Abstract
Ultraviolet (UV) light is a known trigger of skin and possibly systemic inflammation in systemic lupus erythematosus (SLE) patients. Although type I interferons (IFN) are upregulated in SLE skin after UV exposure, the mechanisms to explain increased UVB-induced inflammation remain unclear. This paper compares the role of type I IFNs in regulating immune cell activation between wild-type and lupus-prone mice following UVB exposure. 10-week old female lupus-prone (NZM2328), wild-type (BALB/c) and iNZM mice (lack a functional type I IFN receptor on NZM2328 background) were treated on their dorsal skin with 100mJ/cm2 of UVB for 5 consecutive days. Following UVB treatment, draining lymph node cell populations were characterized via flow cytometry and suppression assays; treated skin was examined for changes in expression of type I IFN genes. Only NZM2328 mice showed an increase in T cell numbers and activation 2 weeks post UVB exposure. This was preceded by a significant increase in UVB-induced type I IFN expression in NZM2328 mice compared to BALB/c mice. Following UVB exposure, both BALB/c and iNZM mice demonstrated an increase in functional T regulatory (TReg) cells; however, this was not seen in NZM2328 mice. These data suggest a skewed UVB-mediated T cell response in lupus-prone mice where activation of T cells is enhanced secondary to a type I IFN-dependent suppression of TReg cells. Thus, we propose type I IFNs are important for UVB-induced inflammation in lupus-prone mice and may be an effective target for prevention of UVB-mediated flares.
Keywords: Systemic lupus, ultraviolet light, TREGs, interferon, T cells
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease in which patients experience devastating organ damage mediated by immune cells and inflammatory cytokine production [1, 2]. Minimal sun exposure, especially ultraviolet (UV) B wavelengths, is a prominent factor that drives cutaneous inflammation in lupus patients [3–7]. However, the mechanisms through which SLE skin is predisposed to persistent inflammation following UVB exposure are unknown.
Much of our knowledge regarding the effects of UVB have been uncovered through studies of healthy skin. Classically, following UVB-mediated damage, resolution of inflammation is promoted via several mechanisms. Langerhans cells phagocytose apoptotic cells and promote dampening of inflammatory responses[8]. In addition, CD11b+Langerin− dendritic cells promote expansion of T regulatory (TReg) cells[9, 10]. In healthy skin, there is also activation of other suppressive populations such as: neutrophils secreting IL-10 and monocytes secreting interferon (IFN) alpha, resulting in an overall suppressive phenotype[11, 12].
UVB exposure may have differential effects in SLE patients compared to healthy controls. In SLE patients, reduced phagocytosis of apoptotic cells results in prolonged autoantigen exposure[13–15]. Reduction of Langerhans cells in SLE skin promotes UVB-mediated inflammation via suppression of epidermal growth factor receptor-mediated signaling[16]. In addition, UVB exposure in SLE patients and lupus-prone mice leads to infiltration of neutrophils, macrophages, dendritic cells, T cells, and mast cells into the skin[8, 17–21]. Intriguingly, despite its immunosuppressive role in healthy skin[8, 11, 12], SLE patients and lupus-prone mice demonstrate a rise in type I IFN signaling following UVB exposure; thus suggesting a potential pro-inflammatory role for type I IFNs in lupus skin[12, 21]. For example, type I IFNs demonstrate a proinflammatory role in keratinocytes and promote cell death following UVB[22].
Because of the unclear role of type I IFNs in UVB-mediated inflammation, this paper seeks to understand the differences in immune cell activation following UVB exposure of lupus-prone and wild-type mice and to elucidate the role of type I IFNs in this process. We found that lupus-prone mice demonstrate increased expansion and prolonged activation of T cells in the draining lymph nodes of UVB exposed skin that is mediated by type I IFN-dependent repression of TReg cells. Thus, in contrast to wild-type mice[12], type I IFNs exhibit a proinflammatory role in lupus-prone mice and are required for skewed immune activation following UVB exposure.
2. Methods and Materials
2.1. Mice
8–10-week-old female wild-type BALB/c mice obtained from Jackson Laboratory were utilized for this study. Wild-type mice were compared to 10-week old female New Zealand Mixed (NZM) 2328 lupus-prone mice and iNZM (knockout of the α chain of the type I IFN receptor) mice. Both NZM 2328 and iNZM mice were a gift from Dr. Chaim Jacob, University of Southern California[23]. All mice were housed in specific pathogen-free facilities at University of Michigan and treated in accordance to our University of Michigan IACUC-approved protocol.
2.2. UVB irradiation
The hair on the backs of the mice was removed via depilation with Veet and mice were placed in a restrainer with facial protection. The mice were treated with 100mJ/cm2 UVB using the UV-2 ultraviolet irradiation system (Tyler Research) for 5 consecutive days and harvested at the times indicated in the experiments. UVB light was provided by cascade-phosphor ultraviolet generators that emit 310nm of UVB radiation.
2.3. Flow Cytometry
10-week old female BALB/c, NZM, and iNZM mice were treated with/without UVB for 5 consecutive days followed by harvesting of 2 inguinal draining lymph nodes (dLN) passed through a 70μm filter to generate a single cell suspension. Cells were then incubated in flow block (1% bovine serum albumin and 1% horse serum in PBS) for 30 minutes, followed by staining with CD8 clone: 53–6.7 (BD Bioscience, San Jose, CA), CD3 clone: 17A2, CD4 clone: GK15, CD69 clone: H12F3, CD25 clone: 3C7, and B220 clone: RA3–6B2(Biolegend, San Diego, CA), Ig (Southern Biotech, Birmingham, AL) for 45 minutes. Following the extracellular staining, cells were intracellularly stained for Foxp3 clone: FJK16s (Biolegend) utilizing the Foxp3 / Transcription Factor Staining Buffer Set from eBiosciences, San Diego, California. The flow cytometry data was collected via a BD LSR II flow cytometer and analyzed using FlowJo VX.0.7 (Tree Star). For analysis, the live cells were gated for: CD4+ T cells: CD3+, CD4+, CD8−; CD8+ T cells: CD3+, CD8+, CD4−; T cell activation: CD69+; B cells: B220+; TReg cells: CD4+, CD3+, CD25+, Foxp3+; Ab secreting cells: CD4−CD8−IgH+LhiB220int-low.
