Abstract
Purpose:
Zika virus (ZIKV) has emerged as an important human pathogen causing ocular complications. There have been reports of the shedding of ZIKV in human as well as animal tears. In this study, we investigated the infectivity of ZIKV in corneal epithelial cells and their antiviral immune response.
Methods:
Primary human corneal epithelial cells (Pr. HCECs) and an immortalized cell line (HUCL) were infected with two different strains of ZIKV (PRVABC59 & BeH823339) or dengue virus (DENV, serotypes 1–4). Viral infectivity was assessed by immunostaining of viral antigen and plaque assay. qRT-PCR and immunoblot analyses were used to assess the expression of innate inflammatory and antiviral genes. Supplementation of recombinant ISG15 (rISG15) and gene silencing approaches were used to elucidate the role of ISG15 in corneal antiviral defense.
Results:
Pr. HCECs, but not the HUCL cells, were permissive to both ZIKV strains and specifically to DENV3 infection. ZIKV induced the expression of viral recognition receptors (TLR3, RIG-I, & MDA5), and genes involved in inflammatory (CXCL10& CCL5) and antiviral (IFNs, MX1, OAS2, ISG15) responses in Pr. HCECs. Furthermore, ZIKV infection caused Pr. HCECs cell death, as evidenced by TUNEL staining. Silencing of ISG15 increased ZIKV infectivity while supplementation with rISG15 reduced ZIKV infection by direct inactivation of ZIKV and inhibiting its entry.
Conclusions:
Our study demonstrates for the first time, that ZIKV virus can readily infect and replicate in Pr. HCECs. Therefore, ZIKV may persist in the cornea and pose the potential risk of transmission via corneal transplantation.
Keywords: antiviral, cornea, dengue virus, innate immune response, ISG15, Zika virus
1. INTRODUCTION
Zika virus (ZIKV) is a mosquito borne flavivirus which has recently emerged as a global threat and poses significant challenges in health care [1]. Apart from its primary mode of transmission via mosquito bites, ZIKV can be transmitted via sexual activities, passed from mother to fetus during pregnancy, and via blood transfusions in humans [2–4]. Although ZIKV infection is generally known to cause mild clinical symptoms in adults, recent outbreaks of ZIKV were linked to an increase in the birth of microcephalic babies from infected mothers [5–12]. Both, clinical studies in humans and animal models have reported that ZIKV primarily targets neuronal cells [13, 14], and cells of the reproductive tract including Sertoli and Leydig cells, spermatogonia, and vaginal epithelium [15, 16] [17]. ZIKV is also capable of infecting placental cells [18–20], skin fibroblast, keratinocytes, dendritic cells [21], and endometrial stromal cells [22].
In addition to microcephaly and fetal death, ZIKV has also been shown to cause hearing loss [23, 24] and a variety of ocular complications in infants including chorioretinal atrophy, microphthalmia, hemorrhagic retinopathy, retinal vasculitis, RPE mottling, optic neuritis, and hypoplasia of the optic nerve [25, 26]. Because of ocular involvement during the ZIKV outbreak in Brazil, our lab and several other groups initiated studies to understand the pathobiology of ocular ZIKV infection [26–31]. We reported that ZIKV causes chorioretinal atrophy in an experimental mouse models, and that cells lining the blood-retinal barrier (BRB) are permissive to ZIKV infection [27]. Consistent with our findings, several independent studies also demonstrated the permissive nature of BRB cells to ZIKV infection [32–35]. How ZIKV modulates the BRB to gain access to the eye remains an active area of investigation in our lab [25]. Recently, Manangeeswaran and co-workers used a one day old neonate pups to show that ZIKV can infect the optic nerve, retinal ganglion cells (RGCs) and inner nuclear layer cells, and caused retinal layer disorganization, retinitis, vitritis and focal choroiditis [26]. Moreover, viral RNA and low levels of ZIKV antigens can be found in the eyes of infected mice up to 75 days post infection [26]. Similarly, ZIKV RNA could be detected in both serum and aqueous humor from infected patients [37, 39–43].
While most ocular ZIKV manifestations have been reported in the posterior segment of the eye, clinical studies have also linked the involvement of the anterior segment, primarily as congenital glaucoma [36–43]. In our experimental mouse models, we have also observed increased intraocular pressure (IOP), trabeculitis, and glaucoma pathology (unpublished data). Along with widespread tissue tropism, recent literature also supports detection of ZIKV in several body fluids, including saliva, semen, cervical mucus, urine, and tears [16, 17, 36, 44, 45]. Since both human and animal studies have shown shedding of ZIKV in tears, we postulated that ZIKV may readily infect ocular surface epithelium and may persist in the cornea. While nearly 200,000 corneal transplants are performed annually in 116 countries [46], there is no routine screening performed for ZIKV detection prior to corneal transplant [47]. Given the widespread epidemic of ZIKV and large number of corneal transplants, it is imperative to investigate the pathobiology of ZIKV in corneal cells.
The ocular pathogenesis of ZIKV and the regulation of the host immune response are not well understood to date. Previously, using a systems biology approach, we have shown that ZIKV alters various host transcriptomes including innate immune receptors and downstream signaling pathways [48]. Toll like receptors (TLRs) and pathogen recognition receptors (PRRs) play an important role in innate antiviral defense against invading pathogens by producing cytokines, interferons (IFNs) and interferons stimulated genes (ISGs) [49–52]. Recently, we reported the production of various inflammatory mediators, IFNs and ISGs, in primary retinal pigmented epithelial (Pr. RPE) and primary human retinal vascular endothelial cells (HRvEC) [48, 53]. Using a mouse model, we demonstrated that ISG15 is essential for antiviral defense against ZIKV ocular infection, as ISG15−/− mice were more susceptible towards ZIKV infection [53]. While the production of ISG15 by human corneal cells [54] and its role in fungal keratitis [55] has been demonstrated, the function of ISG15 in providing corneal innate antiviral defense against ZIKV infection has not been elucidated.
Like ZIKV, dengue virus (DENV) can also cause ocular pathology, leading to the emergence of a new terminology “dengue eye disease” [56]. Clinical studies have shown that DENV can cause retinochoroiditis, vasculitis, choroidal neovascularisation, subconjunctival hemorrhage, ecchymosis, retinal artery occlusion, and in some unusual cases even globe rupture [56, 57]. However, DENV infection in cornea is rarely reported. Recently, the presence of DENV3 has been detected in a donor cornea, indicating that DENV could be transmitted by cornea transplantation [58]. Although DENV and ZIKV belongs to the same family Flaviviridae, one of our recent studies showed a differential modulation of RPE innate responses in DENV versus ZIKV infection [30].
In the present study, we investigated ZIKV infectivity in corneal cells using primary human corneal epithelial cells (Pr. HCEC) as well as an immortalized human corneal epithelial cell line (HUCL). Moreover, we also compared the infectivity of Pr. HCECs to DENV. We also investigated the role played by ISG15 in restricting ZIKV replication in corneal cells.
2. MATERIALS AND METHODS
2.1. Cell Culture
Human Pr. HCEC (ATCC PCS-700–010) were cultured in corneal epithelial cell growth kit media (ATCC PCS-700–040) as per manufacturer’s instructions. Human telomerase-immortalized corneal epithelial (HUCL) cells were maintained in a defined keratinocyte serum-free medium (KSFM; Invitrogen Life Technologies, Carlsbad, CA). Vero (ATCC CCL-81), BHK-21 (ATCC CCL-10) and Aedes albopictus clone C6/36 (ATCC CRL-1660) cells were grown in DMEM and EMEM media, respectively and supplemented with 10% FBS (fetal bovine serum), 10μg/ml L-glutamine, and 1% Penicillin and Streptomycin solution (PS). All cells were maintained at 37°C with 5% CO2, except C6/36, which was maintained at 28°C.
