Abstract
Obesity is a disease that results from an imbalance between energy intake and energy expenditure. Brown adipose tissue (BAT) is a potential therapeutic target to improve the comorbidities associated with obesity due to its inherent thermogenic capacity and its ability to improve glucose metabolism. Multiple studies have shown that activation of BAT using either pharmacological treatments or cold exposure had an acute effect to increase metabolic function and reduce adiposity. Recent preclinical investigations have explored whether increasing BAT mass or activation through transplantation models could improve glucose metabolism and metabolic health. Successful BAT transplantation models have shown improvements in glucose metabolism and insulin sensitivity, as well as reductions in body mass and decreased adiposity in recipients. BAT transplantation may confer its beneficial effects through several different mechanisms, including endocrine effects via the release of ‘batokines’. More recent studies have demonstrated that beige and brown adipocytes isolated from human progenitor cells and transplanted into mouse models result in metabolic improvements similar to transplantation of whole BAT; this could represent a clinically translatable model. In this review we will discuss the impetus for both early and recent investigations utilizing BAT transplantation models, the outcomes of these studies, and review the mechanisms associated with the beneficial effects of BAT transplant to confer improvements in metabolic health.
Keywords: Brown adipose tissue, Transplantation, Obesity, Glucose metabolism, Batokines, Thermogenesis
1. Introduction
The obesity epidemic is increasing at an alarming rate; over the last several decades obesity rates have tripled (WHO Obesity Fact Sheet, 2018). In fact, over 50% of the world’s population has been classified as overweight or obese. With the striking escalation of this pandemic there is a great need to define and identify therapeutic tools to combat obesity and the increased risk of its associated comorbidities including type 2 diabetes, cardiovascular disease, and cancer. Obesity is a disease that results from an imbalance in energy intake and energy expenditure. An important tissue involved in both energy intake and energy expenditure is adipose tissue, an essential organ involved in maintaining systemic metabolic function (Cohen and Spiegelman, 2016; Scherer et al., 1995; Stanford et al., 2013; Thomou et al., 2017)
2. Adipose Tissue
Adipose tissue is composed of various cell types including adipocytes, preadipocytes, adipose-derived stem cells (ADSC’s), and immune cells. During periods of chronic positive energy imbalance adipose tissue expands primarily through an increase in the size of existing mature adipocytes (hypertrophy) and to some extent via an increase in preadipocyte and adipocyte numbers (adipogenesis) (Prins and O’Rahilly, 1997; Rosen and Spiegelman, 2000). However, in the obese state excessive energy intake over a prolonged period overwhelms this capacity resulting in insulin resistance, inflammation, and dysregulation of adipose tissue (McLaughlin et al., 2007; Tchoukalova et al., 2007).
There are two types of adipose tissue in rodents and humans, white adipose tissue (WAT) and brown adipose tissue (BAT). WAT functions to store energy in the form of triglycerides and release energy during fasting or physical activity (Frayn, 2010). WAT is characterized by large unilocular lipid droplets, limited mitochondria and is innervated by the sympathetic and parasympathetic nervous systems (Bamshad et al., 1998; Bartness and Bamshad, 1998; Bowers et al., 2004; Youngstrom and Bartness, 1995). WAT exists in multiple locations in the body and has two major subtypes; visceral and subcutaneous. Visceral WAT (vWAT) is located surrounding the internal organs and is associated with insulin resistance, an increased risk for type 2 diabetes, altered lipid profile, increased incidences of atherosclerosis, and overall mortality (Carey et al., 1997; Nicklas et al., 2006; Ross et al., 2008; Wang et al., 2005). Due to its anatomical location, vWAT directly drains into the portal vein, which allows direct delivery of free fatty acids and cytokines/adipokines to the liver. Additionally, vWAT is more metabolically active than subcutaneous WAT (scWAT) when normalized by total mass (Kraunsoe et al., 2010) and has an increase in lipolytic flux compared to scWAT (Arner, 1995; Lemieux and Despres, 1994).
Subcutaneous WAT (scWAT) is a peripherally located depot and is associated with increased insulin sensitivity and decreased rates of type 2 diabetes, dyslipidemia, and atherosclerosis (Misra et al., 1997; Snijder et al., 2003; Tanko et al., 2003). Healthy scWAT contains small, insulin sensitive adipocytes (Choe et al., 2016) which have a greater affinity for lipid sequestration, thus preventing ectopic lipid storage and insulin resistance in other tissues (Marin et al., 1992; Misra and Vikram, 2003). Studies have also shown functional differences in scWAT and vWAT; transplantation of scWAT into the visceral cavity improves whole-body glucose metabolism and insulin sensitivity but increasing the amount of vWAT into the same depot had no effect (Tran et al., 2008).
The second type of adipose tissue is brown adipose tissue (BAT). In contrast to WAT, BAT is involved in energy expenditure (Lowell and Spiegelman, 2000). BAT is a thermogenic tissue that functions to generate heat in response to cold exposure (Bartelt et al., 2011; Gesta et al., 2007; Rothwell and Stock, 1983; van Marken Lichtenbelt et al., 2009). It does this by uncoupling oxidative phosphorylation in mitochondria through the uniquely expressed uncoupling protein 1 (UCP1) (Aquila et al., 1985; Heaton et al., 1978). BAT is characterized by high levels of mitochondria (Cannon and Nedergaard, 2004), multilocular lipid droplets (Rothwell and Stock, 1983), a high degree of vascularization (Fawcett, 1952), and sympathetic innervation (Cannon and Nedergaard, 2004). UCP1 creates a ‘leaky’ proton channel (Matthias et al., 2000) in the mitochondria that uncouples mitochondrial proton pumping from ATP production, resulting in increased release of heat as part of the process of non-shivering thermogenesis. Activated BAT increases sympathetic signaling through norepinephrine, which stimulates β3-adrenergic receptors (Collins and Surwit, 2001) and initiates lipolytic processes to liberate free fatty acids in the cytosol (Bieber et al., 1975; Kuusela et al., 1986; Nedergaard and Lindberg, 1979). While there are three major types of beta-adrenergic receptors exist: β1, β2 and β3, β3 is the one predominantly expressed in BAT, and thus the most likely to mediate activation by beta-adrenergic stimulation (Collins and Surwit, 2001; Lowell and Flier, 1997; Mund and Frishman, 2013; Muzzin et al., 1991).
