pp. 47–70. [Google Scholar]
- 56.Barbour M. M., Fischer R. A., Sayre K. D., Farquhar G. D., Oxygen isotope ratio of leaf and grain material correlates with stomatal conductance and yield in irrigated, field-grown wheat. Aust. J. Plant Physiol. 27, 625–637 (2000). [Google Scholar]
- 57.Sternberg L., Ellsworth P. F. V., Divergent biochemical fractionation, not convergent temperature, explains cellulose oxygen isotope enrichment across latitudes. PLoS One 6, e28040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reichstein M., et al. , On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Change Biol. 11, 1424–1439 (2005). [Google Scholar]
- 59.Beer C., et al. , Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Global Biogeochem. Cycles 23, GB2018 (2009). [Google Scholar]
- 60.Maire V., et al. , The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS One 7, e38345 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weedon G. P., et al. , The WFDEI meteorological forcing data set: WATCH forcing data methodology applied to ERA-Interim reanalysis data. Water Resour. Res. 50, 7505–7514 (2014). [Google Scholar]
- 62.RStudio Team , RStudio: Integrated development for R (Version 1.1.456, RStudio, Inc., Boston, MA, 2016).
- 63.Pinheiro J., Bates D., DebRoy S., Sarkar D.; R Core Team , nlme: Linear and nonlinear mixed effects models (R Package, Version 3.1-131, 2017). https://CRAN.R-project.org/package=nlme. Accessed 27 May 2019.
- 64.Bartoń K., MuMIn: Multi-model inference (R Package, Version 1.40.0, 2017). https://CRAN.R-project.org/package=MuMIn. Accessed 27 May 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.