2.2. Virus strains and Infection
Zika Virus (ZIKV) strain PRVABC59, NR-50240, originally isolated from human blood in Puerto Rico in December 2015, was obtained through BEI resources, NIAID, NIH. ZIKV strain BeH823339, originally isolated from the brain of a deceased newborn with microcephaly in Brazil in November 2015, was obtained from Dr. Kathy Spindler (University of Michigan, Ann Arbor, MI) which was originally obtained from Dr. Eurico Arruda, University of São Paulo at Ribeirão Preto, São Paulo, Brazil. ZIKV was propagated in C6/36 and Vero cell line, and titers were determined by standard plaque assays. All cells were infected with ZIKV at MOI 5, unless specified.
2.3. Plaque assay
Cells were seeded at confluency in 60-mm tissue culture dishes and washed once with 1X PBS upon adherence. Log10 serial dilutions of the culture supernatant was prepared in serum free DMEM media and added onto the cells for 2h. The virus was removed and an equal mixture of DMEM and Noble Agar (1:1) was added onto the monolayer and allowed to solidify. A nutrient mix of BSA (50mg/ml), MgCl2 (1M), Oxaloacetic acid (400mM) with 1% Penicillin-Streptomycin solution along with DMEM was added onto the overlay and incubated for 5 days (37°C in a CO2 incubator with humidity). The monolayer was fixed with 10% trichloroacetic acid (TCA) for 15 mins followed by careful removal of the solution and the overlay media. The cells were stained using Crystal violet solution for 15 mins and washed with distilled water to visualize the plaques. The plaques were counted and the viral titer was expressed as PFU/ml. Vero, BHK-21 cells were used for estimating the viral titer of ZIKV and DENV, respectively.
2.4. Immunostaining and Immunoblotting
Immunostaining procedures were performed as described previously [48, 53]. Briefly, Pr. HCEC and HUCL cells were cultured in a four-well chamber slide (Fisher Scientific, Rochester, NY) and infected with ZIKV at MOI 5. At desired time points, ZIKV infected and mock-treated cells were fixed overnight with 4% paraformaldehyde in 1X PBS at 4°C. After washing, the cells were permeabilized and blocked with 1 % (w/v) BSA (bovine serum albumin) and 0.4% Triton X-100 made in 1X PBS for 1h at room temperature. Cells were incubated with primary mouse monoclonal antibody 4G2 (1:100) (Millipore, Billerica, MA, Cat # MAB10216), overnight at 4°C. Following removal of the primary antibody, the cells were washed extensively with 1X PBS and incubated for 1h with anti-mouse FITC-conjugated secondary antibody (1:200) at room temperature. Finally, the cells were extensively washed with 1X PBS and slides were mounted in Vectashield anti-fade mounting medium (Vector Laboratories, Burlingame, CA) and visualized using an Eclipse 90i fluorescence microscope (Nikon, Melville, NY). The immunoblotting for TLR3 and ISG15 was performed from ZIKV infected and uninfected control HCEC cell lysates as described earlier [59, 60].
2.5. Terminal dUTP Nick End-Labeling (TUNEL) assay
Pr. HCEC cells were grown and infected with ZIKV in a four well chamber slide (Fisher Scientific, Rochester, NY) for the indicated time points. TUNEL staining was performed using ApopTag® Fluorescein In Situ Apoptosis Detection Kit as per the manufacturer’s instructions (Millipore, Billerica, MA). The TUNEL stained cells were visualized using an Eclipse 90i fluorescence microscope (Nikon, Melville, NY).
2.6. RNA extraction and qRT-PCR
Total RNA was extracted from ZIKV/DENV-infected Pr. HCEC cells using Trizol as per the manufacturer’s recommendations (Thermo scientific, Rockford, IL). cDNA was synthesized using 1μg of total RNA using a Maxima first strand cDNA synthesis kit, as per the manufacturer’s instructions (Thermo scientific, Rockford, IL). The cDNA was amplified using gene-specific PCR primers. qPCR was conducted in a StepOnePlus™ Real-Time PCR system (Applied Biosystems, Grand Island, NY) using TaqMan probes against various inflammatory cytokines/chemokines (TNF-α, IL-1β, IL-6, CXCL10, and CCL5), interferons (IFN1α, IFNβ2, and IFNγ), and toll like receptor-3 (TLR3) and SYBR green based primers against RIG-I, MDA-5, and IFN stimulated genes ISG15, OAS2, and MX1. All primers and Taqman probes (Prime Time Mini qPCR Assay) were purchased from Integrated DNA Technologies (Coralville, IA, USA) and listed in Table 1. The quantification of gene expression was determined via the comparative ΔΔCT method. Gene expression in the test samples were normalized to the endogenous reference β-actin/GAPDH level and were reported as fold change relative to β-actin/GAPDH gene expression.
Table 1:
List of Primers used in the study for qRT-PCR.
| Primer Name | IDT Assay ID or Primer Sequence (5'→3') |
|---|---|
| TNFα | Hs.PT.58.45380900 |
| IL-1β | Hs.PT.58.1518186 |
| CXCL10 | Hs.PT.58.39328322.gs |
| IFN-β1 | Hs.PT.58.39481063.g |
| GAPDH | Hs.PT.39a.22214836 |
| TLR3 F | ACATCCCTGAGCTGTCAAGC |
| TLR3 R | AGAGTTCAAAGGGGGCACTG |
| RIG-I F | TGTGGGCAATGTCATCAAAA |
| RIG-I R | GAAGCACTTGCTACCTCTTGC |
| MDA5 F | GGCACCATGGGAAGTGATT |
| MDA5 R | ATTTGGTAAGGCCTGAGCTG |
| CCL5 F | TGTACTCCCGAACCCATTTC |
| CCL5 R | TACACCAGTGGCAAGTGCTC |
| IFNγ F | TCAGCCATCACTTGGATGAG |
| IFNγ R | CGAGATGACTTCGAAAAGCTG |
| ISG15 F | CAGCGAACTCATCTTTGCCAG |
| ISG15 R | GGACACCTGGAATTCGTTGC |
| MX1 F | GATGATCAAAGGGATGTGGC |
| MX1 R | AGCTCGGCAACAGACTCTTC |
| OAS2 F | ACCATCGGAGTTGCCTCTTA |
| OAS2 R | GGTGAACACCATCTGTGACG |
| β-actin F | CGAGCATCCCCCAAAGTTCA |
| β-actin R | CGCATCTCATATTTGGAATGACTAT |
2.7. ISG15 siRNA Silencing and Recombinant Protein Supplementation
For ISG15 inhibition studies, Pr. HCEC cells were transfected with ISG15 siRNA (Santa Cruz Biotechnology, Dallas TX) using Lipofectamine RNAiMAX transfection reagent (Thermo scientific, Rockford, IL) as per manufacturer’s recommendations. Scrambled control siRNA and ZIKV infected cells were used as siRNA and infection control, respectively. Twenty-four hours following siRNA transfection, cells were infected with ZIKV for 48h and processed for immunostaining, immunoblotting and qPCR to measure the viral replication and ISG15 silencing, respectively. For ISG15 protein supplementation, Pr. HCEC cells were cultured in a 4-well chamber slide and supplemented with 100 ng/ml of recombinant ISG15 (rISG15) (Boston Biochem, Cat # UL-601) 1h prior to infection. Following ISG15 supplementation, cells were infected with ZIKV for 48h. ISG15 protein was present in the media for the entire duration of infection. The effect of rISG15 supplementation on viral replication was measured by immunostaining and plaque assay.
