Abstract
Calcific vascular and valvular disease is widespread and has major health consequences. While coronary artery calcification has long been associated with hyperlipidemia and increased mortality, recent evidence suggests that its progression is increased in association with cholesterol-lowering HMG-CoA reductase inhibitors (“statins”) and with long-term, high intensity exercise. A nationwide trial showed no cardiovascular benefit of vitamin D supplements. Controversy remains as to whether calcium deposits in plaque promote or prevent plaque rupture. CVVD appears to occur through mechanisms similar to those of intramembranous, endochondral, and osteophytic skeletal bone formation. New evidence implicates autotaxin, endothelial-mesenchymal transformation, and micro and Lnc RNAs as newer regulatory factors. New therapeutic options are being developed.
Keywords: calcification, cardiovascular, bone, atherosclerosis, valvular disease
SIGNIFICANCE
Calcific vascular and valvular disease (CVVD; see Glossary) is a well-established marker of atherosclerosis and cardiovascular morbidity and mortality. CVVD is widespread, with a prevalence increasing with age; approximately 60% of 60 year-olds have coronary or aortic calcification [1, 2], which increases the risk for cardiovascular and all-cause mortality [2-4]. It is almost universal in subjects over 70 and in patients on dialysis, in whom it is a major cause of morbidity and mortality [5].
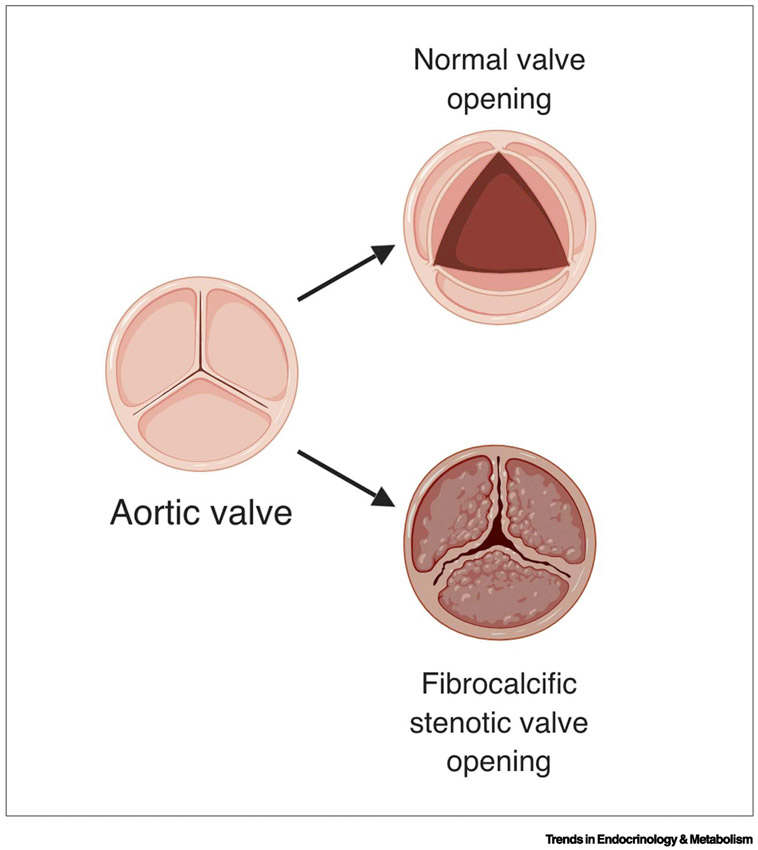
Calcification reduces vascular compliance, leading to pleiotropic clinical consequences: hypertension, coronary ischemia, high pulse pressure, infarction, left ventricular hypertrophy, arrhythmias, syncope and congestive heart failure [6-8]. In the coronary arteries, calcification independently predicts a 1.7-fold increase in mortality [2]. In peripheral arteries, it independently predicts mortality and amputation [9]. In patients with chronic kidney disease, coronary artery calcium score and volume from computed tomography (CT) are directly related with mortality [10]. As for valve leaflets, it is generally accepted that calcification promotes breakdown of the tissue matrix, which causes valve dysfunction, such as flail and regurgitant leaflets, and that advanced calcification renders the valve tissue too stiff to open, resulting in greater risk of cardiovascular events [4] (Fig. 1).
Figure 1. Changes in aortic valve cusps in aortic stenosis.
Fibrocalcific changes in the normally thin cusps reduce the size of the opening and blood flow. This figure was created using BioRender (https://biorender.com/).
Currently there is no established medical therapy for CVVD. In the carotid artery, mineralized plaque may be stented interventionally or removed surgically, and aortic valves may be replaced surgically or by transcatheter intervention. Calcified lesions that completely occlude coronaries have been partially opened through catheter techniques using directional, rotational or orbital atherectomy for purposes of allowing balloon and stent interventions. These latter techniques have been available for almost 3 decades, but they remain in limited use [11].
BIOMECHANICS AND RUPTURE RISK
Plaque rupture stress.
The link between coronary calcification and morbidity/mortality is commonly thought to be plaque rupture. In general, tissue rupture occurs when mechanical (von Mises) stresses exceed tissue strength. By finite element analysis, when a rigid deposit is included in a distensible material, and uniaxial stress is applied, the compliance mismatch leads to high von Mises stress and rupture or debonding at the interface between the rigid deposit and the surrounding compliant tissue [12]. In the form of a calcium deposit in an atherosclerotic plaque, it can lead to intraplaque hemorrhage or plaque rupture into the lumen, each which can cause occlusion and potentially fatal myocardial infarction.
Determinants of rupture stress.
Size and location are key determinants of both the von Mises stress and tissue strength. In theory, the larger the deposit, the greater the rupture stress and the larger the region at risk. Thus, a single macrocalcification (defined as ≥ 50 μm in diameter) is expected to generate higher rupture stress over a larger area than a single microcalcification (defined as < 50 μm in diameter) in the same location in an artery wall. However, given the same location, a single intact calcium deposit is expected to have less surface area (and, hence, less rupture stress) than an identical deposit broken into small pieces. With respect to location, deposits near a free edge distribute the force over a smaller cross-sectional area, which increases the ratio of force to area, i.e. the stress. Thus, a calcium deposit of any size near an edge, such as the luminal surface of an atherosclerotic plaque, carries greater risk than the same deposit located away from the edge. Since the mechanical equivalent of an edge occurs at the interface of vascular tissue with a liquefied pool of lipids found within many atherosclerotic plaques, rupture-prone areas may occur in the cap or at deep sites in the plaque, each of which may cause coronary occlusion, the latter by intraplaque hemorrhage.
