Abstract
Purpose:
To describe and evaluate a comprehensive grading system for meibomian gland (MG) digital infrared images developed by for the Dry Eye Assessment and Management (DREAM) Study.
Methods:
Cross-sectional study. Reading Center (RC) certified readers independently evaluated MG features of both lids from meibographs of dry eye disease subjects. Dropout areas were measured using planimetry software. Inter-reader and grade-regrade agreement and comparison of meiboscale scores (Meiboscale©; Pult) from clinical centers to RC percent dropout and of MG features with clinical parameters were evaluated.
Results:
Among 551 eyes of 277 patients at baseline, 62 (11%) upper lid and 5 (1%) lower lid images were missing. Lid eversion was poor in 63 (13%) of upper lids compared to 15 (3%) of lower lids. Intraclass correlation for inter-reader and grade-regrade agreement was moderate to substantial for most MG features. MG features were more frequent in the upper lid (p<0.001), except for dropout glands, gaps, fluffy gland areas and dropout areas. Clinic meiboscale score was associated with RC percent dropout (p<0.001), a clinic score of 0% having a mean RC score of 19%, and a clinic score of >75% having a mean RC score of 66%. MG plugging was associated with ghost glands (p=0.009), dropout glands (p<0.001) and a composite severity score (p=0.02); turbid and absent secretions were associated with ghost glands (p=0.046).
Conclusion:
RC readers identified MG features with good reproducibility. Upper lids had more MG features. RC dropout areas correlated well with clinic meiboscale scores. Ghost glands were associated with paste like and absent meibomian secretions.
Keywords: Dry eye disease, Meibography, Meibomian glands, Morphology, Reading Center
INTRODUCTION
Meibomian gland dysfunction (MGD) is a leading cause of dry eye disease (DED).1 Clinically, fluorescein tear break up time, lid margin irregularity, vascular engorgement, glandular orifice obstruction, anterior or posterior displacement of the mucocutaneous junction, and the quality of expressed sebum have been used for assessing MGD related to DED.2,3 However, there is no standardized, universal grading system that is in use to evaluate the features of MGs. The introduction of non-invasive, infrared photography of meibomian glands (MG) has been a major step towards allowing assessment of two-dimensional details of the silhouette of the glands.
A variety of scoring systems have been used to quantify the degree of MG dropout in the upper and lower lids and to correlate the gland loss to clinical parameters.4–6 These scoring systems generally are intended for clinical use while examining patients. Further, only a small amount of literature describes the association of MG features seen on meibography with clinical parameters. Individual investigations and reviews have clearly spelt out the need for more exhaustive research to improve the correlation of ocular imaging with clinical findings in DED. There is consensus that the combination of both morphological and functional evaluation would be essential to providing further insights into the pathophysiology of DED.8
The Dry Eye Assessment and Management (DREAM) Study7 was a randomized clinical trial of omega-3 fatty acid supplementation for the treatment of moderate to severe DED. Images of MGs were obtained using infrared photography by centers that had the Oculus Keratograph® 5M (OCULUS Optikgeräte, Wetzlar, Germany). The purpose of this paper is to introduce a comprehensive grading system for meibography images, assess the reproducibility of grading by certified readers, and evaluate the association of the gradings with clinical signs of MGD among participants of the DREAM Study.
1. METHODS
1.1. Study population
From October 2014 through July 2016, 535 subjects from 27 clinical centers in the United States completed a screening and eligibility confirmation visits and were enrolled into the study. A detailed description of the DREAM study design has previously been described.9 Briefly, subjects needed to be ≥18 years with ocular symptoms related to DED for at least 6 months with the use of or a desire to use artificial tears. The patient’s average score from the two visits on the OSDI needed to be between 22 and 80. Additionally, patients had to have at least two of the following four signs in at least one eye: a conjunctival lissamine-green staining score of 1 or more (range 0 to 6, higher scores indicate greater abnormality), a corneal fluorescein staining score of 4 or more (range 0 to 15, higher scores indicate greater abnormality), a tear break-up time of ≤7 seconds, and a result on Schirmer’s test with anesthesia of 1 to 7 mm in 5 minutes. The qualifying signs needed to be the same signs in the same eye at each of the visits. Pregnant or nursing mothers, patients with a history of contact lens wear during 30 days before screening visit, ocular surgery within 6 months of screening visit, using glaucoma medications and having eyelid abnormalities were excluded.
The study protocol was approved by the institutional review board associated with each center and carried out under an Investigational New Drug application for the Food and Drug Administration.5 The research followed the tenets of the Declaration of Helsinki. Ten of the 27 DREAM centers had an Oculus Keratograph 5M and only the 292 patients enrolled through the ten centers were eligible for meibography.
1.2. Imaging
Imaging of MGs was not part of the original DREAM research plan because a commercial device became available only after the application for funding was submitted in January 2012. Imaging was aded to the DREAM clinical assessment for those clinical sites that had the imaging device on site by thebeginning of enrollment in October 2014. Staff from each clinical site received standardized training on meibography imaging as part of the overall instruction on using the keratography for evaluations in the DREAM study which also included non-invasive tear break-up time, measuring tear meniscus height, and obtaining a bulbar redness score. A DREAM standardized protocol was developed with specific instructions for obtaining meibography images using eversion of the upper lid and lower lid. Training for keratography included watching a slide presentation on using the keratograph, viewing a 30-minute training video supplied by the manufacturer of the keratograph, participating in a one-on-one webinar with a representative from the manufacturer and a member of the staff of the study chair, review of a detailed set of instructions for uploading the image files to a central server, and demonstration of proper technique to the principal investigator of each clinical site.
1.3. Development of the grading protocol
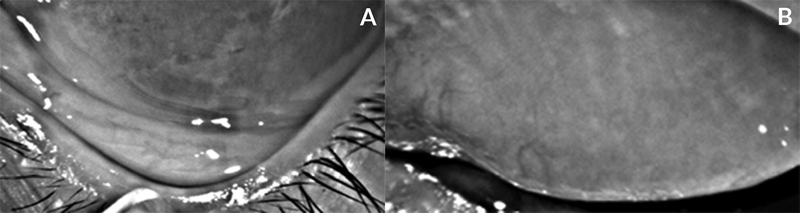
The director of the Reading Center (RC) led the development of the DREAM meibography grading protocol. A comprehensive list of features of MGs was compiled after reviewing publications describing patients with ocular surface disease and normal subjects.10–15 Representative examples of the features were selected from the DREAM Study image database. Specific features of the MGs such as distorted, tortuous, hooked, dropout, shortened, thickened, thinned, overlapping, ghost, tadpoling, abnormal gap, fluffy area, and no extension to lid margin were included in the protocol. These are shown in Figure 1. An example of conjunctival folds, which can masquerade as total MG atrophy, is given in Figure A (appendix).
Figure 1.
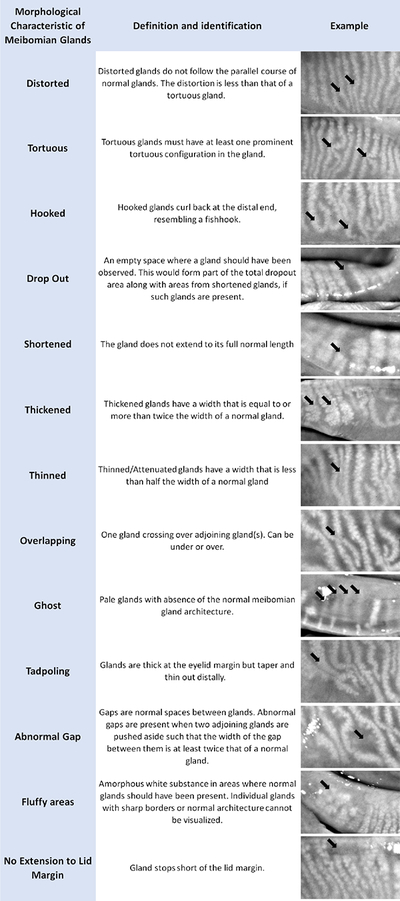
Definitions and representative examples of various meibomian gland features observed in the lids of subjects with moderate to severe dry eye disease.
