Abstract
The expense of production and distribution of snakebite antivenom, as well as its relatively infrequent use, has caused antivenom to be increasingly difficult to obtain and ultimately producing an alarming global shortage. Unused, expired antivenom may represent a significant, untapped resource to ameliorate this crisis. This study examines the efficacy of expired antivenom over time using in vitro, whole blood clotting, and platelet function statistics. Representatives from three years for four different global brands of polyvalent antivenom were chosen and tested against their corresponding venoms as well as other venoms that could display cross-reactivity. These antivenoms include Wyeth Polyvalent (U.S.; exp. 1997, 2001, 2003), Antivipmyn® (Mexico; exp. 2005, 2013, 2017), Biotecfars Polyvalent (Venezuela; exp. 2010, 2014, 2016), and SAIMR (South Africa; exp. 1997, 2005, 2017). Venoms of species tested were Crotalus atrox against Wyeth; C. atrox and Crotalus vegrandis against Antivipmyn®; C. atrox, C. vegrandis and Bothrops colombiensis against Biotecfar; and Bitis gabonica and Echis carinatus against South African Institute for Medical Research (SAIMR). Parameters recorded were activated clotting time (ACT), clotting rate (CR), and platelet function (PF). Preliminary results are encouraging as the antivenoms maintained significant efficacy even 20 y after their expiration date. We anticipate these results will motivate further studies and provide hope in the cases of snakebite emergencies when preferable treatments are unavailable.
Keywords: Expired antivenom, Activated clot time, Clot rate, Platelet Function, Wyeth, Antivipmyn®, Biotecfar, South African Institute for Medical Research (SAIMR), Crotalus atrox, Crotalus vegrandis, Bothrops colombiensis, Bitis gabonica, Echis carinatus, Sonoclot Coagulation & Platelet Function Analyzer
1. Introduction
In 2015, it was estimated that five million people suffered from snakebites annually, causing approximately 100,000 deaths and 400,000 permanent injuries. Of these, 30,000 deaths and 8,000 amputations occurred in sub-Saharan Africa alone (Gutierrez et al., 2010; Kasturiratne et al., 2008). Most bites occur in rural areas where medical care is often inaccessible and antivenom is scarce. Even if antivenom can be obtained, it is often prohibitively expensive (Harrison et al., 2009). Over the past 20 years, the increasing cost of the most effective polyvalent antivenoms has reduced demand causing manufacturers to cease production, thus opening avenues for the introduction of less effective and safe antivenoms (Alirol et al., 2015; Harrison et al., 2017).
Companies that have historically produced antivenom for snakebites are shifting to more profitable pharmaceuticals, or shutting down altogether. A prime example is the 2003 discontinuation of the FDA-approved North American Coral Snake Antivenin® (NACSAV). Even though production has been reported to resume (Wood et al., 2013), supplies are not still available. It has been over 15 years since the termination of this antivenom, however, FDA stability studies have allowed repeated extensions to the expiration date of the remaining supply of NACSAV (FDA, 2019). Although not as many people in the U.S. may be bitten in the staggering numbers as in other countries (Gummin et al., 2017), this is a neglected health issue nonetheless.
Furthermore, in 2014, Sanofi Pasteur stopped the production of Fav-Afrique® with a June 2016 expiration date. This antivenom was considered to be “the world’s only antivenom proven safe and effective to treat envenoming from different types of snakes across Sub-Saharan Africa” (Alirol et al., 2015). Mortality estimates in this area range from 7,000-32,000 deaths per year (Chippaux, 1998; Kasturiratne et al., 2008), but data are fragmented since in West Africa alone mortality has been estimated at 3,557-5,450 deaths per year (Habib et al., 2015). These alarming statistics have triggered the World Health Organization (WHO) to take note and render snakebite as a neglected tropical disease (NTD) (WHO, 2019). Snake envenoming is among the top killers of the 20 NTDs listed by WHO (WHO, 2019; Alirol et al., 2015). Because of the antivenom shortage, there needs to be an alternative avenue to alleviate the human suffering that is a result of snakebites, and one possible short-term alternative could be the use of expired antivenoms that may still be capable of neutralizing venoms far beyond their expiration dates.
Currently, physicians in the U.S. are prohibited from administering medications past their designated expiration date (American College of Medical Technology, 2015). Because the U.S. legislation intended to prevent the diversion of drugs of abuse, criminal sanctions on physicians and facilities that use expired drugs and antivenoms may be proposed. The reluctance of hospitals to purchase medicine that may expire before use, further exacerbate the lack of antivenom.
The stability of lyophilized proteins and anecdotal evidence of the efficacy of expired antivenom allow for the possibility that functional products are being overlooked (O’Leary et al., 2009; Fuchs et al., 2017). This study explores the activity levels of expired antivenom over time. Four brands of expired antivenom (Table 1) were tested for their ability to neutralize the coagulopathic effects of venom from the Western Diamondback Rattlesnake (Crotalus atrox), Uracoan Rattlesnake (Crotalus vegrandis), Venezuelan Lancehead (Bothrops colombiensis), Gaboon Viper (Bitis gabonica), and Saw Scaled Viper (Echis carinatus). The purpose of this study was to evaluate the ability of expired antivenoms to continue neutralizing venom activities in vitro beyond their intended dates.
Table 1.
