Abstract
During pregnancy, the sequential release of progesterone, 17β-estradiol, prolactin, oxytocin and placental lactogens reorganize the female brain. Brain structures such as the medial preoptic area, the bed nucleus of the stria terminalis and the motivation network including the ventral tegmental area and the nucleus accumbens are reorganized by this specific hormonal schedule such that the future mother will be ready to provide appropriate care for her offspring right at parturition. Any disruption to this hormone pattern, notably by exposures to endocrine disrupting chemicals (EDC), is therefore likely to affect the maternal brain and result in maladaptive maternal behavior. Development effects of EDCs have been the focus of intense study, but relatively little is known about how the maternal brain and behavior are affected by EDCs. We encourage further research to better understand how the physiological hormone sequence prepares the mother’s brain and how EDC exposure could disturb this reorganization.
Keywords: Progesterone, Estradiol, Prolactin, MPOA, Motivation, Endocrine disruptors, EDC, Maternal Behavior
1. Introduction
Brain function requires constant plasticity and remodeling. Neurogenesis and neuroplasticity have attracted attention because of the potential long term adverse consequences if these processes are impaired or disturbed by endogenous (hormones) or exogenous (social interaction) factors during development as well as during adulthood. Amongst the best studied examples in adulthood are hormonal-dependent physiological and behavioral changes linked to reproduction, including sexual behavior and parenting.
Parenting can be defined as behavioral interactions directed towards an immature conspecific to improve its survival after birth. In mammals, mothers are usually the most involved in parental care, in part due to the lactating ability of the female. In fact, for more than 90% of mammalian species, parental care is exclusively of maternal origin (Kleinman & Malcom, 1981). However, in the remaining species, parental care can be either paternal or biparental, this last case typically observed in monogamous species (Dulac et al., 2014; Royle, 2014).
Like most behaviors, the performance of parenting behaviors occurs due to changes in the neural connections, number of neurons, activation of neurons, and expression of specific proteins within specific spatially-defined brain nuclei (Dragunow & Faull, 1989; Baez-Mendoza & Schultz, 2013; Galea et al., 2014a; Luft et al., 2014). As we will discuss below, the major drivers of these changes in the parental brain are endogenous hormones, including estrogens (such as 17β-estradiol), progesterone, oxytocin and prolactin. Although the major changes in hormone secretion will impact the whole brain, their main targets to modulate maternal behaviors are the medial preoptic area (MPOA) and ventral tegmental area (VTA) as confirmed by lesion studies, performed mostly in rodents species (Numan, 1974; Gaffori & Le Moal, 1979; Jacobson et al., 1980; Numan & Smith, 1984; Numan et al., 1988; Numan, 1990a). Indeed, experimental lesions of these brain regions in rodents abolishes the onset of maternal behaviors, disrupts nest building, and prevents nursing behaviors. Other brain regions including the bed nucleus of the stria terminalis, the nucleus accumbens, and the arcuate nucleus are also involved in maternal behaviors including lactation (Lonstein et al., 2000; Numan & Insel, 2003; Crowley, 2015).
Because the establishment of parental behaviors and the associated changes in the brain are dependent on the action of specific hormones on hormone receptors, it has been proposed that they also have the potential to be affected by exposures to environmental chemicals that mimic or block the actions of endogenous hormones, i.e. endocrine disrupting chemicals (EDCs) (Walker & Gore, 2011; Catanese et al., 2015; Gore et al., 2015). Our review will describe the steroid-dependent mechanisms involved in the onset of parental brain and behavior and discuss, with the little data available from studies of EDCs, how activation or inhibition of specific hormone signaling pathways affect parental behavior and structures in the brain. Although there is a rich literature demonstrating that EDCs can affect numerous aspects of behaviors (e.g., anxiety-like behaviors, hyperactivity, social behaviors, sex behaviors), we focus our review on the effects of EDCs on maternal behaviors, particularly in females exposed during pregnancy/lactation or in females exposed during perinatal development. Similarly, there are many studies demonstrating that EDCs alter numerous aspects of specific brain regions and nuclei; here, we have focused on the much smaller subset of studies that examine maternally-relevant brain regions during the display of maternal behaviors, i.e. during the lactational period. Although these studies are relatively limited, insights from this work on EDCs could provide new tools to probe the mechanisms by which maternal brain and behaviors are established.
