Abstract
Vagus nerve stimulation (VNS) paired with forelimb training drives robust, specific reorganization of movement representations in the motor cortex. This effect is hypothesized to be mediated by VNS-dependent engagement of neuromodulatory networks. VNS influences activity in the locus coeruleus (LC) and dorsal raphe nucleus (DRN), but the involvement of these neuromodulatory networks in VNS-directed plasticity is unknown. We tested the hypothesis that cortical norepinephrine and serotonin are required for VNS-dependent enhancement of motor cortex plasticity. Rats were trained on a lever pressing task emphasizing proximal forelimb use. Once proficient, all rats received a surgically implanted vagus nerve cuff and cortical injections of either immunotoxins to deplete serotonin or norepinephrine, or vehicle control. Following surgical recovery, rats received half second bursts of 0.8 mA or sham VNS after successful trials. After five days of pairing intracortical microstimulation (ICMS) was performed in the motor cortex contralateral to the trained limb. VNS paired with training more than doubled cortical representations of proximal forelimb movements. Depletion of either cortical norepinephrine or serotonin prevented this effect. The requirement of multiple neuromodulators is consistent with earlier studies showing that these neuromodulators regulate synaptic plasticity in a complimentary fashion.
Keywords: Vagus nerve stimulation, motor cortex, monoamine, plasticity
INTRODUCTION
Vagus nerve stimulation (VNS) delivered during rehabilitation has emerged as a potential strategy to enhance recovery across a wide range of neurological disorders in rodents and humans (Dawson et al., 2016; De Ridder et al., 2014; Engineer et al., 2011; Ganzer et al., 2018; Khodaparast et al., 2014). Brief bursts of VNS timed to coincide with sensory, motor, or cognitive events during rehabilitation are used to enhance plasticity and support lasting functional improvements (Ganzer et al., 2018; Hays et al., 2013b; Peña et al., 2014). Despite its broad potential applications, there is little direct evidence revealing the neural mechanisms by which VNS guides plasticity and enhances functional recovery.
Afferent projections of the vagus nerve innervate the key brainstem nuclei, and electrical stimulation engages multiple downstream neuromodulatory pathways in the central nervous system (Cunningham et al., 2008; Ruffoli et al., 2011). Stimulation rapidly influences neural activity in the noradrenergic locus coeruleus (LC) and chronic stimulation modulates activity in the serotonergic dorsal raphe nucleus (DRN) (Hulsey et al., 2017; Manta et al., 2009). Previous studies point to the role of these and other neuromodulatory networks in the actions of VNS in the central nervous system, but it is not known whether these systems are required for VNS-dependent cortical plasticity (Krahl et al., 1998; Nichols et al., 2011; Shen et al., 2012). Because multiple VNS parameters influence the degree of cortical plasticity and this plasticity is associated with the therapeutic benefits of VNS paired with rehabilitation, a clear understanding of the neural mechanisms that underlie VNS-dependent plasticity may provide a framework to optimize VNS delivery during therapy (Borland et al., 2018, 2016; Buell et al., 2018; Loerwald et al., 2018a, 2018b).
Neuromodulators are known to directly influence cortical plasticity, and cortical cholinergic innervation mediates VNS-based plasticity in the motor cortex. Other neuromodulatory networks, including the noradrenergic and serotonergic systems, may also support plasticity in response to VNS-event pairing. In this study, we assessed whether VNS-dependent targeted plasticity in the motor cortex requires cortical norepinephrine and serotonin.
METHODS
Subjects
All handling, housing, behavioral training, and surgical procedures were approved by the University of Texas Institutional Animal Care and Use Committee. Seventy-four adult female Sprague-Dawley rats weighing 293 ± 41 grams (mean ± standard deviation) were used in this experiment. Female rats were use due to ease of handling, and because the behavioral measures used in this study have been extensively optimized in this sex (Hays et al., 2013a; Seth A. Hays et al., 2014; Hulsey et al., 2016; Khodaparast et al., 2014; Morrison et al., 2019; Porter et al., 2012). Rats were housed in a 12:12 hour reverse light cycle. Rats were food deprived Monday-Friday during behavioral training to increase motivation for food rewards, and provided access to full feed on weekends. Supplemental food was provided on weekdays as necessary to maintain a minimum of 85% original body weight.
Behavioral Task
Rats were trained with MotoTrak (Fig. 1, Vulintus Inc, Richardson, TX) systems on a quantitative, automated, skilled lever pressing task (Hays et al., 2013a). The behavior chamber consisted of an acrylic cage with a slot for access to a lever positioned −1 to 2 cm from the inside edge of the chamber. The slot was positioned next to a divider in the cage, ensuring use of the right forelimb. Reward pellets (45 mg dustless precision pellet, BioServ, Frenchtown, NJ) were delivered to a receptacle on the other side of the divider, forcing rats to travel between areas within the cage to receive rewards thus causing rats to reset their position between trials. The lever was affixed to a potentiometer to record the angle of the lever. The lever had a neutral horizontal position when no force was applied. The lever mechanism allowed a 1.5 cm depression over 13°. A spring provided 28 grams of resistance and returned the lever to its neutral horizontal resting angle. An electronic controller board sampled the potentiometer position at 100 Hz and relayed the information to MotoTrak software that controlled the task criteria and collected data.
Figure 1. Experimental design.
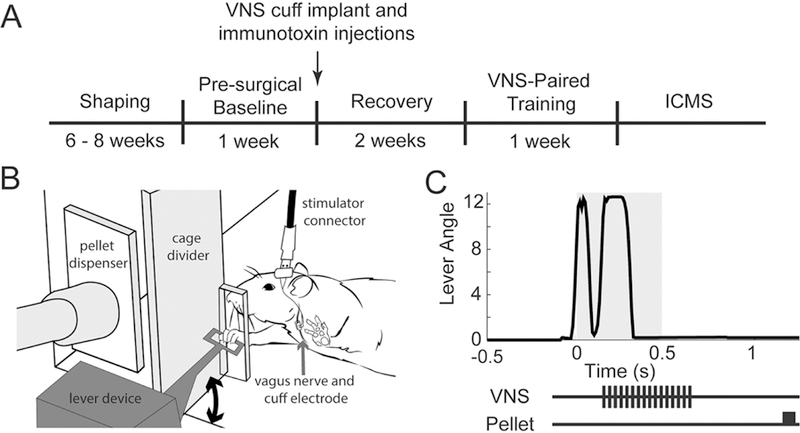
(A) Timeline of experimental procedures. (B) Schematic of behavioral training cage. Rats could access the lever by reaching through a small slot in the cage. Rats were trained to press the lever twice in rapid succession to receive a food reward. (C) Representative data of a trial illustrating a successful double press with VNS and reward pellet delivery timing indicated. The gray shaded area indicates the time window for a double press.