Information of Expired Antivenoms Used For In Vitro Venom Neutralization
| Manufacturer | Proprietary Name |
Lot/Batch (Exp. date) |
Venoms Used (Conc. in mg/ml) | ||
|---|---|---|---|---|---|
| Wyeth, U.S.A | Antivenin (Crotalidae) Polyvalent | 4928049 (Mar 1997) |
4968268 (Aug 2001) |
4978260 (Jul 2003) |
Crotalus atrox (0.4) |
| Instituto Bioclon, Mexico | Antivipmyn® | B-1G-07 (Jul 2005) |
B-9A-21 (Feb 2013) |
B-3B-05 (Feb 2017) |
C. atrox (0.4) C. vegrandis* (0.25) |
| Biotecfar C.A, Venezuela | Suero Antiofidico Polivalente | 140 (Feb 2010) |
165 (Sep 2014) |
176 (Nov 2016) |
Bothrops colombiensis(0.05) C. vegrandis(0.25) C. atrox* (0.4) |
| SAIMR, South Africa | Polyvalent Snakebite Antiserum | G01646A (Apr 1997) |
P01446 (Jul 2005) |
BF 00446 (Nov 2017) |
Bitis gabonica (0.25) Echis carinatus*† (0.0075) |
Venoms were used as negative controls;
Venom was a pooled source purchased from SIGMA
2. Materials and methods
2.1. Antivenoms/Venoms
Inventory was taken of the National Natural Toxin Research Center’s (NNTRC) stock of donated expired antivenoms. Lot numbers, expiration or manufacture dates, and indicated species were recorded. Antivenoms (AV) included were chosen against venoms of species used to produce the antivenom as well as their availability at the NNTRC. In addition, sufficient antivenom stocks to supply the study without exhausting a particular lot, and as wide a range of expiration dates possible were also taken into consideration. The expiration dates of the antivenoms are used throughout the manuscript for identification purposes. With the exception of Echis carinatus venom (SIGMA), venoms were from individual specimens housed at the NNTRC.
Final selection included Antivenin (Crotalidae) Polyvalent from Wyeth tested against the Western Diamondback Rattlesnake (Crotalus atrox, Dimmitt, Co., TX, U.S.A.) venom; Antivipmyn® against C. atrox and Uracoan Rattlesnake (C. vegrandis, State of Monagas, Venezuela) venoms; Suero Antiofidico Polivalente from Biotecfar C.A. against C. vegrandis venom, and the Venezuelan Lancehead (Bothrops colombiensis; captive born) venom; and SAIMR Polyvalent against the Gaboon Viper (Bitis gabonica, captive born) and E. carinatus (Saw-scaled viper) venoms. As negative controls, C. atrox venom was used against Biotecfar antivenom, C. vegrandis venom was used against Antivipmyn antivenom, and E. carinatus venom was used against SAIMR antivenom (Table 1). Biotecfar C.A. and SAIMR antivenoms were in liquid form as distributed by the manufacturer, while Wyeth, and Antivipmyn were lyophilized and reconstituted with 0.85% NaCl solution following the instructions as directed on the antivenom package slip.
2.2. Human Subjects
Blood was collected from healthy adults who had not taken any aspirin or drugs that can alter blood coagulation 14 days prior to blood draw (IRB #2012-196-R6-A1). One donor was used for each experiment, and a total of five donors were used throughout the entire experiment process. Briefly, whole human blood was collected via gravity flow in a 50 mL test tube containing sodium citrate (3.8% sodium citrate/blood, 1:10) using a 19G3/4 Vacutainer needle with 12” tubing.
2.3. Sonoclot Analysis of Venom and Venom/Antivenom Activities
Activated clot time (ACT), clot rate (CR), and platelet function (PF) were measured by a Sonoclot Coagulation & Platelet Function Analyzer (SCPFA) (SIENCO, Inc., Boulder, CO, U.S.A) as described by (Suntravat et al., 2009). A total of 50 μL of venom was incubated with 50 μL of antivenom at 37°C for 30 min. As controls, 50 μL of venom was incubated with 0.85% NaCl and 50 μL of antivenom was incubated with 0.85% NaCl at 37°C for 30 min. A normal saline solution (NSS) was also used as a control. A total of 10 μL of these samples were added to SIENCO gbACT + KIT cuvettes along with 10 μL of 0.3M CaCl2, and 330 μL of citrated, whole human blood. A total of 10 μL of 0.3M CaCl2 was added to one side of a gbACT + KIT cuvette, and then 10 μL of sample (0.85% NaCl, venom/0.85% NaCl, venom/antivenom or antivenom/0.85% NaCl) were added to the opposite side of the cuvette. Upon adding the CaCl2 and the sample, 330 μL of whole human blood sample was added, and the Sonoclot activated. Data were collected by Signature Viewer™ program v.4 provided by Sienco™, Inc. on a Dell computer and analyzed by Microsoft Excel for Mac 2011.
