Abstract
Sepsis is life-threatening systemic dysfunction caused by a deregulated host response to an infectious insult. Currently, the treatment of sepsis is limited to the use of antibiotics, fluids, and cardiovascular/respiratory support. Despite these interventions, septic mortality remains high, with reduced life quality in survivors. For this reason, the identification of novel drug targets is a pressing task of modern pharmacology. Based on recent research, it appears that P2 purinergic receptors, which can regulate the host’s response to infections, have been identified as potential targets for treatment of sepsis. Among P2 receptors, the P2X4 receptor has recently captured the attention of the research community owing to its role in protecting against infections, inflammation, and organ injury. The present review provides an outline of the role played by P2X4 receptors in the modulation of the host response to sepsis and the promise that targeting this receptor holds in the treatment of sepsis.’
Introduction
In 2016, the 3rd International Consensus Conference for Sepsis and Septic Shock defined sepsis as a life-threatening multiorgan dysfunction arising from a dysregulated host response to an infection, which has a high risk of death [1]. According to early estimates in this millennium, there are about 751,000 cases of sepsis (3.0 per 1,000 people) each year, resulting in over 200,000 deaths in the United States of America [2]. More recent studies indicate that sepsis causes, or contributes to, from one-third to one-half of all deaths occurring in hospitals in the United States, with the majority of patients presenting to hospital with sepsis rather than acquiring sepsis in hospital [3]. The overall sepsis-related health care cost in the US has been estimated to be $16-25 billion annually [4]. Taking into account the rapid expansion of the elderly population, which is affected disproportionately by sepsis due to physiologic frailty and immune senescence, the prevalence of sepsis and the associated health care costs are expected to rise significantly over the next two decades [5]
Several risk factors contribute to the development of sepsis, including chronic diseases impairing the immune system (e.g., acquired immunodeficiency syndrome, chronic obstructive pulmonary disease, cancers) and treatment with immunosuppressive drugs [6]. In addition, age, sex, race and ethnic group are important determinants of sepsis prevalence, as it occurs more frequently in infants and elderly persons than in other age groups, at higher rates in males than in females, and at increased frequencies in African Americans as compared to Caucasians [7].
The clinical signs of sepsis are highly variable, depending on the initial site of infection, the pathogen, the pattern of acute organ dysfunction, as well as the underlying health status of the patient, and the interval before initiation of treatment [8]. Septic patients frequently display fever, shock, and respiratory failure [9]. Our understanding about the pathophysiological mechanisms underlying the onset and development of sepsis has evolved over the time. Initially, the development of sepsis was ascribed to a hyper-inflammatory condition characterized by an unrestrained immune response (systemic inflammatory response syndrome or SIRS) with a systemic cytokine storm, which is the result of an overproduction of several pro-inflammatory molecules including TNF-α, IL-1β, IL-2, IL-6, IL-8, and IFN-γ [9]. Subsequent hypotheses refined this original view, and proposed that sepsis develops through a sequential or parallel (SIRS)[10] and compensatory anti-inflammatory response syndrome (CARS), a condition with suppresses immunity and enhances the susceptibility to secondary infections[11].
Despite over 100 clinical trials conducted on sepsis, no FDA-approved treatment options exist that can improve sepsis survival [12]. For this reason, the identification of novel pharmacological approaches to manage sepsis is of extreme interest to the scientific community. Over the past two decades, increasing attention has been paid to the involvement of the purinergic system in the pathophysiology of sepsis [13-19]. The purine ATP is a well-recognized signaling molecule released at sites of infection, inflammation and cell injury. In this setting, extracellular ATP acts as a “danger” signal, which, through the activation of P2 receptors plays a critical role in immune cell migration, chemotaxis, and cytokine release [19-21].
In this brief review article, we have delineated the role of one of the P2 receptors, the P2X4, in regulating immunity during infections, inflammation and sepsis.
Purinergic signalling: receptors, enzymes, transporters
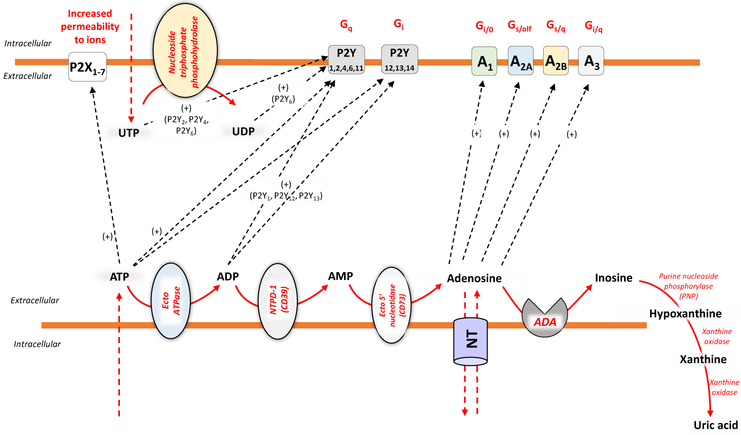
Purinergic signaling is initiated by the release of nucleotides into the extracellular space through volume regulated anion channels, maxi-anion channels, transporters, connexins and pannexins [22], as well as exocytotic pathways and membrane damage (Fig. 1) [23]. Once released into the extracellular space, the nucleotides, which include ATP, ADP, UDP, and UDP-glucose, can trigger a series of cellular responses through the engagement of P2 receptors. These are classified into ionotropic P2X (P2X1–7) and metabotropic P2Y (P2Y1,2,4,6,11–14) receptors (Fig. 1) [23]. P2X receptors display a trimeric structure, where each monomer has two transmembrane domains. They are ion channels and gate primarily Na+, K+ and Ca2+, and, in some cases, Cl− [24]. The stimulation of Gq/11-coupled P2Y1,2,4,6 and P2Y11 receptors causes the activation of phospholipase C, leading to the downstream production of inositol-(1,4,5)-trisphosphate and diacylglycerol (DAG) [25]. Inositol-(1,4,5)-trisphosphate increases intracellular Ca2+ levels were DAG stimulates protein kinase C (PKC) [25]. Moreover, P2Y11 receptor activation can stimulate whereas P2Y12,13 receptor engagement can inhibit, adenylate cyclase [25]. As asserted above, in addition to ATP, UTP and its degradation product, UDP, can play important regulatory roles also in modulating the immune cell activities (i.e. neutrophil and monocyte chemotaxis, activation of granulocytes, dendritic cells, monocytes, neutrophils and lymphocytes)[26]. Indeed, UTP, UDP and UDP-glucose act as agonists on four P2Y receptor subtypes (P2Y2, P2Y4, P2Y6 and P2Y14), whereas no affinity has been observed for P2X receptors [26]. In general, the engagement of P2 receptors has been shown to facilitate immune cell function.