1.4. Training and certification of image readers
Three non-physician image readers in the DREAM RC (Department of Ophthalmology, University of Pennsylvania, Philadelphia) received training in the assessment of MGs from meibography images. These readers had extensive experience in assessing digital retinal color images, fluorescein angiograms, auto-fluorescent images and optical coherence tomography scans for a variety of retinal diseases, but had no prior experience in grading MG images. Training included reading assignments on the anatomy of MGs,1,6 didactic lectures and interactive sessions with the ophthalmologist director of the Reading Center, and a written multiple choice test. Readers reviewed image sets to familiarize themselves with the DREAM images; a set consisted of one meibography image of the upper and lower lids of both eyes. Readers were required to grade 10 image sets independently. The results were compared among readers and discrepancies were discussed; this process was repeated on a second group of 10 image sets. Finally, 10 lid images were given for grading and the reader was certified if the score was more than 80% on key morphological features and areas of dropouts.
1.5. Grading of study image sets
Two readers graded each lid meibography image independently. The readers were masked to all demographic, clinical, and treatment data. The lid image was opened in Adobe Photoshop (Adobe, Inc. San Jose, CA) and an indigenously developed template was dragged and placed on top of the image. The template, comprised of a rectangle with three inner sections, was positioned so that the horizontal outer border was at the lateral canthus and the inner border was on the caruncle. The template allowed the reader to enumerate and record each of the morphological abnormalities within the three similarly spaced sections (lateral, middle and medial). Counting was done systematically from left to right for each eye and the morphological features were counted into the sections where they first appeared regardless of the quantity of the gland present in that section. Extension of the same gland into an adjacent section was not counted.
Readers also measured the following areas using the lasso tool in Adobe® Photoshop in the following order: total area, inclusive dropout area (areas devoid of MGs, ghost glands, fluffy areas) and exclusive dropout area (areas devoid of MGs only). In the upper lid, the superior outline of the total area was just inside the lid margin and followed the curve of the everted lid with its highest point at the center of the lid. The lower margin was either a straight or a gently curving line and was determined by visual cues using the longest MG in the midpoint of the lid. In cases where there was extensive MG atrophy, the reader, with the help of examples of normal lid meibographs, determined the lower border. The total area of the lower lid was measured in a similar manner.
Grading values from the 2 readers were compared using a computer program and features were selected for adjudication when the differences between readers exceeded a certain threshold (Table A appendix). For the morphological features graded on an ordered categorical scale, if both values were >0 (0 denoting absent) and within a difference of 2, the responses were averaged. All other differences were adjudicated by the RC director. Differences for binary (yes/no) features such as gaps, fluffy areas, tadpoling, and glands stopping short of the lid margin were adjudicated if one reader disagreed with the other. If the difference in measurement values for total area and for dropout areas from the two readers was less than 10% from the mean of the two values, they were averaged. All 3 of these area measurements (total and dropout areas including and excluding fluffy areas and ghost glands) were adjudicated by the ophthalmologist if the difference between readers for one or more of the areas exceeded 10% of the mean.
1.6. Quality assurance
Agreement between graders was assessed by comparing the grading values for all image sets for the 3 pairs of 2 readers. For checking the reproducibility of the grading process, a random sample of 20 lids was selected for regrading, including adjudication.
1.7. Clinical assessments of MGD
In both eyes, plugging of the MG opening and lid secretions from the MG openings were evaluated. With mild pressure, the central 5 of the lower eyelid MG openings in the mid-portion of the lower eyelid were observed for plugging and categorized as No plugging, Mild (1–2 glands plugged), Moderate (3–4 glands plugged) and Severe (all 5 MGs were plugged). Lid secretions expressed from the opening of these glands after application of pressure with the MG Evaluator (Tear Science) were defined as clear, mildly cloudy, paste like and absent. The Pult 5-grade meiboscale was used to grade dropout from the meibography images at the clinics.16 Clinical assessments were made by study-certified optometrists or ophthalmologists with the exception that MG dropout area was assessed by study-certified technicians for approximately 50% of patients.
1.8. Developing a composite morphology severity score
Little is known about the diverse morphological features observed in the meibomian glands and whether they relate to the signs and symptoms of dry eye disease. Histopathology of the specific dysmorphic presentations are not available. We created a composite severity score for the morphological features by having three ophthalmologists (ED, VB, GM), two of them experienced external eye specialists, score each of the morphological features independently on a severity scale of 0 to 10, where 10 was judged to be the most severe morphological feature perceived to contribute to dry eye disease. We used the mean score from these three values for each morphological feature (Table B appendix). In each lid a total composite score was generated by adding the mean score of each feature that was present in that lid. This composite score was used to evaluate associations with quality of meibomian secretions, and plugging of the MG openings.
1.9. Statistical Methods
Kappa statistics were calculated to assess agreement for dichotomous MG features, and intraclass correlations were calculated for continuous and ordinal features. The terms suggested by Landis were used as descriptors of the agreement: < 0=poor, 0–0.2 =slight; .21-.40 =fair; .41- .60 = moderate; .61–0.8 = substantial and >.80 = excellent.17 Comparisons of dichotomous MG features by lid or categorical clinical characteristics were made using logistic regression, Comparisons of continuous MG features by lid or categorical clinical characteristics were made using linear regression. Generalized estimating equations were used to accommodate the correlation among lids in the same person.18 In cases where at least one level of the clinical characteristic had only one level of the MG feature, Fisher’s exact tests were used instead. To test for trends across ordered clinical characteristics the characteristic was treated as continuous, where values of 1 through 4 were used for successive categories for plugging and secretion, 1 through 5 for Meiboscale© score, and the actual values were used for RC percent dropout and tear break-up time. Lids with poor quality photos were excluded from the analysis.
2. RESULTS
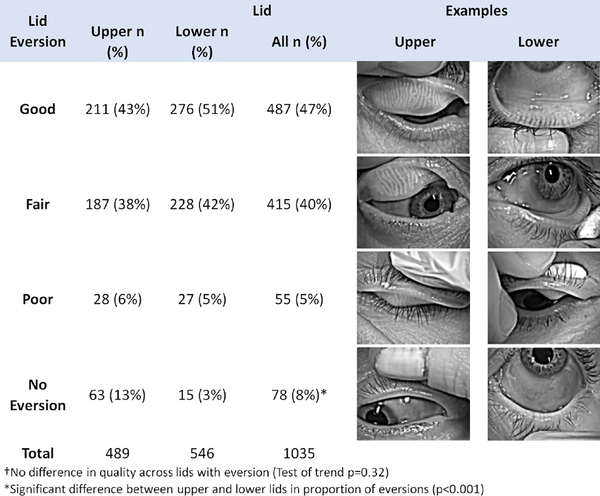
Among the 292 patients enrolled through centers with a keratograph, 277 (98%) patients had at least 1 image of an eyelid submitted to the reading center from the eligibility confirmation visit. Reasons for patients not having any images include machine malfunction and human error. Among the 551 eyes of 277 patients, 62 (11%) upper lid images and 5 (1%) lower lid images were missing. Among available lid images, the quality of lid eversion (good, fair or poor) when it was performed was similar between upper and lower lids (p=0.32; Figure 2); however, the lid was not everted for 63 (13%) of upper lids compared to 15 (3%) of lower lids.
Figure 2.
Quality of lid eversion in subjects with meibography images in the DREAM study
Estimates of the inter-reader and grade-regrade agreement for each of the MG features are shown in Table 1. Agreement on the total number of glands present was excellent for both between readers and on grade-regrade (between the original grading and the repeat grading). There was moderate to substantial agreement between readers and on grade-regrade for most of the other features. However, there was only slight inter-grader agreement on number of thick and thin glands. The grade-regrade agreement for thick, thin, overlap and dropout glands was also slight. The agreement on measurement of percentage dropout area was better when ghost gland and fluffy areas were included in the dropout areas; but not to a statistically significant degree (p=0.41).