2.4. T regulatory cell suppression assay
10-week old female BALB/c, NZM, and iNZM mice were treated with/without UVB for 5 consecutive days. The dLNs were processed into a single cell suspension, as described in flow cytometry. CD4+CD25+ TReg cells and CD4+ T cells were isolated via CD4+ CD25+ Regulatory T Cell Isolation Kit and CD4+ T Cell Isolation Kit, respectively (Miltenyi Biotec, Bergish Gladbach, Germany). TReg cells were labeled with CFSE (ThermoFisher, Eugene, Oregon) and CD4+ cells were labeled with cell proliferation dye 670 (ThermoFisher). Following labeling, the cells were co-incubated at a ratio of 0:1,1:1,1:2,1:4 (TReg: TEffector) with/without anti-CD3/CD28 beads (ThermoFisher) for 72 hrs in a 96-well plate. Cells were then stained, as described in the flow cytometry section, for CD3 clone: 17A2 (BioLegend) for 45 minutes, followed by staining with live/dead cell dye (ThermoFisher) for 30 minutes. After staining, cells were resuspended in PBS and data collected on a BD LSR II flow cytometer and analyzed using FlowJo. For analysis, samples were gated on CFSE negative cells to exclude TRegs, followed by live cell gating, then CD3+ cells and lastly proliferation dye 670 to examine percent proliferation of CD4+ T cells (Supplementary figure 1). Percentage proliferation was calculated using the formula: (100 × 1:1,1:2, or 1:4, samples) /0:1 sample.
2.5. RTqPCR
Biopsies from the backs of mice treated with/without 100mJ/cm2 UVB were taken 3 hrs after the 5th UVB treatment. Skin biopsies were snap frozen in liquid nitrogen and stored at −80°C until further use. Skin was pulverized with the use of a mortar and pestle and placed in TRIzol (life technologies). RNA was isolated using the Direct-zol mini RNA prep kit (Zymo). 100ng of RNA was reverse-transcribed into cDNA, followed by quantitative real-time PCR analysis by the DNA sequencing core at University of Michigan on an ABI PRISM 7900HT (Applied Biosystems). Gene expression was calculated by fold change relative to no UV (control) group. The primers used were as follows (all listed 5′→3′): Myxovirus (influenza virus) resistance 1 (mx1) GATCCGACTTCACTTCCAGATGG (forward), CATCTCAGTGGTAGTCAACCC (reverse); β- Actin TGGAATCCTGTGGCATCCTGAAAC (forward), TAAAACGCAGCTCAGTAACAGTCCG (reverse); Interferon alpha (ifna) ATGGCTAGRCTCTGTGCTTTCCT (forward), AGGGCTCTCCAGAYTTCTGCTCTG(reverse); Interferon beta (ifnb) AGCTCCAAGAAAGGACGAACAT (forward), ATTCTTGCTTCGGCAGTTAC(reverse); Interferon gamma (ifng) AGCGGCTGACTGAACTCAGATTGTA (forward), GTCACAGTTTTCAGCTGTATAGGG (reverse); Interferon kappa (ifnk) ACTCCAAAGTTTTTATGGCTGGT (forward), TACGATAGGAGACGGCGTTTA (reverse); Interferon regulatory factor (irf7) TGCTGTTTGGAGACTGGCTAT(forward), TCCAAGCTCCCGGCTAAGT(reverse).
2.6. Analysis of anti-IgG and dsDNA IgG antibody serum levels
Serum was collected 2 weeks post UVB treatment. Anti-IgG and dsDNA IgG antibody levels were analyzed via ELISA kits (Alpha Diagnostic, San Antonio, TX, and Innovative Research, Novi, MI).
2.7. Statistics
All data was graphed and statistics performed using GraphPad Prism v.6.0. For data comparing multiple groups, ANOVA testing was used. Comparison between two groups was completed via a two-tailed Student’s t-test for normally distributed data. When there was significant difference in variances, Welch’s correction was applied. Comparisons were considered significant with a p value of <0.05.