Interpretation of finite element analyses.
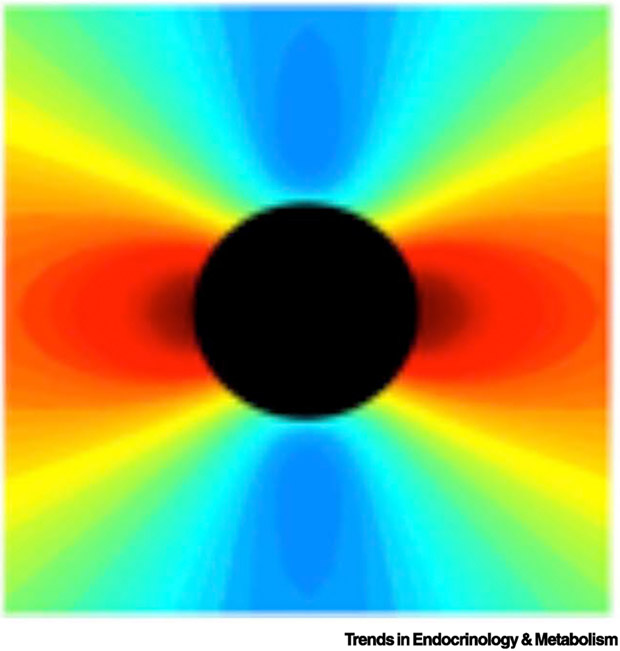
Mechanical analyses of rupture risk at sites of calcification show fundamentally the same result, but their interpretation has differed. For instance, it has been suggested that only microscopic calcium deposits (< 50 μm; “microcalcifications”) increase rupture risk and that larger deposits reduce rupture risk [13]. However, regardless of size, calcium deposits have both effects, increasing rupture stress at the edges facing applied uniaxial load and decreasing rupture stress at the edges perpendicular to the applied load [12] (Fig. 2). When a partial analysis includes only the protected edges facing perpendicular to the applied load, it would lead one to believe that calcium deposits decrease rupture risk, because it would miss the increased risk at the other edges.
Figure 2. Distribution of rupture stress amplitude.
Theoretical analyses showing the degree of rupture (von Mises) stress (scaled by color) in a hypothetical soft tissue in the vicinity of a hypothetical rigid deposit (black circle), which is stretched along the horizontal axis. Rupture risk is greatly increased (red) on the edges facing the direction of pull and reduced (blue) at the perpendicular edges.
Some of the controversy is due to markedly different assumptions about the material properties, isotropy, and strength of plaque tissue at different sites or the complexity of tensor analysis required to describe solid mechanical stresses. At each location on the surface of a given calcium deposit, multiple solid stresses act along multiple axes perpendicular and tangential to the surface at that point. At that interface, the strength of plaque tissue, which is likely anisotropic, would be different along each axis. Multiple parameters must be considered in assessing the effect of rigid deposits on mechanical rupture risk.
Spotty distribution of calcification.
A particular pattern, “spotty” distribution of calcium deposits, has been associated with increased clinical risk compared with continguous deposits of similar mineral content. This is consistent with the theoretical expectations outlined above. Culprit lesions responsible for ischemia as well as risk of acute coronary events have been associated with lesions containing multiple calcium deposits, midsized (on the order of 0.5 mm in diameter and separated by 1-3 mm), based on clinical intravascular ultrasound (IVUS), which has resolution of about 150 μm [14] and CT angiography [15]. This pattern of distribution is now considered synonymous with “high-risk” plaque in coronary and carotid arteries [15-17]. Importantly, these are not microcalcifications since they are larger than 50 μm based on their discernibility by coronary CT angiography and IVUS, which lack the resolution to distinguish individual microcalcifications. It has been noted that sometimes “spotty calcifications” are erroneously referred to as “microcalcifications” [18]. To avoid confusion, the term “spotty” may be reserved to describe a pattern of distribution rather than a deposit size.
REGULATORY FACTORS
The capacity to undergo osteoblastic differentiation and produce a mineralized matrix of hydroxyapatite is a robust and inherent property of vascular [19, 20] and valvular interstitial cells [21-23]. The resulting in vitro matrix mineralization is regulated by a wide range of factors, including modified low density lipoprotein (LDL) [24], inflammatory cytokines [25, 26], transforming growth factor-beta [27], Wnt signaling [28-31], substrate stiffness [32], advanced glycation end-products [33], and glucose [34]. The molecular and cellular processes appear to recapitulate the various forms of embryonic and mature skeletal calcification and remodeling, including intramembranous, endochondral, periosteal, and osteophytic bone formation. The shared mechanisms include overlapping processes of matrix vesicle formation, crystal precipitation on collagen, inhibitor-activator imbalances, apoptosis, and osteoblastic differentiation. At the in vivo and clinical levels, CVVD is regulated by a wide range of factors, and some have corresponding in vitro effects.
Clinically, some regulatory factors have positive associations with all forms - vascular, valvular, and skeletal – calcification, whereas others have opposite effects at the different sites. For instance, hyperlipidemia is positively associated with vascular and valvuar calcification but inversely associated with skeletal mineralization. Serum osteopontin levels are inversely associated with both vascular [35] and valvular [36] calcification, whereas serum fibroblast growth factor 23 (FGF-23) is positively associated with vascular calcification [37], but negatively associated with skeletal bone mineralization [38].
Specific factors and their interrelationships.