A subtype of adipocytes has been identified in both rodents and humans (Ishibashi and Seale, 2010; Min et al., 2016; Nedergaard and Lindberg, 1979; Petrovic et al., 2010; Wu et al., 2012) termed ‘beige’ or ‘brite’ (brown in white) adipocytes. Beige adipocytes express UCP1 and have some functional overlap between WAT and BAT. Beige adipocytes have similar morphology to brown adipocytes but express unique cell surface markers including Tmem26 and Cd137 (Wu et al., 2012). These cells are derived from the Myf5- lineage as opposed to BAT, which is derived from the Myf5+ lineage (Seale et al., 2008; Wu et al., 2012). Various physiological stressors induce beiging of WAT in rodent models including cold exposure (Berry et al., 2017; Hui et al., 2015; Petrovic et al., 2010; Zhu et al., 2016), exercise training (Bostrom et al., 2012; Stanford et al., 2015; Sutherland et al., 2009; Trevellin et al., 2014), intermittent fasting (Li et al., 2017), and noradrenergic stimulation using p3 agonists such as CL316243 (Himms-Hagen et al., 2000; Jiang et al., 2017).
Traditionally, BAT was thought to exist only in rodents and infant humans; the BAT depot was thought to dissipate upon maturation in adult humans. In 2009, multiple retrospective studies using data collected from 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) and computer tomography (CT) led to the ‘rediscovery’ of BAT in adult humans. These seminal studies identified functional BAT in adult humans, concentrated to the neck, supraclavicular, mediastinal, paraspinal, and suprarenal area (Cypess et al., 2009; Li et al., 2017; Nedergaard et al., 2007; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009; Zingaretti et al., 2009). These studies also determined a negative correlation with BAT and BMI, fasting glucose, and age, suggesting that young, lean human subjects had the most active BAT (Cypess et al., 2009; Saito et al., 2009). Interestingly, obese patients with high BAT activity had improved glucose tolerance (Timmons and Pedersen, 2009). Together, these data indicated that BAT is an important tissue to regulate glucose metabolism and adiposity, and a potential therapeutic tool to combat obesity.
3. Increasing BAT mass and activity by transplantation
Strategies harnessing the therapeutic potential of BAT in clinical settings have focused on pharmacological treatment (Baskin et al., 2018; Cypess et al., 2015; Loh et al., 2018) and cold exposure (Chondronikola et al., 2014; Hanssen et al., 2015; Hanssen et al., 2016; Lee et al., 2014b; van der Lans et al., 2013). BAT activation via cold exposure increases glucose uptake in BAT (Chondronikola et al., 2014; van der Lans et al., 2013) and improves whole body insulin sensitivity (Chondronikola et al., 2014; Hanssen et al., 2015; Lee et al., 2014b) in lean and obese subjects (Hanssen et al., 2016) and subjects with type 2 diabetes (Hanssen et al., 2015). Cold exposure in humans (up to 6 weeks) increases BAT activity and energy expenditure with concomitant improvements in body composition (Yoneshiro et al., 2013). These clinical treatments have been centered on activating BAT in humans to take advantage of its metabolic benefits.
Although cold exposure has been shown to be effective in improving metabolism in humans, this treatment is often uncomfortable and requires a considerable time commitment for patients. Importantly, while several studies have looked at the immediate effects of long term exposure to cold, to our knowledge none have conclusively determined how long these beneficial effects of cold on and metabolism persist. Thus, determining how to activate BAT in a safe, sustainable manner to maximize the therapeutic potential of BAT to combat obesity and metabolic disease is of great interest and importance, leading to the idea of increasing BAT mass and activity through a transplantation model.
The first study investigating BAT transplantation took place in the 1960’s, and was primarily conducted to delineate BAT function in vivo (Ferren, 1966). In this study, BAT was isolated from the interscapular region of donor mice and transplanted under the kidney capsule for a period of 8–15 days (Ferren, 1966). Histological analysis of the transplanted BAT revealed that the transplanted tissue survived, but both cell and lipid droplet sizes increased. Interestingly, both unilocular and multilocular cells were seen in the transplanted BAT. Several decades later, a study examined the effects of transplanting undifferentiated hamster BAT into the anterior eye chamber of adult hamsters (Nechad and Olson, 1983). After 20 days the transplants became revascularized and reinnervated by the nerves of the host’s iris and displayed cellular features of BAT including multilocular lipid droplets and highly developed mitochondrial networks. Although the transplanted BAT had low nerve fiber density, it retained the characteristics of BAT (multilocular droplets) and maintained sympathetic innervation. An additional study revealed that transplantation of BAT into lean or obese recipients re-established sympathetic innervation in the transplanted BAT 3 months after transplantation (Ashwell et al., 1986). While these studies did not investigate the metabolic effects of BAT transplantation, such early models indicated that BAT transplant was a viable model and showed that the improved metabolic phenotype in response to BAT transplantation was at least partly related to re-establishment of sympathetic innervation.
4. BAT transplantation reverses type 1 diabetes in a mouse model
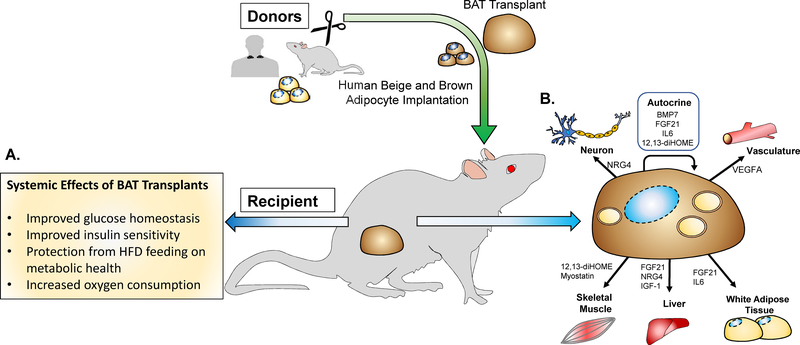
The rediscovery of BAT in humans indicated that BAT played an important role in glucose regulation, but the exact role of BAT in whole-body energy balance had not been established. To address this, multiple studies have investigated the effects of BAT transplantation on glucose metabolism and insulin sensitivity to ascertain its therapeutic potential to regulate metabolic diseases such as diabetes (Figure 1A) (Gunawardana and Piston, 2012, 2015; Liu et al., 2015; Liu et al., 2013; Stanford et al., 2013; Zhu et al., 2014).
Figure 1. Effects of BAT transplantation on metabolic health.