2. Maternal behavior
2.1. The establishment of maternal behavior at parturition
Maternal behavior at birth presents very similar characteristics within many mammalian species (Numan, 2003). For most mammals, maternal behavior emerges at or close to parturition under the influence of changes in circulating hormonal levels (in particular, a drop in circulating levels of progesterone, a rise in circulating estradiol, and intracerebral release of oxytocin due to vagino-cervical stimulation; see (Brunton, 2008; 2010; Bridges, 2015) and section 3.4 of this review). Immediately following birth, the female shows a very rapid interest towards the newborn, and mothers start to lick the amniotic fluid and eat the extra-embryonic membranes covering the offspring. Females also often display placentophagia, i.e. consumption of the placenta. In many mammals, females begin to emit specific vocalizations in response to their young. Within the first hours following parturition, the offspring gets access to the udder or nipples to begin suckling. Nursing behavior is the most important and common pattern of maternal behavior in mammals, defined by obligatory contact with the mammary tissue. In addition, most mothers protect their young from predators or conspecifics by developing maternal aggression towards intruders. Finally, rodent mothers show various forms of retrieval, or carrying behaviors, to place their young in safe places like a nest.
Despite this common scheme, variations in the expression of maternal behaviors occur according to the degree of maturity of the young at birth. In altricial species, females usually have a large litter of young that are immature with regard to both sensory and motor development. In these species, parental behavior is organized around a nest that is used to isolate and protect the litter from the environment. Most rodent species fall into this category. Indeed, rodent pups are rather immobile with poor physiological maturity at birth; they are nude and unable to regulate their body temperature, and cannot defecate or urinate without the help of their mother.
By contrast, in precocial species, the litter is more limited (1–2 offspring) and the young are much more mature. In these species, the offspring can stand and suckle the mother shortly after birth; within a few hours, the young can follow the mother (Nowak, 2000). In this developmental state, maternal behavior is not typically organized around a nest and parental care usually requires a rapid attachment of the mother to its young, often leading to individual recognition of the offspring. Ungulates such as sheep and goats are good examples of this category (Lévy, 2008; Nowak, 2011). Indeed, lambs, once cleaned of fetal fluids by the mother, can regulate their body temperature on their own. They typically reach the mother’s teat within one hour following birth and within a matter of hours can travel long distances. In addition, the mother develops an individual bond with the lamb that is formed within 4h of parturition and is based on the learning of the lamb’s individual olfactory signature (Keller et al., 2003; Keller, 2004). Once this has occurred, any alien lamb attempting to suckle will be rejected at the udder.
Finally, many species of primates, including humans, show an intermediate level of development at birth (semi-precocial), such that the young has relatively developed sensory abilities but is not yet mobile, resulting in a close contact of the mother with the infant when traveling. As the young develop, caregiving behaviors are shared with other conspecifics, especially in new world primates.
2.2. The sensory regulation of parental behavior
It has long been established that parental behavior is regulated by numerous sensory cues and that the sensing of infant cues is greatly enhanced at parturition. Among the various cues involved, olfaction plays a primary role in a very large number of mammalian species (Lévy, 2004; 2009). Indeed, olfaction mediates a shift from aversion to juvenile odors towards attraction to these odors. The typical response of virgin or non-pregnant/non-lactating female rats to pups is avoidance. This avoidance is due to the amniotic/placental fluids that cover the neonate at birth, which induce repellence (Kristal, 1976). Lesioning both the main and accessory olfactory systems eliminates the aversive response of nulliparous females to pups; these females then exhibit a rapid onset of maternal behavior (Fleming & Rosenblatt, 1974b; c; Carretero, 2003)( see also: Fleming et al., 1979). Such an inhibitory influence of olfaction on the onset of maternal behavior in virgin or non-pregnant females has been reported in many other mammalian species including hamsters and sheep (Marques, 1979; Lévy, 1983; Poindron, 1988).