Table 1.
Inter-reader and grade-regrade agreement in evaluating meibomian gland variants and dropout areas.
| Intraclass correlation coefficient (95% confidence interval) | ||
|---|---|---|
| Meibomian Gland Feature | Inter-reader agreement (N=897) | Grade-regrade agreement (N=34) |
| 0.89 (0.88, 0.91) | 0.80 (0.65, 0.90) | |
| Distorted | 0.71 (0.67, 0.74) | 0.59 (0.35, 0.78) |
| Tortuous | 0.43 (0.37, 0.49) | 0.88 (0.79, 0.94) |
| Hooked | 0.61 (0.57, 0.66) | 0.77 (0.62, 0.89) |
| Short | 0.61 (0.56, 0.65) | 0.54 (0.28, 0.75) |
| Thick | 0.32 (0.25, 0.38) | 0.23 (−0.08, 0.55) |
| Thin | 0.22 (0.15, 0.29) | 0.25 (−0.06, 0.56) |
| Overlap | 0.64 (0.60, 0.68) | 0.24 (−0.07, 0.55) |
| Ghost | 0.56 (0.51, 0.60) | 0.78 (0.63, 0.89) |
| Dropout | 0.40 (0.34, 0.46) | 0.26 (−0.05, 0.57) |
| Area Measurements* | ||
| Total area | 0.91 (0.90, 0.92) | 0.98 (0.96, 0.99) |
| Total dropout area including fluffy areas and ghost glands as dropped out. | 0.65 (0.61, 0.69) | 0.70 (0.50, 0.84) |
| Percent dropout area including fluffy areas and ghost glands as dropped out. | 0.52 (0.47, 0.57) | 0.74 (0.56, 0.87) |
| Total dropout area excluding fluffy areas and ghost glands as dropped out. | 0.56 (0.51, 0.61) | 0.39 (0.10, 0.66) |
| Percent dropout area excluding fluffy areas and ghost glands as dropped out | 0.52 (0.47, 0.57) | 0.57 (0.32, 0.77) |
Number of missing values for inter-reader agreement: 4 for total area, 3 for total dropout area (both for including and excluding fluffy areas and ghost glands), 6 for percent dropout area (both for including and excluding fluffy areas and ghost glands)
The distribution of morphological features comparing the upper lid and lower lid are shown in Table 2. Hooked, thick, thin, dropped out, tadpoling glands, gaps between glands and glands with no lid margin extension were uncommon (median number of glands equal to 0) in both the upper and lower lids. Nonetheless, upper lids had more visible MG features than lower lids (all p<0.001) except for lack of extension of MGs to the lid margin. Fluffy areas were present in a much higher proportion of lids and were more common in lower lids than in upper lids (66% vs 46%, p<0.001).
Table 2:
Frequency of meibomian gland features in the lower and upper lids
| Meibomian Gland Features | Lid | p-value* | |
|---|---|---|---|
| Upper (N=392) median (range) | Lower (N=490) median (range) | ||
| Total number | 20.00 (0.0 – 34.0) | 15.50 (0.0 – 29.0) | <0.001 |
| Distorted | 7.00 (0.0 – 20.5) | 2.00 (0.0 – 15.0) | <0.001 |
| Tortuous | 1.00 (0.0 – 7.5) | 0.00 (0.0 – 3.0) | <0.001 |
| Hooked | 0.00 (0.0 – 5.0) | 0.00 (0.0 – 2.5) | <0.001 |
| Short | 5.50 (0.0 – 16.0) | 3.00 (0.0 – 18.5) | <0.001 |
| Thick | 0.00 (0.0 – 5.5) | 0.00 (0.0 – 3.0) | <0.001 |
| Thin | 0.00 (0.0 – 9.0) | 0.00 (0.0 – 5.0) | <0.001 |
| Overlap | 1.00 (0.0 – 5.0) | 0.00 (0.0 – 2.5) | <0.001 |
| Ghost | 1.00 (0.0 – 21.0) | 0.00 (0.0 – 12.0) | <0.001 |
| Drop-out | 0.00 (0.0 – 7.0) | 0.00 (0.0 – 6.0) | <0.001 |
| Other Features | n (%) | n (%) | p-value† |
| Tadpoling (yes) | 28 (7.1%) | 9 (1.8%) | <0.001 |
| Gaps (yes) | 131 (33.4%) | 2 (0.4%) | <0.001 |
| No lid margin extent (yes) | 27 (6.9%) | 39 (8.0%) | 0.55 |
| Fluffy areas (Yes) | 180 (46.0%) | 325 (66.3%) | <0.001 |
P-value from the Kruskal Wallis test
P-value from the chi-square test
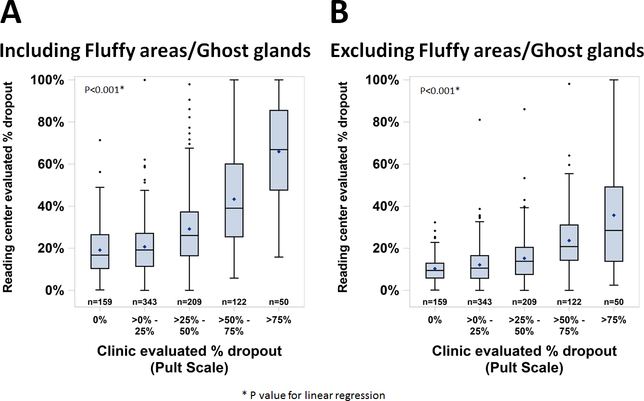
The mean percentage dropout from the RC increased as the clinic’s Pult meiboscale score increased, both when fluffy and ghost glands were included (p<0.001) and excluded (p<0.001) as dropped out areas by the RC (Figure 3). However, the percentage dropout determined by the reading center was not as extreme as the clinical assessment in that when the clinic judged 0% dropout, the mean percentage dropout by the reading center was 19% (including fluffy areas and ghost glands as dropped out) and 10% when excluding them. When the clinic judged >75% dropout, the mean percentage dropout by the reading center was 66% (including fluffy areas and ghost glands) and 36% excluding them. The discrepancy between the clinical judgement and the reading center’s assessment was greater when the readers excluded fluffy areas and ghost glands, with the reading center’s assessment substantially less when the clinic judged dropout to be > 25%.
Figure 3.
Comparison of Reading Center percentage of drop out areas vs the clinic estimated meiboscale (Pult) scores. A – Drop out area includes fluffy areas and ghost glands. B – Drop out area does not include fluffy areas and ghost glands.
Table 3 displays the associations between the morphological features of MGs in the middle third of the lower lid with the clinically assessed MG plugging in the middle 5 glands of the lower lid. Most of the morphological features were not associated with plugging. There was a higher mean number of dropout glands when there was plugging; no dropout glands were noted when plugging was absent (p<0.001). The mean number of ghost glands in a lid was lowest with mild plugging (p=0.009). The composite severity score of MG features was greater when there was moderate and severe plugging (p=0.02).
Table 3.