2.4. Minimum Venom Concentration Using Sonoclot Analysis
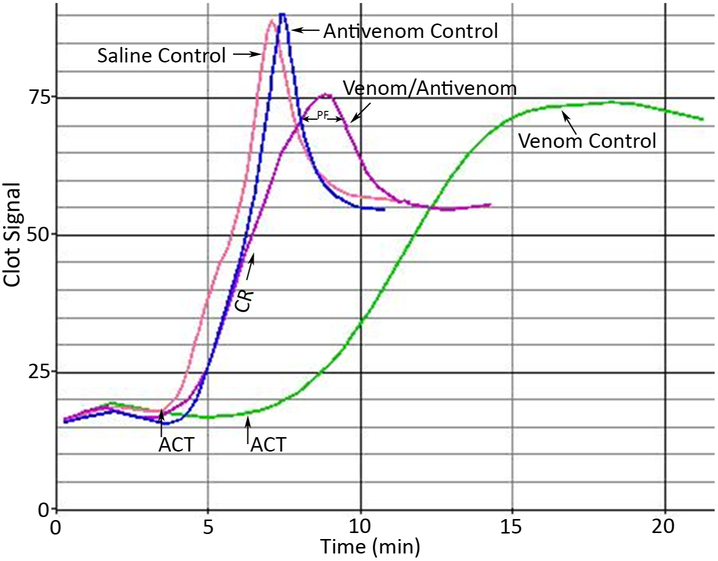
Venom samples were standardized to a minimum concentration of effect on blood samples. The minimum concentration of effect was determined as the lowest venom concentration to provide a statistically significant difference from NSS control. The ACT, CR, and PF were the parameters measured on citrated whole human blood samples (3.8% sodium citrate/blood, 1:10). The ACT is the time it takes to form a clot by contact activation of the coagulation cascade. The contact material for gbACT + KIT is glass beads. The CR is rate of clot formation in clot signal/time, and the PF is a result of clot retraction. The release of multiple coagulation factors from platelets cause the clot to retract. The PF is detected by changes in the viscoelastic measurement of the clot. Fast or robust changes signify stronger platelet function. Figure 1 illustrates suitable platelet function showing significant changes during the clot retraction phase (normal blood control). It is important to recognize that reference values for healthy populations may vary from a specific donor’s normal values. Expected values for healthy adults are 1.67-2.6 min (ACT), 9-35 clot signal /min (CR), and >1.5 (PF). Reference values for citrated blood using gbACT + KIT cuvettes for healthy populations may be different from reference values for specific populations. Concentrations of venoms used in this study are shown in Table 1.
Fig. 1.
Blood Signature Profiles Generated By A Sonoclot Coagulation & Platelet Function Analyzer. The ACT, CR, and PF were the parameters measured on whole human blood samples (3.8% sodium citrate/blood, 1:10). Expected values for healthy adults are 1.67-2.58 min (ACT), 9-35 clot signal units/min (CR), and >1.5 (PF). Reference values for citrated blood using gbACT + KIT cuvettes for healthy populations may be different than reference values for specific populations. These blood signatures represent neutralization of C. atrox venom by Wyeth antivenom. A normal blood signature is represented by the graph labeled as “Saline Control”. The graph labeled “Venom Control” is a blood signature containing C. atrox venom, the graph labeled “Venom/Antivenom” is a blood signature with C. atrox venom and Wyeth antivenom mixture, and the graph labeled “Antivenom Control” contains Wyeth antivenom.
2.5. Statistics
A P<0.05 signifies a significant difference when compared to the saline controls and venom controls. P values were calculated using a t-test, two-tailed P value on Microsoft® Excel® for Mac 2011 v. 17.7.3. A total of 3 trials were evaluated for each sample.
3. Results and Discussion
In June 2017, the WHO included snakebite envenoming in the category A of its list of neglected tropical diseases. In doing so, this will put forth the urgency of the pathology of snake envenoming attracting more attention by governmental agencies and other powers of wills, which in turn, we hope, will provide more resources to allow for the improvements of antivenom availability, accessibility, affordability, and efficacy. Presently, there are antivenoms that are marked with expiration dates that are potentially functional (Tan et al., 2019) and could be an alternative for snakebite therapy when companies cease to produce them due to the vicious cycle of antivenom market decline, which is created by the high prices of antivenoms that drives down their affordability; thereby, reducing the sales of antivenoms. In addition, these expired antivenoms could also be used in the event of emergencies by non-indigenous venomous snakebites in which the specific antivenoms of the country of origin cannot be obtained on time. There is evidence of paraspecific neutralization of snake venoms (Ainsworth et al., 2018; Rogalski et al., 2017); however, this may require higher dosing since pre-clinical neutralization studies done by premixing and incubating venoms as recommended by the WHO could deliver a false sense of security in using an antivenoms from outside the geographical range from which the venomous snakes reside (Visser et al., 2008; Warrel et al., 2008; Rogalski et al., 2017). This study was not to determine how well one lot of antivenom can perform over another lot of antivenom since it is known that manufacturing of antivenoms can be a complicated process especially when dealing with venom variations that occur even within the same species (Salazar et al., 2009; Massey et al., 2012; Cantu et al., 2017; Girón et al., 2018). Venom variation can complicate immunization protocols causing antivenoms to not have the same neutralizing ability as lots produced in earlier years. This study was done to demonstrate the ability for antivenoms to maintain their activities extensively beyond their expiration dates.
Antivenom availability is a valid concern and crisis particularly in sub-Saharan Africa and parts of Asia (Gutiérrez, 2016; Harrison and Gutiérrez, 2016; Gutiérrez et al., 2017; Gutiérrez, 2019). There exist a critical need to make antivenoms available to those countries that lack the resources to provide this much needed therapeutic agent to those who suffer a debilitating snakebite. Availability should consist of providing safe and effective antivenoms in a timely manner and allowing treatments to be inexpensive and accessible to invariably remote, underprivileged people. A deteriorating inclination of antivenoms in disparagingly short supply places patients at increased risk for unfavorable consequences. The allocation of expiration dates, as mandated under federal law (FDA, 2018), to antivenoms and other antidotes, further complicates this issue. Nonetheless, analyses have revealed that some therapeutic agents are stable far past their labeled expiration dates (Lyon et al., 2006; Hoffman et al., 2012; American College of Medical Technology, 2015). Although potency may decline, there is little proof that expired drugs deteriorate to unsafe components. Whereas potency is vital for some drugs that have a limited curative window, many antivenoms are dosed via titrated to reach a desired effect.