2.8. Viral attachment and entry assay
Viral attachment was adapted from a previous publication [61]. Briefly, Vero cells were pre-incubated at 4°C for attachment assay and 37°C for entry assay with rISG15 for an hour and followed by infection with ZIKV at MOI 1. After adsorption for 2h, the cells were washed thrice with fresh medium followed by RNA isolation from whole cells. ZIKV RNA copy number was determined using qPCR.
2.9. Direct inactivation of ZIKV by ISG15
ZIKV stocks were diluted to 106 PFU/mL with serum free medium and rISG15 was added at the final concentration of 100 ng/ml. The virus and rISG15 mixture was incubated at 37°C for 2–4 hours. At each time point, 200μL of the mixture was removed for quantification of virus yield by plaque assay on Vero cells monolayer.
2.10. Statistics
All data are expressed as the mean ± standard deviation (SD) unless indicated otherwise. Statistical differences between experimental groups were determined using unpaired Student’s t-test and one-way ANOVA. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). A value of P<0.05 was considered statistically significant. All experiments were performed at least three times unless indicated otherwise.
3. RESULTS
3.1. ZIKV permissively infects Pr. HCECs and induced cell death
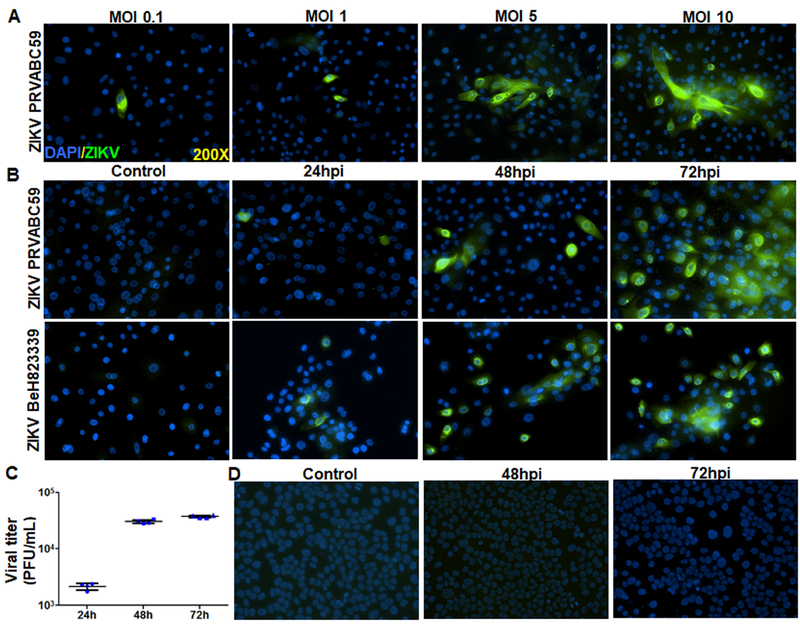
While ZIKV has been shown to be present in the tears of infected humans as well as mice [36, 62], currently, there is no indication of whether it can infect the ocular surface, particularly the corneal epithelium. Hence, we investigated ZIKV interaction with corneal epithelial cells, using both primary and immortalized cell lines [63]. First, a dose response study was performed by infecting Pr. HCECs at various multiplicity of infection (MOI) and we found that ZIKV significantly infects Pr. HCECs at MOI 5 or higher (Fig.1A). Following dosage response, we infected Pr. HCEC with ZIKV using two different strains of ZIKV (Puerto Rican strain, PRVABC59 and a Brazilian strain BeH823339) for various time points (24, 48, and 72h). Our results indicated that ZIKV was capable of infecting Pr. HCEC cells in a time-dependent manner as revealed by increased 4G2 staining, an anti-flavivirus envelope protein antibody which binds to a conserved epitope on the E protein of the flavivirus family (Fig. 1B). Similar to 4G2 immunostaining, plaque assay further confirmed the replication of ZIKV (Brazilian strain) and the production of infectious progeny in Pr. HCEC (Fig. 1C). Interestingly, ZIKV failed to infect immortalized corneal epithelium, i.e., HUCL cells (Fig. 1D).
Figure 1. ZIKV infectivity of HCECs: a dose and time course study.
(A) Human Pr. HCEC cells were infected with ZIKV (PRVABC59, a Puerto Rico strain) with various MOI (0.1–10) for 48h. Infected cells were subjected to immunostaining for anti-flavivirus group antigen 4G2, and representative images show the presence of ZIKV (green) and DAPI (blue, a cell nuclear stain). (B) Time course study was performed by infecting Pr. HCEC with two different strains of ZIKV, PRVABC59, and BeH823339 (Brazilian clinical strain) at MOI 5 for the indicated time points, uninfected cells served as control. Immunostaining for anti-flavivirus group antigen 4G2 was performed to detect ZIKV infectivity. (C) Pr. HCECs were infected with ZIKV (strain BeH823339) at MOI 5. The culture supernatant were collected and used for plaque assay on Vero cells. Dot plot represent the plaque forming units (PFU)/ml of conditioned media (n=4) at various time points. (D) A transformed corneal cell line HUCL, were infected with ZIKV, Brazilian strain BeH823339 at MOI 5 and subjected to immunostaining for 4G2 antibody, representative images (n=3) show the presence of ZIKV (green) and DAPI (blue).
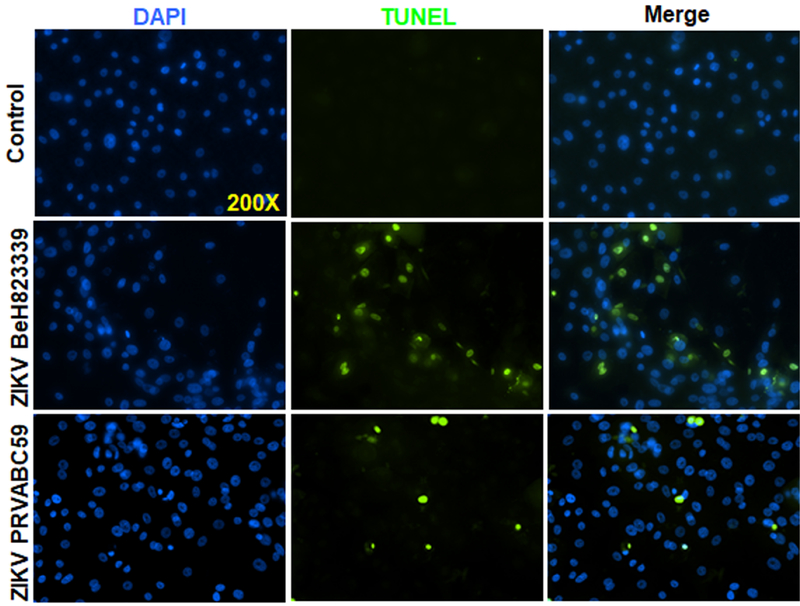
Viral infection often leads to cytopathic effects in infected cells, including cell death. In order to assess ZIKV-induced cell death, we performed TUNEL assay. As shown in figure 2, both ZIKV strains caused Pr. HCECs cell death, with Brazilian strain causing relatively more death at 48h (Fig. 2). Together, these results indicate that only Pr. HCECs are permissive to ZIKV infection and cause cell death. Because the Brazilian strain was found to be more aggressive than the Puerto Rican strain, the Brazilian strain was used for the remainder of the study.