Hyperlipidemia is recognized as a causal factor in cardiovascular calcification in part because osteogenic changes were reduced when hyperlipidemia was terminated in Reversa mice [39]. The underlying factor may be the oxidative stress of accumulation and oxidation of lipids in the artery wall. Oxidative stress, acting through protein kinase B (AKT) [40], farnesyl transferase inhibitors acting through AKT [41], or AKT-independent pathways [42], may be a common pathway. Inflammation may also be a central theme. Inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), were shown to induce endothelial-mesenchymal transition in endothelial cells, which promotes osteogenic differentiation in the presence of bone morphogenetic protein-9 [43]. Lysophosphatidylcholine also promotes vascular cell osteogenesis [44].
Intraplaque hemorrhage.
Although the old view of aortic valve calcification as a disease of “wear-and-tear” is no longer valid, mechanical trauma does have the potential to promote valvular calcification through intra-leaflet hemorrhage. When valvular interstitial cells were exposed to erythrocytes in vitro, it resulted in inflammatory and pro-osteogenic signals, including expression of interleukin-6, interleukin-1β, bone sialoprotein, osteoprotegerin, and bone morphogenetic protein-2 [45]. It is possible that a corresponding event occurs in artery walls when intra-plaque rupture leads to intra-plaque hemorrhage.
Toll-like receptor 2 deficiency.
Lipopolysaccharide (LPS), a powerful inducer of inflammation found on the outer membrane of common bacteria, promotes chondrogenic differentiation of cultured vascular SMC and cartilaginous metaplasia of the aorta in hyperlipidemic Apoe−/− mice [46]. This supports the concept that soft tissue calcification may be an evolutionary response to wall off non-healing bacterial abscesses. LPS, like mildly oxidized LDL, acts through Toll-like receptor 2 (TLR2) to promote inflammation [47], and its deficiency prevented calcification of the aorta in high fat-fed Apoe−/− mice, apparently through inhibition of expression of the cartilage differentiation factors, osterix, SOX9 (sex-determining region Y-box9), and collagen II, but not the bone differentiation factor Runx2 [46].
MicroRNAs (miRs) and long noncoding (Lnc) RNAs.
A number of miRNAs and also regulate calcification of vascular SMC [48]. For example, high glucose treatment of vascular SMC induces LncRNA ES3 and suppresses miR-34c-5p. Since miR-34c-5p ordinarily inhibits Bcl2-modifying factor, suppression of the miR releases expression of BMF, which promotes calcification [34].
CLINICAL ASSOCIATIONS
Effects of agents/conditions on coronary artery calcification and cardiovascular mortality have been summarized in the Table.
Table 1.
Factors Influencing Vascular Calcification
| Agent/Condition | Effects on coronary artery calcification |
Effects on cardiovascular mortality |
|---|---|---|
| Statins | Increase | Decrease |
| High-intensity exercise | Increase | Decrease |
| Vitamin D | Increase | Decrease/Increase |
| Chronic kidney disease | Increase | Increase |
| Diabetes | Increase | Increase |
| Pollution | Increase | Increase |
Statins.
Epidemiological and clinical studies using CT calcium scores and intravascular ultrasound have shown that HMG-CoA reductase inhibitors (“statins”) promote progression of coronary calcification [49, 50], an effect increased with duration of statin use [51, 52]. Such an effect of statins was unexpected, given that they reduce cardiovascular mortality [53] and that hyperlipidemia promotes vascular calcification in mouse models [54] and is associated with calcification in human subjects [55]. This paradoxical effect of statins on calcification indicates a complexity of mechanisms, and it raises important questions about the logic behind use of positive tests for coronary calcification as a clinical indication for statin treatment.
High intensity exercise.
A similar paradox is found with high intensity exercise. The recent findings that a high level of lifelong exercise accelerates coronary artery calcification [56-59] is also unexpected, given that physical activity is associated with reduction in all-cause mortality in patients with coronary artery calcification (CAC) [60] and that history of endurance sports significantly reduced the mortality in male former athletes with ischemic heart disease [61]. In a study of white men, those who participated in physical activity 3 times beyond the guidelines had increased odds of developing coronary calcification [58]. Nevertheless, unlike in less athletic individuals, the coronary calcification found in elite athletes is not associated with increasedmortality [62]. This latter finding again points to a complexity of mechanism or some difference in the nature or microarchitecture of plaque calcification. It may also simply result from the known association of physical fitness with improved survival after myocardial infarction [63].
Vitamin D.
For decades, it has been known that administration of high levels of vitamin D is so effective in inducing vascular calcification in rodents that it is a robust and widely used experimental model [64, 65]. However, in humans, vitamin D was long believed to decrease cardiovascular risk, based on observational studies, until recently, when a nationwide, randomized, placebo-controlled trial (VITAL) involving over 25,000 middle-aged or older subjects, showed that vitamin D supplements did not reduce risk of a major cardiovascular events [66]. With respect to increased risk, in 180,000 Korean subjects, high levels of serum vitamin D were associated with higher risk of coronary calcification in men, but not women [67].
Chronic kidney disease.
The most extensive CVVD is found in patients with chronic kidney disease (CKD) on dialysis. This was previously considered a result of hemodialysis, but a recent cross-sectional cohort study suggests that peritoneal dialysis is associated with at least as much coronary calcification as hemodialysis [68]. The mechanisms are multifold. As described by Towler, CKD is the “perfect storm” for vascular calcification [69]. It includes several risks: hyperphosphatemia, supplementation with active vitamin D, uremia, and, for prevention of dialysis shunt clotting, warfarin (which inhibits the inhibitor of BMP). Vessels from patients with CKD also show increased expression of proatherogenic miR-223 [70], increased osteogenic factors [70], as well as premature oxidative damage [71]. CKD patients also release serum extracellular vesicles that have lower levels of fetuin-A and Gla-rich protein, inhibitors of calcification [72]. These particles are known to promote mineralization of vascular smooth muscle cells [72]. Sodium-dependent phosphate transporters, PiT-1 and PiT-2, are expressed in vascular smooth muscle cells, and they appear to have opposite effects on vascular calcification - PiT-1 promotes [73] whereas PiT-2 protects VSMC calcification [74].
Diabetes.