Figure 1.
Once released into the extracellular environment, through channels or other extrusion systems, ATP, ADP, AMP, and adenosine, are metabolized by a set of nucleotidases [nucleoside triphosphate diphosphohydrolases, ecto-ATPase, NTPD-1 (CD39), ecto-5’nucleotidase (CD73), purine nucleoside phosphorylase PNP), xanthine oxidase], leading to their sequential dephosphorylation and subsequent generation of other bioactive metabolites, which selectively interact with purinergic P1 (A1, A2A, A2B and A3) or P2 (P2X and P2Y) receptors. Several cell types are endowed with nucleoside transporters (NT), which operate the uptake of extracellular adenosine, thus actively participating to the careful regulation and termination of adenosine signaling.
Following their release into the extracellular space, ATP and/or ADP are quickly converted by CD39 (ecto-nucleoside triphosphate diphosphohydrolase 1, E-NTPDase1) into AMP, and then, via CD73 (ecto-5'-nucleotidase) AMP is dephosphorylated into adenosine [27]. The latter is a purine nucleoside, which exerts its cellular actions through the engagement of specific G protein-coupled receptors classified as A1, A2A, A2B and A3. In particular, A1 and A3 receptors are coupled to Gi, Gq and Go proteins, while the A2A and A2B receptors activate adenylyl cyclase via Gs or Golf [28] (Fig. 1). The stimulation of A2B receptors can trigger also phospholipase C via Gq [28,29]. In general adenosine exerts immunosuppressive effects [28,29]. Of note, since the CD39/CD73 enzyme axis is critical in the degradation of ATP, ADP, and AMP into adenosine, it can be considered as a an “immunological switch” which is able to shift an ATP-driven pro-inflammatory immune cell response toward an anti-inflammatory environment steered by adenosine [23] [27,30,31] (Fig. 1).
It is interesting to note that P1 and P2 purinergic receptors have distinct and divergent effects on immunity and inflammation regulation [32]. Whereas adenosine-mediated P1 purinergic receptor activation dampens inflammation and attenuate immune-mediated tissue injury overall [14,15,33], P2 purinergic receptor activation by ATP drives inflammasome activation and stimulates bacterial killing [34,35]. These divergent effects of P1 and P2 purinergic receptors on immunity and inflammation is clinically significant as sepsis is a disease characterized by initial hyper-inflammatory phase followed by hypo-immune state where the subject succumbs from immune paralysis [36]. Depending on the immune state of the septic subject, one can tailor therapy with either P1 (to dampen inflammation) or P2 (to promote immunity) purinergic receptors.
P2X4 receptor: structure and molecular biology
P2X receptors are a family of non-selective trimeric ligand-gated channels, permitting Na+, K+ and Ca2+ ion fluxes upon binding extracellular ATP [37]. Seven subtypes of P2X receptors have been cloned and classified as P2X1 to P2X7, with functional channels assembled as homo- or heterotrimers [37]. Each monomer comprises two transmembrane domains linked by a large extracellular loop and intracellularly located N- and C-termini [38]. The activity of P2X receptors is modified by several factors including the extracellular concentration of ions (Zn2+, Cu2+, Hg2+, Ni2+, Cd2+), protons, lipids, steroids, and ethanol [39].
The P2X4 receptor is one of the most sensitive purinergic receptors, as it is activated by nanomolar concentrations of extracellular ATP [39]. Of note, the Ca2+ permeability of P2X4 is the highest among the P2X family [40].
Immunoprecipitation studies have revealed that the P2X4 subtype can heteromerically assemble with other P2X members, including P2X1, P2X2 and P2X6 [41,42]. In addition, structural interactions have been reported between P2X4 and P2X7 receptors [41,43]. In this regard, Schneider et al. [44] recently demonstrated that P2X4 and P2X7 subunits can form heterotrimeric P2X4/P2X7 receptors. Of note, the P2X4 and P2X7 subunit isoforms are widely co-expressed particularly on immune/inflammatory cells [45].
P2X4 receptors are distributed throughout the body [46]. Indeed, they are widely expressed in central and peripheral neurons, microglia, and various glandular tissues such as pancreatic acinar cells and salivary glands as well as endothelial cells [47]. They are expressed also throughout the gastrointestinal tract, liver, lung, kidney and reproductive system [23][24]. At the cellular level, the P2X4 receptor is located on plasma membrane, but also in intracellular compartments, such as lysosomes, vesicles, vacuoles and lamellar bodies[48].