Clinically assessed plugging of the central 5 meibomian glands of the lower lid and the morphological features in the middle 1/3 of the lower lid assessed by the reading center
| Features in the lower lid middle third | None (n=44) | Mild (1–2 glands) (n=105) | Moderate (3–4 glands) (n=147) | Severe (all 5 glands) (n=172) | p-value | |
|---|---|---|---|---|---|---|
| Total number | Mean (SD) | 7.45 (1.42) | 7.35 (1.62) | 7.03 (2.07) | 7.17 (1.96) | 0.43 |
| Distorted | Mean (SD) | 1.90 (1.57) | 1.38 (1.44) | 1.53 (1.48) | 1.66 (1.43) | 0.19 |
| Tortuous | Mean (SD) | 0.09 (0.43) | 0.05 (0.28) | 0.09 (0.35) | 0.04 (0.24) | 0.45 |
| Hooked | Mean (SD) | 0.05 (0.30) | 0.04 (0.28) | 0.04 (0.25) | 0.06 (0.27) | 0.86 |
| Short | Mean (SD) | 1.47 (1.72) | 1.42 (1.62) | 1.78 (1.70) | 1.75 (1.96) | 0.31 |
| Thick | Mean (SD) | 0.06 (0.27) | 0.05 (0.29) | 0.01 (0.12) | 0.02 (0.19) | 0.51 |
| Thin | Mean (SD) | 0.14 (0.35) | 0.06 (0.28) | 0.06 (0.31) | 0.06 (0.27) | 0.56 |
| Overlap | Mean (SD) | 0.08 (0.30) | 0.06 (0.23) | 0.09 (0.29) | 0.03 (0.21) | 0.33 |
| Ghost | Mean (SD) | 0.66 (1.24) | 0.24 (0.83) | 0.61 (1.54) | 0.61 (1.40) | 0.009 |
| Dropout | Mean (SD) | 0.00 (0.00) | 0.17 (0.45) | 0.25 (0.71) | 0.15 (0.48) | <0.001 |
| Tadpoling | No | 44 (10%) | 105 (23%) | 144 (31%) | 170 (37%) | 0.40 |
| Yes | 0 (0%) | 0(0%) | 3 (60%) | 2 (40%) | ||
| Gaps | No | 44 (9%) | 105 (23%) | 146 (31%) | 171 (37%) | 0.81 |
| Yes | 0 (0%) | 0 (0%) | 1 (50%) | 1 (50%) | ||
| No Lid margin extension | No | 40 (9%) | 102 (23%) | 137 (31%) | 163 (37%) | 0.37 |
| Yes | 4 (15%) | 3 (12%) | 10 (38%) | 9 (35%) | ||
| Fluffy areas | No | 44 (10%) | 97 (22%) | 136 (31%) | 163 (37%) | 0.26 |
| Yes | 0 (0%) | 8 (29%) | 11 (39%) | 9 (32%) | ||
| Severity composite score | Mean (SD) | 18.60 (16.21) | 15.97 (14.43) | 22.74 (20.17) | 21.23 (20.40) | 0.02 |
| Dropout including ghost glands and fluffy areas as dropped out* | Mean (SD) | 28% (14%) | 30% (14%) | 35% (21%) | 33% (21%) | 0.06 |
| Dropout excluding ghost glands and fluffy areas* | Mean (SD) | 17% (13%) | 17% (8%) | 19% (13%) | 20% (17%) | 0.07 |
Missing values:1 in none,3 moderate
Table 4 displays the associations between the morphological features of MGs in the middle third of the lower lid with MG secretions in the middle 5 glands of the lower lid. The ghost glands but not the dropout glands are associated with either pasty secretions or complete obstruction with no secretions (p=0.046). Shortened MGs appear to be associated with a clear secretion from the MGs (p=0.02). All other morphological features and areas of atrophy (p>0.05) do not seem to be significantly associated with MG secretions.
Table 4:
Clinically assessed meibomian gland secretions from the central 5 meibomian glands of the lower lid and the meibomian gland morphological features in the middle 1/3 of the lower lid assessed in the reading center
| Features in the lower lid middle third | Clear secretion (n=82) | Mild haze/cloudiness (n=145) | Paste like secretion (n=71 | No Secretion (N=170) | p-value | |
|---|---|---|---|---|---|---|
| Total number | Mean (SD) | 7.32 (1.66) | 7.08 (2.06) | 7.32 (1.50) | 7.17 (1.97) | 0.76 |
| Distorted | Mean (SD) | 1.51 (1.47) | 1.50 (1.51) | 1.65 (1.44) | 1.66 (1.44) | 0.80 |
| Tortuous | Mean (SD) | 0.10 (0.41) | 0.07 (0.31) | 0.06 (0.32) | 0.04 (0.24) | 0.48 |
| Hooked | Mean (SD) | 0.07 (0.35) | 0.04 (0.25) | 0.03 (0.17) | 0.06 (0.27) | 0.50 |
| Short | Mean (SD) | 2.10 (1.79) | 1.39 (1.60) | 1.52 (1.67) | 1.73 (1.94) | 0.02 |
| Thick | Mean (SD) | 0.04 (0.27) | 0.04 (0.23) | 0.01 (0.12) | 0.02 (0.17) | 0.59 |
| Thin | Mean (SD) | 0.13 (0.44) | 0.06 (0.26) | 0.03 (0.17) | 0.06 (0.27) | 0.25 |
| Overlap | Mean (SD) | 0.11 (0.31) | 0.08 (0.28) | 0.03 (0.17) | 0.04 (0.21) | 0.09 |
| Ghost | Mean (SD) | 0.31 (0.82) | 0.36 (1.14) | 0.83 (1.72) | 0.65 (1.47) | 0.046 |
| Dropout | Mean (SD) | 0.16 (0.69) | 0.17 (0.47) | 0.23 (0.61) | 0.16 (0.48) | 0.87 |
| Tadpoling | No | 82 (18%) | 143 (31%) | 70 (15%) | 168 (36%) | 0.77 |
| Yes | 0 (0%) | 2 (40%) | 1 (20%) | 2 (40%) | ||
| Gaps | No | 82 (18%) | 145 (31%) | 70 (15%) | 169 (36%) | 0.44 |
| Yes | 0 (0%) | 0 (0%) | 1 (50%) | 1 (50%) | ||
| No Lid margin extension of meibomian glands | No | 78 (18%) | 138 (31%) | 65 (15%) | 161 (36%) | 0.67 |
| Yes | 4 (15%) | 7 (27%) | 6 (23%) | 9 (35%) | ||
| Fluffy areas | No | 77 (18%) | 135 (31%) | 67 (15%) | 161 (37%) | 0.95 |
| Yes | 5 (18%) | 10 (36%) | 4 (14%) | 9 (32%) | ||
| Total | 82 (18%) | 145 (31%) | 71 (15%) | 170 (36%) | ||
| Meibomian gland feature composite score | Mean (SD) | 22.18 (16.90) | 16.96 (17.31) | 22.00 (19.25) | 21.46 (20.65) | 0.10 |
| % dropout including ghost glands and fluffy areas* | Mean (SD) | 0.28 (0.15) | 0.31 (0.18) | 0.36 (0.20) | 0.33 (0.22) | 0.08 |
| % dropout excluding ghost glands and fluffy areas* | Mean (SD) | 0.16 (0.11) | 0.18 (0.12) | 0.21 (0.12) | 0.21 (0.17) | 0.06 |
3 lids with mild haze/cloudiness and 1 eye with paste have missing dropout area
3. DISCUSSION
Non-contact infrared photography facilitates assessment of morphological features of the MGs in DED and in other ocular and systemic conditions.19 However, there is not an accepted standard classification of the MG features or method for identifying areas of MG dropout. We have compiled a comprehensive collection of morphological features from several previous studies and added a few more to facilitate investigation of possible associations with the clinical signs and symptoms of DED.
Studying the association of various MG features with DED is complex as it is still unclear which features are associated with pathological conditions and which of them are normal findings in certain ethnic populations or age groups. For example, some of these features have been observed in pediatric age groups with no symptoms of DED.10 In addition increased frequency of the dropout areas have been associated with aging.4,20,21 It has been reported that asymptomatic children in China aged <14 years had distorted, tortuous, hooked and overlapping glands as well as apparent loss of MGs, suggesting that they may be congenital rather than acquired.10 However a study of Japanese children aged 0–12 years showed that morphologically complete MGs were distributed across the entire tarsal plate in both the upper and lower eyelids.22 Other studies have demonstrated increasing MG loss with age and female gender.4,19,20 but there are no longitudinal studies to confirm progression with age.