Shaping of lever press behavior occurred across 5 stages (Fig. S1). The first stage rewarded single lever presses with a sugar pellet. Subsequent stages changed lever position and reward criteria to increase the difficulty of the task. During the final stage rats received a reward after pressing the lever twice within a 500 ms interval. Behavior training was performed in two thirty minute sessions per day, five days a week, with at least two hours between daily training sessions. Proficiency at the task was defined as performing an average of 100 successful trials across 10 behavior sessions with a hit rate of at least 65% on the final stage.
Cortical Noradrenergic and Serotonergic Depletion
Immunotoxins (Advanced Targeting Systems, San Diego, CA, SKU: IT-03 and IT-23) were used to deplete norepinephrine and serotonin in the motor cortex. Rats were anesthetized with ketamine hydrochloride (80 mg/kg, i.p.) and xylazine (10mg/kg, i.p.), and given supplemental doses as necessary to maintain anesthesia throughout surgical procedures. The head was stabilized in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and burr holes were drilled over the left motor cortex (contralateral to the trained forelimb). DBH-Sap, SERT-Sap, CTRL-Sap (0.1 μg/μl), or saline were stereotactically injected with a neuros syringe (Hamilton, Reno, NV) at two stereotaxic coordinates in the caudal forelimb area (from bregma: 3mm lateral, 0 and 2 mm anterior, 1.5 mm ventral to the pial surface; 0.25 μl volume per site, 0.1 μl/minute)(Conner et al., 2010; McGaughy et al., 2008; Milstein et al., 2007). The syringe remained in place for 4 minutes following each injection to allow for diffusion and to prevent backflow of the injection. Burr holes were sealed with Kwik-Cast (WPI, Sarasota, FL) silicone and acrylic.
At the end of the experiment noradrenergic innervation of motor cortex was imaged to confirm depletion with histological methods. Serotonergic depletion was not confirmed due to technical difficulties (see discussion section). At the end of the experiment, animals were transcardially perfused with 0.01 M PBS followed by 4% paraformaldehyde. After cryoprotection by immersion in 20% sucrose, the brains were sectioned with a cryostat through the extent of forelimb cortex (40μm sections, +4mm to −1mm from bregma). Every sixth section was stained for DBH positive fibers. Sections were placed in a PBS solution of 0.5% Triton X-100 (TX) and 2% normal horse serum (NHS). After 1 hour, sections were transferred into the primary antibody (mouse anti-DBH, 1:1000 dilution, EMD Millipore MAB308) in PBS with 0.5% TX and 2% NHS and incubated overnight in a humidified chamber. Following 3×5 minute rinses in PBS sections were placed in biotinylated secondary antibody (biotinylated universal antibody - horse anti-mouse/rabbit, 1:50 dilution, Vector Laboratories, Burlingame, CA, USA) for 30 minutes. Following 3×5 minute PBS washes, tissue was placed in an avidin-biotin complex solution (Vectastain Elite ABC, Vector Laboratories) for 30 minutes. After 3×5 minute washes, visualization was accomplished with a solution of 3,3-diaminobenzidine (ImmPACT DAB, Vector Laboratories). Sections were mounted on slides and air dried before coverslipping.
Slides were imaged with an Olympus VS120 scanner at 2× for overview, and at 20× detail in regions of interest over motor cortex in left and right hemispheres. Images from three cortical sections (approximately 0, 1.2, and 2.4 mm from bregma) were overlaid with a 5×5 grid with 50μm spacing and randomly coded for blinded analysis of fiber crossings to determine innervation.
Vagus Nerve Cuff Implantation
A vagus nerve cuff and headcap connector were implanted following cortical injections using methods detailed in previous studies (Hulsey et al., 2016; Khodaparast et al., 2014). While in the stereotaxic frame, four bone screws were placed into the parietal and supraoccipital bones. A two-channel connector was attached to the cranial screws with acrylic. Rats were transferred to a supine position, and an incision and blunt dissection of the neck muscles exposed the left vagus nerve. The vagus nerve was isolated and placed in a bipolar stimulating cuff electrode with platinum iridium leads (~5 kΩ impedance). Cuff leads were tunneled subcutaneously, attached to the skull mounted connector, and encapsulated with acrylic. Neck and scalp incisions were sutured and treated with topical antibiotic ointment. Rats received an injection of buprenorphine (0.03 mg/kg, s.c) and provided carprofen and enroflaxcin tablets (1mg each) for 3 days following surgery to provide analgesia and prevent infection. Rats recovered for one week before returning to behavioral training.
Vagus nerve stimulation procedure
Upon returning to behavioral training, rats were habituated to the stimulator connection cables while performing the lever press task. VNS pairing began once rats consistently performed 200 successful trials per day while connected to the inactive stimulator. During VNS pairing, an isolated pulse stimulator (A-M Systems, Sequim, WA, Model 2100) was triggered by the MotoTrak software immediately after successful trials. VNS consisted of a 500 ms train of 100 μs biphasic pulses at 30 Hz at 0.8 mA. The stimulation parameters are identical to previous studies (Engineer et al., 2011; Hulsey et al., 2016; Khodaparast et al., 2014). Rats received VNS paired with behavior training for 5 days before ICMS. Sham stimulation rats underwent the same habituation and training protocol but did not receive stimulation.