Acute kidney injury and inflammation is a major complication of sepsis and has extremely high mortality (>70% in severe sepsis) [49]. Although several renal cells express P2X4 purinergic receptor, the role for P2X4 receptor in acute kidney injury and inflammation is unclear unlike the better-characterized pro-inflammatory role of P2X7 receptor [50]. It appears that P2X4 dampens renal fibrosis response during recovery from kidney injury as mice deficient in P2X4 receptors have exacerbated renal fibrosis in a mouse model of chronic interstitial inflammation [51]. Furthermore, renal tubular P2X4 receptor activation may promote kidney injury in early sepsis by promoting NOD-like receptor 3 inflammasome activation and facilitating IL-1β and IL-18 maturation [52].
P2X4 receptor and immune system
All immune cells, whether from the myeloid or lymphoid lineage, express at least one P2X or P2Y receptor subtype (see Table 1) [53]. Consistently with this knowledge, an increasing body of evidence supports the critical role played by both P2X and P2Y receptors in immune cell biology [54-57]. In accordance with its wide distribution, the P2X4 receptor has been shown to regulate a variety of pathophysiological processes, such as neuropathic pain and autoimmune diseases [39,58,59]. Many of these modulating roles are mediated by cells of the immune system.
Table 1.
Expression of P2 receptors on immune cells
| Immune cell population | Purinergic receptors | References |
|---|---|---|
| Monocyte/macrophage | P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14 P2X1, P2X4, P2X7 | [55,96] |
| Dendritic cells | P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y14, P2X1, P2X4, P2X7 | [76,97] |
| Neutrophils | P2Y1, P2Y4, P2Y6, P2Y11 P2X1, P2X4, P2X7 | [56] |
| Eosinophils | P2Y1, P2Y4, P2Y6, P2Y11, P2Y14, P2X1, P2X4, P2X7 | [98,99] |
| Mast cells | P2Y1, P2Y12, P2Y13, P2Y14 P2X1, P2X3, P2X4, P2X7, | [100,101] |
| T lymphocytes | P2Y1, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14, P2X1, P2X4, P2X7 | [102] |
| B lymphocytes | P2Y1, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14 P2X1, P2X4, P2X7 | [57] |
| Natural killer cells | P2Y11, P2X1, P2X4, P2X7 | [103] |
Monocytes, and macrophages.
Monocytes and macrophages play a pivotal role in the immune response against micro-organisms and the pathogenesis of sepsis [60] [61]. These cells phagocyte and kill micro-organisms, release cytokines, and present pathogens to T cells, thus triggering both cellular and humoral immune responses [60]. All monocyte and macrophage cell types have been found to express P2X and P2Y receptors. In monocytes, the most abundant P2X receptor transcripts are P2X4, followed by transcripts for P2X7 and P2X1 [62], thus suggesting an important role for these receptor subtypes in monocyte functions.
Murine peritoneal macrophages express functionally active P2X4 and P2X7 receptors [63,64]. In particular, the exposure of these cells to low concentrations of ATP evoked a small P2X4-driven ion current, while higher ATP concentrations evoked a large P2X7-driven ion current [64]. However, the physiological role of the P2X4 receptor-driven current was not explored in this study [64]. P2X4 expression has been detected also on both human and rat alveolar macrophages [65], and the authors described a dynamic regulation of P2X4 receptors during distinct phases of macrophage activation [65]. In resting macrophages, functional P2X4 receptors as measured using ATP-evoked currents were maintained at very low levels, whereas they underwent a rapid up-regulation in response to phagocytosis [65]. By contrast, classical activation of macrophages elicited by their incubation with IFN- γ and TNF-α or IFN- γ and LPS, reduced the surface expression of P2X4 receptors and decreased ATP-evoked currents without altering total P2X4 receptor protein levels [65]. Of note, the alternative activation of these cells with IL- 4 or IL- 13 did not alter the total surface or the functional expression of these receptors [65]. Based on these observations, the authors hypothesized a scenario in which an initial bacterial or inflammatory stimulus elicits rapid trafficking of P2X4 receptors to the macrophage cell surface causing increased Ca2+ influx, thus promoting their activity [65]. After the termination of macrophage activation, a feedback mechanism develops to curb P2X4 receptor trafficking to the cell membrane and function, probably aimed at facilitating the resolution inflammation [65].
Recent observations highlighted molecular and functional interactions between P2X4 and P2X7 receptors on macrophages [66-68]. In particular, a physical protein–to protein interaction takes place between P2X4 and P2X7 receptors, where the two receptors are bridged by the C-terminus of P2X7 receptor [66]. This physical interaction was facilitated by the presence of extracellular ATP [66]. Kawano et al. provided evidence about a role of P2X4 receptor in modulating P2X7 receptor-dependent inflammatory functions [67]. Indeed, by means of in vitro assay, it was observed that treatment of RAW264.7 cells with ATP elicited a P2X7-dependent release of HMGB1 and IL-1β [67]. Of note, this event was blunted by the genetic ablation of P2X4 receptor via short hairpin RNA transfection as well as by removing extracellular Ca2+[67], thus confirming a functional interplay between P2X4 and P2X7 receptors, driven by a modulation of intracellular Ca2+ concentrations.