At parturition, the mother becomes highly responsive to olfactory cues from the young. This olfactory shift is important for the transition from a non-maternal state to a maternal one. In rats, rabbit and sheep, parturient females consume the placenta and amniotic fluid, and lick the offspring who are covered, while this behavior is not typically displayed by non-pregnant females (Kristal, 1980; Lévy, 1983; 2009). In addition, females begin to show a preference for bedding soiled by pups, over clean bedding, around the peripartum period (Kinsley, 1990). Various experiments aimed at disrupting the olfactory systems confirmed the importance of olfaction for the expression of maternal behavior.
Finally, neurobiological evidence supports the important role of olfactory cues in the onset and maintenance of maternal behavior. For example, in sheep, the mitral cells of the main olfactory bulb show a profound shift in response after parturition and become highly responsive to the lamb’s odor (Kendrick et al., 1992b). The importance of olfactory cues has been confirmed by studying the effects of lesions of the main olfactory neurons (zinc sulfate-induced lesion of the olfactory epithelium, including olfactory neurons); these lesions produce disturbances in the establishment of maternal behavior in primiparous mothers and affects lamb recognition regardless of maternal experience (Lévy, 1995).
Other sensory cues are also involved in maternal behavior depending on the species. Maternal behavior in sheep offers a model to understand multisensory interactions between the ewe and her lamb. While the ewe develops a selective olfactory bond with the lamb within a few hours of parturition (Keller, 2003), within one day the mother becomes able to recognize her offspring own’s vocalizations (Sèbe, 2008). A recognition of the lamb face occurs within a few weeks following birth (Kendrick, 1994). In rodents, female searching behavior, to locate the pups, is facilitated by the playback of ultrasonic pup vocalizations (Smotherman, 1974). Electrophysiological responses in the auditory cortex undergo significant plasticity in maternal females (Liu & Schreiner, 2007; Cohen, 2011).
Finally, somato-sensory cues coming from the vagina and the nipple are also important for the establishment of maternal behavior. First, the vagino-cervical stimulation caused by expulsion of the fetus triggers an intracerebral release of oxytocin (see section 3.4) that is responsible for a cascade of behavioral changes, including the interest towards amniotic fluid consumption and behaviors directed at the offspring at parturition, a mechanism especially well studied in sheep (Keverne et al., 1983; Kendrick, 1987; Lévy, 1992). Interestingly, an artificial vagino-cervical stimulation performed during the early post-partum period allows for the establishment of a new olfactory recognition process for the lamb (Lévy, 2010). Second, the stimulation of the mammary gland through the pup’s suckling activity induces an increase in the electrophysiological receptive field of the ventral skin surrounding the nipple area in the somatosensory cortex during lactation in rats (Xerri, 1994). More generally, teat or nipple stimulation induces a wide pattern of neuronal activity in the maternal brain, a result confirmed by the pattern of fMRI activation in post-partum female rats when suckled (Febo, 2011).
2.3. Hormones and maternal behavior
Work conducted in the 1930s (Wiesner & Sheard, 1933) showed that immediate maternal care and sensory responsiveness to pups cues are only present in parturient female rats whereas non-pregnant nulliparous or pregnant primiparous females during the first week of pregnancy do not show such behaviors. The initial response of nulliparous females is avoidance of the pups but infanticide can be observed in various strains (Fleming & Rosenblatt, 1974b; Jakubowski & Terkel, 1985; Mennella & Moltz, 1989). The classical work of Terkel and Rosenblatt (Terkel & Rosenblatt, 1968; 1972) showed that blood transfusion from a parturient female into virgin female significantly reduced latency to retrieve pups, suggesting the implication of humoral factor(s) to facilitate maternal behavior.