A number of morphological features have been reported in earlier studies, including total normal glands, distorted glands, tortuous glands and shortened glands.12,22,23–26 In addition to the morphological features described in earlier studies, we added the following additional features: thin or attenuated glands, ghost glands, dropout glands, abnormal gaps between glands, no lid margin extension of glands and fluffy areas as defined in Figure 1. In the present study thin glands and gaps between glands were rare while ghost glands were plentiful. The amalgamated mass we had termed fluffy areas were more common in the lateral and medial portions of the lid and could represent a unique form of atrophy that is different from ghost glands, dropout glands or shortened glands. Correlation between the clinic staff grading of the meiboscale score was better when ghost glands and fluffy areas were included rather than excluded from the total atrophic areas. Our grading excluded the whole gland if it was identified as a ghost gland; however, it is possible that ghost glands and fluffy areas represent regions where atrophy is incomplete and some functionality of the MG persists. Long term longitudinal studies would be helpful in answering these questions.
Our study demonstrated that obtaining good quality images from multiple centers was feasible, but we did experience some challenges. Lack of funding at the time of acquiring the DREAM images did not permit review and feedback on image quality by the reading center. Despite this, more than 90% of images received by the reading center were either of fair or good quality. Sufficient eversion to expose the entire palpebral conjunctiva without the lid being drawn to one side or the imager’s fingers obscuring the view is necessary to obtain an accurate assessment. To standardize the procedures and obtain good images it is important to train and certify the imagers irrespective of their prior training or experience. Feedback to the imagers on the quality of the meibography images could have reduced the number of poorly everted images that precluded assessments.
Worse agreement for certain morphological features such as thick, thin, and dropout glands suggest a need for a more robust definition of these features with reference images covering the full spectrum of the feature. Few previous studies have included evaluation of the reproducibility of grading. One study showed substantial inter-reader agreement in counting the number of whole glands (ICC of 0.75 (95% CI = 0.69–0.79).27 Our results on the total number of glands in each lid had an ICC of 0.89 (95% CI = 0.87, 0.90). Another study found the inter-grader reliability to be fair for acini appearance (weighted Kappa (Kw) 0.23) and moderate for gland dropout (Kw 0.50).28 Both these studies included only lower lids. Another study found reproducibility for 30 right eyes to be better in the upper lid (Kw 0.516 to 0.650) than the lower (K 0.212 to 0.530).29
Features such as large gaps, gland shortening, ghost glands and fluffy areas influence the meiboscore; however, few investigators have elaborated on the management of these features in measuring the dropout area. The variability of grading results observed in our study as well as in previous studies warrants dual independent grading that is more robust than results from a single reader.30,31
In agreement with previous reports, we found that the upper eyelid is more difficult to evert than the lower lid, as demonstrated by a higher percentage of upper lid images that were not obtained or had insufficient lid eversion.32,33 The upper lid glands are more in number, thinner and longer than the lower lid glands.1,34 Our study confirms that the presumably abnormal morphological features of the MGs, in general, are more common in the upper lid. Our finding of more dropout glands in the lower than the upper lid is consistent with similar results from previous studies.4,35 MG loss was significantly less in the upper lid than in the lower lid in patients with DED.12,36 Therefore it is important to evaluate both the upper and lower eyelids as assuming that findings from the upper lid are the same as those from the lower lid is not warranted.
Optical coherence tomography and confocal microscopy have been used to visualize and study meibomian glands.37,38 However, infrared meibography is the commonly used imaging modality and several models from several companies such as the Cobra® Fundus Camera (CSO and bon Optic VertriebsgmbH), TOPCON® Slitlamp Microscope BG-4M, EyeTop® Topographer and the Sirius® Scheimpflug Camera are available. Refinements to earlier meibography models have been aimed at making imaging easier to perform. For example, everting the upper eyelid, holding it in place and taking the image without hindrance from the keratograph has been made easier in some models. A pen shaped meibography system captures images without the need for a slitlamp (Meibopen; Japan Focus Company, Tokyo, Japan).39
Several meiboscale scores have been used in previous studies, including using the proportion of shortened glands or using areas of dropout scored along a four- or five-point scale.4,13,36,40 The calculation of meiboscores require the total area (denominator) and the dropout area (numerator). The handling of clarity and focus of the images, large areas of reflections, inadequate and incorrect eversion of the lids, loose folds of conjunctiva obscuring the glands (Figure 2), inadvertent lid distortion and an altered vertical globe gaze direction during meibography in deriving the meiboscale score is unclear.41 Also, even with a well-everted lid, the location of the borders of the total area of the tarsal plate can be difficult to delineate. All of these difficulties have a negative impact on accuracy and reproducibility.
When external features, orifice plugging and secretions of the 5 MGs in the center lower lid were compared to the morphological features, there were few morphological features related to the external features. However, dropout glands were absent in eyes that did not have plugging but were present in eyes with plugged glands and the mean composite severity of morphological features was found to be higher in eyes that had moderate and severe plugging. In addition, ghost glands appear to influence lid secretions from MG. Eyelids that expressed sebum with a thick paste like consistency or did not express any secretions at all were associated with larger numbers of ghost glands. A study investigating clinical factors associated with MG dropout among contact lens wearers found that lower eyelid MG atrophy was not associated with upper or lower MG plugging or upper or lower meibum quality while upper eyelid atrophy was associated with both gland plugging and meibum quality in both eyelids.42 The study, however, did not identify ghost glands specifically. It is possible that ghost glands are sick glands progressing towards atrophy. Conversely, they may be recovering glands. Longitudinal observations are needed to better understand the course of these features of glands that appear to influence the function of the MG.
Limitations of the study include insufficient hands-on training in everting the lids and no feedback given to the imagers on the quality of images, resulting in a number of missing and ungradable images.
4. CONCLUSIONS
We have catalogued various morphological features among the MGs present in moderate to severe dry eye disease patients that will allow further investigation into their associations with demographic, clinical and laboratory tests common to DED. We have shown good agreement among readers in identifying different morphological features as well as measuring the percentage areas of MG dropout. There is good correlation between the clinic meiboscale scores and the RC drop out percentages. More MG structural features and dropout areas were observed in the upper lids. We have identified a distinct category made up of substantial amounts of ghost glands and fluffy areas that can alter the meiboscale score depending on whether they are added or eliminated from the MG dropout areas. Dropout glands, ghost glands and the composite MG structural severity score were associated with MG plugging. Ghost glands were associated with pasty and scanty meibomian secretions.
Acknowledgments
Financial Support: Grants U10EY022881, U10EY022879 and R01 EY026972 from the National Eye Institute; Supplemental funding from the Office of Dietary Supplements, National Institutes of Health; Prevention of blindness
Table A.