The P2X4/P2X7 linkage has been shown to hold a critical role also in eliciting macrophage death[68]. In particular, the recruitment of P2X7 receptors in RAW264.7 cells with high concentrations of ATP determined an increase in Ca2+ influx, pore formation, and activation of ERK1/2 and p38MAPK, thus leading to cell death [68]. In this context, despite the activation of P2X4 receptor alone did not induce cell death, the P2X4 receptor-dependent acute-phase Ca2+ influx, elicited by the high levels of ATP, contributed to P2X7 receptor-dependent cell death in activated macrophages [68].
Recently, our experiments revealed a role of ATP in regulating bacterial killing in macrophages via P2X4 receptors [18]. We observed that the selective P2X4 receptor antagonist 5-BDBD and the shRNA-mediated receptor silencing, prevented the stimulant effect of ATP on E. coli killing by macrophages [18]. In addition, in macrophages isolated from P2X4 receptor knockout mice, ATP failed to stimulate bacterial killing [18]. Thus, we concluded that P2X4 receptors augment bacterial killing by macrophages. Mechanistically, we found that P2X4 receptor activation specifically increased mitochondrial, but not cellular, ROS production, which was a likely mediator of the antibacterial effect of P2X4 receptor activation [18].
In parallel, using the murine cecal ligation and puncture model, considered by several investigators as the “gold standard” mouse model of sepsis [69], we demonstrated also that macrophage P2X4 receptors control bacterial spread and inflammation in sepsis [18]. That is, P2X4 receptor knockout mice showed an increased bacterial load and a decreased survival in comparison with wild type animals [18], indicating that endogenous ATP released during sepsis, exerts a protective effect through P2X4 receptor engagement [18]. Of note, we also provided evidence that protective effect of P2X4 receptor activation was mediated though P2X4 receptor signaling on macrophages, as both adoptive transfer of P2X4−/− receptor macrophages or myeloid-specific P2X4−/− receptor mice emulated the deleterious phenotype of P2X4 deficient animals.
Dendritic cells.
Dendritic cells (DCs) are the primary antigen-presenting cells in the body and therefore they are pivotal in linking innate and adaptive immunity [70]. Dendritic cell function is compromised during sepsis [71,72] and sepsis is associated with widespread dendritic cell depletion [73,74]. DCs are endowed with both P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11 and P2Y14) and P2X receptors (P2X1, P2X4, P2X7) [75,76]. Our understanding of the role of P2X4 receptors in regulating dendritic cell function is in its infancy. Sakaki et al. demonstrated recently that P2X4 receptor activation elicited a rapid and substantial initial Ca2+ influx in dendritic cells. This Ca2+ influx was required for optimal P2X7-dependent IL-1β and IL-18 release from DCs [77]. Clearly, further studies are required to unravel the precise role of P2X4 receptors in regulating DC function during immune responses and sepsis.
Neutrophils.
Neutrophils are professional phagocytes that play a central role in host defense against extracellular bacteria and fungi [78]. These cells are endowed with a unique capacity to very efficiently and rapidly engulf and thereby eliminate pathogens and cell debris [79]. Patients with sepsis display aberrant neutrophil activity, characterized by an impaired recruitment of these cells to sites of infection, abnormal longevity, and overproduction of toxic mediators leading to bystander organ injury [80].
A substantial body of evidence supports the notion that ATP regulates tightly several neutrophil functions, such as chemotaxis, rolling, adhesion, transmigration, phagocytosis, oxidative burst, extracellular trap formation, degranulation and apoptosis [56]. The role of P2X4 receptors in neutrophils is incompletely understood. The only study dealing with this issue showed that P2X4 knockout mice demonstrated reduced infiltration of neutrophils to the injury site in a murine spinal cord injury model [81]. It was however unclear from this study whether P2X4 receptor signaling in neutrophils was required for optimal neutrophil infiltration or P2X4 signaling indirectly affected neutrophil infiltration.
Considering our data, showing a stimulant role of P2X4 receptors on macrophages in bacterial killing [18], it would be interesting to test whether P2X4 receptors have a similar role in neutrophils.
T lymphocytes.
Sepsis results in a deluge of pro- and anti-inflammatory cytokines and immunosuppressive mediators, leading to CD4+ and CD8+ T cell dysfunction and death [82]. Although the precise mechanisms, underlying this condition of T cell suppression, are not well defined, several lines of evidence indicate that purinergic signaling may control T cells during sepsis in many different ways [20,83]. Indeed, the release of ATP via pannexin-1 pores during lymphocyte activation, enhances IL-2 release, in an autocrine manner, through the engagement of P2X1, P2X4 and P2X7 receptors [84,85]. Mechanistically, ATP elicits Ca2+ influx through these receptors and stimulates the nuclear factor of activated T cells and MAPK signaling resulting in increased IL-2 production [84,86,87]. The accumulation of several purinergic components, including pannexin 1 channels, P2X1 and P2X4 receptors, at the immune synapse facilitates T cell–accessory cell communication, antigen recognition and T cell activation [87]. Mitochondria also translocate to the immune synapse where by directly providing the ATP to be released, fuel the autocrine purinergic signaling in the synaptic cleft [87]. P2X4 receptors colocalize with mitochondria in clusters at the front of cells, facilitating the Ca2+ influx to maintain local mitochondrial ATP production at the levels needed for cell migration [88].
In patients with sepsis, purinergic signaling appears defective [89]. Indeed, resting cells from septic patients lack the autocrine purinergic feedback loops that maintain basal cytosolic Ca2+ levels and mitochondrial function thus, leading to a condition of cellular paralysis that impairs vigilance and precludes proper functional responses to T cell stimulation [89]. However, the suppression of purinergic signaling is not due to a reduction of P2X receptor expression, since the mRNA levels of these receptors appear unchanged (P2X4 and P2X7) or elevated (P2X1) in patients with sepsis [89]. Further studies will be needed to unravel the precise role of purinergic signaling in T cells in sepsis.