(A) BAT tissue and cell transplantation causes systemic effects on metabolism (B) Endocrine activity of ‘Batokines’ to directly affect BAT and other tissues.”
Some of the first studies to investigate the role of BAT on glucose metabolism used a preclinical model of type I diabetes (Gunawardana and Piston, 2012, 2015). Embryonic BAT was transplanted into both immune competent and immune deficient streptozotocin-treated recipient mice that had severely impaired glucose tolerance and dramatic loss of WAT. Increasing BAT improved fasting glucose and normalized glucose tolerance, reduced adipose tissue inflammation, and reversed several clinical markers of diabetes including polyuria, polydipsia, and polyphagia over a 6-month period post-transplantation (Gunawardana and Piston, 2012). Because of the model used, these effects were independent of insulin concentration and insulin sensitivity. Inhibiting the insulin receptor diminished the effects of the BAT transplant, indicating the importance of the insulin receptor in this model. Circulating IGF-1, leptin, and adiponectin were all increased as a result of the BAT transplantation. Since IGF-1 directly activates the insulin receptor, the authors hypothesized that BAT-released IGF-1 was a primary mechanism to improve glucose homeostasis in this model. In a follow up study (Gunawardana and Piston, 2015), embryonic BAT was transplanted into non-obese diabetic mice. This transplantation resulted in the complete reversal of type I diabetes in parallel with a rapid and enduring improvement in glucose homeostasis for 3–6 months after transplantation. In both models, transplantation of embryonic BAT increased IGF-1, which may be an important mechanism to improve glucose homeostasis.
5. BAT transplantation improves glucose metabolism and insulin sensitivity
Other investigations examined the role of BAT transplantation to combat obesity and improve glucose metabolism (Liu et al., 2013; Misra and Vikram, 2003; Stanford et al., 2013; Zhu et al., 2014). In our laboratory, we transplanted BAT from wild-type donor mice into the visceral cavity of chow fed wild-type recipient mice (Stanford et al., 2013). Twelve weeks after transplantation recipient mice had a marked improvement in glucose tolerance and insulin sensitivity. Insulin-stimulated glucose uptake was increased into endogenous BAT, visceral WAT, and the heart. Circulating IL-6 and FGF21 were increased in mice receiving BAT. In order to elucidate the role of IL-6 to improve glucose metabolism, BAT from IL-6−/− mice was transplanted into wild-type recipient mice. Transplantation of BAT from IL-6−/− mice into recipients did not confer the same metabolic improvements compared to transplantation of wild-type BAT, indicating that IL-6 is necessary for the marked improvements in glucose homeostasis and insulin sensitivity.
Transplantation of wild-type BAT into the visceral cavity of mice fed a high-fat diet revealed that increasing BAT could negate the detrimental effects of a high-fat diet. Transplantation of BAT into mice fed a high-fat diet resulted in a complete reversal of insulin resistance, improved glucose tolerance, decreased body weight, and reduced fat mass. These data establish the role of BAT to regulate glucose tolerance and insulin sensitivity and demonstrate its potential to act as a therapeutic tool to ameliorate the detrimental effects of obesity.
Other studies investigated the role of BAT transplantation as a tool to prevent obesity. (Liu et al., 2013; Timmons and Pedersen, 2009; Zhu et al., 2014). In one study BAT was transplanted into the dorsal interscapular region of recipient mice and then the mice were immediately placed on a high-fat diet for 20 weeks (Liu et al., 2013). Mice that received BAT transplantation had a reduction in weight gain and adiposity, decreased liver mass, and improved glucose tolerance and insulin sensitivity compared to sham-operated mice. Mice receiving BAT also had elevated oxygen consumption rates and an increased expression of genes involved in fatty acid oxidation in endogenous BAT and skeletal muscle. In another study the ability of BAT transplant to prevent weight gain in a high-fat diet model was confirmed (Zhu et al., 2014). The authors determined an amplified sympathetic drive to skeletal muscle and WAT in the mice receiving BAT, indicating a role for BAT to increase energy expenditure in response to both cold exposure and a norepinephrine challenge.
To further elucidate the mechanisms associated with BAT transplantation, BAT from wild-type mice was transplanted into leptin deficient (ob/ob) mice. Mice transplanted with BAT had a reduced weight gain, decreased fat mass, improved insulin sensitivity, and decreased liver steatosis compared to sham-operated mice (Liu et al., 2015). Additionally, this model resulted in higher oxygen consumption rates and increased expression of genes involved in fatty acid oxidation markers in WAT.
In each of these studies, the transplanted BAT was re-vascularized and re-innervated in the recipient mouse (Liu et al., 2013; Stanford et al., 2013; Zhu et al., 2014), similar to what had been observed in previous studies (Ashwell et al., 1986; Nechad and Olson, 1983). Re-innervation of transplanted BAT was confirmed by IHC staining of tyrosine hydroxylase, an essential enzyme for the production of catecholamines. Importantly, these improvements in glucose metabolism and energy expenditure were observed regardless of strain (Gunawardana and Piston, 2012; Liu et al., 2015), rodent species (mice, rat, or hamster) (Gunawardana and Piston, 2012; Nechad and Olson, 1983; Stanford et al., 2013; Yuan et al., 2016), gender (Gunawardana and Piston, 2012, 2015; Stanford et al., 2013) and location of transplant (Gunawardana and Piston, 2012; Nechad and Olson, 1983; Stanford et al., 2013). In our study, markers of BAT (UCP1, PRDM16, citrate synthase activity) and glucose uptake into BAT, were detected in the transplanted BAT, but reduced compared to that of endogenous BAT. Additionally, the transplanted BAT had fewer multilocular droplets over time as evidenced by histological staining (Stanford et al., 2013), similar to what was observed in previous studies (Ashwell et al., 1986; Ferren, 1966). To confirm that the transplanted BAT still retained its thermogenic capacity, both sham-operated mice and mice transplanted with BAT underwent a cold challenge. Mice transplanted with BAT were more cold-tolerant compared to sham-operated mice, indicating that the transplanted BAT still maintained some of its thermogenic activity.
Collectively, these BAT transplant models demonstrate that increasing BAT mass through transplantation improves glucose metabolism, increases insulin sensitivity, and reduces adiposity and body mass. The mechanism through which BAT transplantation improves metabolic health is not clear, but data indicate that IL-6 (Stanford et al., 2013) or IGF-1 (Gunawardana and Piston, 2012, 2015) could be potential drivers of this phenotype. Regardless, these data indicate that BAT is an important therapeutic target to improve glucose metabolism and combat obesity.