In this current study, a total of four antivenoms consisting of three different years per antivenom were tested with whole human blood for their efficacy to neutralize venom activities affecting ACT, CR, and PF. The goal of this study was to utilize an in vitro assay that would be able to determine and compare the efficacy of older antivenoms to that of newer ones. Additionally, there is a strong initiative to minimize the use of animal studies whenever possible; and although Sonoclot Analysis may not give information on how well antivenoms can neutralize neurotoxins, it can determine neutralization of hemotoxins by antivenoms which more than likely can infer the ability to neutralize lethal activity.
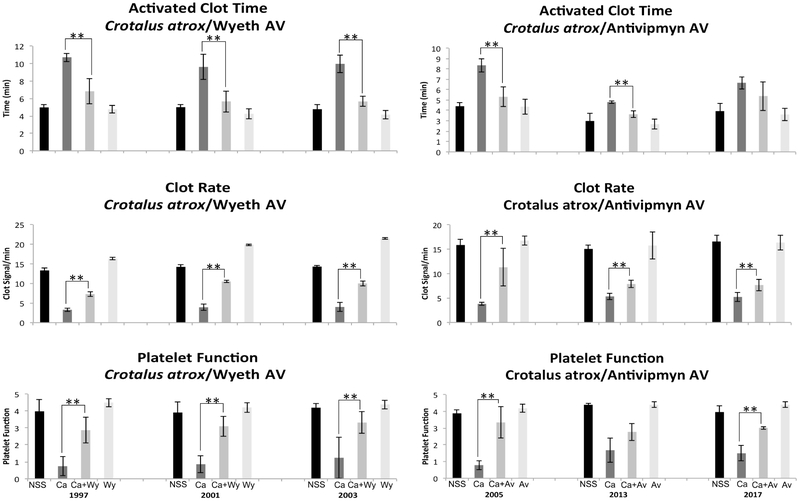
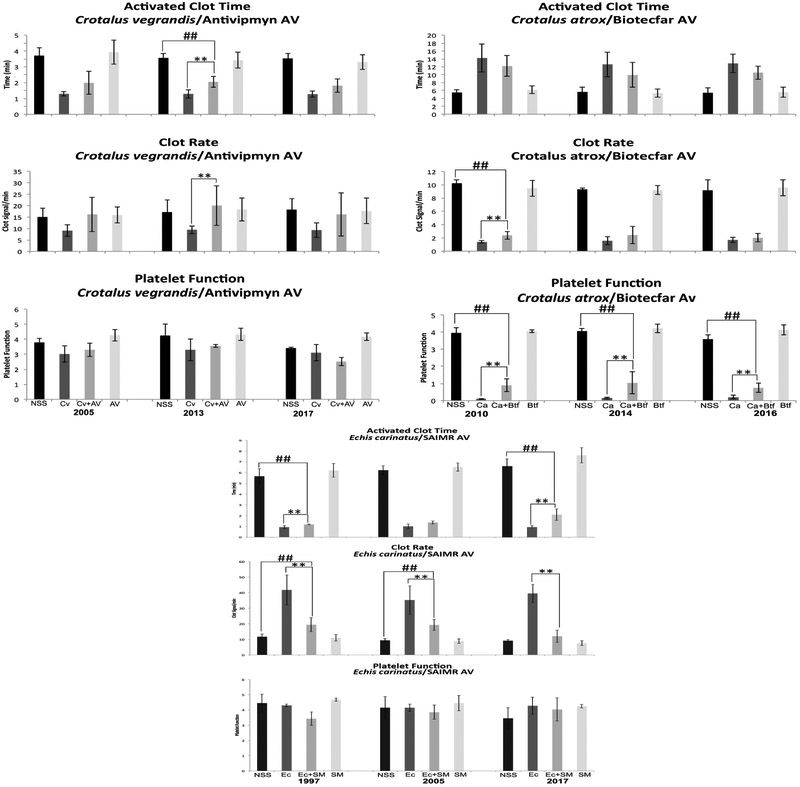
Crotalus atrox venom was used first for evaluating antivenoms from the U.S. since it was used in the immunization protocols to produce Wyeth and Antivipmyn antivenoms. Wyeth antivenom significantly neutralized C. atrox venom’s activities on ACT and PF, while the CR, although neutralized, did not fully recover to normal basal values (Fig. 2). The data suggest that the year 1997 antivenom was just as effective as those from 2001 and 2003. These results, especially for year 1997, were positive since they suggest that these antivenoms are potentially viable over 20 years after their expiration date. Antivipmyn, an antivenom similar to Anivip (the newest antivenom in the U.S. market), has the ability to neutralize C. atrox venom as well (Fig. 2). Even though the three lots were able to neutralize at least one of the activities tested, year 2005 was the most effective since it neutralized all the three activities. The year 2013 was effective in neutralizing the venom’s activity on ACT, it showed significant neutralizing activity for CR even though the numbers did not return to normal basal values, and no significant neutralization was seen for PF (Fig. 2). There were similar findings for the year 2017, although there was some neutralization, the values were not restored to the range of the normal controls as in the 2005 antivenom. This study was merely to test efficacy in older antivenoms, which was accomplished with these particular antivenoms, but it also appears to show that there are differences between lots of the Antivipmyn antivenoms. These differences could arise from a number of factors ranging from the condition of the immunization hosts, the use of different lots of venom in the immunization protocols, and changes in manufacturing protocols, to name a few. Nonetheless, the fact that the 2005 antivenom was still significantly effective in neutralizing C. atrox venom indicates the potency of these expired antivenoms. As a negative control, C. atrox venom was used with Biotecfar since this venom is not used in the immunization protocol to produce the Venezuelan antivenom. Although there is some neutralization that occurred on the venom’s activity on PF for all the years tested and on CR for only year 2010, values did not return to NSS values, which was to be expected (Fig. 5).