Conclusions
Although antibiotic therapy still represents an irreplaceable part of sepsis management, this therapeutic approach is facing hard challenges due to the development of multi-resistant bacterial strains [90,91]. In addition, antibiotic treatment does not prevent the various immune abnormalities occurring during sepsis. For these reasons, it is necessary to foster an integrated approach in the treatment of sepsis, which might take advantage of our increasing understanding of the role of purinergic signaling is sepsis [92].
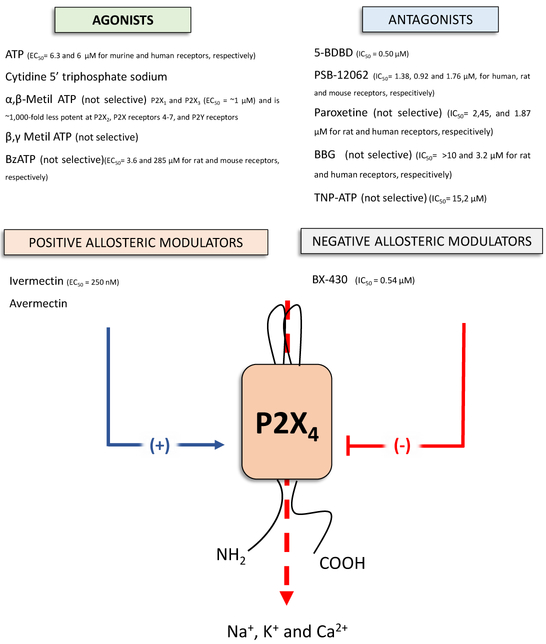
So far, in the arena of purinergic research much attention has been focused on P2X7 receptors, as they have been shown to be involved in a plethora of inflammatory disorders (i.e. osteoarthritis, rheumatoid arthritis, chronic obstructive pulmonary disease, and Crohn’s disease), and thus they appear to be one of the most appealing potential drug target [93]. However, recently there has been also growing interest on the P2X4 receptors. Indeed, this receptor subtype, which is widely investigated in the context of pain transmission [94,95], also has a role in mediating the effects of ATP in shaping immune/inflammatory responses. For this reason, it is striking that the P2X4 receptor, which is being widely expressed on cells of both the innate and adaptive arms of the immune system (see Table 1) remains poorly characterized in sepsis. Our pioneering preclinical studies demonstrated that P2X4 receptor engagement is critical in limiting the dissemination of bacteria by enhancing killing of these pathogens[18]. This indicates that P2X4 receptors are a potential target for antibacterial drug discovery. Unfortunately, the study of P2X4 receptors has been seriously hindered by the lack of selective pharmacological tools. Indeed, for a long time, researchers had to rely on non-selective P2X receptor antagonists such as paroxetine, TNP-ATP, and BBG to characterize P2X4 receptors (Fig. 2). We are hopeful that in the coming years medicinal chemists will generate selective and potent ligands for P2X4 receptors
Figure 2.
Schematic structure of the P2X4 receptor and the main commercially available ligands acting on this receptor subtype with indicated the IC50 and EC50 values.
Highlights.
Sepsis is an organ dysfunction caused by a deregulated immune response to an infection
At present, no approved treatment options exist that can improve sepsis survival
Increasing attention has been paid to the role of the purinergic system in sepsis pathophysiology
Acknowledgements
This work was supported by National Institutes of Health grants R01GM066189 (G.H.) and R01DK113790 (G.H.); the Intramural Research Program of the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (P.P.).
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- ••1.Cecconi M, Evans L, Levy M, Rhodes A: Sepsis and septic shock. Lancet 2018, 392:75–87.This is a comprehensive review that presents up-to-date evidence and controversies about sepsis, and highlights the importance of driving quality improvement through initiatives
- 2.Angus DC, Wax RS: Epidemiology of sepsis: an update. Crit Care Med 2001, 29:S109–116. [DOI] [PubMed] [Google Scholar]
- 3.Finfer S, Machado FR: The Global Epidemiology of Sepsis. Does It Matter That We Know So Little? Am J Respir Crit Care Med 2016, 193:228–230. [DOI] [PubMed] [Google Scholar]
- 4.Alvaro-Meca A, Jimenez-Sousa MA, Micheloud D, Sanchez-Lopez A, Heredia-Rodriguez M, Tamayo E, Resino S, Group of Biomedical Research in Critical Care M: Epidemiological trends of sepsis in the twenty-first century (2000-2013): an analysis of incidence, mortality, and associated costs in Spain. Popul Health Metr 2018, 16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn JM, Le T, Angus DC, Cox CE, Hough CL, White DB, Yende S, Carson SS, ProVent Study Group I: The epidemiology of chronic critical illness in the United States*. Crit Care Med 2015, 43:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayr FB, Yende S, Angus DC: Epidemiology of severe sepsis. Virulence 2014, 5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC: Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med 2008, 177:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remick DG: Pathophysiology of sepsis. Am J Pathol 2007, 170:1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG: Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev 2013, 93:1247–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R: Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015, 372:1629–1638. [DOI] [PubMed] [Google Scholar]
- 11.Adib-Conquy M, Cavaillon JM: Compensatory anti-inflammatory response syndrome. Thromb Haemost 2009, 101:36–47. [PubMed] [Google Scholar]