3. Results
3.1. UVB exposure increases the number of activated T cells in the dLN of lupus-prone mice
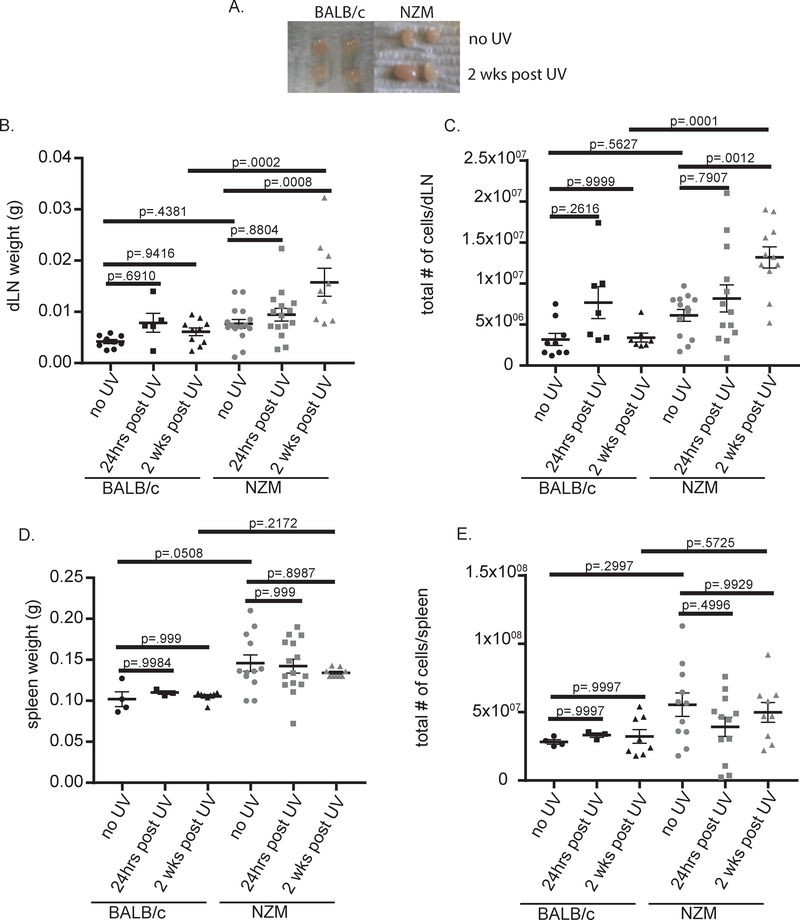
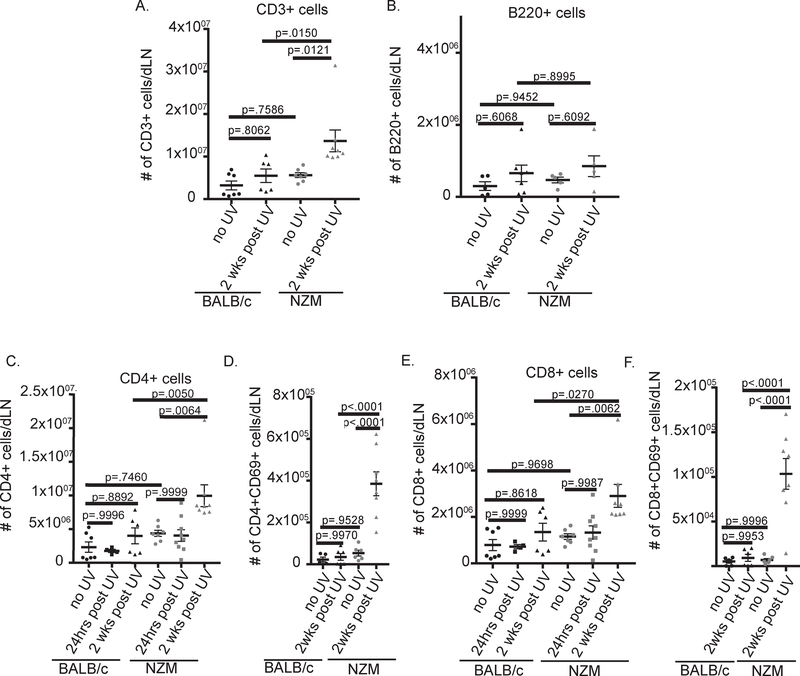
Ten-week-old NZM2328 (lupus-prone) and BALB/c (wild-type) mice were treated with 100mJ/cm2 UVB on their dorsum for 5 days, followed by harvest of the draining lymph nodes (dLN) 24 hrs or 2 weeks after the last UVB treatment. Intriguingly, the size of the draining lymph nodes (dLN) was increased 2 weeks following treatment in NZM2328 compared to BALB/c mice (fig.1A–C). This response was not systemic, indicated by a lack of increase in spleen size following UVB exposure in either strain (fig.1D and E). In order to determine the cellular contribution to the expanded LNs, we next examined changes in adaptive immune cell populations in the dLN and observed a significant increase in T cells 2 weeks post treatment in NZM2328 mice (fig.2 A). This UVB dosage did not significantly increase total B cells (Fig. 2B) or antibody secreting cells (Supplementary Figure 2A). In addition, no significant increase in total IgG or anti-dsDNA antibodies were detected in the serum 2 weeks after UVB exposure (Supplementary Figure 2B, C). Further exploration of changes in the subsets of T cells revealed increases in both CD4+ and CD8+ T cells (fig.2 C and E). In addition to expansion, we also observed increased activation of both CD4+ and CD8+ T cell subsets in NZM, but not WT mice, as indicated by increased CD69+ expression (fig.2D and F). These results suggest that UVB exposure induces expansion and activation of T cells in the dLN of lupus-prone but not wild-type mice.
Figure 1:
UVB- induces an increase in dLN, but not splenic size, 2 weeks post treatment in lupus-prone compared to wild-type mice. Ten-week-old NZM2328 and BALB/c mice treated with 100mJ/cm2 on their dorsum for 5 days were analyzed 24hrs and 2 weeks post treatment. (A) Representative dLN 2 weeks post treatment. (B) dLN weight. (C) Total number of dLN cells. (D) Spleen weight. (E) Total number of splenocytes. (B-E) Each dot represents an individual mouse. ANOVA testing was used to determine significance.
Figure 2:
Lupus-prone mice have increased T cell activation in dLN 2 weeks post UVB treatment compared to wild-type mice. Changes in immune cell populations in the dLN were evaluated by flow cytometry 24hrs and 2 weeks post UVB treatment. (A) Total T cells per dLN: CD3+. (B) Total B cells: B220+. (C) CD4+ T cells: CD3+CD4+CD8−. (D) CD4+ T cell activation: CD69+. (E) CD8+ T cells: CD3+CD4−CD8+. (F) CD8+ T cell activation: CD69+. (A-F) Each dot represents an individual mouse. ANOVA testing was used to determine significance.