Patients with diabetes also have a greater degree of vascular calcification, though not as advanced as that of patients with CKD. By ultrasound, macrocalcifications in a spotty pattern are associated with the need for amputation [75]. One mechanism for the link to diabetes may be an effect of hyperglycemia on vascular cells. In cell culture, hyperglycemic media increase calcification [34]. Advanced glycation endproducts (AGEs) and the receptor for AGEs are also associated with vascular calcification in diabetic patients, and they have crossover effects in CKD, where AGEs accumulate, and mRNA expression for their receptor (RAGE) increases. A high-phosphate diet, used to induce CKD, was found to increase the degree of calcific valve disease in Apoe−/− mice with CKD, but not in mice lacking the receptor for RAGE. One potential mechanism is that AGE ligands significantly increase expression of osteogenic factors Runx2, collagen 1, and bone morphogenetic protein-2; another is that RAGE is required for Pit-1 induction, which contributes to vascular calcification and possibly valvular calcification in these mice [33].
Environmental factors.
Ultrafine air pollution particles may also affect CVVD. The MESA study of Air Pollution monitored air quality conditions at residential intersections during commuting hours in a large city and found that coronary calcification progressed at higher rates per unit of particulate matter exposure among people living in areas with high ratios of ultrafine particle counts relative to NOX concentrations [76].
Circulating factors
Several circulating factors have been associated with CVVD. In the general population as well as in high-risk populations, high circulating levels of osteoprotegerin (OPG), a decoy receptor for RANKL, are robustly associated with incident cardiovascular disease [77]. In renal transplant patients, high levels of serum fetuin have been shown to associate with aortic valve calcification [78], whereas low fetuin-A levels is associated with abdominal aortic calcification, independent of renal impairment [79]. Sirtuin1 levels are reduced by 75% in diabetic patients and in SMC undergoing osteogenic differentiation under hyperglycemic conditions, and inhibition of sirtuin1 via Sirtinol and siRNA increased the osteogenic differentiation factor, RUNX2, by over 50% [80].
Optimization.
The effects of some circulating factors that regulate biomineralization depend greatly on dose and timing. For instance, chronically elevated parathyroid hormone (PTH) levels associated with chronic kidney disease lead to bone loss, whereas intermittent elevations in PTH by daily injections or cyclic regimens with PTH analogs are beneficial for bone health [81] and potential benefit to cardiovascular health [54]. It has been proposed that this same dependence on timing or physiologic optimization may apply to osteopontin [82], and other regulatory factors as well.
IMAGING
CT imaging
Electron beam, ultrafast, and gated CT imaging have allowed visualization of the location of calcium deposits and quantification of the calcium content in the coronary vascular tree. EBCT, which has a spatial resolution of 800 μm [83], has been widely used as a simple diagnostic, monitoring, and screening tool. It is used in major clinical trials as a subclinical marker for atherosclerosis. It has been suggested that accuracy could be improved by eliminating unnecessary truncation, thresholding, and simplification steps in the software algorithms used to quantify calcium content [84]. These steps introduce random noise and distortion into the calcium score. For example, the algorithms could lead a clinician to miss a 4-fold increase or a 4-fold decrease in the mineral content of a patient’s calcium deposit, which would likely to affect clinical management.
PET imaging
Recent studies using positron emission tomographic (PET) imaging of 18F-NaF uptake have introduced the exciting possibility that surface area of calcium deposits can be determined noninvasively [85]. The fluoride ion replaces the hydroxyl groups in hydroxyapatite crystals forming fluoroapatite [86]. It preferentially labels the exposed surfaces, and/or actively mineralizing surfaces, of calcium deposits. Initial clinical studies indicate that increased fluoride uptake identifies culprit plaques in coronary arteries [87]. Thus, PET imaging, which has the resolution of 3 mm [88], appears to label only the surface area whereas CT reflects calcium mineral content regardless of shape/surface area or mineral metabolic activity. It has been suggested that 18F uptake in arteries correlates with the amount of surface area [13]. Roughly speaking, the 18F signal is expected to depend on the total surface area (or active mineralizing surface area) of all calcium deposits of any size. A valuable experiment would be to determine the linear or nonlinear relationships between the size or surface area of calcium mineral particles and their 18F uptake, as proposed by Tavakoli and Sadeghi [18]. If it continues to be validated, PET imaging may be useful in determining whether surface area or morphology of calcium deposits is altered by statins or high intensity exercises.
It may also have clinical application for bioprosthetic valve calcification, a major cause of valve dysfunction leading to heart failure, especially in pediatric patients, who have the most rapid of calcific degeneration. Evidence suggests that 18F-NaF PET has the sensitivity to detect early mineralization in bioprosthetic valves before it causes mechanical degeneration, allowing time for replacement before permanent ventricular damage leading to heart failure [89]. As with any high sensitivity test, the frequency of false positive scans will need to be assessed before widespread application.
Whether an imaging modality can detect and discern certain sizes of calcium deposits depends on multiple factors. Ability to detect depends on imaging sensitivity (as opposed to diagnostic sensitivity), and ability to discern depends on resolution. Factors that determine imaging sensitivity include signal strength (tracer activity in the case of PET), sensitivity of the detector instruments, tissue attenuation, and the signal-to-noise ratio. A key determinant of resolution is pixel (or voxel) size. For instance, although objects smaller than 50 μm, such as microcalcifications, would not be distinguishable by clinical PET (since its resolution is 3 mm), it is still theoretically possible to detect collections of such small objects, as long as the signal strength, detector sensitivity, density, and signal-to-noise ratio can be made sufficiently high. There may be physical limits to these parameters. In other words, with sufficiently high sensitivity, objects smaller than the pixel/voxel size may show up as a proportionately small increase in intensity of the corresponding pixel/voxel. But, due to limits of resolution, one would not be able to discern if the increase in intensity is due to a single object with high signal strength or multiple objects of lower signal strength.
POTENTIAL THERAPEUTIC OPTIONS
On the horizon are a number of proposed treatment approaches: medical,interventional and surgical. One example of a medical therapeutic approach is vitamin K supplementation. This has been under consideration for decades. The rationale is that a post-translational modification of matrix GLA protein (MGP), by gamma-glutamyl carboxylase, which enables MGP to inhibit bone morphogenetic protein-2, requires a vitamin K dependent reaction [90]. Failure of this post-translational modification, termed functional vitamin K insufficiency, as well as low vitamin K intake, were more common in patients with cardiovascular and renal disease and associated with increased risk of cardiovascular and all-cause mortality in the PREVEND study from the Netherlands [91].