Figure 2. ZIKV Induced cell death in Pr. HCECs.
Human Pr. HCECs were infected with either ZIKV strain PRVABC59 or BeH823339 at MOI 5 for 48h, uninfected cells served as control. Control and infected cells were subjected to TUNEL staining to detect cell death. Representative images showing TUNEL positive cells (green).
3.2. ZIKV infected Pr. HCECs elicited innate inflammatory and antiviral response
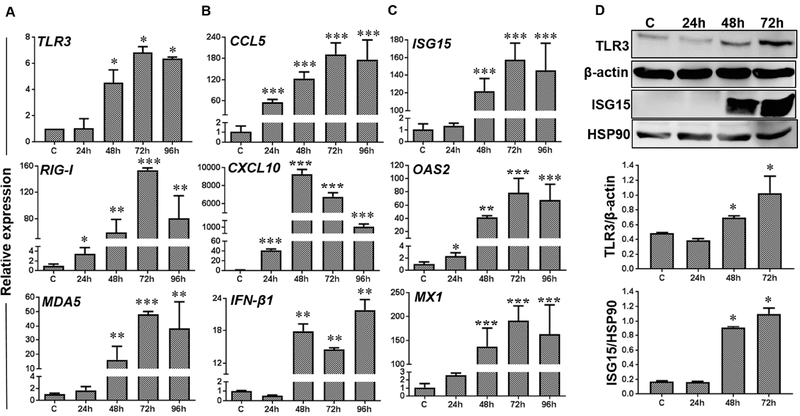
Next, we sought to determine whether ZIKV-infected corneal cells elicit innate immune response. We checked the expression of several pathogen recognition receptors (PPRs) and Toll like receptors (TLRs), which are known to initiate innate immune signaling in response to viral infection. Our data showed a time-dependent upregulation of transcripts of various PRRs including TLR3, MDA5, and RIG-I in ZIKV infected Pr. HCEC (Fig. 3A). Concomitant with induced PRR expression, mRNA levels of inflammatory cytokines: TNFα, and IL-1β, chemokines: CCL5 and CXCL10, and interferons: IFN-β1 were increased in infected Pr. HCECs (Fig. 3B). Concurring with IFN expression, ZIKV-infected Pr. HCEC showed a significant upregulation of interferon-stimulated antiviral response genes, ISG15, OAS2, and MX1 (Fig. 3C). Immunoblot analysis further confirmed the ZIKV-induced expression of TLR3 and ISG15 at protein level (Fig. 3D). These results indicate that Pr. HCEC cells possess the ability to respond to ZIKV infection by evoking inflammatory and antiviral responses.
Figure 3. Induction of innate inflammatory and antiviral responses upon ZIKV infection of Pr. HCEC.
Pr. HCECs were infected with ZIKV (Brazilian strain BeH823339 at MOI 5 for indicated time points. Infected and mock treated cells and subjected to qRT-PCR or western blotting. (A-C) qRT-PCR results (n=3) showing expression of PRRs (TLR3, RIG-I, and MDA5), inflammatory mediators (CCL5, and CXCL10), interferons (IFN-β1), and interferon induced genes (ISG15, OAS 2, and MX1) represented as mean ± SD, * p<0.05, ** p<0.005, *** p<0.0005, Student’s t-test. (D) Western blot analysis showing induced expression of TLR3 and ISG15 in ZIKV infected Pr. HCEC in time-dependent manner. Bar graph represents densitometry analysis of Western blots using Image J software with respect to housekeeping genes β-actin for TLR3 and HSP90 for ISG15, respectively. Mean ± SD; n=3, * p<0.05; Student’s t-test.
3.3. ISG15 restricted ZIKV replication in Pr. HCECs
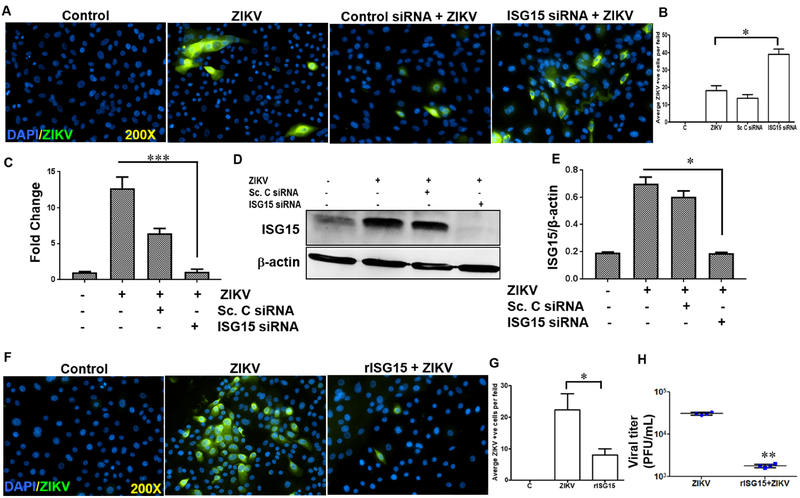
Previously, we showed that ISG15−/− mice are more susceptible to ocular ZIKV infection, resulting in severe chorioretinal atrophy as compared to WT mice [53]. Moreover, ZIKV-infected Pr. HCECs were found to express ISG15 both at mRNA and protein level (Fig. 3). To investigate the functional role of ISG15 on corneal innate immunity, we used siRNA knockdown and rISG15 supplementation approaches. We observed that siRNA silencing of ISG15 increased ZIKV replication as evidenced by increase in 4G2 positive cells compared to control siRNA-treated cells (Fig. 4A & B). Silencing of ISG15 was confirmed by qRT-PCR (Fig. 4C) and immunoblotting (Fig. 4D & E). Supplementation of Pr. HCEC with rISG15 reduced ZIKV replication as revealed by fewer 4G2 positive cells (Fig. 4F&G) and the reduction in plaque counts (Fig. 4H). These findings indicates an anti-ZIKV role of ISG15 in corneal epithelium.
Figure 4. Effect of ISG15 in modulating ZIKV replication in Pr. HCECs.
Pr. HCEC cells were transfected with scrambled or ISG15 siRNA followed by ZIKV infection (Brazilian strain BeH823339) at MOI 5. (A) Immunostaining for 4G2 antigen (green) was performed to detect ZIKV infectivity. (B) Quantification of ZIKV antigen (4G2) positive (+ve) cells per field upon ISG15 silencing. Silencing of ISG15 was confirmed by (C) qRT-PCR and (D&E) western blot followed by densitometry analysis. The values on bar graph (qPCR and densitometry) have been represented as mean ± SD; n=3, *** p<0.0005; * p<0.05. (F) Pr. HCEC cells were exogenously supplemented with rISG15 (100ng/ml) one hour prior to ZIKV infection. At 48h post infection, cells were stained using anti-flavivirus 4G2 antibody. (G) Quantification of average ZIKV antigen (4G2) positive (+ve) cells per field upon rISG15 supplementation (H) The culture supernatant was used for plaque assay and the values have been presented as mean ± SD; n=4, ** p<0.005; Student’s t-test.