Intracortical Microstimulation
Within 24 hours of the final training session, rats underwent intracortical microstimulation to derive functional representation maps using standard procedures (Ganzer et al., 2018; Hulsey et al., 2016; Morrison et al., 2019; Porter et al., 2012). Rats were anesthetized by intraperitoneal injections of ketamine hydrochloride (75 mg/kg) and xylazine (5 mg/kg) and received supplemental doses of ketamine (25 mg/kg) and xylazine (1.5 mg/kg) as necessary throughout the procedure in order to maintain a consistent level of anesthesia as indicated by breathing rate, vibrissae whisking, and foot reflex. To prevent cortical swelling, a small incision was made in the cisterna magna. A craniotomy and duratomy exposed the left motor cortex, contralateral to the trained forelimb. A tungsten electrode (459 ± 41 kΩ, FHC Inc., Bowdin, MD, Cat#: UEWMEGSEBN3M) was inserted to a depth of 1.8 mm following a 0.5 mm grid, with subsequent penetrations at least 1 mm apart when possible. Stimulation consisted of a 40 ms train of twelve 200 μs monophasic cathodal pulses delivered at 300 Hz. Stimulation intensity was gradually increased from 20 to 200 μA until a movement was observed. If no movement was observed at maximal intensity, the site was deemed nonresponsive. Borders of the motor cortex were defined based on nonresponsive sites and vibrissae responses in the posterior-lateral edge of the map.
Motor mapping procedures were conducted with two experimenters. The first experimenter placed the electrode and recorded data for each site. The second experimenter, who was blind to the experimental group and electrode position, delivered stimulation, and observed and classified motor movements. Observed movements were classified as “proximal forelimb” (shoulder and elbow), “distal forelimb” (wrist and digits), “hindlimb” (hindlimb, trunk, and tail), and “head” (neck, vibrissa, jaw, nose, and eye). Cortical area was calculated by multiplying the number of sites eliciting a response by the area surrounding the site (0.25 mm2). Complete borders were determined when possible, but some maps do not have medial or posterior borders due to proximity to the median sinus or headcap placement. The contiguous forelimb region was always mapped completely. Raw ICMS maps from all subjects can be found in the supplemental material (Fig. S2–5).
Statistics
All data are reported in the main text as mean ± SEM except as noted. All comparisons were planned in the experimental design a priori and preregistered (https://osf.io/xf3yj/). Significant differences were determined using ANOVA and t-tests where appropriate using the MATLAB 2018a statistics toolbox (MathWorks, Natick, MA). Statistical tests for each comparison are noted in the text.
Exclusions and criteria
Twenty-seven rats out of seventy-four were excluded from analysis based on pre-determined criteria listed in the study registration. Six rats failed to acquire the lever pressing task. Seven rats died due to surgical complications. Two rats were excluded due to mechanical failure of the head mounted connector. Nine rats were excluded due to high or inconsistent stimulation voltages (>20V peak to peak intensity), consistent with electrical failure of the stimulating cuff. To confirm effective stimulation of the vagus nerve, we measured rapid stimulation-dependent reduction in oxygen saturation at the conclusion of ICMS. This well described biomarker of VNS in rats is mediated by vagal A-fibers and is ascribed to activation of the Hering-Breuer reflex (Chang et al., 2015; McAllen et al., 2018). Three rats were excluded after ICMS because VNS did not reliably elicit this characteristic drop in oxygen saturation.
RESULTS
Serotonergic or Noradrenergic depletion prevents VNS-dependent cortical plasticity
VNS paired with proximal forelimb movements resulted in a significant expansion of proximal forelimb representation in neurologically intact animals (Fig. 2). One-way ANOVA of proximal representation revealed a significant difference between groups (F[3,32] = 8.73, p < 0.001). VNS paired with lever pressing more than doubled the proximal forelimb representations in animals with vehicle injections compared to sham stimulated animals. (VNS-control: 2.78 mm2 ± 0.41, Sham-control: 1.08 mm2 ± 0.24, unpaired t-test, p < 2.4 × 10−3). This finding replicates three previous studies in motor cortex and corroborates numerous studies in the auditory system showing stimulus-specific cortical plasticity in response to paired VNS (Borland et al., 2016; Engineer et al., 2015, 2011; Hulsey et al., 2016; Morrison et al., 2019; Porter et al., 2012; Shetake et al., 2012).
Figure 2. Noradrenergic or serotonergic depletion prevent VNS-directed cortical plasticity.
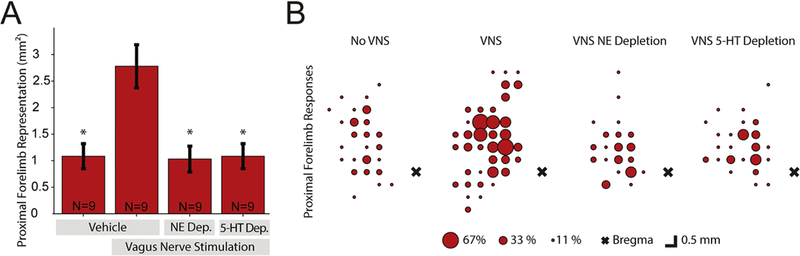
(A) Total area of proximal forelimb presentation for each experimental group. VNS paired with lever training more than doubles proximal forelimb representation of vehicle injected rats compared to rats that underwent equivalent lever training without VNS. Noradrenergic or serotonergic depletion prevent this VNS-dependent expansion. (B) Bubble plots indicating the percentage of rats with a proximal forelimb response at each location in motor cortex. Note the increase in cortical representation of proximal forelimb representation throughout motor cortex in rats that received VNS paired with training. * denotes Bonferroni-corrected p < 8.3 × 10−3 compared to VNS-control.
Next, we evaluated whether cortical noradrenergic innervation was required for VNS-dependent plasticity. Noradrenergic depletion with DBH-Sap immunotoxin injections prevented VNS-mediated expansion of proximal forelimb representation. Rats that received DBH-Sap injections and underwent VNS paired with forelimb training had a significantly smaller proximal forelimb representation than rats that received vehicle injections and VNS paired with forelimb training, and representations comparable to sham stimulated rats (VNS-DBH: 1.03 mm2 ± 0.25, unpaired t-tests, vs. VNS-control p = 2.0 × 10−3, vs. Sham-control, p = 0.87). Similarly, rats which received SERT-Sap injections had significantly smaller proximal forelimb representations than VNS-controls and were comparable to sham stimulated and VNS-DBH rats (VNS-SERT: 1.08 ± 0.24, unpaired t-tests, vs. VNS-control p = 2.4 × 10−3, vs. Sham-control p = 1, vs. VNS-DBH p = 0.87). Additional analysis of the composition of forelimb representations revealed proximal forelimb constitutes a larger percent of forelimb movements after VNS paired training in neurologically intact animals when compared to VNS-DBH and VNS-SERT groups (Fig S6, VNS-control: 34.85% ± 3.98; unpaired t-tests, vs. VNS-DBH: 16.11% ± 4.07, p= 4.6 × 10−3; vs. VNS-SERT: 16.57% ± 3.38, p= 3.0 × 10−3; vs. Sham-control: 19.59% ± 4.69, p= 2.45 × 10−2). These results demonstrate that cortical norepinephrine and serotonin are each required for VNS-directed plasticity. Along with the previously published results on acetylcholine (Hulsey et al., 2016), this is the third type of specific neuromodulatory lesion to block cortical plasticity caused by VNS-movement pairing.