Adjudication rules for dealing with discrepancies between two readers
| DREAM Study Meibomian Gland Baseline Grading Form Adjudication Rules | ||
|---|---|---|
| Question | Value Testing | Final Record |
| 1. Image Present | Values equal | Retain value |
| Values not equal | Adjudicate | |
| Image no. | Values equal | Retain value |
| Values not equal | Adjudicate | |
| 2. Lid Eversion | Values agree | Retain value |
| Good and Fair | Good | |
| Good and Poor | Fair | |
| Fair and Poor | Fair | |
| (Good or Fair or Poor) and None | Adjudicate complete form | |
| Al Overall focus | Values agree | Retain value |
| Good and Fair | Good | |
| Good and Poor | Fair | |
| Fair and Poor | Fair | |
| (Good or Fair or Poor) and CG* | Adjudicate complete form | |
| A2 Reflections | Values equal | Retain value |
| Values not equal | Adjudicate | |
| A3 Exposure | Values equal | Retain value |
| Values not equal | Adjudicate | |
| B. Morphology | Both values zero | Retain zero |
| Both values CG | Retain CG | |
| One value zero, other value >0 | Adjudicate | |
| One value CG, other value>0 | Adjudicate | |
| One value zero, other value CG | Adjudicate | |
| Both values > 0 and within difference of 2 | Average responses | |
| Both values > 0 and difference > 2 | Adjudicate | |
| 11. Tadpoling | Values equal | Retain value |
| 12. Gaps | Values not equal | Adjudicate |
| 13. No Lid Margin | Values not equal | Adjudicate |
| 14. Fluffy Areas | Values not equal | Adjudicate |
| C1. Total Area | Calculate mean between values | |
| If each value is within 10% of mean | Average responses | |
| If absolute value is greater than 10% different than then mean | Adjudicate | |
| If one value is CG and the other value measured | Adjudicate | |
| C2. Total drop out includes | Calculate mean between values | |
| If each value is within 10% of mean | Average responses | |
| If absolute value is greater than 10% different than then mean | Adjudicate | |
| If one value is CG and the other value measured | Adjudicate | |
| C3. Total drop out excludes | Calculate mean between values | |
| If each value is within 10% of mean | Average responses | |
| If absolute value is greater than 10% different than then mean | Adjudicate | |
| If one value is CG and the other value measured | Adjudicate | |
CG* - Cannot Grade
Table B.
Composite score values for each meibomian gland feature derived as an average from 3 ophthalmologists.
| Meibomian Gland Feature | Value |
|---|---|
| Distorted | 0.67 |
| Tortuous | 2.67 |
| Hooked | 4.00 |
| Dropout | 10.00 |
| Short | 7.33 |
| Thick | 4.67 |
| Thin | 4.33 |
| Overlap | 2.67 |
| Ghost | 8.00 |
| Tadpoling | 3.67 |
| Gap | 3.67 |
| Fluffy | 5.67 |
| No Extension to Lid Margin | 5.00 |
Figure A.
Meibography image of the lower lid in which a large fold of conjunctiva covers the meibomian glands (A) and only a close inspection reveals the meibomian glands seen near the lid margin. Meibography image of the upper lid with a representative example of almost complete meibomian gland atrophy throughout the tarsal region (B). Conjunctival folds (A) can be mistaken for complete atrophy (B).
Credit Roster for the DRy Eye Assessment And Management (DREAM) Trial
Certified Roles at Clinical Centers: Clinician (CL); Clinic Coordinator (CC), Data Entry Staff (DE) Principal Investigator (PI), Technician (T).
Milton M. Hom (Azusa, CA): Milton M. Hom, OD FAAO (PI); Melissa Quintana (CC/T); Angela Zermeno (CC/T).
Pendleton Eye Center (Oceanside, CA): Robert Pendleton, MD, PhD. (PI); Debra McCluskey (CC); Diana Amador (T); Ivette Corona (CC/T); Victor Wechter, MD (CL).
University of California School of Optometry, Berkeley (Berkeley, CA): Meng C. Lin, OD PhD FAAO (PI); Carly Childs (CC); Uyen Do (CC); Mariel Lerma (CC); Wing Li, OD (T); Zakia Young (CC); Tiffany Yuen, OD (CC/T).
Clayton Eye Center (Morrow, GA): Harvey Dubiner, MD (PI); Heather Ambrosia, OD (C); Mary Bowser (CC/T); Peter Chen, OD (CL); Helen Dubiner, PharmD, CCRC (CC/T); Cory Fuller (CC/T); Kristen New (DE); Tu Vy Nguyen (C); Ethen Seville (CC/T); Daniel Strait, OD (CL); Christopher Wang (CC/T); Stephen Williams (CC/T); Ron Weber, MD (CL).
University of Kansas (Prairie Village, KS) John Sutphin, MD (PI); Miranda Bishara, MD (CL); Anna Bryan (CC); Asher Ertel (CC/T); Kristie Green (T); Gloria Pantoja, Ashley Small (CC); Casey Williamson (T).
Clinical Eye Research of Boston (Boston, MA): Jack Greiner, MS OD DO, PhD (PI); EveMarie
DiPronio (CC/T); Michael Lindsay (CC/T); Andrew McPherson (CC/T); Paula Oliver (CC/T); Rina Wu (T).
Mass Eye & Ear Infirmary (Boston, MA): Reza Dana, MD (PI); Tulio Abud (T): Lauren Adams (T); Marissa Arnofsky (T); Jillian Candlish, COA (T); Pranita Chilakamarri (DE); Joseph Ciolino, MD (CL); Naomi Crandall (T); Antonio Di Zazzo (T); Merle Fernandes (T); Mansab Jafri (T); Britta Johnson (T); Ahmed Kheirkhah (T); Sally Kiebdaj (CC/T); Andrew Mullins (CC/T); Milka Nova (T); Vannarut Satitpitakul (T); Chunyi Shao (T); Kunal Suri (T); Vijeeta Tadla (CC); Saboo Ujwala (T); Jia Yin MD, PhD (T); Man Yu (T).
Kellogg Eye Center, University of Michigan (Ann Arbor, MI): Roni Shtein, MD (PI); Christopher Hood, MD (CL); Munira Hussain, MS, COA, CCRP (CC/T); Erin Manno, COT (T); Laura Rozek, COT (T/DE).
Minnesota Eye Consultants (Bloomington, MN): David R. Hardten, MD FACS (PI); Kimberly Baker (T); Alex Belsaas (T); Erich Berg (CC/T); Alyson Blakstad, OD (CL); Ken DauSchmidt (T); Lindsey Fallenstein (CC/T); Ahmad M. Fahmy OD (CL); Mona M. Fahmy OD FAAO (CL); Ginny Georges (T); Deanna E. Harter (CL); Scott G. Hauswirth, OD (CL); Madalyn Johnson (T); Ella Meshalkin (T); Rylee Pelzer (CC/T); Joshua Tisdale (CC/T); JulieAnn C. Wick (CL).
Tauber Eye Center (Kansas City, MO): Joseph Tauber, MD, PHD (PI); Megan Hefter (CC/T).
Silverstein Eye Centers (Kansas City, MO): Steven Silverstein, MD (PI); Cindy Bentley (CC/T); Eddie Dominguez (CC/T); Kelsey Kleinsasser, OD (CL).
Icahn School of Medicine at Mt. Sinai, (New York, NY): Penny Asbell, MD, FACS, MBA (PI); Brendan Barry (CC/T); Eric Kuklinski (CC/T); Afsana Amir (CC/T); Neil Chen (CC/T); Marko Oydanich (CC/T); Viola Spahiu (CC/T); An Vo, MD (T); Matthew Weinstein, DO (T).
University of Rochester Flaum Eye Institute (Rochester, NY): Tara Vaz, OD (PI); Holly Hindman, MD (PI); Rachel Aleese (CC/T); Andrea Czubinski (CC/T); Gary Gagarinas, COMT CCRA (CC/T); Peter McDowell (CC); George O’Gara (DE); Kari Steinmetz (CC/T).
University of Pennsylvania Scheie Eye Institute (Philadelphia, PA): Vatinee Bunya, MD (PI); Michael Bezzerides (CC/T); Dominique Caggiano (CC/T); Sheri Drossner (T); Joan Dupont (CC); Marybeth Keiser (CC/T); Mina Massaro, MD (CL); Stephen Orlin, MD (CL); Ryan O’Sullivan (CC/T).
Southern College of Optometry (Memphis, TN): Michael Christensen, OD PhD (PI); Havilah Adkins (CC); Randy Brafford (CC/T); Cheryl Ervin (CL); Rachel Grant OD (CL); Christina Newman (CL).
Shettle Eye Research (Largo, FL): Lee Shettle, DO (PI); Debbie Shettle (CC).
Stephen Cohen, OD, PC (Scottsdale, AZ): Stephen Cohen, OD (PI); Diane Rodman (CC/T).