3.4. ISG15 inhibits ZIKV infection by direct inactivation and reduced viral binding to cells
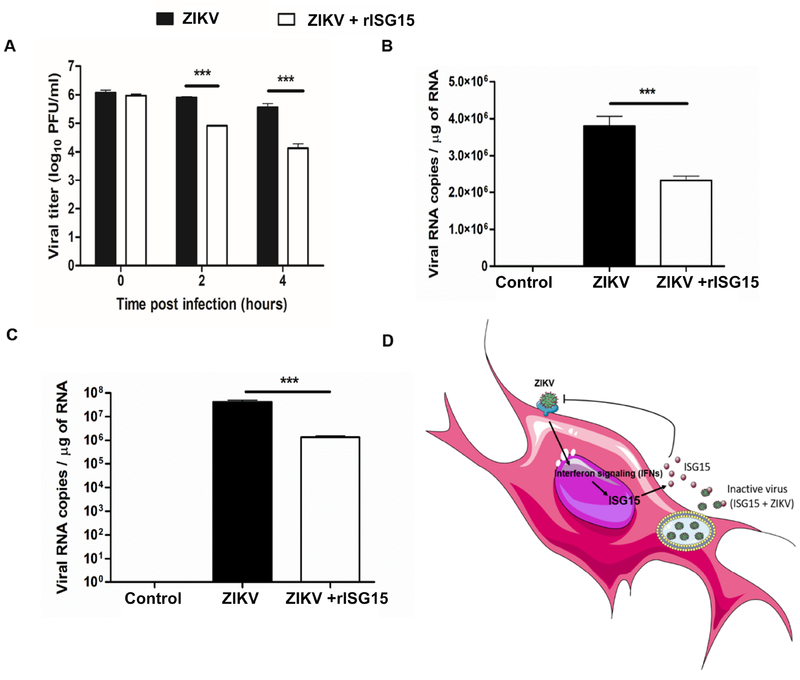
To determine the mechanism of ISG15 mediated ZIKV inhibition, we investigated ZIKV attachment and direct virus inactivation by plaque assay and RNA copy number estimation. The time points were chosen as per a prior report on the action of anti-microbial peptides on ZIKV replication [61]. The incubation of rISG15 with ZIKV at 37°C decreased the viral infectivity in a time-dependent manner as evidenced by reduction of viral titer one log10 PFU at 2h and two log10 PFU at 4h post incubation (Fig. 5A). Similarly, the viral attachment assay revealed that cells incubated with rISG15 at 4°C for an hour followed by ZIKV infection for 2h resulted in decreased viral RNA copy number (Fig. 5B). For the viral entry assay, Vero cells were incubated with rISG15 at 37°C for an hour followed by ZIKV infection for 2h showed a reduction in viral RNA copy number in rISG15-treated cells (Fig. 5C). Collectively, these results indicate that the antiviral action of ISG15 produced by host cells could be due to the combined actions of direct inactivation and inhibition of ZIKV binding to host cells (Fig. 5D).
Figure 5: Modes of action of ISG15 to inhibit ZIKV infection.
(A) rISG15 was incubated with ZIKV (106 PFU/ml) for specified time points at 37°C followed by viral titer estimation by plaque assay. The values have been expressed as PFU/ml. (B) The Vero cells were treated with rISG15 for 1h prior to ZIKV infection at 4°C. The cells were infected at 4°C followed by RNA isolation and qPCR for ZIKV Envelope gene. The values have been expressed as RNA copies/μg. (C) The Vero cells were treated with rISG15 for 1h prior to ZIKV infection followed by challenge with ZIKV at 37°C. qPCR was performed for ZIKV envelope gene and the values have been expressed as RNA copies/μg of total RNA. (D) Schematic representation of the role of ISG15 in inhibiting ZIKV replication in mammalian cells. Data is presented as mean ± SD, * p<0.05, ** p<0.005, *** p<0.0005, One way-ANOVA.
3.5. Dengue virus shows limited infectivity to Pr. HCECs.
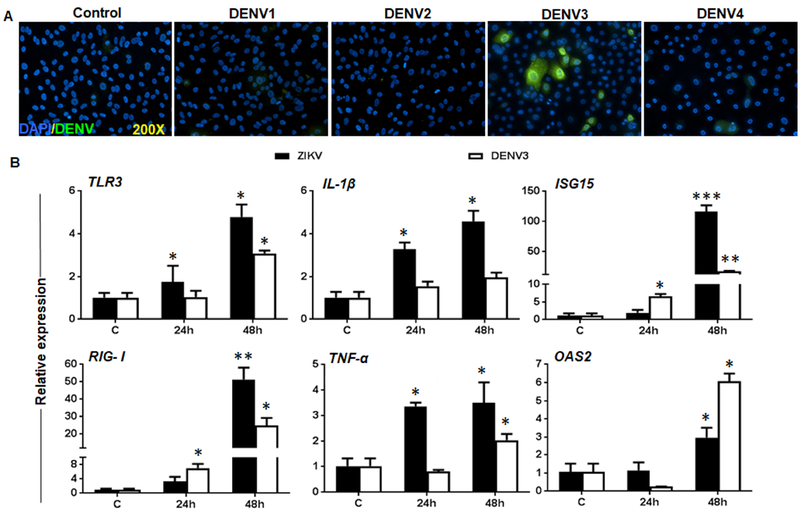
Being a member of the flavivirus family, ZIKV is closely related to DENV, which has also been implicated in causing ocular complications [25]. Moreover, the presence of DENV has recently been reported in the donor cornea [64]. Thus, we sought to test the infectivity of all four DENV serotypes in Pr. HCEC and compared the innate response against ZIKV infection. Among all serotypes, only DENV3 showed limited infectivity in Pr. HCECs as revealed by few 4G2 positive cells (Fig. 6A). Next, we compared the host innate response against DENV3 versus ZIKV in infected Pr. HCEC. Our data showed that similar to ZIKV, DENV3 infection induced an innate immune response in Pr. HCECs, as evidenced by increased expression of PRRs (e.g., TLR3, RIG-I), inflammatory cytokines (e.g., IL-1β, TNFα), and interferon stimulated genes (e.g., ISG15, OAS2). Comparative analysis revealed differential expression of TNFα, IL-1β and ISG15 being higher in ZIKV infected cells whereas OAS2 levels were higher in DENV3 infected cells (Fig. 6B). These results indicate that Pr. HCECs are not permissive to all the flaviviruses with a similar degree of infection, and both of these viruses could modulate host immune system differentially.
Figure 6. Infectivity and innate response of Pr. HCECs with Dengue virus.
(A) Pr. HCECs were infected with DENV serotypes 1, 2, 3, and 4 at MOI 5 for 48h. Infected cells were subjected to immunostaining using 4G2 antibody, and representative images (n=3) showed the presence of DENV (green) and DAPI (blue). (B) Pr. HCECs were infected with either ZIKV Brazilian strain or DENV3 at MOI: 5 for indicated time points and qRT-PCR was performed to assess the expression of indicated PRRs (TLR3, and RIG-I), inflammatory mediators (IL-1β and TNF-α), and interferon induced genes (ISG15, and OAS2). The values have been presented as mean ± SD, * p<0.05, ** p<0.005, *** p<0.0005, Student’s t-test.