6. BAT and ‘Batokines’
Adipokines released from BAT have been termed ‘batokines’ (Stanford et al., 2013; Townsend and Tseng, 2012) that in addition to other functions, play a major role in the contribution of BAT to metabolic health through improved glucose and lipid homeostasis. Batokines can act in several ways and possess autocrine, paracrine, and endocrine functions. Models of BAT transplantation have indicated that the improvements in body mass, glucose control, and insulin sensitivity are likely a result of an endocrine effect from the transplanted tissue (Gunawardana and Piston, 2012, 2015; Marin et al., 1992; Stanford et al., 2013). Here, we will discuss factors that: 1) are released from multiple tissues, including BAT; ‘Batokines that have been shown to affect BAT activity (FGF21, IL-6, VEGFA); 2) are secreted from BAT, considered ‘batokines’ and can affect other tissues (IGF-1, NRG4, BMPs). These factors have all been identified to be increased after BAT transplantation, or have the potential to be released from BAT and could be important regulators of glucose metabolism in a transplantation model (Figure 1B).
6.1. Fibroblast Growth Factor 21 (FGF21)
FGF21 is a peptide hormone secreted by several organs, including BAT, and has the ability to regulate systemic metabolism. FGF21 is a complicated protein to study in metabolism due to its plethora of metabolic effects and its ability to function in an autocrine, paracrine, and endocrine manner. Specifically, FGF21 functions to increase glucose uptake in adipose tissue and increase fatty acid oxidation in the liver (Fisher and Maratos-Flier, 2016). While the liver is the main source of systemic FGF21, activated brown adipocytes can release significant amounts of this peptide (Chartoumpekis et al., 2011; Hondares et al., 2011) and contribute to systemic FGF21 levels. Interestingly, mice that are cold exposed have an increase in FGF21 release from BAT, and a decrease in liver FGF21 expression (Hondares et al., 2011). In BAT, cAMP-related mechanisms simultaneously regulate the expression and release of FGF21 and induce thermogenic gene expression (Hondares et al., 2011). FGF21 was identified as an important batokine after BAT transplantation (Stanford et al., 2013), and increased FGF21 production from activated BAT may be a key mechanism of BAT transplant models as FGF21 metabolic effects such as improving glucose intolerance and increased free fatty acid levels are consistent with the metabolic benefits of activated BAT.
FGF21 is one of the few batokines that has been investigated in humans and was found to be highly expressed in human BAT (Di Franco et al., 2016). In a clinical model, FGF21 in the plasma increases upon cold exposure (Lee et al., 2014a); additionally, several investigations have attempted to link FGF21 plasma levels to BAT activity. Since FGF21 can be released from multiple sources, additional research is needed to confirm whether human BAT is the major source of increases in plasma FGF21 upon cold exposure.
6.2. Interleukin-6 (IL-6)
IL-6 has traditionally been viewed as a pro-inflammatory cytokine linked to certain disease states and pathological mechanisms. However, that view was altered in the early 2000’s when IL-6 was discovered to be released from contracting skeletal muscle (Febbraio and Pedersen, 2002), functioned to promote glucose uptake in skeletal muscle (Glund et al., 2007) and impacted hepatic gluconeogenesis after an acute bout of exercise (Coker et al., 2001). As a response to this work and the identification of IL-6 as a major player in metabolism, this adipokine is now considered distinct from traditional pro-inflammatory cytokines. IL-6 appears to play a major endocrine role in the effects of BAT transplant on improving metabolic health (Stanford et al., 2013). Both cold exposure and noradrenergic stimulation of BAT increase IL-6 gene expression and secretion (Burysek and Houstek, 1997; Hanssen et al., 2015). Data from our previous work reveals that the metabolic effects of BAT transplant when performed with IL-6−/− mice were lost compared to transplant with wild type mice (Burysek and Houstek, 1997; Stanford et al., 2013). Furthermore, transplantation of IL-6−/− BAT diminished the associated FGF21 plasma increase seen with the wild type mice.
6.3. Insulin-like Growth Factor 1 (IGF-1)
IGF-1 is a batokine that acts in an endocrine manner (Gunawardana and Piston, 2012, 2015). In rats, cold exposure increases IGF-1 gene expression and IGF-1 peptide content in BAT (Duchamp et al., 1997), however cold-exposure did not increase IGF-1 in humans (Cypess et al., 2012). In both genetic and pharmacological mouse models of type 1 diabetes, transplantation of BAT reverses diabetes and improves glucose metabolism, effects attributed to an increase in IGF-1 (Gunawardana and Piston, 2012, 2015). IGF-1 mediates glucose metabolism through modulation of the insulin receptor in BAT because inhibition the insulin receptor after BAT transplantation negated the improvement in glucose metabolism (Gunawardana and Piston, 2012).
6.4. Neuregulin 4 (NRG4)
NRG4 is a member of the epidermal growth factor (EGF) family of extracellular ligands. In addition to its paracrine effects NRG4 has also been shown to have substantial endocrine effects. NRG4 is most highly expressed in BAT (Pfeifer, 2015). When NRG4 is released from BAT, it can target the liver and increase hepatic fatty acid oxidation and repression of de novo lipogenesis (Wang et al., 2014), therefore potentially providing protection from nonalcoholic fatty liver disease (NAFLD). Transgenic overexpression of NRG4 in mice fed a high-fat diet resulted in a decrease in weight gain and improved glucose tolerance and insulin sensitivity (Wang et al., 2014). To this point, studies investigating the effects of BAT transplantation on metabolic health have not measured NRG4 but it is possible that it could be an important driver of improved metabolic health.
6.5. Bone Morphogenetic Proteins
One of the most well studied batokines with a major role in BAT function is bone morphogenetic protein 7 (BMP7). BMP7 is an extracellular signaling protein and is a member of the transforming growth factor β (TGFβ) family (Zamani and Brown, 2011). Production of BMP7 is largely attributed to stromal vascular cells in BAT (Schulz et al., 2011) and results in increased expression of PRDM16 and PGC1α (Seale et al., 2009). Genetic models elegantly illustrated the importance of this batokine in regards to brown adipocyte differentiation (Tseng et al., 2008); the absence BMP7 reduces BAT mass and function while overexpression leads to increased metabolic rate and a reduction in body weight. In addition to its role in differentiation, BMP7 has important functions in regards to thermogenesis including activating mitochondrial networks to enhance fatty acid oxidation in differentiated mature adipocytes, a role that is dependent on fatty acid transporters CD36 and CPT1 (Townsend et al., 2013).