3.2. UVB exposure fails to induce functional TReg cells in the dLN of lupus-prone mice
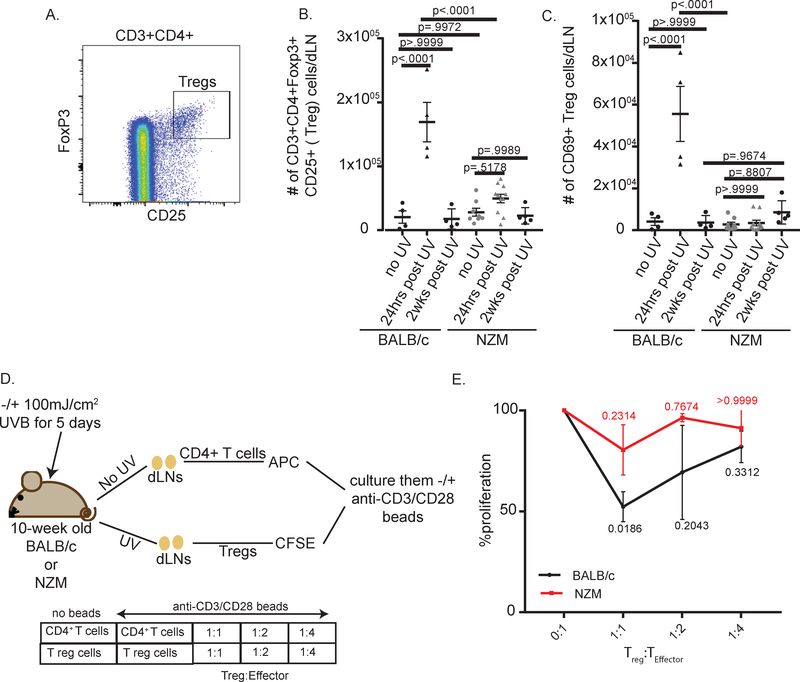
UV exposure is known to induce TReg activation [9, 24], which is critical for preventing aberrant T cell activation, so we next examined changes in TReg populations following UVB exposure. Interestingly, BALB/c but not NZM mice demonstrated a significant increase in TReg cell numbers 24hrs post UVB treatment (fig.3A, B). Strikingly, a significant increase in activated, CD69+ TReg cells was also noted in BALB/c mice following UVB exposure. In contrast, no increase in CD69 expression was noted on Foxp3+ cells in NZM2328 mice, suggesting that TReg cells were activated only in wild-type mice after UVB (fig.3C). In order to confirm aberrant TReg suppressive function in NZM mice, we performed a TReg suppression assay (fig 3D, E) using TRegs isolated from UVB-exposed mice. While TRegs from BALB/c mice were able to suppress CD4+ T cell proliferation, TRegs from NZM mice did not significantly inhibit proliferation at any ratio. These data indicate that TReg cells from dLN of UVB-treated lupus-prone mice have reduced functionality thus setting the stage for skewing of T cell activation following UVB exposure.
Figure 3:
UVB exposure fails to induce T regulatory cell activation in lupus-prone mice. (A-D) T regulatory cell changes were examined via flow cytometry in the dLN 24hrs and 2 weeks post UVB treatment. Each dot represents an individual mouse. (A) Changes in T regulatory cells: CD3+CD4+CD25+FoxP3+. (B) T regulatory cell activation: CD69+. (C) Diagram of protocol for T regulatory suppression assay. (D) Percent proliferation of CD4+ cells in TReg suppression assay. n=three independent experiments in duplicate. ANOVA testing was used to determine significance.
3.3. Type I IFN signaling is increased in NZM skin and is required for activation of T cells following UVB exposure
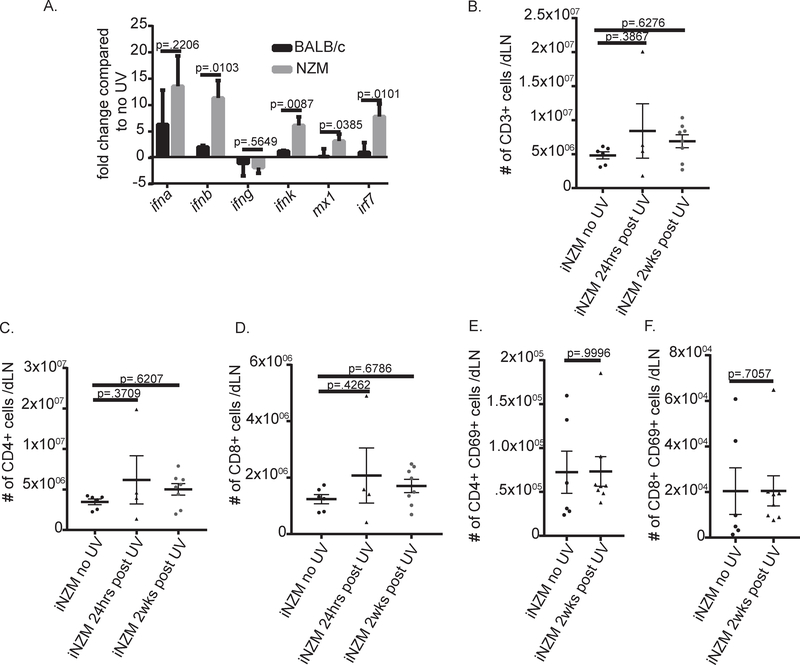
Anti-inflammatory effects of type I IFNs have been described in wild-type mice post UVB exposure[12], yet SLE skin has elevated type I IFNs after UVB [12, 21]. Thus, we next characterized induction of IFNs after UVB exposure in NZM2328 vs. BALB/c mice. Examination of transcriptional changes in the skin of mice 3 hrs after UVB exposure resulted in an upregulation of type I IFNs and their downstream signaling genes in the skin of both wild-type and lupus-prone mice. Interestingly, NZM2328 mice had significantly higher expression of ifnb and ifnk as well as downstream IFN-regulated genes, indicating an elevated type I IFN response in lupus-prone mice vs. WT following UVB exposure (fig.4A).