4. DISCUSSION
In this study, we report that Pr. HCECs are highly permissive to ZIKV but not DENV infections, with the exception of DENV serotype 3 (DENV3), which showed limited infectivity. We demonstrated that ZIKV-infected Pr. HCECs elicited innate immune response characterized by the induction of TLRs, inflammatory cytokines and antiviral response genes regulated by type 1 interferon signaling. To understand the functional role of the induced antiviral molecule, ISG15, we used siRNA and supplementation approaches and showed that ISG15 plays an important role in restricting ZIKV replication by direct inactivation and inhibition of viral entry. To the best of our knowledge, this is the first study to demonstrate that HCECs possess the ability to recognize and respond to ZIKV, and ISG15 attenuates ZIKV infection in HCECs.
Corneal epithelium provides the first line of defense against invading pathogens and their virulence factors at the ocular surface. Prior studies from our group and others have demonstrated that apart from making a physical barrier, HCECs actively participate in evoking an innate response, in part through the expression of several PRRs, including TLRs [65, 66]. These receptors are programmed to sense viral entry, and control viral replication and spread by triggering an antiviral response. Our data showed induced expression of, TLR3, RIGI and MDA5 in Pr. HCEC indicating the potential role of these PRRs in regulating innate inflammatory and antiviral responses to ZIKV infection. These results corroborate with previous reports in the literature showing ZIKV and other flaviviruses, including DENV, West Nile virus (WNV), and Yellow fever virus (YFV), evoke immune responses by modulating the expression of these PRRs in various ocular [33, 53, 67, 68] and non-ocular cell types [19, 49, 51, 52, 69]. Because our prior study had demonstrated the role of TLR3 in regulating antiviral response in HCECs, [70] and ZIKV was found to induce TLR3 both at mRNA and protein level, we propose that TLR3 signaling might play a role in corneal innate defense. However, further studies are needed to dissect the role of TLR3 in ZIKV infection in HCECs.
Upon binding with specific viral ligands, PRRs become activated and trigger signaling pathways, leading to the production of various inflammatory mediators and interferons. Our data showed induction of inflammatory cytokines and chemokines (CCL5 & CXCL10), and interferon (IFNβ1) in ZIKV-infected Pr. HCECs. These findings imply that enhanced production of cytokines/chemokines by infected HCECs may assist in the recruitment of innate immune cells (PMNs, macrophages, NK cells) to the infection site to limit viral replication in cells. Similarly, the induction of IFNs triggers the production of several ISGs, which promote antiviral response and exert direct antiviral properties to limit viral spread. Our data showed increased expression of IFNβ1, as well as the induction of several ISGs, including ISG15, OAS2, and MX1. These results support our prior finding that ZIKV elicits antiviral immune response in retinal cells [27]
Among the various antiviral molecules, ISG15 is of significant interest, as it is one of the most abundant transcripts induced upon TLR activation or viral infection, and plays an important role in modulating immune response against several flaviviruses [71, 72]. Studies including the overexpression or knockdown of ISG15 have indicated its involvement in the regulation of influenza virus, vaccinia, vesicular stomatitis virus (VSV), Sendai virus, Japanese encephalitis virus (JEV), as well as in the release of virus-like particles (VLPs) derived from HIV-1 and avian sarcoma leukosis virus (ASLV) [73, 74]. The mechanism by which ISG15 alters the viral replication is unknown for the majority of these pathogens. Moreover, corneal cells have been shown to produce ISG15 in both in vivo as well as in vitro models of microbial infection [75] and in response to interferon challenge [76]. Previously, we have demonstrated the expression of ISG15 in various retinal cells infected with ZIKV, and ISG15 deficiency caused increased susceptibility towards ZIKV-induced ocular pathology [53]. As ZIKV was found to induce the expression of ISG15 in Pr. HCEC at both mRNA and protein levels, we evaluated its antiviral role and mode of action. We observed that inhibition of ISG15 expression by siRNA resulted in enhanced viral replication, whereas the exogenous supplementation of the recombinant ISG15 protein restricted ZIKV replication in Pr. HCECs. ZIKV susceptibility varies among ocular cell types; for example, RPE and retinal vascular endothelium were more susceptible to ZIKV as compared to photoreceptors cells [26, 27]. Similarly, Pr. HCEC cells were permissive to ZIKV infection, however, a higher MOI (MOI 5) was required to infect these cells in comparison with other retinal cell types (MOI 1). Therefore, in order to understand the mechanism of antiviral action of ISG15, Vero cells were used to obtain uniform infection for viral attachment and entry assays [61]. This data showed that ISG15 exerts its antiviral activities by attenuating ZIKV and inhibiting its entry. Collectively, these findings indicate that ISG15 plays a role in inducing antiviral response of HCECs.
While the literature on corneal infection due to flaviviruses is sparse, a recent study reported the presence of DENV3 in human donor’s cornea [67] [64] and the ability of WNV to infect mouse and human cornea [77]. The closely related DENV is more prevalent worldwide as compared to ZIKV, and its emergence as an ocular pathogen has lead to the term “dengue eye disease” [56]. However, the literature in regards to DENV infection in ocular cells, and corneal cells in particular, is sparse. Our data showing DENV infectivity in Pr. HCEC provides additional insight into the potential risks of flaviviruses in causing ocular surface complications. Interestingly, among all four serotypes of DENV, only DENV3 showed infectivity in Pr. HCECs. Moreover, DENV3 infection induced the expression of PRRs (TLR3 & RIG-I), inflammatory cytokines (IL-1β, & TNF-α), and ISGs (ISG15, and OAS2) in Pr. HCECs. However, the innate response was lower when compared to the response against ZIKV infection. This indicates that ZIKV may differ from DENV in modulating the host immune response, as reported in our recent study using RPE cells [30].
Although the genetic material of ZIKV has been shown to be present in tears and cornea of infected mice [41], currently there are no reports showing the presence of ZIKV in human cornea. Our study demonstrating the ability of two different strains of ZIKV to infect HCECs indicates the possibility of the cornea being a reservoir of ZIKV in the eye. It is well known that donor tissues may harbor microorganisms that can persist despite sterile procedures and the use of appropriate antiseptics and antibiotics. Therefore, the transmission of infectious agents during transplantation remains a major concern for surgeons and tissue banks. Corneal transplantation is the world’s most frequent type of transplantation, the incidence of corneal graft rejection increases when the donor cornea carries infectious agents [78]. Indeed, several studies have demonstrated the transmission of pathogens including viruses via corneal transplant [79–81]. However, there is no evidence of whether ZIKV can be transmitted via corneal graft.
In summary, our study shows that human Pr. HCECs support ZIKV replication and induces an innate immune response by activating various PRRs and producing inflammatory cytokines and interferons. Moreover, ISG15 was found to play a critical role in corneal innate response by restricting ZIKV replication. Together these findings provide valuable insight into the pathogenesis of ZIKV in causing ocular complications, and highlight the potential risks associated with corneal transplantation, suggesting for the screening of ZIKV and DENV in the cornea prior to transplantation.
5. Acknowledgments
This study was supported by NIH grants R21AI135583, R01EY026964, & R01EY02738 (to A.K.). Our research is also supported in part by an unrestricted grant to the Department of Ophthalmology/Kresge Eye Institute from Research to Prevent Blindness Inc. The immunology resource core is supported by an NIH center grant P30EY004068. The authors would like to thank other members of the lab for their helpful discussion and editing the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no financial conflict of interest.