Bone morphogenetic protein-4 (BMP4) was previously thought to regulate WAT differentiation (Bowers and Lane, 2007), however, a recent study indicated that BMP4 is also an important regulator of beige fat differentiation (Qian et al., 2013). BMP4 is secreted from preadipocytes during differentiation (Bowers and Lane, 2007) and treatment of adipose-derived progenitor cells with BMP4 can induce BAT development in both mouse (Xue et al., 2014) and human (Elsen et al., 2014) in vitro models. Overexpression of BMP4 in mice induces a beiging of subcutaneous WAT, while BMP4 knockout mice have enlarged adipocytes in WAT and increased insulin resistance (Qian et al., 2013). Future studies are needed to investigate the role of BMP7 or BMP4 to mediate the effects of BAT transplantation.
6.6. Vascular Endothelial Growth Factor A (VEGFA)
VEGFA is a batokine that regulates BAT function and can induce thermogenesis. Secreted VEGFA promotes vascularization (Asano et al., 1999; Xue et al., 2009). Interestingly, VEGFA has been shown to increase in WAT in response to exercise in both mice (Ludzki et al., 2018; Stanford et al., 2015) and humans (Van Pelt et al., 2017). Increased perfusion through elevated vascularization has been show to increase thermogenic activity in BAT in both rodent (Foster and Frydman, 1979) and human models (Orava et al., 2011). Genetic models clearly show the importance of this secreted factor in the activation of thermogenesis; overexpression of BAT-VEGFA increases vascularization in BAT and UCP1, thus increasing thermogenesis during cold exposure (Sun et al., 2014). Additionally, adipocyte specific overexpression of VEGFA also results in beiging of WAT indicating a potential role in increased thermogenesis, although this has not been thoroughly examined (Sun et al., 2012). In contrast, VEGFA-null mice have impaired thermogenic capacity (Mahdaviani et al., 2016). Studies have shown that the transplanted BAT becomes re-vascularized (Stanford et al., 2013), but the role of VEGFA has not been established.
6.7. Other Endocrine Factors
Other factors are released from BAT in response to various stimuli, such as cold or exercise. These include retinol binding protein 4 (RBP4) (Rosell et al., 2012), angiopoietin-like 8 (Fu et al., 2013) and angiopoietin-like 6 (Verdeguer et al., 2015) (ANGPTL8 and ANGPTL6), growth/differentiation factor 15 (GDF15), neuromedin B, and nesfatin (Verdeguer et al., 2015), myostatin (Kong et al., 2018), as well as the lipokine 12,13-diHOME (Lynes et al., 2017; Stanford et al., 2018). Further work is required to determine if these factors are released from BAT after transplantation of the tissue, and how they work to affect metabolic health.
7. Beige and Brown Adipocyte Implantation: A Translational Model of BAT Transplant
BAT transplant studies indicate that in a rodent model, increasing BAT mass and activity have a striking effect to improve glucose tolerance, insulin sensitivity, and combat obesity. The potential translational relevance is exceedingly important. Recent studies have investigated the effects of adipocyte implantation, which may represent a more clinically translatable therapeutic tool. Human beige adipocytes are found in lean, metabolically healthy humans (Nedergaard et al., 2007) and their precursor cells are associated with expanding capillary networks (Min et al., 2016). Due to the high yield of progenitor cells that exist in adipose tissue depots, it is possible that even a small amount of biological material acquired from a patient could provide material for human adipocyte implantation therapies.
One potential BAT-centered therapeutic treatment that is gaining prominence is the utilization of an ex vivo cellular approach to develop beige and brown adipocytes from human precursor cells and re-implant them into the donor to increase thermogenic capacity. The impetus for this treatment stems from previous work performed in rodents involving transplantation of preadipocytes or adipose-derived progenitor cells from WAT, as well as, BAT cell implantation studies that have already been conducted in rodent models (Kishida et al., 2015; Lee et al., 2017; Min et al., 2016; Silva et al., 2014).
Several clinically translatable models exist for the implantation of thermogenic adipocytes into mice. In one investigation, beige progenitors were isolated from the capillary network of human adipose tissue fragments and differentiated in vitro with the use of the adenylate cyclase activator forskolin (Min et al., 2016; van der Lans et al., 2013). Forskolin activation increased Ucp1-positive gene expression in the beige progenitors, and transplantation of these cells into either normal chow-fed and high-fat fed NOD-sciJ IL2rgnul1 (NSG) mice improved glucose tolerance. Another study utilized adipose-derived stem cells isolated from human mediastinal adipose tissue. Cells were isolated and differentiated in porous extracellular matrix-derived scaffolds fabricated from human WAT depots and subsequently transplanted into normal weight diabetic/severe combined immunodeficiency (NOD/SCID) mice fed a high-fat diet. The scaffolds remained functional for a period of up to 6 weeks and resulted in a reduced body weight and improved glucose levels (Silva et al., 2014). The mechanism for the improvements in body weight and glucose tolerance has not been identified.
In another study, human adipose-derived stem cells were injected into mice every other week over a 10-week period. These human cells were differentiated into brown adipocytes with rosiglitazone and then injected into high-fat fed, wild-type mice (Lee et al., 2017). Injection of the thermogenic cells reduced body weight and improved glucose tolerance. Importantly, this model may be the closest representation of how a clinical model would function in humans as WAT progenitor cells could be isolated from a human adipose tissue biopsy, thermogenically enhanced in vitro, and re-implanted into the recipient. Together these exciting data indicate that transplantation of beige or brown adipocytes improves metabolic health, thus increasing the translational relevance of BAT transplantation.
8. Conclusions and Future Perspectives
There is growing evidence for BAT as a potential therapeutic target in the prevention or treatment of obesity and diabetes and the associated metabolic derangements of insulin resistance, glucose intolerance, and hyperlipidemia. Transplantation of BAT, regardless of the type of BAT (embryonic or adult), or the location of the transplantation (visceral or subcutaneous cavity), consistently results in dramatic improvements in glucose tolerance and reductions in adiposity in recipient mice. These improvements are mediated, at least in part, through the release of various batokines that act in an endocrine manner to improve metabolic health. The clinical relevance of this model has increased with the successful transplantation of brown or beige adipocytes which also improve glucose metabolism and reduce adiposity in recipient mice.