Figure 4:
UVB induced T cell activation in lupus-prone mice is type I IFN dependent. (A) RNA isolated from the skin of NZM or BALB/c mice 3hrs post UVB treatment. Real-time PCR was performed using primers of the genes listed. Graph displays the fold change for each gene compared with the respective no UV group (n = 4 NZM no UV; n = 4 NZM UV; n= 4 BALB/c no UV; n=4 BALB/c UV). A two-tailed student’s t-test was used for normally distributed data and for comparisons with significant difference in variances, Welch’s correction was applied. (B-F) Ten- week-old iNZM mice treated with 100mJ/cm2 UVB on their dorsum for 5 days were analyzed via flow cytometry 24hrs or 2 weeks post treatment. Each dot represents an individual mouse. (B) Total T cells: CD3+. (C) CD4+ T cells: CD3+CD4+CD8−. (D) CD8+ T cells: CD3+CD4−CD8+. (E) CD4+ T cell activation: CD69+. (F) CD8+ T cell activation: CD69+. ANOVA testing was used to determine significance.
In order to understand the role of type I IFNs in UVB-induced T cell activation in lupus-prone mice we studied iNZM mice, which lack a functional type I IFN receptor. Intriguingly, UVB treatment of 10-week old iNZM mice failed to induce an increase in T cell numbers 2 weeks post UVB (fig.4B). No difference in T cell subset numbers or activation (via CD69+) were identified in iNZM mice (fig. 4C–F), similar to BALB/c mice (fig.2 C and E). These results suggest that in the absence of type I IFN signaling, activation of dLN T cells is prevented.
3.4. Type I IFNs regulate TReg functionality in lupus-prone mice following UVB exposure
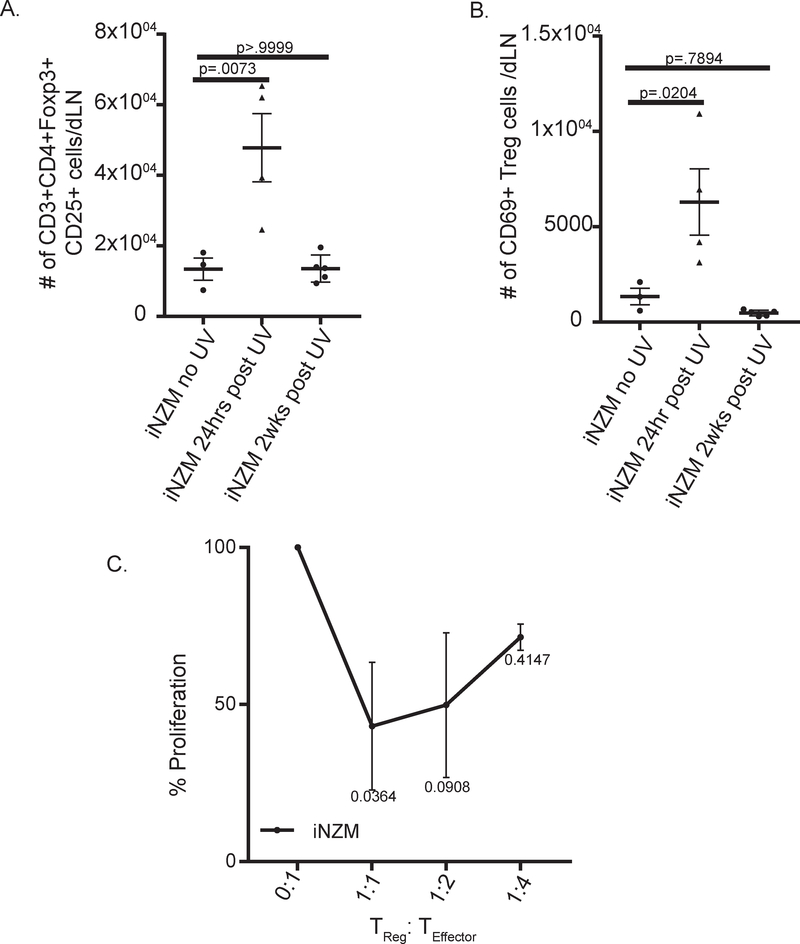
Past work in other disease models has shown that type I IFNs can manipulate TReg cells directly or indirectly[25, 26], so we next examined the effect of type I IFN signaling on TReg cells following UVB treatment. Consistent with a role for type I IFNs in suppression of T Regs, we observed a significant increase in the number and activation of TReg cells in iNZM mice 24hrs post UVB exposure (figs.5A, B). Further characterization of TReg cells in iNZM mice showed strong suppressive function, and even suggest enhancement of suppressive function in the absence of type I IFN signaling (fig.5C). Taken together, these data indicate that lupus-prone mice display an enhanced type I IFN response to UVB that inhibits TReg function and promotes T cell activation and expansion.