To further determine the specificity of VNS-dependent changes, we also compared the area of other movement representations and total map size across groups. Because motor training specifically emphasized proximal forelimb movements, we predicted that the representation of other movement types would not change. As expected, ANOVA did not reveal differences between groups in representations of distal forelimb, demonstrating that VNS-dependent plasticity is specific to the paired movement (Fig. S7, One-way ANOVA, F[3,32] = 0.48, p = 0.70). Additionally no differences in representational area of the head or hindlimb were observed across groups (Fig. S8–9, One-way ANOVA, F[3,32] = 2.27, p = 0.10; F[3,32] = 1.6, p = 0.21). Gross topography of primary motor cortex remains unchanged across groups (Fig. 3). Additionally, the total motor representations in cortex and ICMS thresholds were not significantly different between groups (S10–11, One-way ANOVA, F[3,32] = 2.03, p = 0.13; F[3,32] = 1.24, p = 0.31). These results corroborate previous studies and support the specificity of VNS-directed plasticity (Hulsey et al., 2016; Porter et al., 2012).
Figure 3. Composite ICMS map of each experimental group.
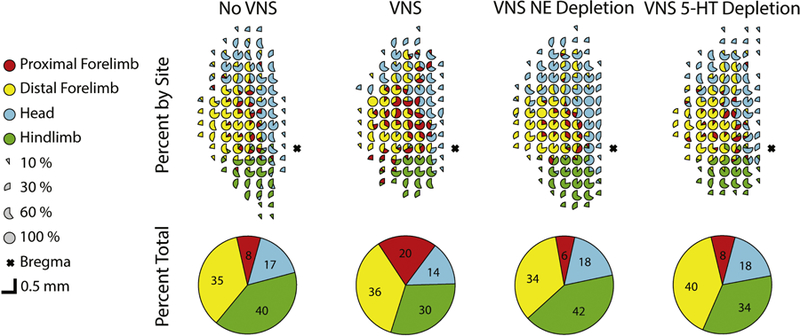
Percentage of rats with each response type reported at every electrode site. The top row represents percentage of responses by site. The bottom row represents total representation in motor cortex. Colors denote evoked movement categories. Incomplete or absent circles indicate non-responsive sites. The VNS-control group has additional proximal forelimb responses throughout motor cortex. Gross topography of primary motor cortex remains unchanged across groups.
To assess the depletion of noradrenergic innervation, DBH positive fiber crossings over a digitally overlaid grid were counted in the right and left motor cortex in sham (n=7) and DBH-sap injected rats (n=8) (Fig. 4). There was no significant difference in the number of DBH-positive fiber crossings between groups in the uninjected right hemisphere (Sham-R: 118.24 ± 10.01, DBH-R: 122.67 ± 5.58, unpaired t-test, p = 0.70). The targeted left hemisphere had substantial reduction of noradrenergic fibers in DBH-Sap injected animals as compared to vehicle controls (Sham-L: 107.64 ± 13.58, DBH-L: 17 ± 9.07, unpaired t-test p = 7.52 × 10−5). This depletion constituted a significant 87% ± 6% loss of noradrenergic innervation in the targeted left hemisphere of DBH-Sap animals compared to unaffected right hemisphere (paired t-test, DBH-R vs DBH-L, p = 2.74 × 10−6). No significant difference was observed between hemispheres in vehicle injected controls (paired t-test, Sham-R vs Sham-L, p = 0.26). These results confirm depletion of noradrenergic innervation in DBH-Sap animals.
Figure 4. Cortical DBH-Sap injections produce targeted noradrenergic deafferentation.
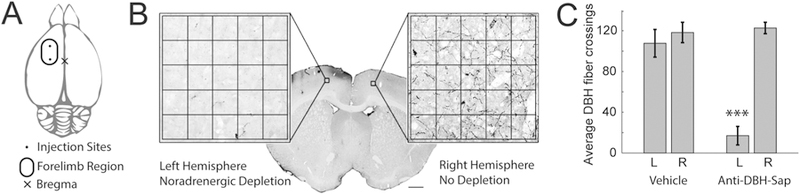
(A) Schematic of injections targeted to the forelimb region of left motor cortex. (B) Example section from a DBH-Sap injected animal with expanded region of interest of left (SAP-injected) and right (control) motor cortex, and fiber counting grid overlaid. There is extensive depletion of DBH positive fibers in the targeted left, and preserved innervation in the right motor cortex. (C) Quantitative analysis of DBH fibers in the cortex reveals a significant reduction only in the targeted left hemisphere. *** denotes p < 0.001
Neuromodulatory depletion and VNS do not influence established behavior
Differences in duration, performance, or total VNS delivered during behavioral training could potentially account for observed changes in cortical representations. We tested whether any of these parameters were influenced by VNS or neuromodulatory depletion. ANOVA revealed no significant difference between groups in training duration (One-way ANOVA, F[3,32] = 1.25, p = 0.3). Additionally, Two-way ANOVA revealed no significant main effect or interaction between experimental groups of training with or without VNS on success rate (Fig. 5, Two-way ANOVA, Group, F[3,64] = 1.06, p = 0.37; VNS vs no VNS, F[1,64] = 0, p=0.97; interaction, F[3,64] = 0.48, p = 0.69) or inter-press-interval (Two-way ANOVA, Group, F[3,63] = 1.56, p = 0.20; VNS vs no VNS, F[1,63] = 0.06, p=0.81; interaction, F[3,63] = 0.37, p = 0.78). Additionally, all VNS groups received comparable amounts of stimulation (One-way ANOVA, F[3,32] = 0.14, p = 0.94). Together, these results exclude the possibility that differences in task performance or amount of VNS could account for the observed differences in cortical representation between groups.