As the rates of obesity and type 2 diabetes reach epidemic levels, the development of new therapies and treatments to combat obesity become increasingly important. BAT is a unique tissue with the potential to improve whole-body metabolism. Increasing BAT mass via transplantation of BAT or of beige/brown adipocytes is an important therapeutic tool to improve metabolic health.
Acknowledgements
This work was supported by National Institutes of Health Grants R01-HL138738 to K.I.S. and 5T32HL08039-09 to J.D.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aquila H, Link TA, and Klingenberg M (1985). The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. The EMBO journal 4, 2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P (1995). Differences in lipolysis between human subcutaneous and omental adipose tissues. Annals of medicine 27, 435–438. [DOI] [PubMed] [Google Scholar]
- Asano A, Kimura K, and Saito M (1999). Cold-induced mRNA expression of angiogenic factors in rat brown adipose tissue. The Journal of veterinary medical science 61, 403–409. [DOI] [PubMed] [Google Scholar]
- Ashwell M, Wells C, and Dunnett SB (1986). Brown adipose tissue: contributions of nature and nurture to the obesity of an obese mutant mouse (ob/ob). International journal of obesity 10, 355–373. [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, and Bartness TJ (1998). Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. The American journal of physiology 275, R291–299. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. (2011). Brown adipose tissue activity controls triglyceride clearance. Nature medicine 17, 200–205. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, and Bamshad M (1998). Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. The American journal of physiology 275, R1399–1411. [DOI] [PubMed] [Google Scholar]
- Baskin AS, Linderman JD, Brychta RJ, McGehee S, Anflick-Chames E, Cero C, Johnson JW, O’Mara AE, Fletcher LA, Leitner BP, et al. (2018). Regulation of Human Adipose Tissue Activation, Gallbladder Size, and Bile Acid Metabolism by a beta3-Adrenergic Receptor Agonist. Diabetes 67, 2113–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, Jiang Y, Arpke RW, Close EL, Uchida A, Reading D, Berglund ED, Kyba M, and Graff JM (2017). Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell metabolism 25, 166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber LL, Pettersson B, and Lindberg O (1975). Studies on norepinephrine-induced efflux of free fatty acid from hamster brown-adipose-tissue cells. European journal of biochemistry 58, 375–381. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. (2012). A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, and Bartness TJ (2004). Sympathetic innervation of white adipose tissue and its regulation of fat cell number. American journal of physiology. Regulatory, integrative and comparative physiology 286, R1167–1175. [DOI] [PubMed] [Google Scholar]
- Bowers RR, and Lane MD (2007). A role for bone morphogenetic protein-4 in adipocyte development. Cell cycle (Georgetown, Tex.) 6, 385–389. [DOI] [PubMed] [Google Scholar]
- Burysek L, and Houstek J (1997). beta-Adrenergic stimulation of interleukin-1 alpha and interleukin-6 expression in mouse brown adipocytes. FEBS letters 411, 83–86. [DOI] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiological reviews 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, and Manson JE (1997). Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. American journal of epidemiology 145, 614–619. [DOI] [PubMed] [Google Scholar]
- Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, and Papavassiliou AG (2011). Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Molecular medicine (Cambridge, Mass) 17, 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe SS, Huh JY, Hwang IJ, Kim JI, and Kim JB (2016). Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Frontiers in endocrinology 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. (2014). Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, and Spiegelman BM (2016). Cell biology of fat storage. Molecular biology of the cell 27, 2523–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker RH, Simonsen L, Bulow J, Wasserman DH, and Kjaer M (2001). Stimulation of splanchnic glucose production during exercise in humans contains a glucagon-independent component. American journal of physiology. Endocrinology and metabolism 280, E918–927. [DOI] [PubMed] [Google Scholar]
- Collins S, and Surwit RS (2001). The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent progress in hormone research 56, 309–328. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, Holman AR, Tal I, Palmer MR, Kolodny GM, et al. (2012). Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proceedings of the National Academy of Sciences of the United States of America 109, 10001–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. (2009). Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine 360, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, et al. (2015). Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell metabolism 21, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Franco A, Guasti D, Squecco R, Mazzanti B, Rossi F, Idrizaj E, Gallego-Escuredo JM, Villarroya F, Bani D, Forti G, et al. (2016). Searching for Classical Brown Fat in Humans: Development of a Novel Human Fetal Brown Stem Cell Model. Stem cells (Dayton, Ohio) 34, 1679–1691. [DOI] [PubMed] [Google Scholar]
- Duchamp C, Burton KA, Geloen A, and Dauncey MJ (1997). Transient upregulation of IGF-I gene expression in brown adipose tissue of cold-exposed rats. The American journal of physiology 272, E453–460. [DOI] [PubMed] [Google Scholar]
- Elsen M, Raschke S, Tennagels N, Schwahn U, Jelenik T, Roden M, Romacho T, and Eckel J (2014). BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. American journal of physiology. Cell physiology 306, C431–440. [DOI] [PubMed] [Google Scholar]
- Fawcett DW (1952). A comparison of the histological organization and cytochemical reactions of brown and white adipose tissues. Journal of morphology 90, 363–405. [Google Scholar]
- Febbraio MA, and Pedersen BK (2002). Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 16, 1335–1347. [DOI] [PubMed] [Google Scholar]
- Ferren L (1966). Morphological differentiation of implanted brown and white fats. Transactions of the Kansas Academy of Science. Kansas Academy of Science 69, 350–353. [PubMed] [Google Scholar]
- Fisher FM, and Maratos-Flier E (2016). Understanding the Physiology of FGF21. Annual review of physiology 78, 223–241. [DOI] [PubMed] [Google Scholar]
- Foster DO, and Frydman ML (1979). Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Canadian journal of physiology and pharmacology 57, 257–270. [DOI] [PubMed] [Google Scholar]
- Frayn KN (2010). Fat as a fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta physiologica (Oxford, England) 199, 509–518. [DOI] [PubMed] [Google Scholar]
- Fu Z, Yao F, Abou-Samra AB, and Zhang R (2013). Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochemical and biophysical research communications 430, 1126–1131. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, and Kahn CR (2007). Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256. [DOI] [PubMed] [Google Scholar]
- Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K, Zierath JR, and Krook A (2007). Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 56, 1630–1637. [DOI] [PubMed] [Google Scholar]
- Gunawardana SC, and Piston DW (2012). Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 61, 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardana SC, and Piston DW (2015). Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. American journal of physiology. Endocrinology and metabolism 308, E1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, Jorgensen JA, Boekschoten MV, Hesselink MK, Havekes B, et al. (2015). Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nature medicine 21, 863–865. [DOI] [PubMed] [Google Scholar]