- 12.Delano MJ, Ward PA: The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev 2016, 274:330–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasko G, Nemeth ZH, Vizi ES, Salzman AL, Szabo C: An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur J Pharmacol 1998, 358:261–268. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Hasko G: Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol 2006, 176:5616–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscso B, Himer L, Vizi ES, et al. : A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol 2010, 185:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasko G, Csoka B, Koscso B, Chandra R, Pacher P, Thompson LF, Deitch EA, Spolarics Z, Virag L, Gergely P, et al. : Ecto-5'-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol 2011, 187:4256–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csoka B, Nemeth ZH, Toro G, Koscso B, Kokai E, Robson SC, Enjyoji K, Rolandelli RH, Erdelyi K, Pacher P, et al. : CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB J 2015, 29:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••18.Csoka B, Nemeth ZH, Szabo I, Davies DL, Varga ZV, Paloczi J, Falzoni S, Di Virgilio F, Muramatsu R, Yamashita T, et al. : Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight 2018, 3.In this article, the authors demonstrated that the endogenous purine ATP augments the killing of sepsis-causing bacteria by macrophages through P2X4 receptors
- 19.Csoka B, Nemeth ZH, Toro G, Idzko M, Zech A, Koscso B, Spolarics Z, Antonioli L, Cseri K, Erdelyi K, et al. : Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J 2015, 29:3626–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••20.Ledderose C, Bao Y, Kondo Y, Fakhari M, Slubowski C, Zhang J, Junger WG: Purinergic Signaling and the Immune Response in Sepsis: A Review. Clin Ther 2016, 38:1054–1065.In this article, the authors provide a comprehensive and authoritative overview abot the roles of ATP as a key regulator of immune cell function and as a disruptive signal that contributes to immune dysfunction in sepsis
- 21.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC: Adenosine 5'- triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 2006, 112:358–404. [DOI] [PubMed] [Google Scholar]
- 22.Taruno A: ATP Release Channels. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonioli L, Colucci R, Pellegrini C, Giustarini G, Tuccori M, Blandizzi C, Fornai M: The role of purinergic pathways in the pathophysiology of gut diseases: pharmacological modulation and potential therapeutic applications. Pharmacol Ther 2013, 139:157–188. [DOI] [PubMed] [Google Scholar]
- 24.Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Southan C, et al. : The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res 2014, 42:D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke H, Krugel U, Illes P: P2 receptors and neuronal injury. Pflugers Arch 2006, 452:622–644. [DOI] [PubMed] [Google Scholar]
- ••26.Cekic C, Linden J: Purinergic regulation of the immune system. Nat Rev Immunol 2016, 16:177–192.In this article, the authors provide a comprehensive and authoritative overview about the potential of targeting purinergic signalling pathways for the immunotherapeutic treatment of ischaemia, organ transplantation, autoimmunity or cancer
- 27.Antonioli L, Pacher P, Vizi ES, Hasko G: CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013, 19:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonioli L, Fornai M, Blandizzi C, Pacher P, Hasko G: Adenosine signaling and the immune system: When a lot could be too much. Immunol Lett 2018. [DOI] [PubMed] [Google Scholar]
- 29.Antonioli L, Blandizzi C, Pacher P, Hasko G: Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 2013, 13:842–857. [DOI] [PubMed] [Google Scholar]
- 30.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES: Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 1996, 157:4634–4640. [PubMed] [Google Scholar]
- 31.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marion A, Szabo C: Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J 2000, 14:2065–2074. [DOI] [PubMed] [Google Scholar]
- 32.Idzko M, Ferrari D, Eltzschig HK: Nucleotide signalling during inflammation. Nature 2014, 509:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallos G, Ruyle TD, Emala CW, Lee HT: A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol 2005, 289:F369–376. [DOI] [PubMed] [Google Scholar]
- 34.Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R: The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front Pharmacol 2018, 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hechler B, Gachet C: Purinergic Receptors in Thrombosis and Inflammation. Arterioscler Thromb Vase Biol 2015, 35:2307–2315. [DOI] [PubMed] [Google Scholar]
- 36.Delano MJ, Ward PA: Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest 2016, 126:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Yu Y: Insights into the channel gating of P2X receptors from structures, dynamics and small molecules. Acta Pharmacol Sin 2016, 37:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stojilkovic SS, Yan Z, Obsil T, Zemkova H: Structural insights into the function of P2X4: an ATP-gated cation channel of neuroendocrine cells. Cell Mol Neurobiol 2010, 30:1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •39.Suurvali J, Boudinot P, Kanellopoulos J, Ruutel Boudinot S: P2X4: A fast and sensitive purinergic receptor. Biomed J 2017, 40:245–256.This work demonstrates that P2X4 and P2X7 subunits can form heterotrimeric P2X4/P2X7 receptors.