Fig. 2.
A Sonoclot Coagulation & Platelet Function Analyzer was used to determine the neutralization of C. atrox venom effects on ACT, CR, and PF by Wyeth and Antivipmyn expired antivenoms. Black bars: NSS 0.85% NaCl, Dark grey bars: venom controls (venom+NaCl), Medium grey bars: venom/antivenom samples, and Light grey bars: antivenom controls (antivenom+NaCl). NSS: normal control, Ca: C. atrox venom, Wy: Wyeth antivenom, and AV: Antivipmyn antivenom. ** Indicates significant differences between the venom controls and the venom/antivenom samples.
Fig. 5.
A Sonoclot Coagulation & Platelet Function Analyzer was used to determine paraspecific neutralization of C. vegrandis, C. atrox and E. carinatus venom effects on ACT, CR, and PF by expired Biotecfar, Antivipmyn, and SAIMR antivenoms. Black bars: NSS 0.85% NaCl, Dark grey bars: venom controls (venom+NaCl), Medium grey bars: venom/antivenom samples, and Light grey bars: antivenom controls (antivenom+NaCl). NSS: normal control, Cv: C. vegrandis venom, Av: Antivipmyn antivenom, Ca: C. atrox venom, Btf: Biotecfar antivenom, Ec: E. carinatus venom, and SM: SAIMR antivenom. ** Indicates significant differences between the venom controls and the venom/antivenom samples. ##/** Indicates significant differences between venom controls and venom/antivenom samples and significant differences between normal controls and venom/antivenom samples, but the venom/antivenom values did not return to basal values of the normal controls.
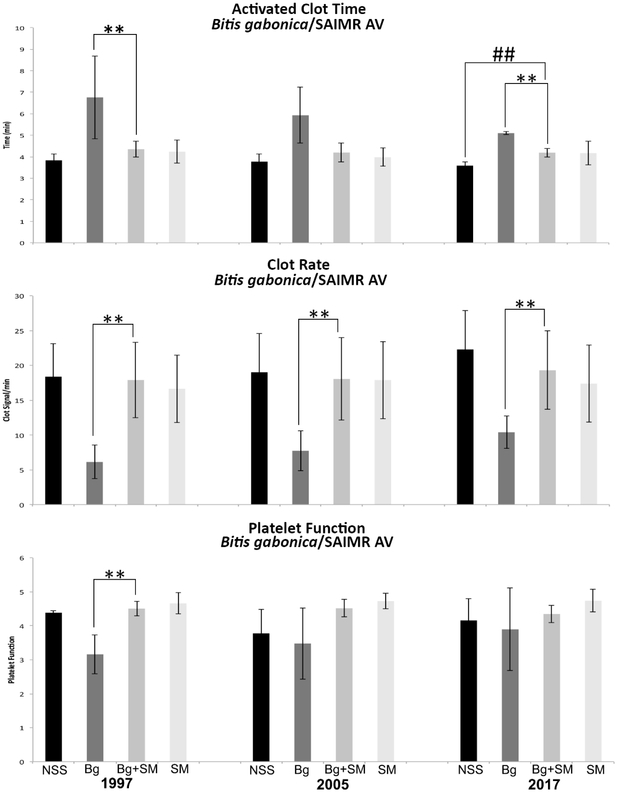
We then sought to test the SAIMR antivenom from South Africa. The ability to neutralize the ACT activities of B. gabonica venom by SAIMR antivenom proved to be significant for all three years, 1997, 2005 and 2017. In this particular antivenom, ACT and CR were significantly restored to normal control values with antivenom that had expired 20 years after the expiration date. Although B. gabonica venom did not have a substantial effect on platelet function while testing the 2005 and 2017 antivenoms, the venom appeared to affect platelet function when testing year 1997. There was a significant venom response in PF as compared to the saline control, which was significantly neutralized by the antivenom (Fig. 3). In the subsequent years, 2005 and 2017, the donors’ blood platelets did not respond significantly to the venom. It may have been that the platelets of that individual donor included in the year 1997 experiment were more susceptible to the venom than the rest of the donor population. Medication, sampling discrepancy, and operator technique can alter reference values. In this study, all donors had platelet functions >3 for their normal controls. As a negative control, E. carinatus venom was used with the SAIMR antivenom since E. ocellatus venom was used to develop this antivenom. Significant venom neutralization was detected on ACT for antivenom years 1997 and 2017 when compared to the venom controls; however, the values did not return to the values of the normal controls. No venom neutralization was detected on ACT for year 2005. All three years of SAIMR were able to significantly neutralize CR when compared to the venom controls, but years 1997 and 2005 were not able to return to normal basal levels as were achieved with year 2017 (Fig. 5). Echis carinatus venom did not affect PF.
Fig. 3.