Figure 5. VNS and neuromodulator depletion do not influence motor performance.
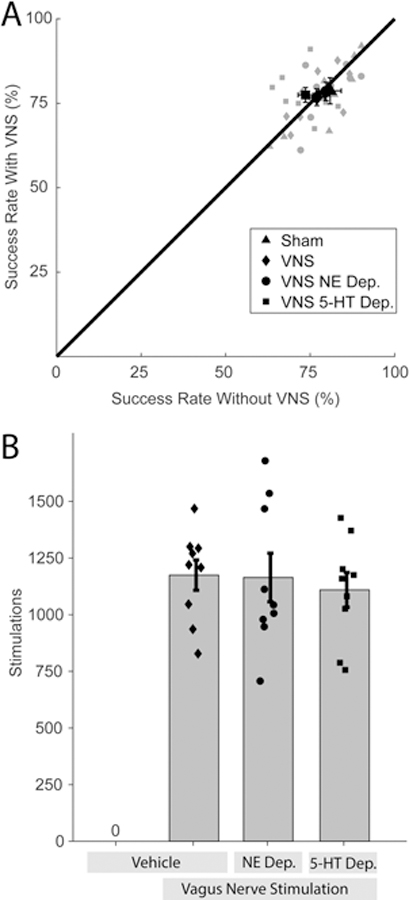
(A) There is no significant difference in behavioral performance on the lever press task across groups before or after VNS. (B) All groups received similar amounts of VNS. Together, these findings indicate that differences in motor performance or number of stimulations cannot account for the VNS-dependent enhancement of plasticity in motor cortex.
DISCUSSION
In this study, we tested the requirement for norepinephrine and serotonin in VNS-dependent enhancement of plasticity. Noradrenergic depletion prevented plasticity of motor representations in the cortex driven by pairing VNS with motor training. In conjunction with evidence of LC activation by VNS, these results provide a clear mechanistic pathway for VNS effects (Hulsey et al., 2017). We also found that serotonergic depletion blocked VNS-dependent enhancement of plasticity. Along with previous findings that document the necessity of cortical cholinergic innervation, this study demonstrates that multiple neuromodulatory systems are required for VNS pairing to facilitate cortical plasticity (Hulsey et al., 2016).
Previous studies have established that pairing VNS with sensory, motor, or cognitive events drives robust, specific plasticity, raising the prospect that VNS engages a common mechanism to support plasticity across a range of training paradigms (Childs et al., 2017; Engineer et al., 2011; Hulsey et al., 2016; Peña et al., 2014; Porter et al., 2012). In this study, we provide the fourth independent replication that VNS paired with a lever pressing task drives expansion of proximal forelimb representation in motor cortex of neurologically intact rats (Hulsey et al., 2016; Morrison et al., 2019; Porter et al., 2012). These findings mirror previous studies in the auditory system, where VNS paired with tones or complex sounds drives stimulus specific plasticity in auditory cortex (Engineer et al., 2015; Shetake et al., 2012). Plasticity in motor and auditory pathways after VNS-event pairing is associated with behavioral recovery from tinnitus, stroke, traumatic brain injury, and spinal cord injury (Engineer et al., 2011; Ganzer et al., 2018; Hays et al., 2016; S.A. Hays et al., 2014; Seth A. Hays et al., 2014; Khodaparast et al., 2016, 2014, 2013; Meyers et al., 2018; Pruitt et al., 2016). Additionally, VNS delivered during extinction of fear memories or drug-seeking behaviors enhances learning and modulates plasticity in key pathways between the prefrontal cortex and basolateral amygdala, indicating VNS can drive plasticity in cognitive networks (Childs et al., 2017; Peña et al., 2014). In each case, pairing a specific stimulus or training paradigm with brief bursts of VNS drives substantial reorganization in modality-specific circuits. The present study provides evidence that VNS-dependent engagement of neuromodulatory networks likely represents the common mechanism by which VNS drives plasticity across a broad range of training paradigms and in multiple brain regions.
Several lines of evidence establish a link between VNS and activation of neuromodulatory networks in the central nervous system. Long trains of VNS delivered chronically increases neural activity in the DRN and LC (Manta et al., 2009). The LC is required for the beneficial effects on epilepsy seen with extended stimulation (Krahl et al., 1998; Manta et al., 2009). In addition to changes after extensive stimulation, VNS provides rapid activation of neuromodulatory pathways. VNS induces immediate changes in cortical synchrony, an effect dependent on activation of muscarinic acetylcholine receptors (Nichols et al., 2011). Additionally, brief bursts of VNS drive rapid, phasic neural activity of the LC, providing a direct characterization of noradrenergic engagement by VNS (Hulsey et al., 2017). The LC projects to the DRN and potentiation of DRN activity by chronic VNS is mediated by the LC, revealing a pathway by which VNS could regulate serotonergic activity. In the context of VNS-directed plasticity, individual blockade of noradrenergic, serotonergic, and cholinergic pathways each completely prevented cortical reorganization (Hulsey et al., 2016).
Norepinephrine, serotonin, and acetylcholine are all known to influence cortical plasticity (Bear and Singer, 1986; Gu, 2002; Gu and Singer, 1995; Kilgard and Merzenich, 1998). Boosting levels of each enables plasticity in response to stimuli generally unable to alter cortical representations (Kasamatsu et al., 1979; Kilgard and Merzenich, 1998; Vetencourt et al., 2008). Individual neuromodulator availability dramatically influences plasticity, as they act through multiple pathways to guide outcomes. Removing individual neuromodulators in spike timing dependent plasticity (STDP) preparations influences the polarity of synaptic plasticity responses (Seol et al., 2007). Further, the concentration of neuromodulators can alter plasticity, as different receptors are activated at high and low concentrations and have opposing actions during STDP (Salgado et al., 2012). Increases in VNS amplitude drive monotonically increasing activity in the LC, indicating that varied VNS intensities may differentially influence plasticity. Indeed, moderate intensity VNS produces robust cortical plasticity, while lower and higher stimulation intensities are less effective (Borland et al., 2016; Loerwald et al., 2018a, 2018b). Moderate stimulation intensity may sufficiently activate receptors necessary to drive plasticity, while high stimulation intensities activate a set of low affinity receptors that inhibit plasticity responses. These findings support the role of neuromodulatory engagement in VNS-dependent plasticity and suggest that the composition of postsynaptic receptor activation influences plasticity enhancement. Moreover, individual depletion of three distinct neuromodulators blocks VNS-directed reorganization of motor cortex, indicating that each is integrally involved in facilitating plasticity. Together, these findings highlight the importance of considering the level and interactions of multiple neuromodulators to optimize VNS stimulation parameters (Hulsey et al., 2016).