A second potential medical approach is the use of targeted nanoparticles. Karamched et al have reported use of elastin antibody-conjugated albumin nanoparticles with a calcium chelator that targets degraded elastin in a rat model of mitral annular calcification induced by dietary renal failure [92]. A third approach is the use of antisense oligonucleotides. Viney et al. reported on development of antisense oligonucleotides to lower serum lipoprotein(a) levels in patients with existing cardiovascular disease and high levels [93].
One potential interventional therapy is intravascular lithotripsy. Extracorporeal lithotripsy is currently used to disintegrate gallstones and kidney stones by focusing ultrasonic energy on them. The technology is now adapted to intravascular catheters and is being tested for its ability to disrupt coronary calcium deposits into smaller pieces without removal. It is currently used only to allow stent deployment and expansion [94], but it would be interesting to test whether it also restores vasomotility and alters plaque vulnerability to rupture, given the likely increase in surface area of the mineral deposits. Although, in early studies, concerns were raised about mineral debris entering the circulation, however, most calcium deposits are embedded within the wall -- not exposed on the luminal surface – mineral is unlikely to dislodge into the lumen unless the artery wall is severely breached. A second approach would be targeted cell therapy. One promising approach is the induction of osteoclastic differentiation in circulating monocytes that are osteoclastic precursors, using an engineered fusion protein containing the RANK intracellular signaling domain and the FK506-derived dimerization domain, where the fusion protein is inducible only by a small molecule chemical inducer of dimerization [95].
CONCLUDING REMARKS
Calcific vascular and valvular diseases are widespread and are associated with increased morbidity and mortality. The calcium mineral, hydroxyapatite, is the same mineral in skeletal bone, which appears to arise from matrix vesicles released from vascular cells that undergo osteochondrogenic differentiation. As in endochondral, osteophytic, or intramembranous bone mineralization, amorphous mineral appears to be gradually replaced by true bone tissue as microvessels invade.
Due to their biomechanical consequences, both vascular and valvular calcification cause surgical complications, including dissection in coronary interventions and aortic regurgitation in transcatheter aortic valve replacement. It is generally accepted that the biomechanical consequences in aortic valve cusps cause morbidity and mortality, and that aortic calcification, which alters standing pressure reflectance waves, promotes systolic hypertension, left ventricular hypertrophy, and heart failure. However, controversy surrounds the issue of whether calcium deposits confer risk or benefit with respect to atherosclerotic plaque rupture. Engineers generally avoid allowing rigid inclusions of any size in a flexible material because of rupture risk.
Numerous factors have been shown to initiate and regulate CVVD, ranging from the molecular to the population levels. These include transcriptional, post-translational, nanoparticulate, paracrine, exocrine, endocrine, matricrine, developmental, solid mechanical, fluid mechanical, systemic, vesicular, soluble decoy, protein, immunological, gaseous, atmospheric, and beyond. There are some distinctions between regulations of vascular calcification and valvular calcification; they share many, but not all, regulatory mechanisms. There is also substantial overlap between these CVVD regulatory mechanisms and those that regulate bone mineralization in the skeleton. Diabetes, chronic kidney disease, vitamin D supplementation, statins, serum fetuin, and other clinical factors also strongly influence CVVD.
Depending on location and application, CVVD may be imaged clinically by x-ray techniques (flat, fluoroscopic, angiographic, and computed tomographic) and ultrasound techniques (transcutaneous, transthoracic, transesophageal, and intravascular), and PET. An important new imaging approach, for clinical and pre-clinical applications, is the use of 18F-sodium fluoride as a tracer in PET, which may have the important capacity to identify high-risk coronary atherosclerotic plaque. For in vitro and ex vivo studies, calcification may be detected by a variety of radiographic, histochemical, fluorescence, and indirect immunological methods. Current therapeutic options are limited, invasive, expensive, and high-risk, but new technologies, including medical, interventional, and surgical approaches, are under active investigation and development (see Outstanding Questions Box). It remains to be determined whether calcification, like cholesterol, comes in “good” and “bad” forms; whether any medical treatment can reduce vascular calcification without harmful effects on skeletal mineralization; and how statins and high-intensity exercise promote coronary calcification.
OUTSTANDING QUESTIONS.
Do calcium deposits promote or prevent mechanical plaque rupture?
What treatment(s) can beneficially alter vascular and valvular calcification without harming skeletal mineralization?
Are there “good” and “bad” forms of calcification in arteries?
What mechanism accounts for the paradoxical association of statins and high-intensity exercise with progression of coronary calcification?
HIGHLIGHTS.
Although hyperlipidemia is established as a risk factor for vascular calcification, lipid-lowering drugs (statins) are clinically associated with progression of coronary calcification.
At the cellular and molecular level, endothelial-mesenchymal transformation, microRNA and LncRNA, and autotaxin have been implicated in CVVD.
At the clinical level, intense exercise and elite athleticism have been implicated, paradoxically, with increased coronary calcification.
Acknowledgement
This work was supported in part by funding from the National Institutes of Health [HL121019, AG061586 and HL137647].
GLOSSARY
- CVVD
Calcific vascular and valvular disease, a form of ectopic mineralization, in some cases evolving to form true bone and cartilage tissue in animal models and humans.
- von Mises stress
Parameter that determines whether a given material will rupture. When it reaches a critical value equal to or greater than the yield limit (tolerance) of the material, then the material will give
- Finite element analysis
A numerical method used for predicting mechanical behavior of a material under given mechanical loads. It helps engineers to find areas of weakness and high stress
- Debonding
Mechanical or rupture at an interface
- Reversa mice
A model for atherosclerosis regression based on reversal of hyperlipidemia. This mouse model harbors several genetic modifications, including LDL receptor deficiency, apolipoprotein B-100 overexpression and conditional deletion of microsomal triglyceride transfer protein.