Figure 5:
Type I IFNs suppress TReg activation and functionality following UVB exposure. (A and B) Ten-week-old iNZM mice were treated with 100mJ/cm2 on their dorsum for 5 days and dLNs were analyzed via flow cytometry 24hrs or 2 weeks post treatment. Each dot represents an individual mouse. (A) Changes in T regulatory cells: CD3+CD4+CD25+FoxP3+. (B) T regulatory cell activation: CD69+. (C) Percent proliferation of CD4+ cells in TReg suppression assay. n=three independent experiments in duplicate. ANOVA testing was used to determine significance.
4. Discussion
SLE-associated skin inflammation is characterized by increased apoptosis, increased type I IFN expression, and the presence of inflammatory infiltrates, including T cells. However, the mechanisms to explain the propensity for UVB-induced inflammation remain unclear. In this paper, we examined the mechanisms involved in UVB-induced immune cell activation in wild-type vs. lupus-prone mice. UVB exposure results in increased T cell activation and decreased TReg induction in a type I IFN-dependent manner in lupus-prone vs. WT mice. Intriguingly, we also noted enhanced cutaneous type I IFN responses to UVB in lupus-prone mice, consistent with previous observations in human SLE skin[27–29].
To our knowledge, we demonstrate for the first time a differential activation of Tregs in wild-type vs. lupus-prone mice following UVB treatment. In healthy skin, migratory UVB-induced Tregs contribute, along with resident T cells, to skewing of the immune cell response towards a suppressive phenotype and thus possibly limiting DNA damage[9, 30]. Our data also support a role for Tregs in inhibition of T cell activation in the dLN following UVB exposure. Thus, in SLE, where Tregs are suppressed secondary to increased IFNs, activated T cells may contribute to apoptosis induction through increased expression of FasL[20, 31, 32]. Whether activated T cells in the dLN are able to migrate to the skin and contribute to inflammation following UVB exposure remains to be determined.
Some types of photosensitive cutaneous lupus lesions (especially discoid lupus) are associated with T cell infiltrates[33] and may present without significant autoantibody positivity[34]. Similarly, treatment of our lupus-prone mice with 100mJ/cm2 UVB was able to significantly activate T cells, but we did not identify an induction of B cell activation or antibody production. Thus, our model may reflect scenarios where T cells are the dominant contributors following UVB stimulation. Alternatively, our results may indicate a need for higher doses of UV to induce B cell activation, as UVB-driven autoantibody production in BXSB mice was induced at higher daily dosages of UVB (500 mJ/cm2)[35]. In addition, because we studied UVB treatment in pre-autoimmune lupus-prone mice, the autoreactive B cell populations may not have developed sufficiently to be rapidly induced following UVB stimulation. While production of auto-antibodies can be driven via B cells in the draining lymph nodes[36], the type of stimulation (especially utilizing TLR7 activation) may be relevant as well.
Similar to others, we found an upregulation of type I IFNs in the skin of wild-type and lupus-prone mice following UVB exposure[12, 37]. The expression of type I IFNs, especially ifnb and ifnk are enhanced in lupus-prone compared to wild-type mice following UVB treatment. Sources of the IFN production may include infiltrating inflammatory monocytes[12]; however, in lupus skin, both infiltrating plasmacytoid dendritic cells and keratinocytes exhibit upregulation of type I IFNs following UVB as well[18, 22], possibly secondary to UVB effects on immunostimulatory nucleic acids[38, 39]. Keratinocyte production of IFNκ has been shown to prime lupus skin for a more inflammatory response through promotion of other proinflammatory cytokines, such as IL- 6[28]. It has also been demonstrated that chronic exposure to type I IFNs results in enhanced immune cell activation, suggesting an inflammatory role for type IFNs in the skin and for priming of monocytes and dendritic cells migrating to dLNs[22, 40]. Intriguingly, we also demonstrate that type I IFNs have a proinflammatory role in T cell expansion in lupus-prone mice through the repression of TReg activation in the dLN. This is contrary to their protective role demonstrated in wild-type mice [12]. This differential effect could be due to T cells in lupus patients having decreased DNA methylation allowing for sensitization to type I IFN effects[41]. Alternatively, in lupus-prone mice or SLE patients, migratory dendritic cell populations may bring enhanced IFN production into the dLN and provide focal inhibition of TRegs through type I IFNs. This is the focus of ongoing studies.
5. Conclusions
In conclusion, we demonstrate UVB-induced differential immune cell activation in lupus-prone vs. wild-type mice. Lupus-prone mice exhibit prolonged T activation following UVB exposure compared to wild-type mice. This skewing is driven by upregulation of Type I IFNs in lupus-prone mice which are required for repression of TReg cells following UVB exposure. Future studies should address the source of type I IFNs following UVB in the skin and dLN. Overall, our work suggests that targeting of type I IFNs may be an important strategy to prevent skin inflammation and systemic immune activation following UV exposure in SLE patients.
Supplementary Material
Supplementary Figure 1: Gating strategy for CD4+ T cell proliferation in TReg suppression assay. Samples were gated on CFSE negative cells to exclude TReg cells, followed by live cell gating, then CD3+ cells and lastly proliferation dye 670 to examine percent proliferation of CD4+ T cells (Supplementary figure 2). Percent proliferation was calculated using the formula: (100 × 1:1,1:2, or 1:4, samples) /0:1 sample.
Supplementary Figure 2: UVB fails to induce ab secreting cells and antibody production. (A) Ten-week-old iNZM mice treated with 100mJ/cm2 on their dorsum for 5 days and dLNs were analyzed via flow cytometry 2 weeks post treatment. Each dot represents an individual mouse. Changes in ab secreting cells: CD4−CD8−IgH+LhiB220int-low (B) IgG abs in the serum (C) dsDNA IgG abs in the serum. ANOVA testing was used to determine significance.