Phasic neuromodulation enables synaptic plasticity by providing temporally-precise feedback during the synaptic eligibility trace (He et al., 2015). Optogenetically triggered release of neuromodulators after priming neural activity transforms synaptic eligibility traces, leading to STDP (He et al., 2015). VNS similarly activates the LC prompting noradrenergic release, and repeated stimulation following sensory, motor, and cognitive events directs lasting plasticity. Neuromodulatory feedback must occur within seconds of synaptic activation to fall within the eligibility trace. Similarly, a number of studies describe a similar temporal requirement for VNS, such that stimulation must be delivered within seconds of an event to enhance plasticity. Delivery of VNS coincident with presentations of a tone leads to expansion of the representation of the paired tone in auditory cortex. However, if VNS is delayed 15 seconds after tone presentation, no cortical reorganization is observed (Engineer et al., 2011). Similarly, pairing VNS with forelimb movement during rehabilitation enhances plasticity and recovery, but delaying stimulation by ~20 seconds from relevant neural circuit activation prevented VNS-dependent benefits (Ganzer et al., 2018; Hulsey et al., 2016; Porter et al., 2012). In this study depleting cortical noradrenergic signaling prevented plasticity responses. Together these findings suggest VNS phasically activates neuromodulatory networks to provide a temporally-precise feedback signal during the synaptic eligibility trace to direct plasticity specific to paired events.
One limitation of the present study is the absence of direct histological verification of serotonergic depletion. Despite this, several pieces of evidence provide support that serotonin was effectively depleted in the appropriate group. First, saporin-conjugated immunotoxins have been widely used to generate localized depletion of neuromodulatory innervation after axonal endocytosis (Blessing et al., 1998; Conner et al., 2010; McGaughy et al., 2008; Milstein et al., 2007; Radley et al., 2008). SERT-Sap has been used in previous studies to generate targeted ablation of serotonergic cells in the Dorsal Raphe Nucleus, Raphe Obscurus, and Raphe Magnus Nucleus (da Silva et al., 2011; Dias et al., 2007; Nattie et al., 2004; Su et al., 2014). SERT is expressed by serotonergic axons within the cortex as well as in raphe nuclei, and it is therefore reasonable to expect endocytosis of the toxin after cortical SERT-Sap injections, leading to serotonergic denervation (Amilhon et al., 2010). Second, VNS pairing was delivered during the optimal window for the presence of a serotonergic deficit. Saporin toxins generate maximal and typically persistent depletion within two weeks of injection, which is the minimum duration between surgical procedures and VNS pairing (Blessing et al., 1998; Waite et al., 1994). Serotonergic fibers are known to regenerate within the cortex after denervation, but recovery of half of original innervation takes three months, while all ICMS procedures in the present study were completed within one month of saporin injections (Jin et al., 2016). Finally, the clearest interpretation of the experimental effect of SERT-Sap injections on proximal forelimb representations is that a serotonergic deficit prevented VNS-directed plasticity. The present study provides initial demonstration that both serotonin and norepinephrine are required for VNS-directed plasticity. Future studies are necessary to investigate specific mechanisms and interactions between neuromodulatory systems. Such studies would require confirmation of graded and combined alterations of neuromodulation, which could include more dilute saporin injections which induce less extensive deficits, or chronic antidepressant administration known to enhance cortical innervation (McGaughy et al., 2008; Nakamura, 1991; Walsh et al., 1996; Zhou et al., 2006).
The reliance of VNS on multiple neuromodulators suggests that a wide range of pharmacological manipulations could alter VNS efficacy. Increasing neuromodulator availability with reuptake inhibitors may increase overall plasticity responses to VNS pairing protocols (Vetencourt et al., 2008). However, changes in neuromodulator levels may require different stimulation parameters, as the balance and interaction between them are affected. Alternatively, influencing the temporal dynamics of neuromodulator reuptake may diminish VNS efficacy by impacting important timing relationships required for plasticity effects. Any factors impacting neuromodulatory signaling must be carefully considered while translating targeted plasticity therapies to clinical settings. In one clinical trial utilizing VNS paired with tones to treat tinnitus half of the subjects saw improvement in their symptoms, while a group taking pharmacological agents influencing neuromodulatory pathways saw no improvement (De Ridder et al., 2014). The current study highlights the importance of multiple neuromodulatory pathways which act in conjunction to facilitate changes in response to VNS-event pairings, opening broad possibilities for future investigation on the effects of neuromodulatory alterations on VNS-dependent effects.
Supplementary Material
Research Highlights.
VNS paired with motor training directs cortical plasticity
Depletion of norepinephrine or serotonin blocks VNS effects on cortical plasticity
Consideration of neuromodulator systems is important for translation of VNS therapy
ACKNOWLEDGEMENTS
We would like to thank Aaron Kuo, Ayushi Bisaria, Yun-Yee Tsang, Prathima Kandukuri, Joy Mong, Stephanie Abe, Naser Asfoor, Eric Bellinghausen, and Joanna John for behavioral training. We would also like to thank Robert Morrison, Joy Mong, Yun-Yee Tsang, and Tanya Danaphongse for ICMS support. Further thanks to Kimi Rahebi, Lena Lynn Sadler, and Matt Buell for vagus nerve cuff construction.
FUNDING
This work was supported by the National Institutes of Health R01NS094384 (SAH), R01NS085167 (MPK), and R01NS103803 (MPK) and by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Eric Van Gieson through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. N66001-15-2-4057 and the DARPA BTO Targeted Neuroplasticity Training (TNT) program under the auspices of Dr. Tristan McClure-Begley through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001-17-2-4011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
MPK is a consultant for, and has a financial interest in, MicroTransponder, Inc., which is developing therapies using VNS. DRH, CMS, SFS, and SAH report no financial conflict of interests.