Case Western Reserve University (Cleveland, OH): Loretta Szczotka-Flynn, OD PhD (PI); Tracy Caster (T); Pankaj Gupta MD MS (CL); Sangeetha Raghupathy (CC/T); Rony Sayegh, MD (CL).
Mayo Clinic Arizona (Scottsdale, AZ): Joanne Shen, MD (PI); Nora Drutz, CCRC (CC); Lauren Joyner, COA (T); Mary Mathis, COA (T); Michaele Menghini, CCRP (CC); Charlene Robinson, CCRP (CC).
Wolston & Goldberg Eye Associates (Torrance, CA): Damien Goldberg, MD (PI); Lydia Jenkins (T); Brittney Rodriguez (CC/T); Jennifer Picone Jones (CC/T); Nicole Thompson (T), Barry Wolstan, MD (CL).
Northeast Ohio Eye Surgeons (Stow, OH): Marc Jones, MD (PI); April Lemaster (CC/T); Julie Ransom-Chaney (T); William Rudy, OD (CL).
Tufts Medical Center (Boston, MA): Pedram Hamrah, MD (PI); Mildred Commodore (CC); Christian Iyore (T); Lioubov Lazarev (T): Leah Mullen (T); Nicholas Pondelis (T); Carly Satsuma (CC).
University of Illinois at Chicago (Chicago, IL): Sandeep Jain, MD (PI); Peter Cowen (CC/T); Joelle Hallak (CC);Christine Mun (CC/T); Roxana Toh (CC).
The Eye Centers of Racine & Kenosha (Racine, WI): Inder Singh, MD (PI); Pamela Lightfield (CC/T); Eunice Lowery (T); Sarita Ornelas (T); R. Krishna Sanka, MD (CL); Beth Saunders (T).
Mulqueeny Eye Centers (St. Louis, MO): Sean P. Mulqueeny, OD (PI); Maggie Pohlmeier (CC/T).
Oculus Research at Garner Eyecare Center (Raleigh, NC): Carol Aune, OD (PI); Hoda Gabriel (CC); Kim Major Walker, RN MS (CC/T); Jennifer Newsome (CC/T).
Resource Centers
Chairman’s Office (Icahn School of Medicine at Mount Sinai, New York, NY):Penny Asbell, MD, FACS, MBA (Study Chair); Brendan Barry (Clinical Research Coordinator); Eric Kuklinski (Clinical Research Coordinator); Shir Levanon (Clinical Research Coordinator); Michael Farkouh, MD FRCPC, FACC, FAHA (Medical Safety Monitor); Seunghee Kim-Schulze, PhD (Consultant); Robert Chapkin, PhD, MSc. (Consultant); Giampaolo Greco, PhD (Consultant); Artemis Simopoulos, MD (Consultant); Ines Lashley (Administrative Assistant); Peter Dentone, MD (Clinical Research Coordinator); Neha Gadaria-Rathod, MD (Clinical Research Coordinator); Morgan Massingale, MS (Clinical Research Coordinator); Nataliya Antonova (Clinical Research Coordinator).
Coordinating Center (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA): Maureen G. Maguire, PhD (PI); Mary Brightwell-Arnold, SCP (Systems Analyst) John Farrar, MD PhD (Consultant); Sandra Harkins (Staff Assistant); Jiayan Huang, MS (Biostatistician); Kathy McWilliams, CCRP (Protocol Monitor); Ellen Peskin, MA, CCRP (Director); Maxwell Pistilli, MS, MEd (Biostatistician); Susan Ryan (Financial Administrator); Hilary Smolen (Research Fellow); Claressa Whearry (Administrative Coordinator); Gui-Shuang Ying, PhD (Senior Biostatistician) Yinxi Yu (Biostatistician).
Biomarker Laboratory (Icahn School of Medicine at Mount Sinai, New York, NY): Yi Wei, PhD, DVM (co-Director, Biomarker Laboratory); Neeta Roy, PhD (co-Director, Biomarker Laboratory); Seth Epstein, MD (Former co-Director; Biomarker Laboratory); Penny A. Asbell, MD, FACS, MBA (Director and Study Chair).
Investigational Drug Service (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA): Kenneth Rockwell, Jr., PharmD MS (Director).
Peroxisomal Diseases Laboratory at the Kennedy Krieger Institute, Johns Hopkins University Baltimore MD: Ann Moser (Co-Director/Consultant); Richard O. Jones, PhD (Co-Director/Consultant)
Meibomian Gland Reading Center (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA): Ebenezer Daniel, MBBS, MPH, PhD, (PI); E. Revell Martin (Image Grader); Candace Parker Ostroff, (Image Grader); Eli Smith (Image Grader); Pooja Axay Kadakia (Student Researcher).
National Eye Institute, National Institutes of Health, Department of Health and Human Services: Maryann Redford, DDS, MPH (Program Officer).
Office of Dietary Supplements/National Institutes of Health, Department of Health and Human Services
Committees
Executive Committee (Members from all terms of appointment): Penny Asbell, MD FACS, MBA (Chair); Brendan Barry, MS; Munira Hussain, MS, COA, CCRP; Jack Greiner, MS OD PhD; Milton Hom, OD, FAAO; Holly Hindman, MD, MPH; Eric Kuklinski, BA; Meng C. Lin OD, PhD. FAAO; Maureen G. Maguire, PhD; Kathy McWilliams, CCRP; Ellen Peskin, MA, CCRP; Maryann Redford, DDS, MPH; Roni Shtein, MD, MS; Steven Silverstein, MD; John Sutphin, MD.
Operations Committee: Penny Asbell, MD FACS, MBA (Chair); Brendan Barry, MS; Eric Kuklinski, BA; Maureen G. Maguire, PhD; Kathleen McWilliams, CCRP, Ellen Peskin, MA, CCRP; Maryann Redford, DDS, MPH.
Clinic Monitoring Committee: Ellen Peskin, MA, CCRP (Chair); Mary Brightwell-Arnold, SCP, Maureen G. Maguire, PhD; Kathleen McWilliams, CCRP.
Data and Safety Monitoring Committee: Stephen Wisniewski, PhD (Chair); Tom Brenna, PhD; William G. Christen Jr, SCD, OD, PhD; Jin-Feng Huang, PhD; Cynthia S. McCarthy, DHCE, MA; Susan T. Mayne, PhD; Mari Palta, PhD; Oliver D. Schein, MD, MPH, MBA.
Industry Contributors of Products and Services
Access Business Group, LLC (Ada, MI) Jennifer Chuang, PhD. CCRP; Maydee Marchan, M.Ch.E; Tian Hao, PhD; Christine Heisler; Charles Hu, PhD; Clint Throop, Vikas Moolchandani, PhD.