Highlights.
Interferon responses to UVB are greater in lupus prone mice.
UVB induces TREG production to restrain T cell activation in wild type mice.
UVB induces T cell activation in lupus-prone mice secondary inhibition of TREGs.
Type I IFN is required for TREG inhibition in lupus-prone mice.
Acknowledgments
Funding Sources
This work was supported by the Stone Family Foundation, and the National Institutes of Health via the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) under Award Numbers K08AR063668 and R01AR071384 (to JMK) and the National Institute of Allergy and Infectious Diseases Research Training in Experimental Immunology Training Grant T32AI007413.
Competing Interests Statement
J.E.G. serves as an Advisory Board member for Novartis and MiRagen, and has received research support from AbbVie, SunPharma, and Genentech. J.M.K serves on advisory boards for AstraZeneca and Eli Lilly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- [1].Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R et al. Systemic lupus erythematosus. Nature reviews Disease primers, 2016;2:16039. [DOI] [PubMed] [Google Scholar]
- [2].Bagavant H, Fu SM Pathogenesis of kidney disease in systemic lupus erythematosus. Current opinion in rheumatology, 2009;21:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sanders CJ, Van Weelden H, Kazzaz GA, Sigurdsson V, Toonstra J, Bruijnzeel-Koomen CA Photosensitivity in patients with lupus erythematosus: a clinical and photobiological study of 100 patients using a prolonged phototest protocol. The British journal of dermatology, 2003;149:131–7. [DOI] [PubMed] [Google Scholar]
- [4].Furukawa F Photosensitivity in cutaneous lupus erythematosus: lessons from mice and men. Journal of dermatological science, 2003;33:81–9. [DOI] [PubMed] [Google Scholar]
- [5].Foering K, Goreshi R, Klein R, Okawa J, Rose M, Cucchiara A et al. Prevalence of self-report photosensitivity in cutaneous lupus erythematosus. Journal of the American Academy of Dermatology, 2012;66:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wysenbeek AJ, Block DA, Fries JF Prevalence and expression of photosensitivity in systemic lupus erythematosus. Annals of the rheumatic diseases, 1989;48:461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kuhn A, Sonntag M, Richter-Hintz D, Oslislo C, Megahed M, Ruzicka T et al. Phototesting in lupus erythematosus tumidus--review of 60 patients. Photochem Photobiol, 2001;73:532–6. [DOI] [PubMed] [Google Scholar]
- [8].Hatakeyama M, Fukunaga A, Washio K, Taguchi K, Oda Y, Ogura K et al. Anti-Inflammatory Role of Langerhans Cells and Apoptotic Keratinocytes in Ultraviolet-B-Induced Cutaneous Inflammation. Journal of immunology, 2017;199:2937–47. [DOI] [PubMed] [Google Scholar]
- [9].Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y et al. Ultraviolet radiation- induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. Journal of immunology, 2004;172:1036–43. [DOI] [PubMed] [Google Scholar]
- [10].Yamazaki S, Odanaka M, Nishioka A, Kasuya S, Shime H, Hemmi H et al. Ultraviolet B-Induced Maturation of CD11b-Type Langerin(−) Dendritic Cells Controls the Expansion of Foxp3(+) Regulatory T Cells in the Skin. Journal of immunology, 2018;200:119–29. [DOI] [PubMed] [Google Scholar]
- [11].Aubin F Mechanisms involved in ultraviolet light-induced immunosuppression. Eur J Dermatol, 2003;13:515–23. [PubMed] [Google Scholar]
- [12].Sontheimer C, Liggitt D, Elkon KB Ultraviolet B Irradiation Causes Stimulator of Interferon Genes-Dependent Production of Protective Type I Interferon in Mouse Skin by Recruited Inflammatory Monocytes. Arthritis & rheumatology (Hoboken, NJ), 2017;69:826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis and rheumatism, 1998;41:1241–50. [DOI] [PubMed] [Google Scholar]
- [14].Ren Y, Tang J, Mok MY, Chan AW, Wu A, Lau CS Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis and rheumatism, 2003;48:2888–97. [DOI] [PubMed] [Google Scholar]
- [15].Schaper F, de Leeuw K, Horst G, Bootsma H, Limburg PC, Heeringa P et al. High mobility group box 1 skews macrophage polarization and negatively influences phagocytosis of apoptotic cells. Rheumatology (Oxford, England), 2016;55:2260–70. [DOI] [PubMed] [Google Scholar]
- [16].Shipman WD, Chyou S, Ramanathan A, Izmirly PM, Sharma S, Pannellini T et al. A protective Langerhans cell-keratinocyte axis that is dysfunctional in photosensitivity. Sci Transl Med, 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. Journal of immunology, 2011;187:538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. The American journal of pathology, 2001;159:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mawrin C, Brunn A, Rocken C, Schroder JM Peripheral neuropathy in systemic lupus erythematosus: pathomorphological features and distribution pattern of matrix metalloproteinases. Acta neuropathologica, 2003;105:365–72. [DOI] [PubMed] [Google Scholar]
- [20].Kind P, Lehmann P, Plewig G Phototesting in lupus erythematosus. The Journal of investigative dermatology, 1993;100:53S–7S. [DOI] [PubMed] [Google Scholar]
- [21].Yin Q, Xu X, Lin Y, Lv J, Zhao L, He R Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: a possible role for chemerin. Autoimmunity, 2014;47:185–92. [DOI] [PubMed] [Google Scholar]