A Sonoclot Coagulation & Platelet Function Analyzer was used to determine the neutralization of B. gabonica venom effects on ACT, CR, and PF by SAIMR expired antivenoms. Black bars: NSS 0.85% NaCl, Dark grey bars: venom controls (venom+NaCl), Medium grey bars: venom/antivenom samples, and Light grey bars: antivenom controls (antivenom+NaCl). NSS: normal control, Bg: B. gabonica venom, SM: SAIMR antivenom, Ec: E. carinatus venom. ** Indicates significant differences between the venom controls and the venom/antivenom samples. ##/** Indicates significant differences between venom controls and venom/antivenom samples and significant differences between normal controls and venom/antivenom samples, but the venom/antivenom values did not return to basal values of the normal controls.
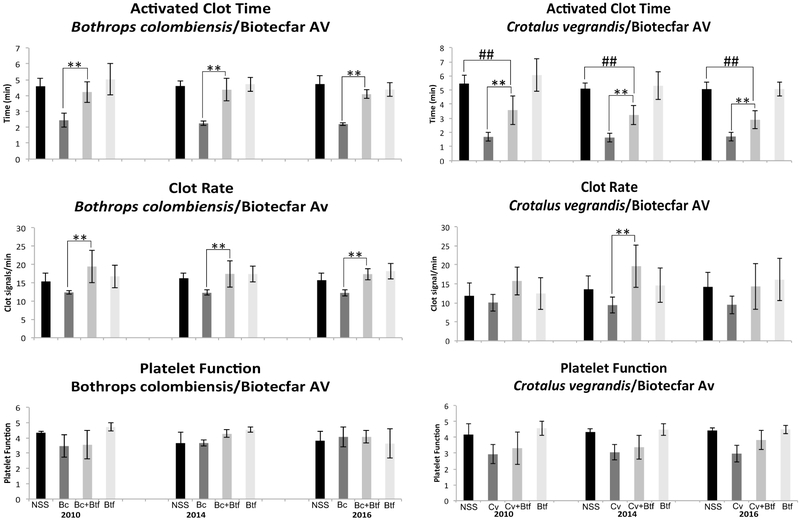
We next evaluated Biotecfar antivenom for years 2010, 2014 and 2016 all which had significant neutralizing ability on the activities of B. colombiensis venom on ACT and CR. All three years were able to restore values to that of the normal saline controls (Fig. 4). This venom did not affect PF for all donors. Although it has been described that B. colombiensis venom does contain disintegrins (Calvete et al., 2015) that affect platelet function (Sanchez et al., 2009; Suntravat et al., 2016a), the abundance of these disintegrins in this particular venom sample may not have been sufficient to affect this parameter in this specific assay (Suntravat et al., 2016b), thus causing no alterations as compared with the normal saline controls when whole blood was tested. When using C. vegrandis venom, the ability of Biotecfar to neutralize its effect on ACT was significant, but the ACT values were not restored to the levels of the normal saline control with all three antivenom years. These two Venezuelan venoms along with E. carinatus are procoagulant since they all accelerated the ACT as compared to the normal saline controls (Figs. 4 & 5). These venoms are in contrast to the other venoms (C. atrox and B. gabonica) used in this study that contained anticoagulant activity as demonstrated by the delayed ACT (Figs. 2 & 3). Crotalus vegrandis venom did not significantly affect CR. Although the venom significantly affected PF in the experiments done with years 2014 and 2016, no significant neutralization occurred. While the insert for Biotecfar antivenom indicates that it can neutralize Crotalus venom, it did not have the ability to fully neutralize C. vegrandis venom components that affect blood factors. This may explain why the Venezuelans produced another antivenom (Suero Anticrotalico) solely for Crotalid species. It is known that C. vegrandis venom contains a mixture of hemotoxins and neurotoxins (Viala et al., 2015), and because neutralization of this venom was not impressive does not imply that Biotecfar antivenom does not have the ability to neutralize those important neurotoxins that can lead to death. However, if lethal components are not efficiently neutralized, it may not be because the antivenoms are expired but because they initially never had the capacity to do so. As another negative control, Antivipmyn antivenom was used to test its neutralizing abilities on the activities of C. vegrandis venom, and it was not unexpected that this antivenom performed poorly. There was no significant neutralization of both ACT and CR by antivenom years 2005 and 2017. Year 2013 had significant neutralization on the venom activities affecting ACT and CR when compared to the venom controls but was still proven to be weak. Crotalus vegrandis venom did not appear to have affected the platelet activities of this population of donors (Fig. 5). While C. vegrandis venom significantly affected the PF of the population of donors during the Biotecfar assay, the values were still not as impressive as those obtained by C. atrox venom in which the values for PF were consistently low in all tests (Figs. 2 & 5).
Fig. 4.
A Sonoclot Coagulation & Platelet Function Analyzer was used to determine the neutralization of B. colombiensis and C. vegrandis venoms effect on ACT, CR, and PF by Biotecfar expired antivenoms. Black bars: NSS 0.85% NaCl, Dark grey bars: venom controls (venom+NaCl), Medium grey bars: venom/antivenom samples, and Light grey bars: antivenom controls (antivenom+NaCl). NSS: normal control, Bc: B. colombiensis venom, Btf: Biotecfar antivenom, and Cv: C. vegrandis venom. ** Indicates significant differences between the venom controls and the venom/antivenom samples. ##/** Indicates significant differences between venom controls and venom/antivenom samples and significant differences between normal controls and venom/antivenom samples, but the venom/antivenom values did not return to basal values of the normal controls.