7. REFERENCES
- [1].Peters R, Stevenson M. Zika Virus Diagnosis: Challenges and Solutions. Clin Microbiol Infec. 2018. [DOI] [PubMed] [Google Scholar]
- [2].Yakob L, Kucharski A, Hue S, Edmunds WJ. Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect Dis. 2016;16:1100–2. [DOI] [PubMed] [Google Scholar]
- [3].Moreira J, Peixoto TM, Siqueira AM, Lamas CC. Sexually acquired Zika virus: a systematic review. Clin Microbiol Infect. 2017;23:296–305. [DOI] [PubMed] [Google Scholar]
- [4].Magnus MM, Esposito DLA, Costa VAD, Melo PS, Costa-Lima C, Fonseca B, et al. Risk of Zika virus transmission by blood donations in Brazil. Hematol Transfus Cell Ther. 2018;40:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sejvar JJ. Zika Virus and Other Emerging Arboviral Central Nervous System Infections. Continuum (Minneap Minn). 2018;24:1512–34. [DOI] [PubMed] [Google Scholar]
- [6].Joob B, Wiwanitkit V. Regarding Zika Microcephaly. Innov Clin Neurosci. 2018;15:12. [PMC free article] [PubMed] [Google Scholar]
- [7].Grazel R, Harris-Haman P. Zika Virus Infection: A Vector-Borne Threat to Pregnant Women and Infants. Adv Neonatal Care. 2018;18:350–9. [DOI] [PubMed] [Google Scholar]
- [8].Franca TLB, Medeiros WR, Souza NL, Longo E, Pereira SA, Franca TBO, et al. Growth and Development of Children with Microcephaly Associated with Congenital Zika Virus Syndrome in Brazil. Int J Environ Res Public Health. 2018;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Krow-Lucal ER, de Andrade MR, Cananea JNA, Moore CA, Leite PL, Biggerstaff BJ, et al. Association and birth prevalence of microcephaly attributable to Zika virus infection among infants in Paraiba, Brazil, in 2015–16: a case-control study. Lancet Child Adolesc Health. 2018;2:205–13. [DOI] [PubMed] [Google Scholar]
- [10].Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S, et al. Zika Virus as a Cause of Neurologic Disorders. N Engl J Med. 2016;374:1506–9. [DOI] [PubMed] [Google Scholar]
- [11].Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med. 2016;375:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moshfeghi DM, de Miranda H, Ii, Costa M. ZIka virus, microcephaly, and ocular findings. JAMA Ophthalmology. 2016. [DOI] [PubMed] [Google Scholar]
- [13].Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell stem cell. 2016;18:587–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, et al. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell. 2016;19:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell Rep. 2016;17:3091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miner JJ, Diamond MS. Zika Virus Pathogenesis and Tissue Tropism. Cell Host & Microbe. 2017;21:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matusali G, Houzet L, Satie AP, Mahe D, Aubry F, Couderc T, et al. Zika virus infects human testicular tissue and germ cells. The Journal of clinical investigation. 2018;128:4697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr., et al. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host & Microbe. 2016;19:705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, et al. Zika Virus Infects Human Placental Macrophages. Cell Host & Microbe. 2016;20:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, et al. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell host & microbe. 2016;20:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of Zika Virus Infection in Human Skin Cells. Journal of virology. 2015;89:8880–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pagani I, Ghezzi S, Ulisse A, Rubio A, Turrini F, Garavaglia E, et al. Human Endometrial Stromal Cells Are Highly Permissive To Productive Infection by Zika Virus. Scientific reports. 2017;7:44286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Peloggia A, Ali M, Nanda K, Bahamondes L. Zika virus exposure in pregnancy and its association with newborn visual anomalies and hearing loss. Int J Gynaecol Obstet. 2018. [DOI] [PubMed] [Google Scholar]
- [24].Mittal R, Fifer RC, Liu XZ. A Possible Association Between Hearing Loss and Zika Virus Infections. JAMA Otolaryngol Head Neck Surg. 2017. [DOI] [PubMed] [Google Scholar]
- [25].Singh S, Kumar A. Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier. Viruses. 2018;10:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Manangeeswaran M, Kielczewski JL, Sen HN, Xu BC, Ireland DDC, McWilliams IL, et al. ZIKA virus infection causes persistent chorioretinal lesions. Emerg Microbes Infect. 2018;7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh PK, Guest JM, Kanwar M, Boss J, Gao N, Juzych MS, et al. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight. 2017;2:e92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhao Z, Yang M, Azar SR, Soong L, Weaver SC, Sun J, et al. Viral Retinopathy in Experimental Models of Zika Infection. Invest Ophthalmol Vis Sci. 2017;58:4355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Simonin Y, Erkilic N, Damodar K, Cle M, Desmetz C, Bollore K, et al. Zika virus induces strong inflammatory responses and impairs homeostasis and function of the human retinal pigment epithelium. EBioMedicine. 2019;39:315–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Singh PK, Khatri I, Jha A, Pretto CD, Spindler KR, Arumugaswami V, et al. Determination of system level alterations in host transcriptome due to Zika virus (ZIKV) Infection in retinal pigment epithelium. Sci Rep. 2018;8:11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Singh S, Kumar A. Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier. Viruses. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Salinas S, Erkilic N, Damodar K, Moles JP, Fournier-Wirth C, Van de Perre P, et al. Zika Virus Efficiently Replicates in Human Retinal Epithelium and Disturbs Its Permeability. J Virol. 2017;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Roach T, Alcendor DJ. Zika virus infection of cellular components of the blood-retinal barriers: implications for viral associated congenital ocular disease. J Neuroinflammation. 2017;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhao Z, Yang M, Azar SR, Soong L, Weaver SC, Sun J, et al. Viral Retinopathy in Experimental Models of Zika Infection. Investigative Ophthalmology & Visual Science. 2017;58:4075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Simonin Y, Erkilic N, Damodar K, Clé M, Desmetz C, Bolloré K, et al. Zika virus induces strong inflammatory responses and impairs homeostasis and function of the human retinal pigment epithelium. EBioMedicine. 2019;39:315–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016;16:3208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Furtado JM, Espósito DL, Klein TM, Teixeira-Pinto T, da Fonseca BA. Uveitis Associated with Zika Virus Infection. New England Journal of Medicine. 2016;375:394–6. [DOI] [PubMed] [Google Scholar]
- [38].De Moraes CG, Pettito M, Yepez JB, Sakuntabhai A, Simon-Loriere E, Zaidi MB, et al. Optic neuropathy and congenital glaucoma associated with probable Zika virus infection in Venezuelan patients. JMM Case Rep. 2018;5:e005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yepez JB, Murati FA, Pettito M, Penaranda CF, de Yepez J, Maestre G, et al. Ophthalmic Manifestations of Congenital Zika Syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017;135:440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].de Paula Freitas B, Ko AI, Khouri R, Mayoral M, Henriques DF, Maia M, et al. Glaucoma and Congenital Zika Syndrome. Ophthalmology. 2017;124:407–8. [DOI] [PubMed] [Google Scholar]