- Hanssen MJ, van der Lans AA, Brans B, Hoeks J, Jardon KM, Schaart G, Mottaghy FM, Schrauwen P, and van Marken Lichtenbelt WD (2016). Short-term Cold Acclimation Recruits Brown Adipose Tissue in Obese Humans. Diabetes 65, 1179–1189. [DOI] [PubMed] [Google Scholar]
- Heaton GM, Wagenvoord RJ, Kemp A Jr., and Nicholls DG (1978). Brown-adipose- tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. European journal of biochemistry 82, 515–521. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, and Cinti S (2000). Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. American journal of physiology. Cell physiology 279, C670–681. [DOI] [PubMed] [Google Scholar]
- Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, and Villarroya F (2011). Thermogenic activation induces FGF21 expression and release in brown adipose tissue. The Journal of biological chemistry 286, 12983–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, Feng T, Zhong C, Wang Y, Lam KS, et al. (2015). Adiponectin Enhances Cold-Induced Browning of Subcutaneous Adipose Tissue via Promoting M2 Macrophage Proliferation. Cell metabolism 22, 279–290. [DOI] [PubMed] [Google Scholar]
- Ishibashi J, and Seale P (2010). Medicine. Beige can be slimming. Science (New York, N.Y.) 328, 1113–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, and Graff JM (2017). Distinct cellular and molecular mechanisms for P3 adrenergic receptor-induced beige adipocyte formation. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida T, Ejima A, Yamamoto K, Tanaka S, Yamamoto T, and Mazda O (2015). Reprogrammed Functional Brown Adipocytes Ameliorate Insulin Resistance and Dyslipidemia in Diet-Induced Obesity and Type 2 Diabetes. Stem cell reports 5, 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Yao T, Zhou P, Kazak L, Tenen D, Lyubetskaya A, Dawes BA, Tsai L, Kahn BB, Spiegelman BM, et al. (2018). Brown Adipose Tissue Controls Skeletal Muscle Function via the Secretion of Myostatin. Cell metabolism 28, 631–643.e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraunsoe R, Boushel R, Hansen CN, Schjerling P, Qvortrup K, Stockel M, Mikines KJ, and Dela F (2010). Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. The Journal of physiology 588, 2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P, Nedergaard J, and Cannon B (1986). Beta-adrenergic stimulation of fatty acid release from brown fat cells differentiated in monolayer culture. Life sciences 38, 589–599. [DOI] [PubMed] [Google Scholar]
- Lee CW, Hsiao WT, and Lee OK (2017). Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet. Translational research : the journal of laboratory and clinical medicine 182, 61–74.e68. [DOI] [PubMed] [Google Scholar]
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et al. (2014a). Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell metabolism 19, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, and Celi FS (2014b). Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63, 3686–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux S, and Despres JP (1994). Metabolic complications of visceral obesity: contribution to the aetiology of type 2 diabetes and implications for prevention and treatment. Diabete & metabolisme 20, 375–393. [PubMed] [Google Scholar]
- Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, Ma Y, Brocker CN, Yan T, et al. (2017). Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell metabolism 26, 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, Lin J, Zhao Q, Zhang C, Yuan X, et al. (2015). Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology 156, 2461–2469. [DOI] [PubMed] [Google Scholar]
- Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen Y, Chi Q, Wang D, Zhang Z, Li C, et al. (2013). Brown adipose tissue transplantation improves whole-body energy metabolism. Cell research 23, 851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh RKC, Formosa MF, La Gerche A, Reutens AT, Kingwell BA, and Carey AL (2018). Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes, obesity & metabolism. [DOI] [PubMed] [Google Scholar]
- Lowell BB, and Flier JS (1997). Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annual review of medicine 48, 307–316. [DOI] [PubMed] [Google Scholar]
- Lowell BB, and Spiegelman BM (2000). Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660. [DOI] [PubMed] [Google Scholar]
- Ludzki AC, Pataky MW, Cartee GD, and Horowitz JF (2018). Acute endurance exercise increases Vegfa mRNA expression in adipose tissue of rats during the early stages of weight gain. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 43, 751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, Takahashi H, Hirshman MF, Schlein C, Lee A, et al. (2017). The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nature medicine 23, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdaviani K, Chess D, Wu Y, Shirihai O, and Aprahamian TR (2016). Autocrine effect of vascular endothelial growth factor-A is essential for mitochondrial function in brown adipocytes. Metabolism: clinical and experimental 65, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, Kvist H, Holm G, Sjostrom L, and Bjorntorp P (1992). The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism: clinical and experimental 41, 1242–1248. [DOI] [PubMed] [Google Scholar]
- Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, and Cannon B (2000). Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. The Journal of biological chemistry 275, 25073–25081. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, and Cushman SW (2007). Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 50, 1707–1715. [DOI] [PubMed] [Google Scholar]
- Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, Noh HL, Kim JK, Cooper MP, Fitzgibbons T, et al. (2016). Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nature medicine 22, 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, and Grundy SM (1997). Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obesity research 5, 93–99. [DOI] [PubMed] [Google Scholar]
- Misra A, and Vikram NK (2003). Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition (Burbank, Los Angeles County, Calif.) 19, 457–466. [DOI] [PubMed] [Google Scholar]
- Mund RA, and Frishman WH (2013). Brown adipose tissue thermogenesis: beta3-adrenoreceptors as a potential target for the treatment of obesity in humans. Cardiology in review 21, 265–269. [DOI] [PubMed] [Google Scholar]
- Muzzin P, Revelli JP, Kuhne F, Gocayne JD, McCombie WR, Venter JC, Giacobino JP, and Fraser CM (1991). An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. The Journal of biological chemistry 266, 24053–24058. [PubMed] [Google Scholar]
- Nechad M, and Olson L (1983). Development of interscapular brown adipose tissue in the hamster. II - Differentiation of transplants in the anterior chamber of the eye: role of the sympathetic innervation. Biology of the cell 48, 167–174. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, and Cannon B (2007). Unexpected evidence for active brown adipose tissue in adult humans. American journal of physiology. Endocrinology and metabolism 293, E444–452. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, and Lindberg O (1979). Norepinephrine-stimulated fatty-acid release and oxygen consumption in isolated hamster brown-fat cells. Influence of buffers, albumin, insulin and mitochondrial inhibitors. European journal of biochemistry 95, 139–145. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, and Harris TB (2006). Abdominal obesity is an independent risk factor for chronic heart failure in older people. Journal of the American Geriatrics Society 54, 413–420. [DOI] [PubMed] [Google Scholar]
- Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, et al. (2011). Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell metabolism 14, 272–279. [DOI] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, and Nedergaard J (2010). Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of biological chemistry 285, 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer A (2015). NRG4: an endocrine link between brown adipose tissue and liver. Cell metabolism 21, 13–14. [DOI] [PubMed] [Google Scholar]