- [22].Sarkar MK, Hile GA, Tsoi LC, Xing X, Liu J, Liang Y et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Ann Rheum Dis, 2018;77:1653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Agrawal H, Jacob N, Carreras E, Bajana S, Putterman C, Turner S et al. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. Journal of immunology, 2009;183:6021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bruhs A, Schwarz T Ultraviolet Radiation-Induced Immunosuppression: Induction of Regulatory T Cells. Methods in molecular biology (Clifton, NJ, 2017;1559:63–73. [DOI] [PubMed] [Google Scholar]
- [25].Glick AB, Wodzinski A, Fu P, Levine AD, Wald DN Impairment of regulatory T-cell function in autoimmune thyroid disease. Thyroid : official journal of the American Thyroid Association, 2013;23:871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mao C, Wang S, Xiao Y, Xu J, Jiang Q, Jin M et al. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves’ disease. Journal of immunology, 2011;186:4734–43. [DOI] [PubMed] [Google Scholar]
- [27].Sarkar M, Hile G, Tsoi L, Xing X, Liu J, Liang Y et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal derived interferon kappa. Annals of Rheumatic Diseases, 2018;77:1653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stannard JN, Reed TJ, Myers E, Lowe L, Sarkar MK, Xing X et al. Lupus Skin Is Primed for IL-6 Inflammatory Responses through a Keratinocyte-Mediated Autocrine Type I Interferon Loop. The Journal of investigative dermatology, 2017;137:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zahn S, Graef M, Patsinakidis N, Landmann A, Surber C, Wenzel J et al. Ultraviolet light protection by a sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Experimental Dermatology, 2014;23:516–8. [DOI] [PubMed] [Google Scholar]
- [30].MacLeod AS, Rudolph R, Corriden R, Ye I, Garijo O, Havran WL Skin-resident T cells sense ultraviolet radiation-induced injury and contribute to DNA repair. J Immunol, 2014;192:5695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sole C, Gimenez-Barcons M, Ferrer B, Ordi-Ros J, Cortes-Hernandez J Microarray study reveals a transforming growth factor-beta-dependent mechanism of fibrosis in discoid lupus erythematosus. The British journal of dermatology, 2016;175:302–13. [DOI] [PubMed] [Google Scholar]
- [32].Mande P, Zirak B, Ko WC, Taravati K, Bride KL, Brodeur TY et al. Fas ligand promotes an inducible TLR-dependent model of cutaneous lupus-like inflammation. J Clin Invest, 2018;128:2966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sole C, Gimenez-Barcons M, Ferrer B, Ordi-Ros J, Cortes-Hernandez J Microarray study reveals a TGFbeta-Dependent mechanism of fibrosis in discoid lupus erythematosus. Br J Dermatol, 2016. [DOI] [PubMed] [Google Scholar]
- [34].Callen JP, Fowler JF, Kulick KB Serologic and clinical features of patients with discoid lupus erythematosus: relationship of antibodies to single-stranded deoxyribonucleic acid and of other antinuclear antibody subsets to clinical manifestations. J Am Acad Dermatol, 1985;13:748–55. [DOI] [PubMed] [Google Scholar]
- [35].Ansel JC, Mountz J, Steinberg AD, DeFabo E, Green I Effects of UV radiation on autoimmune strains of mice: increased mortality and accelerated autoimmunity in BXSB male mice. The Journal of investigative dermatology, 1985;85:181–6. [DOI] [PubMed] [Google Scholar]
- [36].Wolf SJ, Theros J, Reed TJ, Liu J, Grigorova IL, Martinez-Colon G et al. TLR7-Mediated Lupus Nephritis Is Independent of Type I IFN Signaling. J Immunol, 2018;201:393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xin H, D’Souza S, Jorgensen TN, Vaughan AT, Lengyel P, Kotzin BL et al. Increased expression of Ifi202, an IFN-activatable gene, in B6.Nba2 lupus susceptible mice inhibits p53- mediated apoptosis. Journal of immunology, 2006;176:5863–70. [DOI] [PubMed] [Google Scholar]
- [38].Scholtissek B, Zahn S, Maier J, Klaeschen S, Braegelmann C, Hoelzel M et al. Immunostimulatory Endogenous Nucleic Acids Drive the Lesional Inflammation in Cutaneous Lupus Erythematosus. Journal of Investigative Dermatology, 2017;137:1484–92. [DOI] [PubMed] [Google Scholar]
- [39].Cadet J, Wagner JR DNA Base Damage by Reactive Oxygen Species, Oxidizing Agents, and UV Radiation. Cold Spring Harbor Perspectives in Biology, 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu J, Berthier CC, Kahlenberg JM Enhanced Inflammasome Activity in Systemic Lupus Erythematosus Is Mediated via Type I Interferon-Induced Up-Regulation of Interferon Regulatory Factor 1. Arthritis & rheumatology, 2017;69:1840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jeffries MA, Dozmorov M, Tang Y, Merrill JT, Wren JD, Sawalha AH Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics, 2011;6:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Gating strategy for CD4+ T cell proliferation in TReg suppression assay. Samples were gated on CFSE negative cells to exclude TReg cells, followed by live cell gating, then CD3+ cells and lastly proliferation dye 670 to examine percent proliferation of CD4+ T cells (Supplementary figure 2). Percent proliferation was calculated using the formula: (100 × 1:1,1:2, or 1:4, samples) /0:1 sample.
Supplementary Figure 2: UVB fails to induce ab secreting cells and antibody production. (A) Ten-week-old iNZM mice treated with 100mJ/cm2 on their dorsum for 5 days and dLNs were analyzed via flow cytometry 2 weeks post treatment. Each dot represents an individual mouse. Changes in ab secreting cells: CD4−CD8−IgH+LhiB220int-low (B) IgG abs in the serum (C) dsDNA IgG abs in the serum. ANOVA testing was used to determine significance.