- [41].Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell reports. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].De Moraes CG, Pettito M, Yepez JB, Sakuntabhai A, Simon-Loriere E, Zaidi MB, et al. Optic neuropathy and congenital glaucoma associated with probable Zika virus infection in Venezuelan patients. JMM Case Rep. 2018;5:e005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yepez JB, Murati FA, Pettito M, Penaranda CF, de Yepez J, Maestre G, et al. Ophthalmic Manifestations of Congenital Zika Syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017;135:440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hou W, Armstrong N, Obwolo LA, Thomas M, Pang X, Jones KS, et al. Determination of the Cell Permissiveness Spectrum, Mode of RNA Replication, and RNA-Protein Interaction of Zika Virus. BMC Infect Dis. 2017;17:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tan JJL, Balne PK, Leo YS, Tong L, Ng LFP, Agrawal R. Persistence of Zika virus in conjunctival fluid of convalescence patients. Sci Rep. 2017;7:11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmology. 2016;134:167–73. [DOI] [PubMed] [Google Scholar]
- [47].Bandyopadhyay D, Qureshi A, Ashish K, Hajra A, Chakraborty S. Effect of ZIKA virus on adult eyes. Eur J Intern Med. 2018;47:e18–e9. [DOI] [PubMed] [Google Scholar]
- [48].Singh PK, Khatri I, Jha A, Pretto CD, Spindler KR, Arumugaswami V, et al. Determination of system level alterations in host transcriptome due to Zika virus (ZIKV) Infection in retinal pigment epithelium. Scientific reports. 2018;8:11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Modhiran N, Watterson D, Blumenthal A, Baxter AG, Young PR, Stacey KJ. Dengue virus NS1 protein activates immune cells via TLR4 but not TLR2 or TLR6. Immunol Cell Biol. 2017;95:491–5. [DOI] [PubMed] [Google Scholar]
- [50].Chen J, Ng MM, Chu JJ. Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection. PLoS pathogens. 2015;11:e1005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chugh PE, Damania BA, Dittmer DP. Toll-like receptor-3 is dispensable for the innate microRNA response to West Nile virus (WNV). PLoS One. 2014;9:e104770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sabouri AH, Marcondes MC, Flynn C, Berger M, Xiao N, Fox HS, et al. TLR signaling controls lethal encephalitis in WNV-infected brain. Brain Res. 2014;1574:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Singh PK, Guest J-M, Kanwar M, Boss J, Gao N, Juzych MS, et al. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Taylor JL, D’Cunha J, Tom P, O’Brien WJ, Borden EC. Production of ISG-15, an interferon-inducible protein, in human corneal cells. J Interferon Cytokine Res. 1996;16:937–40. [DOI] [PubMed] [Google Scholar]
- [55].Dong C, Gao N, Ross BX, Yu F-SX. ISG15 in Host Defense Against Candida albicans Infection in a Mouse Model of Fungal Keratitis. Investigative Ophthalmology & Visual Science. 2017;58:2948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kularatne SA. Dengue fever. BMJ. 2015;351:h4661. [DOI] [PubMed] [Google Scholar]
- [57].Nagaraj KB, Jayadev C, Yajmaan S, Prakash S. An unusual ocular emergency in severe dengue. Middle East Afr J Ophthalmol. 2014;21:347–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Janani MK, Durgadevi P, Padmapriya J, Malathi J, Kulandai LT, Rao Madhavan HN. First Report on Detection of Dengue Virus in the Donor Cornea. Cornea. 2018;37:1586–9. [DOI] [PubMed] [Google Scholar]
- [59].Singh PK, Kumar A. Mitochondria mediates caspase-dependent and independent retinal cell death in Staphylococcus aureus endophthalmitis. Cell Death Discov. 2016;2:16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Singh PK, Kumar A. Retinal Photoreceptor Expresses Toll-Like Receptors (TLRs) and Elicits Innate Responses Following TLR Ligand and Bacterial Challenge. PLoS One. 2015;10:e0119541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].He M, Zhang H, Li Y, Wang G, Tang B, Zhao J, et al. Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway. Front Immunol. 2018;9:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Manangeeswaran M, Kielczewski JL, Sen HN, Xu BC, Ireland DDC, McWilliams IL, et al. ZIKA virus infection causes persistent chorioretinal lesions. Emerg Microbes Infect. 2018;7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kumar A, Zhang J, Yu F-SX. Innate Immune Response of Corneal Epithelial Cells to Staphylococcus aureus Infection: Role of Peptidoglycan in Stimulating Proinflammatory Cytokine Secretion. Investigative ophthalmology & visual science. 2004;45:3513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Janani MK, Durgadevi P, Padmapriya J, Malathi J, Kulandai LT, Rao Madhavan HN. First Report on Detection of Dengue Virus in the Donor Cornea. Cornea. 2018. [DOI] [PubMed] [Google Scholar]
- [65].Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Eslani M, Movahedan A, Afsharkhamseh N, Sroussi H, Djalilian AR. The Role of Toll-Like Receptor 4 in Corneal Epithelial Wound Healing. Investigative Ophthalmology & Visual Science. 2014;55:6108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Parthasarathy D, Madhuravasal JK, Jayavel P, Kulandai LT, Narahari Rao MH, Jambulingam M. Expression analysis of toll-like receptors of Dengue-infected cornea by real-time polymerase chain reaction. Inflamm Res. 2018;67:555–8. [DOI] [PubMed] [Google Scholar]
- [68].Zhu S, Luo H, Liu H, Ha Y, Mays ER, Lawrence RE, et al. p38MAPK plays a critical role in induction of a pro-inflammatory phenotype of retinal Muller cells following Zika virus infection. Antiviral Res. 2017;145:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Luo H, Winkelmann ER, Fernandez-Salas I, Li L, Mayer SV, Danis-Lozano R, et al. Zika, dengue and yellow fever viruses induce differential anti-viral immune responses in human monocytic and first trimester trophoblast cells. Antiviral Res. 2018;151:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kumar A, Zhang J, Yu F-SX. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hishiki T, Han Q, Arimoto K, Shimotohno K, Igarashi T, Vasudevan SG, et al. Interferon-mediated ISG15 conjugation restricts dengue virus 2 replication. Biochemical and biophysical research communications. 2014;448:95–100. [DOI] [PubMed] [Google Scholar]
- [72].Dai J, Pan W, Wang P. ISG15 facilitates cellular antiviral response to dengue and west nile virus infection in vitro. Virology journal. 2011;8:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lenschow DJ. Antiviral Properties of ISG15. Viruses. 2010;2:2154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Morales DJ, Lenschow DJ. The antiviral activities of ISG15. J Mol Biol. 2013;425:4995–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dong C, Gao N, Ross BX, Yu FX. ISG15 in Host Defense Against Candida albicans Infection in a Mouse Model of Fungal Keratitis. Investigative Ophthalmology & Visual Science. 2017;58:2948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Taylor JL, D’Cunha J, Tom P, O’Brien WJ, Borden EC. Production of ISG-15, an interferon-inducible protein, in human corneal cells. J Interferon Cytokine Res. 1996;16:937–40. [DOI] [PubMed] [Google Scholar]
- [77].Blitvich BJ, Wang T, Saxena V, Zeng S, Harmon KM, Raymond MD, et al. West Nile Virus Infection in Human and Mouse Cornea Tissue. Am J Trop Med Hyg. 2016;95:1185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Robert PY, Adenis JP, Denis F, Ranger-Rogez S. Transmission of viruses through corneal transplantation. Clin Lab. 2005;51:419–23. [PubMed] [Google Scholar]
- [79].Couderc T, Gangneux N, Chrétien F, Caro V, Le Luong T, Ducloux B, et al. Chikungunya Virus Infection of Corneal Grafts. The Journal of Infectious Diseases. 2012;206:851–9. [DOI] [PubMed] [Google Scholar]
- [80].Hassan SS, Wilhelmus KR, Dahl P, et al. Infectious disease risk factors of corneal graft donors. Arch Ophthalmol-Chic. 2008;126:235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Remeijer L, Maertzdorf J, Doornenbal P, Verjans GMGM, Osterhaus ADME. Herpes simplex virus 1 transmission through corneal transplantation. The Lancet. 2001;357:442. [DOI] [PubMed] [Google Scholar]