- Lnc RNAs
Long noncoding RNAs that modulate specific gene expressions in a cell/tissue selective manner. They are implicated in diverse roles, and their dysrgulation leads to pathology and diseases
- CAC
Coronary artery calcification is calcium buildup in the arteries that supply blood to the heart muscle; a reliable marker for assessing cardiovascular risk
- PiT1 and PiT2
Related multiple transmembrane proteins, which function as high-affinity, sodium-dependent, phosphate transporters; they may also serve as phosphate sensors through their heterodimerization induced by phosphate binding
- RANKL
Receptor activator of nuclear factor kappa B ligand (also known as TNFSF11, OPGL, ODF) is secreted by osteoblastic cells. RANKL binds to RANK that is expressed on osteoclast precursors to induce osteoclastic differentiation and bone resorption; promotes vascular cell calcification in vitro
- Microcalcification
Term coined by other investigators, referring to a calcium deposit smaller than 50 μm in diameter.
- Macrocalcification
Term coined by other investigators, referring to a calcium deposit greater than or equal to 50 μm in diameter.
- Spotty calcification
Term originally coined to describe a scattered distribution of calcium deposits found by intravascular ultrasound imaging (in distinction to larger coalescent deposits) to be a feature of vulnerable plaque. Given the resolution of IVUS, and the images shown in the reports, these deposits range in size, but they would be on the order of 1 mm in diameter.
- Anisotropic
Having directional dependence with respect to physical properties, such as stiffness and strength. An atherosclerotic plaque is anisotropic since it is composed of fibrous tissue, extracellular lipids, calcium deposits and smooth muscle and inflammatory cells that are randomly aligned.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Budoff MJ et al. (2007) Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 49 (18), 1860–70. [DOI] [PubMed] [Google Scholar]
- 2.Rennenberg RJ et al. (2009) Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag 5 (1), 185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Rourke RA et al. (2000) American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation 102 (1), 126–40. [DOI] [PubMed] [Google Scholar]
- 4.Gondrie MJ et al. (2011) The association of incidentally detected heart valve calcification with future cardiovascular events. Eur Radiol 21 (5), 963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SC et al. (2016) Association of Ankle-Brachial Index and Aortic Arch Calcification with Overall and Cardiovascular Mortality in Hemodialysis. Sci Rep 6, 33164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ et al. (2018) Association of Exercise Capacity, Cardiac Function, and Coronary Artery Calcification with Components for Metabolic Syndrome. Biomed Res Int 2018, 4619867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowell SJ et al. (2004) Calcific aortic stenosis: same old story? Age Ageing 33 (6), 538–44. [DOI] [PubMed] [Google Scholar]
- 8.Shao JS et al. (2010) Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension 55 (3), 579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blacher J et al. (2001) Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38 (4), 938–42. [DOI] [PubMed] [Google Scholar]
- 10.Mukai H et al. (2018) Inverse J-shaped relation between coronary arterial calcium density and mortality in advanced chronic kidney disease. Nephrol Dial Transplant. [DOI] [PubMed] [Google Scholar]
- 11.Tomey MI et al. (2014) Current status of rotational atherectomy. JACC Cardiovasc Interv 7 (4), 345–53. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino T et al. (2009) Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol 297 (2), H802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creager MD et al. (2019) (18)F-Fluoride Signal Amplification Identifies Microcalcifications Associated With Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ Cardiovasc Imaging 12 (1), e007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki Y et al. (2008) In vivo comparison between optical coherence tomography and intravascular ultrasound for detecting small degrees of in-stent neointima after stent implantation. JACC Cardiovasc Interv 1 (2), 168–73. [DOI] [PubMed] [Google Scholar]
- 15.Nerlekar N et al. (2018) Computed Tomographic Coronary Angiography-Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Circ Cardiovasc Imaging 11 (1), e006973. [DOI] [PubMed] [Google Scholar]
- 16.Williams MC et al. (2019) Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J Am Coll Cardiol 73 (3), 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F et al. (2019) Spotty Calcium on Cervicocerebral Computed Tomography Angiography Associates With Increased Risk of Ischemic Stroke. Stroke 50 (4), 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavakoli S and Sadeghi MM (2019) (18)F-Sodium Fluoride Positron Emission Tomography and Plaque Calcification. Circ Cardiovasc Imaging 12 (1), e008712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bostrom K et al. (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 91 (4), 1800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schor AM et al. (1990) Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci 97 (Pt 3), 449–61. [DOI] [PubMed] [Google Scholar]
- 21.Albanese I et al. (2017) Role of Noncanonical Wnt Signaling Pathway in Human Aortic Valve Calcification. Arterioscler Thromb Vasc Biol 37 (3), 543–552. [DOI] [PubMed] [Google Scholar]
- 22.Lim J et al. (2016) Inflammation Drives Retraction, Stiffening, and Nodule Formation via Cytoskeletal Machinery in a Three-Dimensional Culture Model of Aortic Stenosis. Am J Pathol 186 (9), 2378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X et al. (2015) Protective Role of Smad6 in Inflammation-Induced Valvular Cell Calcification. J Cell Biochem 116 (10), 2354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nsaibia MJ et al. (2017) OxLDL-derived lysophosphatidic acid promotes the progression of aortic valve stenosis through a LPAR1-RhoA-NF-kappaB pathway. Cardiovasc Res 113 (11), 1351–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tintut Y et al. (2002) Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 105 (5), 650–5. [DOI] [PubMed] [Google Scholar]
- 26.Al-Aly Z et al. (2007) Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol 27 (12), 2589–96. [DOI] [PubMed] [Google Scholar]