REFERENCES
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S, 2010. VGLUT3 (Vesicular Glutamate Transporter Type 3) Contribution to the Regulation of Serotonergic Transmission and Anxiety. J. Neurosci 30, 2198–2210. 10.1523/JNEUROSCI.5196-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Singer W, 1986. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320, 172–176. 10.1038/320172a0 [DOI] [PubMed] [Google Scholar]
- Blessing W, Lappi D, Wiley R, 1998. Destruction of locus coeruleus neuronal perikarya after injection of anti-dopamine-B-hydroxylase immunotoxin into the olfactory bulb of the rat. Neurosci. Lett 243, 85–88. 10.1016/S0304-3940(98)00090-1 [DOI] [PubMed] [Google Scholar]
- Borland MS, Engineer CT, Vrana WA, Moreno NA, Engineer ND, Vanneste S, Sharma P, Pantalia MC, Lane MC, Rennaker RL, Kilgard MP, 2018. The Interval Between VNS-Tone Pairings Determines the Extent of Cortical Map Plasticity. Neuroscience 369, 76–86. 10.1016/j.neuroscience.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, Engineer CT, Kilgard MP, 2016. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul 9, 117–123. 10.1016/j.brs.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell EP, Loerwald KW, Engineer CT, Borland MS, Buell JM, Kelly CA, Khan II, Hays SA, Kilgard MP, 2018. Cortical map plasticity as a function of vagus nerve stimulation rate. Brain Stimul 9, 117–123. 10.1016/j.brs.2018.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD, 2015. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell 161, 622–633. 10.1016/j.cell.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs JE, DeLeon J, Nickel E, Kroener S, 2017. Vagus nerve stimulation reduces cocaine seeking and alters plasticity in the extinction network. Learn. Mem 24, 35–42. 10.1101/lm.043539.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Kulczycki M, Tuszynski MH, 2010. Unique Contributions of Distinct Cholinergic Projections to Motor Cortical Plasticity and Learning. Cereb. Cortex 20, 2739–2748. 10.1093/cercor/bhq022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Mifflin SW, Gould GG, Frazer A, 2008. Induction of c-Fos and ΔFosB Immunoreactivity in Rat Brain by Vagal Nerve Stimulation. Neuropsychopharmacology 33, 1884–1895. 10.1038/sj.npp.1301570 [DOI] [PubMed] [Google Scholar]
- da Silva GSF, Giusti H, Benedetti M, Dias MB, Gargaglioni LH, Branco LGS, Glass ML, 2011. Serotonergic neurons in the nucleus raphe obscurus contribute to interaction between central and peripheral ventilatory responses to hypercapnia. Pflügers Arch. - Eur. J. Physiol 462, 407–418. 10.1007/s00424-011-0990-x [DOI] [PubMed] [Google Scholar]
- Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, Hilmi O, McLean J, Forbes K, Kilgard MP, Rennaker RL, Cramer SC, Walters M, Engineer N, 2016. Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke 47, 143–150. 10.1161/STROKEAHA.115.010477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Engineer ND, Kilgard MP, 2014. Safety and Efficacy of Vagus Nerve Stimulation Paired With Tones for the Treatment of Tinnitus: A Case Series. Neuromodulation Technol. Neural Interface 17, 170–179. 10.1111/ner.12127 [DOI] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglione LH, Branco LGS, 2007. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J. Appl. Physiol 103, 1780–1788. 10.1152/japplphysiol.00424.2007 [DOI] [PubMed] [Google Scholar]
- Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP, 2015. Pairing Speech Sounds With Vagus Nerve Stimulation Drives Stimulus-specific Cortical Plasticity. Brain Stimul 8, 637–644. 10.1016/j.brs.2015.01.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP, 2011. Reversing pathological neural activity using targeted plasticity. Nature 470, 101–104. 10.1038/nature09656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzer PD, Darrow MJ, Meyers EC, Solorzano BR, Ruiz AD, Robertson NM, Adcock KS, James JT, Jeong HS, Becker AM, Goldberg MP, Pruitt DT, Hays SA, Kilgard MP, Rennaker RL, 2018. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife 7, 1–19. 10.7554/eLife.32058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, 2002. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111, 815–835. 10.1016/S0306-4522(02)00026-X [DOI] [PubMed] [Google Scholar]
- Gu Q, Singer W, 1995. Involvement of Serotonin in Developmental Plasticity of Kitten Visual Cortex. Eur. J. Neurosci 7, 1146–1153. 10.1111/j.1460-9568.1995.tb01104.x [DOI] [PubMed] [Google Scholar]
- Hays Seth A., Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, Kilgard MP, 2014. Vagus Nerve Stimulation During Rehabilitative Training Improves Functional Recovery After Intracerebral Hemorrhage. Stroke 45, 3097–3100. 10.1161/STROKEAHA.114.006654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, Kilgard MP, 2014. The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport 25, 682–688. 10.1097/WNR.0000000000000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Khodaparast N, Sloan AM, Fayyaz T, Hulsey DR, Ruiz AD, Pantoja M, Kilgard MP, Rennaker RL, 2013a. The bradykinesia assessment task: An automated method to measure forelimb speed in rodents. J. Neurosci. Methods 214, 52–61. 10.1016/j.jneumeth.2012.12.022 [DOI] [PubMed] [Google Scholar]
- Hays SA, Rennaker RL, Kilgard MP, 2013b. Targeting Plasticity with Vagus Nerve Stimulation to Treat Neurological Disease, in: Progress in Brain Research Elsevier B.V., pp. 275–299. 10.1016/B978-0-444-63327-9.00010-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, Rennaker RL, Kilgard MP, 2016. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol. Aging 43, 111–118. 10.1016/j.neurobiolaging.2016.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Huertas M, Hong SZ, Tie X, Hell JW, Shouval H, Kirkwood A, 2015. Distinct Eligibility Traces for LTP and LTD in Cortical Synapses. Neuron 88, 528–538. 10.1016/j.neuron.2015.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, Kilgard MP, 2016. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul 9, 174–181. 10.1016/j.brs.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA, 2017. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol 289, 21–30. 10.1016/j.expneurol.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Dougherty SE, Wood K, Pletnikov M, Hashemi P, Linden DJ, Jin Y, Dougherty SE, Wood K, Sun L, Cudmore RH, Abdalla A, Kannan G, 2016. Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury Article Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury. Neuron 91, 748–762. 10.1016/j.neuron.2016.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu T, Pettigrew JD, Ary M, 1979. Restoration of visual cortical plasticity by local microperfusion of norepinephrine. J. Comp. Neurol 185, 163–181. 10.1002/cne.901850110 [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, Kilgard MP, 2014. Vagus Nerve Stimulation Delivered During Motor Rehabilitation Improves Recovery in a Rat Model of Stroke. Neurorehabil. Neural Repair 28, 698–706. 10.1177/1545968314521006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker RL, Kilgard MP, 2013. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol. Dis 60, 80–88. 10.1016/j.nbd.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, Rennaker RL, Hays SA, 2016. Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabil. Neural Repair 30, 676–684. 10.1177/1545968315616494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM, 1998. Cortical Map Reorganization Enabled by Nucleus Basalis Activity. Science (80-. ) 279, 1714–1718. 10.1126/science.279.5357.1714 [DOI] [PubMed] [Google Scholar]