- Prins JB, and O’Rahilly S (1997). Regulation of adipose cell number in man. Clinical science (London, England : 1979) 92, 3–11. [DOI] [PubMed] [Google Scholar]
- Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, et al. (2013). BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America 110, E798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell M, Hondares E, Iwamoto S, Gonzalez FJ, Wabitsch M, Staels B, Olmos Y, Monsalve M, Giralt M, Iglesias R, et al. (2012). Peroxisome proliferator-activated receptors- alpha and -gamma, and cAMP-mediated pathways, control retinol-binding protein-4 gene expression in brown adipose tissue. Endocrinology 153, 1162–1173. [DOI] [PubMed] [Google Scholar]
- Rosen ED, and Spiegelman BM (2000). Molecular regulation of adipogenesis. Annual review of cell and developmental biology 16, 145–171. [DOI] [PubMed] [Google Scholar]
- Ross R, Berentzen T, Bradshaw AJ, Janssen I, Kahn HS, Katzmarzyk PT, Kuk JL, Seidell JC, Snijder MB, Sorensen TI, et al. (2008). Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obesity reviews : an official journal of the International Association for the Study of Obesity 9, 312–325. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, and Stock MJ (1983). Effects of age on diet-induced thermogenesis and brown adipose tissue metabolism in the rat. International journal of obesity 7, 583–589. [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. (2009). High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, and Lodish HF (1995). A novel serum protein similar to C1q, produced exclusively in adipocytes. The Journal of biological chemistry 270, 26746–26749. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. (2011). Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proceedings of the National Academy of Sciences of the United States of America 108, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, and Spiegelman BM (2009). Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes & development 23, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FJ, Holt DJ, Vargas V, Yockman J, Boudina S, Atkinson D, Grainger DW, Revelo MP, Sherman W, Bull DA, et al. (2014). Metabolically active human brown adipose tissue derived stem cells. Stem cells (Dayton, Ohio) 32, 572–581. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, Yudkin JS, Heine RJ, Nijpels G, and Seidell JC (2003). Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. The American journal of clinical nutrition 77, 1192–1197. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, et al. (2018). 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell metabolism 27, 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, et al. (2013). Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. The Journal of clinical investigation 123, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, et al. (2015). A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64, 20022014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Luby-Phelps K, Spurgin SB, An YA, Wang QA, Holland WL, and Scherer PE (2014). Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Molecular metabolism 3, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, and Scherer PE (2012). Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proceedings of the National Academy of Sciences of the United States of America 109, 5874–5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, and Wright DC (2009). Exercise and adrenaline increase PGC-1 {alpha} mRNA expression in rat adipose tissue. The Journal of physiology 587, 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, and Christiansen C (2003). Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation 107, 1626–1631. [DOI] [PubMed] [Google Scholar]
- Tchoukalova Y, Koutsari C, and Jensen M (2007). Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia 50, 151–157. [DOI] [PubMed] [Google Scholar]
- Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, et al. (2017). Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, and Pedersen BK (2009). The importance of brown adipose tissue. The New England journal of medicine 361, 415–416; author reply 418–421. [DOI] [PubMed] [Google Scholar]
- Townsend K, and Tseng YH (2012). Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte 1, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, and Tseng YH (2013). Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxidants & redox signaling 19, 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, and Kahn CR (2008). Beneficial effects of subcutaneous fat transplantation on metabolism. Cell metabolism 7, 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, et al. (2014). Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 63, 2800–2811. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. (2008). New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. (2013). Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. The Journal of clinical investigation 123, 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, and Teule GJ (2009). Cold-activated brown adipose tissue in healthy men. The New England journal of medicine 360, 1500–1508. [DOI] [PubMed] [Google Scholar]
- Van Pelt DW, Guth LM, and Horowitz JF (2017). Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. Journal of applied physiology (Bethesda, Md. : 1985) 123, 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdeguer F, Soustek MS, Hatting M, Blattler SM, McDonald D, Barrow JJ, and Puigserver P (2015). Brown Adipose YY1 Deficiency Activates Expression of Secreted Proteins Linked to Energy Expenditure and Prevents Diet-Induced Obesity. Molecular and cellular biology 36, 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. (2009). Functional brown adipose tissue in healthy adults. The New England journal of medicine 360, 1518–1525. [DOI] [PubMed] [Google Scholar]
- Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, et al. (2014). The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nature medicine 20, 1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rimm EB, Stampfer MJ, Willett WC, and Hu FB (2005). Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. The American journal of clinical nutrition 81, 555–563. [DOI] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R, Wan Y, Zhang S, Zhang Q, Ye H, and Li Y (2014). Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. American journal of physiology. Endocrinology and metabolism 306, E363–372. [DOI] [PubMed] [Google Scholar]
- Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, et al. (2009). Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell metabolism 9, 99–109. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, and Saito M (2013). Recruited brown adipose tissue as an antiobesity agent in humans. The Journal of clinical investigation 123, 3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom TG, and Bartness TJ (1995). Catecholaminergic innervation of white adipose tissue in Siberian hamsters. The American journal of physiology 268, R744–751. [DOI] [PubMed] [Google Scholar]
- Yuan X, Hu T, Zhao H, Huang Y, Ye R, Lin J, Zhang C, Zhang H, Wei G, Zhou H, et al. (2016). Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the United States of America 113, 2708–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani N, and Brown CW (2011). Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocrine reviews 32, 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Gao Y, Tao C, Shao M, Zhao S, Huang W, Yao T, Johnson JA, Liu T, Cypess AM, et al. (2016). Connexin 43 Mediates White Adipose Tissue Beiging by Facilitating the Propagation of Sympathetic Neuronal Signals. Cell metabolism 24, 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Spicer EG, Gavini CK, Goudjo-Ako AJ, Novak CM, and Shi H (2014). Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation). Physiology & behavior 125, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, and Cinti S (2009). The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 23, 3113–3120. [DOI] [PubMed] [Google Scholar]