- 27.Borland SJ et al. (2017) Regulation of vascular smooth muscle cell calcification by syndecan-4/FGF-2/PKCalpha signalling and cross-talk with TGFbeta. Cardiovasc Res 113 (13), 1639–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W et al. (2018) IGFBP7 regulates the osteogenic differentiation of bone marrow-derived mesenchymal stem cells via Wnt/beta-catenin signaling pathway. FASEB J 32 (4), 2280–2291. [DOI] [PubMed] [Google Scholar]
- 29.Cheng SL et al. (2015) Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR−/− mice by restraining noncanonical Wnt signals. Circ Res 117 (2), 142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng SL et al. (2010) Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res 107 (2), 271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramachandran B et al. (2018) A GTPase-activating protein-binding protein (G3BP1)/antiviral protein relay conveys arteriosclerotic Wnt signals in aortic smooth muscle cells. J Biol Chem 293 (21), 7942–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yip CY et al. (2009) Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol 29 (6), 936–42. [DOI] [PubMed] [Google Scholar]
- 33.Belmokhtar K et al. (2019) Receptor for advanced glycation end products: a key molecule in the genesis of chronic kidney disease vascular calcification and a potential modulator of sodium phosphate co-transporter PIT-1 expression. Nephrol Dial Transplant. [DOI] [PubMed] [Google Scholar]
- 34.Lin X et al. (2019) lncRNA-ES3/miR-34c-5p/BMF axis is involved in regulating high-glucose-induced calcification/senescence of VSMCs. Aging (Albany NY) 11 (2), 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tousoulis D et al. (2013) Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int J Cardiol 167 (5), 1924–8. [DOI] [PubMed] [Google Scholar]
- 36.Ganidagli B et al. (2019) The relationship between serum osteopontin and FGF 23 levels with valvular calcification in hemodialysis patients. Clin Nephrol 91 (1), 9–16. [DOI] [PubMed] [Google Scholar]
- 37.Turan MN et al. (2016) FGF-23 levels are associated with vascular calcification, but not with atherosclerosis, in hemodialysis patients. Int Urol Nephrol 48 (4), 609–17. [DOI] [PubMed] [Google Scholar]
- 38.Guo YC and Yuan Q (2015) Fibroblast growth factor 23 and bone mineralisation. Int J Oral Sci 7 (1), 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JD et al. (2009) Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation 119 (20), 2693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byon CH et al. (2008) Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 283 (22), 15319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponnusamy A et al. (2018) FTI-277 inhibits smooth muscle cell calcification by up-regulating PI3K/Akt signaling and inhibiting apoptosis. PLoS One 13 (4), e0196232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y et al. (2018) AKT-independent activation of p38 MAP kinase promotes vascular calcification. Redox Biol 16, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Duffhues G et al. (2019) Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J Pathol 247 (3), 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vickers KC et al. (2010) Lyso-phosphatidylcholine induces osteogenic gene expression and phenotype in vascular smooth muscle cells. Atherosclerosis 211 (1), 122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morvan M et al. (2019) Relationship of Iron Deposition to Calcium Deposition in Human Aortic Valve Leaflets. J Am Coll Cardiol 73 (9), 1043–1054. [DOI] [PubMed] [Google Scholar]
- 46.Lee GL et al. (2019) TLR2 Promotes Vascular Smooth Muscle Cell Chondrogenic Differentiation and Consequent Calcification via the Concerted Actions of Osteoprotegerin Suppression and IL-6-Mediated RANKL Induction. Arterioscler Thromb Vasc Biol 39 (3), 432–445. [DOI] [PubMed] [Google Scholar]
- 47.Chavez-Sanchez L et al. (2010) Activation of TLR2 and TLR4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Hum Immunol 71 (8), 737–44. [DOI] [PubMed] [Google Scholar]
- 48.Liao XB et al. (2013) MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology 154 (9), 3344–52. [DOI] [PubMed] [Google Scholar]
- 49.Banach M et al. (2015) Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med 13, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puri R et al. (2015) Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol 65 (13), 1273–1282. [DOI] [PubMed] [Google Scholar]
- 51.Dykun I et al. (2016) Statin Medication Enhances Progression of Coronary Artery Calcification: The Heinz Nixdorf Recall Study. J Am Coll Cardiol 68 (19), 2123–2125. [DOI] [PubMed] [Google Scholar]
- 52.Henein M et al. (2015) High dose and long-term statin therapy accelerate coronary artery calcification. Int J Cardiol 184, 581–6. [DOI] [PubMed] [Google Scholar]
- 53.Chou R et al. (2016) Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 316 (19), 2008–2024. [DOI] [PubMed] [Google Scholar]
- 54.Hsu JJ et al. (2018) Effects of teriparatide on morphology of aortic calcification in aged hyperlipidemic mice. Am J Physiol Heart Circ Physiol 314 (6), H1203–H1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orakzai SH et al. (2009) Non-HDL cholesterol is strongly associated with coronary artery calcification in asymptomatic individuals. Atherosclerosis 202 (1), 289–95. [DOI] [PubMed] [Google Scholar]
- 56.Aengevaeren VL et al. (2017) Relationship Between Lifelong Exercise Volume and Coronary Atherosclerosis in Athletes. Circulation 136 (2), 138–148. [DOI] [PubMed] [Google Scholar]
- 57.Mohlenkamp S et al. (2008) Running: the risk of coronary events : Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J 29 (15), 1903–10. [DOI] [PubMed] [Google Scholar]
- 58.Laddu DR et al. (2017) 25-Year Physical Activity Trajectories and Development of Subclinical Coronary Artery Disease as Measured by Coronary Artery Calcium: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Mayo Clin Proc 92 (11), 1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz RS et al. (2014) Increased Coronary Artery Plaque Volume Among Male Marathon Runners. Mo Med 111 (2), 89–94. [PMC free article] [PubMed] [Google Scholar]
- 60.Arnson Y et al. (2017) Comparison of the Coronary Artery Calcium Score and Number of Calcified Coronary Plaques for Predicting Patient Mortality Risk. Am J Cardiol 120 (12), 2154–2159. [DOI] [PubMed] [Google Scholar]
- 61.Kettunen JA et al. (2015) All-cause and disease-specific mortality among male, former elite athletes: an average 50-year follow-up. Br J Sports Med 49 (13), 893–7. [DOI] [PubMed] [Google Scholar]
- 62.DeFina LF et al. (2019) Association of All-Cause and Cardiovascular Mortality With High Levels of Physical Activity and Concurrent Coronary Artery Calcification. JAMA Cardiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maessen MF et al. (2017) Vascular Function and Structure in Veteran Athletes after Myocardial Infarction. Med Sci Sports Exerc 49 (1), 21–28. [DOI] [PubMed] [Google Scholar]