The three negative controls were important to include in this study because they were not only an indication that using Sonoclot Analysis is sufficient in measuring the parameters to evaluate antivenom efficacy but to determine paraspecific neutralization. Another important observation was that antivenoms that are manufactured and distributed in liquid form (SAIMR and Biotecfar) were able to maintain their neutralizing abilities even 20 years after their expiration date. It is important to note that this study was to examine and evaluate antivenom efficacy of expired antivenoms, and it is apparent that if antivenoms are maintained at proper conditions, they have the potential to retain their pharmacotherapeutic efficacy.
4. Conclusion
This study demonstrated the ability of expired antivenoms to sustain their pharmacological actions up to 20 years beyond their expiration date, and with the alarming global situation of antivenom shortages, expired antivenoms could provide an alternative option for snakebite treatment.
Highlights.
We tested the neutralizing efficacy of four global expired antivenoms.
Efficacy was tested in vitro using Sonoclot Coagulation & Platelet Function Analyzers.
Neutralization of venom activities on activated clot time, clot rate and platelet function were significant.
Antivenoms can maintain their efficacy 20 years after expiration.
Acknowledgments
Funding for this project was provided by Grant from the NIH/ORIP, Viper Resource Grant # P40OD01960-15 (National Natural Toxins Research Center (NNTRC), Texas A&M University-Kingsville, Dr. E.E. Sánchez), and the Robert A. Welch Foundation Department, grant # AC-0006 (TAMUK-Department of Chemistry). We also thank Mark Hockmuller and Juan Salinas, curator and animal room technician of the NNTRC and Nora Diaz De Leon for her administrative assistance.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alirol E, Lechevalier P, Zamatto F, Chappuis F, Alcoba G, Potet J (2015) Antivenoms for snakebite envenoming: What is in the research pipeline? PLoS Negl Trop Dis. 9(9), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Medical Toxicology & American Academy of Clinical Toxicology (2015) Antidote Shortages in the USA: Impact and Response. J. Med. Toxicol. 11, 144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth S, Slagboom J, Alomran N, Pla D, Alhamdi Y, King SI, Bolton FMS, Gutierrez JM, Vonk FJ, Toh C-H, Calvete JJ, Kool J, Harrison RA, Casewell NR (2018) The paraspecific neutralization of snake venom induced coagulopathy by antivenoms. Comm Bio. 34, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ, Borges A, Segura A, Flores-Diaz M, Alape-Giron A, Gutierrez JM, Diez N, De Sousa L, Kiriakos D, Sánchez E, Faks JG, Escolano J, Sanz L (2009) Snake venomics and antivenomics of Bothrops colombiensis, a medically important pitviper of the Bothrops atrox-asper complex endemic to Venezuela: Contributing to its taxonomy and snakebite management. J Proteomics. 72, 227–240. [DOI] [PubMed] [Google Scholar]
- Cantu E Jr., Mallela S, Nyguen M, Báez R, Parra V, Johnson R, Wilson K, Suntravat M, Lucena S Rodriguez-Acosta A, Sánchez EE (2017) The binding effectiveness of anti-disintegrin polyclonal antibodies against disintegrins and PII and PIII metalloproteases: An immunological survey of type A, B and A+B venoms from Mohave rattlesnakes. Comp. Biochem. Physiol C Toxicol Pharmacol. 191, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux JP (1998) Snake-bites: appraisal of the global situation. Bull. World Health Organ. 76, 515–524. [PMC free article] [PubMed] [Google Scholar]
- Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W (2017) 2016 Annual Report of the American Association of Poison Control Centers’ National Data System (NPDS): 34th Annual Report. Clin. Toxicol. 55, 1072–1254. [DOI] [PubMed] [Google Scholar]
- de Queiroz MR, de Sousa BB, da Cunha Pereira DF, Mamede CCN, Matias MS, de Morais NCG, de Oliveira Costa J, de Oliveira F (2017) The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon. 133, 33–47. [DOI] [PubMed] [Google Scholar]
- FDA Code of Federal Regulations. Title 21. Chapter 1 (Food and Drug Administration Department of Health and Human Services). Subpart G (Packaging and Labeling Control). Part 211 (Current Good Manufacturing Practices for Finished Pharmaceuticals. Available at: http://www.acessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=211&showFR=1 [accessed January 20, 2019].