- 40.Egan TM, Khakh BS: Contribution of calcium ions to P2X channel responses. J Neurosci 2004, 24:3413–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonio LS, Stewart AP, Xu XJ, Varanda WA, Murrell-Lagnado RD, Edwardson JM: P2X4 receptors interact with both P2X2 and P2X7 receptors in the form of homotrimers. Br J Pharmacol 2011, 163:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonio LS, Stewart AP, Varanda WA, Edwardson JM: Identification of P2X2/P2X4/P2X6 heterotrimeric receptors using atomic force microscopy (AFM) imaging. FEBS Lett 2014, 588:2125–2128. [DOI] [PubMed] [Google Scholar]
- 43.Boumechache M, Masin M, Edwardson JM, Gorecki DC, Murrell-Lagnado R: Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem 2009, 284:13446–13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider M, Prudic K, Pippel A, Klapperstuck M, Braam U, Muller CE, Schmalzing G, Markwardt F: Interaction of Purinergic P2X4 and P2X7 Receptor Subunits. Front Pharmacol 2017, 8:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surprenant A, North RA: Signaling at purinergic P2X receptors. Annu Rev Physiol 2009, 71:333–359. [DOI] [PubMed] [Google Scholar]
- 46.Bo X, Kim M, Nori SL, Schoepfer R, Burnstock G, North RA: Tissue distribution of P2X4 receptors studied with an ectodomain antibody. Cell Tissue Res 2003, 313:159–165. [DOI] [PubMed] [Google Scholar]
- 47.Burnstock G, Kennedy C: P2X receptors in health and disease. Adv Pharmacol 2011, 61:333–372. [DOI] [PubMed] [Google Scholar]
- 48.Xu J, Chai H, Ehinger K, Egan TM, Srinivasan R, Frick M, Khakh BS: Imaging P2X4 receptor subcellular distribution, trafficking, and regulation using P2X4-pHluorin. J Gen Physiol 2014, 144:81–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM: Sepsis-associated acute kidney injury. Semin Nephrol 2015, 35:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS: Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 2011, 63:641–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim MJ, Turner CM, Hewitt R, Smith J, Bhangal G, Pusey CD, Unwin RJ, Tam FW: Exaggerated renal fibrosis in P2X4 receptor-deficient mice following unilateral ureteric obstruction. Nephrol Dial Transplant 2014, 29:1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, He Y: ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 2013, 45:932–943. [DOI] [PubMed] [Google Scholar]
- 53.Burnstock G: P2X ion channel receptors and inflammation. Purinergic Signal 2016, 12:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••54.Junger WG: Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 2011, 11:201–212.This article provides a comprehensive overview of the roles of autocrine purinergic signalling in the regulation of both innate and adaptive immune responses and discusses the potential of targeting purinergic receptors for treating immune-mediated disease
- 55.Di Virgilio F, Vuerich M: Purinergic signaling in the immune system. Auton Neurosci 2015,191:117–123. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Chen D: Purinergic Regulation of Neutrophil Function. Front Immunol 2018,9:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Przybyla T, Sakowicz-Burkiewicz M, Pawelczyk T: Purinergic signaling in B cells. Acta Biochim Pol 2018, 65:1–7. [DOI] [PubMed] [Google Scholar]
- 58.Li F, Guo N, Ma Y, Ning B, Wang Y, Kou L: Inhibition of P2X4 suppresses joint inflammation and damage in collagen-induced arthritis. Inflammation 2014, 37:146–153. [DOI] [PubMed] [Google Scholar]
- 59.Sadovnick AD, Gu BJ, Traboulsee AL, Bernales CQ, Encarnacion M, Yee IM, Criscuoli MG, Huang X, Ou A, Milligan CJ, et al. : Purinergic receptors P2RX4 and P2RX7 in familial multiple sclerosis. Hum Mutat 2017, 38:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haveman JW, Muller Kobold AC, Tervaert JW, van den Berg AP, Tulleken JE, Kallenberg CG, The TH: The central role of monocytes in the pathogenesis of sepsis: consequences for immunomonitoring and treatment. Neth J Med 1999, 55:132–141. [DOI] [PubMed] [Google Scholar]
- 61.Kumar V: Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. Int Immunopharmacol 2018, 58:173–185. [DOI] [PubMed] [Google Scholar]
- 62.Layhadi JA, Fountain SJ: P2X4 Receptor-Dependent Ca(2+) Influx in Model Human Monocytes and Macrophages. Int J Mol Sci 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sim JA, Park CK, Oh SB, Evans RJ, North RA: P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharmacol 2007, 152:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brone B, Moechars D, Marrannes R, Mercken M, Meert T: P2X currents in peritoneal macrophages of wild type and P2X4 −/− mice. Immunol Lett 2007, 113:83–89. [DOI] [PubMed] [Google Scholar]
- 65.Stokes L, Surprenant A: Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur J Immunol 2009, 39:986–995. [DOI] [PubMed] [Google Scholar]
- •66.Perez-Flores G, Levesque SA, Pacheco J, Vaca L, Lacroix S, Perez-Cornejo P, Arreola J: The P2X7/P2X4 interaction shapes the purinergic response in murine macrophages. Biochem Biophys Res Commun 2015, 467:484–490.This study highlights that the P2X4/P2X7 interaction is important for shaping the physiological response to ATP in macrophages.
- ••67.Kawano A, Tsukimoto M, Mori D, Noguchi T, Harada H, Takenouchi T, Kitani H, Kojima S: Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem Biophys Res Commun 2012, 420:102–107.This work demonstrates that the co-expression of P2X4 receptorwith P2X7 receptor enhances P2X7-mediated inflammation through both facilitation of release of cytokines and suppression of autophagy
- 68.Kawano A, Tsukimoto M, Noguchi T, Hotta N, Harada H, Takenouchi T, Kitani H, Kojima S: Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem Biophys Res Commun 2012, 419:374–380.This article provides demonstration that P2X4 receptor plays a significant role in P2X7 receptor-dependent cell death in macrophages.