- 64.Ellam T et al. (2014) Vitamin D deficiency and exogenous vitamin D excess similarly increase diffuse atherosclerotic calcification in apolipoprotein E knockout mice. PLoS One 9 (2), e88767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price PA et al. (2001) The amino bisphosphonate ibandronate prevents vitamin D toxicity and inhibits vitamin D-induced calcification of arteries, cartilage, lungs and kidneys in rats. J Nutr 131 (11), 2910–5. [DOI] [PubMed] [Google Scholar]
- 66.Manson JE et al. (2019) Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med 380 (1), 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sung KC et al. (2016) High levels of serum vitamin D are associated with a decreased risk of metabolic diseases in both men and women, but an increased risk for coronary artery calcification in Korean men. Cardiovasc Diabetol 15 (1), 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jansz TT et al. (2018) Coronary Artery Calcification in Hemodialysis and Peritoneal Dialysis. Am J Nephrol 48 (5), 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Towler DA (2013) Chronic kidney disease: the "perfect storm" of cardiometabolic risk illuminates genetic diathesis in cardiovascular disease. J Am Coll Cardiol 62 (9), 799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavallari C et al. (2019) Online Hemodiafiltration Inhibits Inflammation-Related Endothelial Dysfunction and Vascular Calcification of Uremic Patients Modulating miR-223 Expression in Plasma Extracellular Vesicles. J Immunol 202 (8), 2372–2383. [DOI] [PubMed] [Google Scholar]
- 71.Sanchis P et al. (2019) Arterial "inflammaging" drives vascular calcification in children on dialysis. Kidney Int 95 (4), 958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viegas CSB et al. (2018) Chronic Kidney Disease Circulating Calciprotein Particles and Extracellular Vesicles Promote Vascular Calcification: A Role for GRP (Gla-Rich Protein). Arterioscler Thromb Vasc Biol 38 (3), 575–587. [DOI] [PubMed] [Google Scholar]
- 73.Chavkin NW et al. (2015) Phosphate uptake-independent signaling functions of the type III sodium-dependent phosphate transporter, PiT-1, in vascular smooth muscle cells. Exp Cell Res 333 (1), 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamada S et al. (2018) PiT-2, a type III sodium-dependent phosphate transporter, protects against vascular calcification in mice with chronic kidney disease fed a high-phosphate diet. Kidney Int 94 (4), 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Z et al. (2016) CML/RAGE signal induces calcification cascade in diabetes. Diabetol Metab Syndr 8, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keller JP et al. (2018) Pollutant composition modification of the effect of air pollution on progression of coronary artery calcium: the Multi-Ethnic Study of Atherosclerosis. Environ Epidemiol 2 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tschiderer L et al. (2018) Osteoprotegerin and Cardiovascular Events in High-Risk Populations: Meta-Analysis of 19 Prospective Studies Involving 27 450 Participants. J Am Heart Assoc 7 (16), e009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kocyigit I et al. (2015) Association Between Cardiac Valvular Calcification and Serum Fetuin-A Levels in Renal Transplant Recipients. Transplant Proc 47 (5), 1398–401. [DOI] [PubMed] [Google Scholar]
- 79.Schoppet M et al. (2015) Serum fetuin-A levels and abdominal aortic calcification in healthy men - The STRAMBO study. Bone 79, 196–202. [DOI] [PubMed] [Google Scholar]
- 80.Bartoli-Leonard F et al. (2019) Suppression of SIRT1 in Diabetic Conditions Induces Osteogenic Differentiation of Human Vascular Smooth Muscle Cells via RUNX2 Signalling. Sci Rep 9 (1), 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dempster DW et al. (2016) Effects of Daily or Cyclic Teriparatide on Bone Formation in the Iliac Crest in Women on No Prior Therapy and in Women on Alendronate. J Bone Miner Res 31 (8), 1518–26. [DOI] [PubMed] [Google Scholar]
- 82.Lok ZSY and Lyle AN (2019) Osteopontin in Vascular Disease. Arterioscler Thromb Vasc Biol 39 (4), 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinitsyn V. a.A., S (2004) Electron Beam Computed Tomography (EBCT) In Coronary Radiology (Oudkerk M ed), pp. 137–162, Springer. [Google Scholar]
- 84.Demer LL et al. (2017) Rigor and Reproducibility in Analysis of Vascular Calcification. Circ Res 120 (8), 1240–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Irkle A et al. (2015) Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 6, 7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Czernin J et al. (2010) Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med 51 (12), 1826–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joshi NV et al. (2014) 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 383 (9918), 705–13. [DOI] [PubMed] [Google Scholar]
- 88.Moses WW (2011) Fundamental Limits of Spatial Resolution in PET. Nucl Instrum Methods Phys Res A 648 Supplement 1, S236–S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cartlidge TRG et al. (2019) Detection and Prediction of Bioprosthetic Aortic Valve Degeneration. J Am Coll Cardiol 73 (10), 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Villa JKD et al. (2017) Effect of vitamin K in bone metabolism and vascular calcification: A review of mechanisms of action and evidences. Crit Rev Food Sci Nutr 57 (18), 3959–3970. [DOI] [PubMed] [Google Scholar]
- 91.Riphagen IJ et al. (2017) Prevalence and Effects of Functional Vitamin K Insufficiency: The PREVEND Study. Nutrients 9 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karamched SR et al. (2019) Site-specific chelation therapy with EDTA-loaded albumin nanoparticles reverses arterial calcification in a rat model of chronic kidney disease. Sci Rep 9 (1), 2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Viney NJ et al. (2016) Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 388 (10057), 2239–2253. [DOI] [PubMed] [Google Scholar]
- 94.Yeoh J et al. (2019) Intravascular lithotripsy assisted chronic total occlusion revascularization with reverse controlled antegrade retrograde tracking. Catheter Cardiovasc Interv. [DOI] [PubMed] [Google Scholar]
- 95.Rementer CW et al. (2013) An inducible, ligand-independent receptor activator of NF-kappaB gene to control osteoclast differentiation from monocytic precursors. PLoS One 8 (12), e84465. [DOI] [PMC free article] [PubMed] [Google Scholar]