- Krahl SE, Clark KB, Smith DC, Browning RA, 1998. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 39, 709–14. [DOI] [PubMed] [Google Scholar]
- Loerwald KW, Borland MS, Rennaker RL, Hays SA, Kilgard MP, 2018a. The interaction of pulse width and current intensity on the extent of cortical plasticity evoked by vagus nerve stimulation. Brain Stimul 11, 271–277. 10.1016/j.brs.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerwald KW, Buell EP, Borland MS, Rennaker RL, Hays SA, Kilgard MP, 2018b. Varying Stimulation Parameters to Improve Cortical Plasticity Generated by VNS-tone Pairing. Neuroscience 388, 239–247. 10.1016/j.neuroscience.2018.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P, 2009. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J. Psychiatry Neurosci 34, 272–280. [PMC free article] [PubMed] [Google Scholar]
- McAllen RM, Shafton AD, Bratton BO, Trevaks D, Furness JB, 2018. Calibration of thresholds for functional engagement of vagal A, B and C fiber groups in vivo. Bioelectron. Med 1, 21–27. 10.2217/bem-2017-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H, 2008. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 153, 63–71. 10.1016/j.neuroscience.2008.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, Kilgard MP, Hays SA, 2018. Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke 49, 710–717. 10.1161/STROKEAHA.117.019202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein JA, Lehmann O, Theobald DEH, Dalley JW, Robbins TW, 2007. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase–saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology (Berl) 190, 51–63. 10.1007/s00213-006-0594-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RA, Hulsey DR, Adcock KS, Rennaker RL, Kilgard MP, Hays SA, 2019. Vagus nerve stimulation intensity influences motor cortex plasticity. Brain Stimul 12, 256–262. 10.1016/j.brs.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, 1991. Axonal sprouting of noradrenergic locus coeruleus neurons following repeated stress and antidepressant treatment, in: Progress in Brain Research Elsevier B.V., pp. 587–598. 10.1016/S0079-6123(08)63836-4 [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA, 2004. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J. Physiol 556, 235–253. 10.1113/jphysiol.2003.059766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M, 2011. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 189, 207–214. 10.1016/j.neuroscience.2011.05.024 [DOI] [PubMed] [Google Scholar]
- Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S, 2014. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci 8, 1–8. 10.3389/fnbeh.2014.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, Rennaker RL, Kilgard MP, 2012. Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cereb. Cortex 22, 2365–2374. 10.1093/cercor/bhr316 [DOI] [PubMed] [Google Scholar]
- Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL, 2016. Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J. Neurotrauma 33, 871–879. 10.1089/neu.2015.3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE, 2008. Noradrenergic Innervation of the Dorsal Medial Prefrontal Cortex Modulates Hypothalamo-Pituitary-Adrenal Responses to Acute Emotional Stress. J. Neurosci 28, 5806–5816. 10.1523/JNEUROSCI.0552-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F, 2011. The chemical neuroanatomy of vagus nerve stimulation. J. Chem. Neuroanat 42, 288–296. 10.1016/j.jchemneu.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Salgado H, Köhr G, Treviño M, 2012. Noradrenergic ‘Tone’ Determines Dichotomous Control of Cortical Spike-Timing-Dependent Plasticity. Sci. Rep 2, 417 10.1038/srep00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee H-K, Kirkwood A, 2007. Neuromodulators Control the Polarity of Spike-Timing-Dependent Synaptic Plasticity. Neuron 55, 919–929. 10.1016/j.neuron.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Fuchino Y, Miyamoto D, Nomura H, Matsuki N, 2012. Vagus nerve stimulation enhances perforant path-CA3 synaptic transmission via the activation of β-adrenergic receptors and the locus coeruleus. Int. J. Neuropsychopharmacol 15, 523–530. 10.1017/S1461145711000708 [DOI] [PubMed] [Google Scholar]
- Shetake JA, Engineer ND, Vrana WA, Wolf JT, Kilgard MP, 2012. Pairing tone trains with vagus nerve stimulation induces temporal plasticity in auditory cortex. Exp. Neurol 233, 342–349. 10.1016/j.expneurol.2011.10.026 [DOI] [PubMed] [Google Scholar]
- Su J, Wang W, Sun L, Li T, Kong D, Kang J, 2014. Raphe serotonergic neurons modulate genioglossus corticomotor activity in intermittent hypoxic rats. Respir. Res 25, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetencourt JFM, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E, Maffei L, 2008. The Antidepressant Fluoxetine Restores Plasticity in the Adult Visual Cortex. Science (80-. ) 320, 385–388. 10.1126/science.1150516 [DOI] [PubMed] [Google Scholar]
- Waite JJ, Wardlow ML, Chen a C., Lappi D. a Wiley RG, Thal LJ, 1994. Time course of cholinergic and monoaminergic changes in rat brain after immunolesioning with 192 IgG-saporin. Neurosci. Lett 169, 154–8. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Herzog CD, Gandhi C, Stackman RW, Wiley RG, 1996. Injection of IgG 192-saporin into the medial septum produces cholinergic hypofunction and dose-dependent working memory deficits. Brain Res 726, 69–79. 10.1016/0006-8993(96)00271-5 [DOI] [PubMed] [Google Scholar]
- Zhou L, Huang KX, Kecojevic A, Welsh AM, Koliatsos VE, 2006. Evidence that serotonin reuptake modulators increase the density of serotonin innervation in the forebrain. J. Neurochem 96, 396–406. 10.1111/j.1471-4159.2005.03562.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