- 69.Ruiz S, Vardon-Bounes F, Merlet-Dupuy V, Conil JM, Buleon M, Fourcade O, Tack I, Minville V: Sepsis modeling in mice: ligation length is a major severity factor in cecal ligation and puncture. Intensive Care Med Exp 2016, 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rossi M, Young JW: Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol 2005, 175:1373–1381. [DOI] [PubMed] [Google Scholar]
- 71.Flohe SB, Agrawal H, Schmitz D, Gertz M, Flohe S, Schade FU: Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol 2006, 79:473–481. [DOI] [PubMed] [Google Scholar]
- 72.Ding Y, Chung CS, Newton S, Chen Y, Carlton S, Albina JE, Ayala A: Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock 2004, 22:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pene F, Courtine E, Ouaaz F, Zuber B, Sauneuf B, Sirgo G, Rousseau C, Toubiana J, Balloy V, Chignard M, et al. : Toll-like receptors 2 and 4 contribute to sepsis-induced depletion of spleen dendritic cells. Infect Immun 2009, 77:5651–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE: Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol 2002, 168:2493–2500. [DOI] [PubMed] [Google Scholar]
- 75.Berchtold S, Ogilvie AL, Bogdan C, Muhl-Zurbes P, Ogilvie A, Schuler G, Steinkasserer A: Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett 1999, 458:424–428. [DOI] [PubMed] [Google Scholar]
- 76.Ferrari D, La Sala A, Chiozzi P, Morelli A, Falzoni S, Girolomoni G, Idzko M, Dichmann S, Norgauer J, Di Virgilio F: The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J 2000, 14:2466–2476. [DOI] [PubMed] [Google Scholar]
- ••77.Sakaki H, Fujiwaki T, Tsukimoto M, Kawano A, Harada H, Kojima S: P2X4 receptor regulates P2X7 receptor-dependent IL-1beta and IL-18 release in mouse bone marrow-derived dendritic cells. Biochem Biophys Res Commun 2013, 432:406–411.In this article, the authors describe that co-expression of P2X4 receptor with P2X7 receptor in dendritic cells leads to enhancement of inflammation through facilitation of P2X7-dependent release of pro-inflammatory cytokines.
- 78.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG: The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 2017, 17:407–420. [DOI] [PubMed] [Google Scholar]
- 79.Leiding JW: Neutrophil Evolution and Their Diseases in Humans. Front Immunol 2017, 8:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kovach MA, Standiford TJ: The function of neutrophils in sepsis. Curr Opin Infect Dis 2012, 25:321–327. [DOI] [PubMed] [Google Scholar]
- 81.de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S: P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci 2012, 32:3058–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jensen IJ, Sjaastad FV, Griffith TS, Badovinac VP: Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J Immunol 2018, 200:1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burnstock G, Boeynaems JM: Purinergic signalling and immune cells. Purinergic Signal 2014, 10:529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG: Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J 2009, 23:1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y, Junger WG: Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol 2009, 88:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F: Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 2008, 1:ra6. [DOI] [PubMed] [Google Scholar]
- 87.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG: Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 2010, 116:3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ledderose C, Liu K, Kondo Y, Slubowski CJ, Dertnig T, Denicolo S, Arbab M, Hubner J, Konrad K, Fakhari M, et al. : Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest 2018, 128:3583–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ledderose C, Bao Y, Ledderose S, Woehrle T, Heinisch M, Yip L, Zhang J, Robson SC, Shapiro NI, Junger WG: Mitochondrial Dysfunction, Depleted Purinergic Signaling, and Defective T Cell Vigilance and Immune Defense. J Infect Dis 2016, 213:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turnidge J: Impact of antibiotic resistance on the treatment of sepsis. Scand J Infect Dis 2003, 35:677–682. [DOI] [PubMed] [Google Scholar]
- 91.Figueiredo Costa S: Impact of antimicrobial resistance on the treatment and outcome of patients with sepsis. Shock 2008, 30 Suppl 1:23–29. [DOI] [PubMed] [Google Scholar]
- 92.Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL: Sepsis and septic shock. Nat Rev Dis Primers 2016, 2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S: The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47:15–31. [DOI] [PubMed] [Google Scholar]
- 94.Bernier LP, Ase AR, Seguela P: P2X receptor channels in chronic pain pathways. Br J Pharmacol 2018, 175:2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stokes L, Layhadi JA, Bibic L, Dhuna K, Fountain SJ: P2X4 Receptor Function in the Nervous System and Current Breakthroughs in Pharmacology. Front Pharmacol 2017, 8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Desai BN, Leitinger N: Purinergic and calcium signaling in macrophage function and plasticity. Front Immunol 2014, 5:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferrari D, Gorini S, Callegari G, la Sala A: Shaping immune responses through the activation of dendritic cells' P2 receptors. Purinergic Signal 2007, 3:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, Norgauer J, Chiozzi P, Di Virgilio F, Luttmann W: P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett 2000, 486:217–224. [DOI] [PubMed] [Google Scholar]
- 99.Kobayashi T, Soma T, Noguchi T, Nakagome K, Nakamoto H, Kita H, Nagata M: ATP drives eosinophil effector responses through P2 purinergic receptors. Allergol Int 2015, 64 Suppl:S30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bulanova E, Bulfone-Paus S: P2 receptor-mediated signaling in mast cell biology. Purinergic Signal 2010, 6:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao ZG, Jacobson KA: Purinergic Signaling in Mast Cell Degranulation and Asthma. Front Pharmacol 2017, 8:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang L, Jacobsen SE, Bengtsson A, Erlinge D: P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 2003, 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gorini S, Callegari G, Romagnoli G, Mammi C, Mavilio D, Rosano G, Fini M, Di Virgilio F, Gulinelli S, Falzoni S, et al. : ATP secreted by endothelial cells blocks CX(3)CL 1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y(1)(1) receptor activation. Blood 2010, 116:4492–4500. [DOI] [PubMed] [Google Scholar]