- Fuchs J, Casado Diaz JI, Schefer RJ, Rauber-Lüthy C (2017) Expired antivenom: good efficacy in a severely envenomed cat bitten by Sistrurus miliarius miliarius (Carolina Pigmy Rattlensnake). Clinical Toxicology. 55, 613–614. [DOI] [PubMed] [Google Scholar]
- Girón ME, Padrón V, Ramos MI, Sánchez EE, Guerrero B, García A, Uzcátegui NL, Navarrete LF, Rodríguez-Acosta A (2018) Intraspecies geographical variability in South American tigra mariposa (Bothrops venezuelensis Sandner 1952) snake venom activities. Toxicon. 144, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez JM, Williams D, Fan HW, Warrell DA (2010) Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon. 56, 1223–1235. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM (2016) Improving antivenom availability and accessibility: science, technology, and beyond. Toxicon. 60, 676–687. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrel DA (2017) Snakebite envenoming. Primer. 3, 1–20. [Google Scholar]
- Gutiérrez JM (2019) Global availability of antivenoms: the relevance of public manufacturing laboratories. Toxins. 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib AG (2015) Snakebite is underappreciated: appraisal of burden from West Africa. PLoS Negl. Trop. Dis. 9, e0004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG (2009) Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 3, e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R, Gutiérrez J (2016) Priority actions and progress to substantially and sustainably reduce the mortality, morbidity and socioeconomic burden of tropical snakebite. Toxins (Basel). 8, E351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RA, Oluoch GO, Ainsworth S, Alsolaiss J, Bolton F, Arias AS, Gutierrez JM, Rowley P, Kalya S, Ozwara H, Casewell NR (2017) Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Negl. Trop Dis. 11(1): e0005969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RS, Mercurio-Zappala M, Bouchard N, Ravikumar P, Goldfrank L (2012) Preparing for chemical terrorism: a study of the stability of expired pralidoxime (2-PAM). Disaster Med Public Health Prep. 6, 20–25. [DOI] [PubMed] [Google Scholar]
- Kasturiratne A, Wichremasinghe AR, De Silva N, Gunawardena NK, De Silva N, Pathmeswaran A, Premaratna R Savioli L, Lalloo DG De Silva HJ (2008) The global burden of snakebite: A literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 5, e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon RC, Taylor JS, Porter DA, Prasanna HR, Hussain AS (2006) Stability profiles of drug products extended beyond labeled expiration dates. J. Pharm Sci. 95, 1549–1560. [DOI] [PubMed] [Google Scholar]
- Massey DJ, Calvete JJ, Sánchez EE, Sanz L, Richards K, Curtis R, Boesen K (2012) Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J Proteomics. 75, 2576–2587. [DOI] [PubMed] [Google Scholar]
- O’Leary MA, Kornhauser RS, Hodgson WC, Isbister GK, (2009) An examination of the activity of expired and mistreated commercial Australian antivenoms. Transaction of the Royal Society of Tropical Medicine and Hygiene. 103, 937–942. [DOI] [PubMed] [Google Scholar]
- Rogalski A Soerensen C, op den Brouw B, Lister C, Dashevsky D, Arbuckle K, Goria A, Zdenek CN, Casewell NR, Gutierrez JM, Wüster W, Ali SA, Masci P, Rowley P, Frank N, Fry BG (2017) Differential procoagulant effects of saw-scaled viper (Serpentes: Viperidae: Echis) snake venoms on human plasma and the narrow taxonomic ranges of antivenom efficacies. Toxicol Lett. 280, 159–170. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Guerrero B, Cantu B, Cantu E, Rodríguez-Acosta A, Perez JC, Galan JA, Tao A, Sánchez EE (2009) Venom variation in hemostasis of the southern Pacific rattlesnake (Crotalus oreganus helleri): Isolation of hellerase. Comp Biochem Physiol C Toxicol Pharmacol. 149, 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez EE, Rodríguez-Acosta A, Palomar R, Lucena SE, Bashir S, Soto JG Perez JC (2009) Colombistatin: a disintegrin isolated from the venom the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-Mel-28 cell adhesion. Arch Toxicol. 83, 731–739. [DOI] [PubMed] [Google Scholar]
- Suntravat M, Helmke TJ, Atphaisit C, Cuevas E, Lucena SE, Uzcátegui NL, Sánchez EE, Rodriguez-Acosta A (2016a). Expression, purification, and analysis of three recombinant ECD disintegrins (r-colombistatins) from P-III class snake venom metalloproteinases affecting platelet aggregation and SKMEL-28 cell adhesion Toxicon. 122, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntravat M, Uzcategui NL, Atphaisit C, Helmke TJ, Lucena SE, Sánchez EE, Acosta AR (2016b) Gene expression profiling of the venom gland from the Venezuelan mapanare (Bothrops colombiensis) using expressed sequence tags (ESTs). BMC Mol Biol. 17, 7. Erratum in: BMC Mol Biol. (2016) 17, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YK, Liew ST, Tan QY, Abdul-Rahman FN, Azmi NI, Sim SM, Tan NH, Khomvilai S, Sitprija V, Tan CH (2019) Evaluating the physicochemical properties and efficacy of recently expired and aged antivenom products from Thailand and Taiwan. Toxicon. 160, 55–58. [DOI] [PubMed] [Google Scholar]
- Viala V, Hildebrand D, Fucase TM, Sciani JM, Prezotto-Neto JP, Riender M, Sanches L, Nishimura PJ, Oguiura N, Pimenta DC, Schlüter H, Betzel C, Ami RK, Spencer PJ (2015) Proteomic analysis of the rare Uracoan rattlesnake Crotalus vegrandis venom: Evidence of a broad arsenal of toxins. Toxicon. 107, 234–251. [DOI] [PubMed] [Google Scholar]
- Visser I,E, Kyei-Faried S, Belcher DW, Geelhoed DW, van Leeuwen JS, van Roosmalen J (2008) Failure of a new antivenom to treat Echis ocellatus snake bite in rural Ghana: the importance of quality surveillance. Trans. R. Soc. Trop. Med. Hyg. 102, 445–450. [DOI] [PubMed] [Google Scholar]
- Warrel DA (2008) Unscrupulous marketing of snake bite antivenoms in Africa and Papua New Guinea: choosing the right product-’what’s in a name? Trans. R. Soc. Trop. Med. Hyg. 102, 607–609. [DOI] [PubMed] [Google Scholar]
- Wood A, Schauben J Thundiyil J, Kunisaki T, Sollee D, Lewis-Younger C, Bernstein J, Weisman R (2013) Review of Eastern coral snake (Micrurus fulvius fulvius) exposures managed by the Florida Poison Information Center Network: 1998-2010. Clinical Toxicology. 51, 783–788. [DOI] [PubMed] [Google Scholar]
- WHO. Neglected Tropical Diseases. [cited 26 April 2019]. http://www.who.int/neglecteddiseases/diseases/en/