Compounded Solutions in Pharmacy (Monroe, CT)
Leiter’s (San Jose, CA)
Immco Diagnostics Inc. (Buffalo NY
OCULUS Inc. (Arlington, WA)
RPS Diagnostics, Inc. (Sarasota, FL)
TearLab Corporation (San Diego, CA)
TearScience Inc. (Morrisville, NC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presentation at ARVO May 2019
Conflict of Interest:
Dr. Asbell:consultant for Sun Pharma, Dompe, Novaliq, Senju, Santen, Shire, Alcon, Kala, CLAO, Allakon, Medscape and Regeneron. Dr Bunya: Grant recipient from Bausch &Lomb/Immco Diagnostics and consultant for Celularity. Dr Massaro-giordano is consultant for GSK, Celularity and PRN. Ebenezer Daniel, Maureen Maguire, Maxwell Pistilli, Giacomina Massaro-giordano, Eli Smith and Pooja Kadakia have no conflicts of interest
References:
- 1.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci 2011;52:1922–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf 2017;15:539–574. [DOI] [PubMed] [Google Scholar]
- 3.McGinnigle S, Naroo SA, Eperjesi F. Evaluation of dry eye. Surv Ophthalmol 2012;57:293–316. [DOI] [PubMed] [Google Scholar]
- 4.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008;115:911–5 [DOI] [PubMed] [Google Scholar]
- 5.Dry Eye Assessment and Management Study Research Group, Asbell PA, Maguire MG, Pistilli M, Ying GS, Szczotka-Flynn LB, Hardten DR, et al. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N Engl J Med 2018;378:1681–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finis D, Ackermann P, Pischel N, König C, Hayajneh J, Borrelli M, et al. Evaluation of Meibomian Gland Dysfunction and Local Distribution of Meibomian Gland Atrophy by Non-contact Infrared Meibography. Curr Eye Res 2015;40:982–9 [DOI] [PubMed] [Google Scholar]
- 7.Chan TCY, Wan KH, Shih KC, Jhanji V. Advances in dry eye imaging: the present and beyond. Br J Ophthalmol 2018;102:295–301 [DOI] [PubMed] [Google Scholar]
- 8.Arita R, Fukuoka S, Morishige N. New insights into the morphology and function of meibomian glands. Exp Eye Res 2017;163:64–71 [DOI] [PubMed] [Google Scholar]
- 9.Asbell PA, Maguire MG, Peskin E, Bunya VY, Kuklinski EJ; Dry Eye Assessment and Management (DREAM©) Study Research Group. Dry Eye Assessment and Management (DREAM©) Study: Study design and baseline characteristics. Contemp Clin Trials 2018;71:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Chen S, Wang S, Chen Y, Li J, Fu Y, et al. The significance of meibomian gland changes in asymptomatic children. Ocul Surf 2018;16:301–305 [DOI] [PubMed] [Google Scholar]
- 11.Robin JB, Nobe JR, Suarez E, Jester JV, Smith RE. Meibomian gland evaluation in patients with extended wear soft contact lens deposits. CLAO J 1986;12:95–8 [DOI] [PubMed] [Google Scholar]
- 12.Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Fukuoka S, et al. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology 2009;116:2058–63 [DOI] [PubMed] [Google Scholar]
- 13.Pult H, Riede-Pult BH, Nichols JJ. Relation between upper and lower lids’ meibomian gland morphology, tear film, and dry eye. Optom Vis Sci 2012;89:E310–15 [DOI] [PubMed] [Google Scholar]
- 14.Koh YW, Celik T, Lee HK, Petznick A, Tong L. Detection of meibomian glands and classification of meibography images. J Biomed Opt 2012;17:086008. [DOI] [PubMed] [Google Scholar]
- 15.Arita R, Suehiro J, Haraguchi T, Shirakawa R, Tokoro H, Amano S. Objective image analysis of the meibomian gland area. Br J Ophthalmol 2014;98:746–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pult H, Riede-Pult BH. Non-contact meibography: keep it simple but effective. Cont Lens Anterior Eye 2012;35:77–80 [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174 [PubMed] [Google Scholar]
- 18.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health 1993;14:43–68 [DOI] [PubMed] [Google Scholar]
- 19.Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf 2004;2:149–164 [DOI] [PubMed] [Google Scholar]
- 20.Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea 2006;25:651–5 [DOI] [PubMed] [Google Scholar]
- 21.Yeotikar NS, Zhu H, Markoulli M, Nichols KK, Naduvilath T, Papas EB. Functional and Morphologic Changes of Meibomian Glands in an Asymptomatic Adult Population. Invest Ophthalmol Vis Sci 2016;57:3996–4007 [DOI] [PubMed] [Google Scholar]
- 22.Shirakawa R, Arita R, Amano S. Meibomian gland morphology in Japanese infants, children, and adults observed using a mobile pen shaped infrared meibography device. Am J Ophthalmol 2013;155:1099–1103 [DOI] [PubMed] [Google Scholar]
- 23.Fukuoka S, Arita R, Shirakawa R, Morishige N. Changes in meibomian gland morphology and ocular higher-order aberrations in eyes with chalazion. Clin Ophthalmol 2017;11:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arita R, Itoh K, Maeda S, Maeda K, Tomidokoro A, Amano S. Association of contact lens-related allergic conjunctivitis with changes in the morphology of meibomian glands. Jpn J Ophthalmol 2012;56:14–9 [DOI] [PubMed] [Google Scholar]
- 25.Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Tomidokoro A, et al. Meibomian gland duct distortion in patients with perennial allergic conjunctivitis. Cornea 2010;29:858–60 [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Wang MTM, Craig JP. Exploring the Asian ethnic predisposition to dry eye disease in a pediatric population. Ocul Surf 2019;17:70–77 [DOI] [PubMed] [Google Scholar]
- 27.Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea 2005;24:382–8 [DOI] [PubMed] [Google Scholar]
- 28.Powell DR, Nichols JJ, Nichols KK. Inter-examiner reliability in meibomian gland dysfunction assessment. Invest Ophthalmol Vis Sci 2012;53:3120–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dogan AS, Kosker M, Arslan N, Gurdal C. Interexaminer Reliability of Meibography: Upper or Lower Eyelid? Eye Contact Lens 2018;44:113–117 [DOI] [PubMed] [Google Scholar]
- 30.Gupta PK, Stevens MN, Kashyap N, Priestley Y. Prevalence of Meibomian Gland Atrophy in a Pediatric Population. Cornea 2018;37:426–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palamar M, Degirmenci C, Ertam I, Yagci A. Evaluation of dry eye and meibomian gland dysfunction with meibography in patients with rosacea. Cornea 2015;34:497–9 [DOI] [PubMed] [Google Scholar]
- 32.Pult H Relationships Between Meibomian Gland Loss and Age, Sex, and Dry Eye. Eye Contact Lens 2018;44 Suppl 2:S318–S324 [DOI] [PubMed] [Google Scholar]
- 33.Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea 2008. 27:1142–7 [DOI] [PubMed] [Google Scholar]
- 34.Butovich IA. Meibomian glands, meibum, and meibogenesis. Exp Eye Res 2017;163:2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eom Y, Choi KE, Kang SY, Lee HK, Kim HM, Song JS. Comparison of meibomian gland loss and expressed meibum grade between the upper and lower eyelids in patients with obstructive meibomian gland dysfunction. Cornea 2014;33:448–52 [DOI] [PubMed] [Google Scholar]
- 36.Pflugfelder SC, Tseng SC, Sanabria O, Kell H, Garcia CG, Felix C, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea 1998;17:38–56 [DOI] [PubMed] [Google Scholar]
- 37.Randon M, Aragno V, Abbas R, Liang H, Labbé A, Baudouin C. In vivo confocal microscopy classification in the diagnosis of meibomian gland dysfunction. Eye (Lond) 2018. December 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo YS, Na KS, Byun YS, Shin JG, Lee BH, Yoon G, et al. Examination of Gland Dropout Detected on Infrared Meibography by Using Optical Coherence Tomography Meibography. Ocul Surf 2017;15:130–138 [DOI] [PubMed] [Google Scholar]
- 39.Arita R, Itoh K, Maeda S, Maeda K, Amano S. A newly developed noninvasive and mobile pen-shaped meibography system. Cornea 2013;32:242–7 [DOI] [PubMed] [Google Scholar]
- 40.Pult H, Nichols JJ. A review of meibography. Optom Vis Sci 2012;89:760–9 [DOI] [PubMed] [Google Scholar]
- 41.Maskin SL, Testa WR. Infrared Video Meibography of Lower Lid Meibomian Glands Shows Easily Distorted Glands: Implications for Longitudinal Assessment of Atrophy or Growth Using Lower Lid Meibography. Cornea 2018;37:1279–86 [DOI] [PubMed] [Google Scholar]
- 42.Pucker AD, Jones-Jordan LA, Marx S, Powell DR, Kwan JT, Srinivasan S, et al. Contact Lens Assessment of Symptomatic Subjects (CLASS) Study Group. Clinical factors associated with contact lens dropout. Cont Lens Anterior Eye 2018. December 8 [DOI] [PubMed] [Google Scholar]