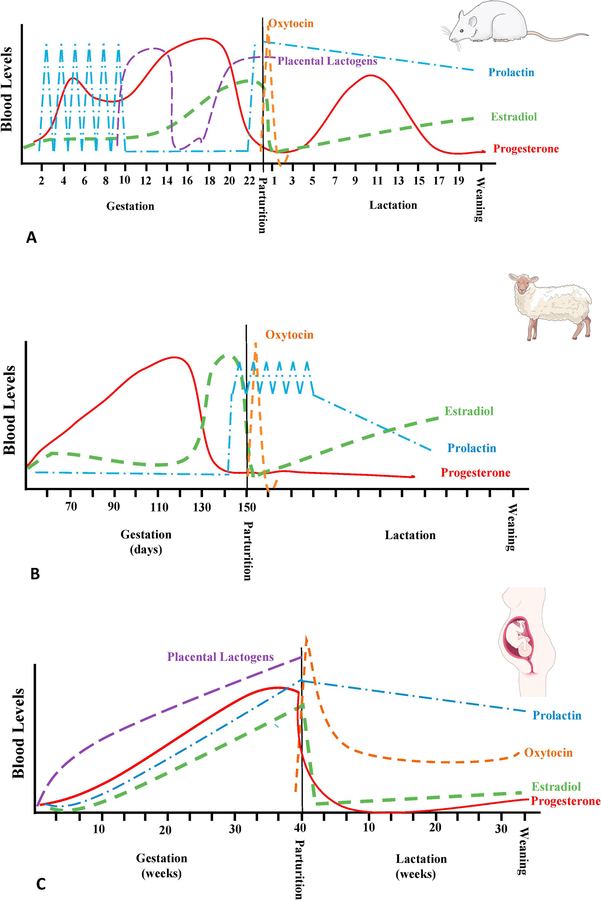
Consequently, additional studies have identified the changes in serum hormone concentrations that occur during pregnancy, at parturition, and to some extent during lactation, with focus on progesterone, 17β-estradiol, prolactin, oxytocin and placental lactogens (see figure 1). In rats (see figure 1A), estrogens (including 17β-estradiol, estrone and/or estriol) concentrations are relatively low during the first 10 days of pregnancy and then rapidly increase to reach a plateau 3–4 days before parturition; they then drop after parturition. In parallel, progesterone increases during the first part of pregnancy to reach a peak at around 15 days post-conception, before rapidly declining. The high level of progesterone and low level of estradiol is required for the maintenance of pregnancy, but interestingly, as will be discussed below, the shift in hormone concentration is the pre-requisite for the timely induction of maternal behaviors. A few days following parturition, circulating progesterone rises again, peaking on postpartum day 10–12 and waning again to reach basal levels 7 days later (Morishige et al., 1973; Bridges, 1984). In addition, prolactin is secreted in daily surges during the first 10 days of pregnancy and then again after parturition while placental lactogens I and II are secreted the last 2 weeks of pregnancy and can also be found in the cerebrospinal fluid where they affect the central nervous system (Robertson & Friesen, 1981; Peake et al., 1983; Bridges et al., 1996). Parturition itself is also associated with a high peak of oxytocin, important to stimulate contraction of the uterine wall. These hormonal variations during pregnancy and lactation occur in all species studied to date albeit there are variations in the sequential release pattern (see figure 1 B and C respectively for comparison with sheep and human). These changes not only suggest a role for hormones in sustaining pregnancy, but also in the modification of the brain network and resulting maternal behaviors. As we will discuss below, it is not any individual hormone that is able to facilitate a specific sequence of the maternal behavior but the precise and sequential exposure to this complex hormone mixture that allows for the parturient female to rapidly express maternal behavior.
Figure 1.
Schematic representation of relative variation of Estradiol (----), Progesterone (—), prolactin (−.−.), oxytocine (……) and placental lactogens (----) during gestation, parturition and lactation. These variations are presented for rats (A), sheep (B) and human (C) for comparison. Images of rat, sheep and human were obtained from Servier Medical Art (https://smart.servier.com/)
The role of these hormones, or more precisely the tissues responsible for hormonal secretion, was first highlighted by the seminal work of Rosenblatt and his colleagues (Rosenblatt & Siegel, 1975; Siegel & Rosenblatt, 1975a; b). They developed a pregnancy termination model in rats to explore the endocrine basis of maternal behavior. Upon the presentation of foster pups to a primigravid female Sprague Dawley rat on day 17 of pregnancy (pregnancy lasts 20–22 days in rats), no maternal behavior was observed. However, hysterectomy (removal of the uterus, including placenta and pups) on day 15 of pregnancy, followed by presentation with foster pups 2 days later, induced the display of maternal behaviors 24 hours following pup exposure. Hysterectomy mimics parturition as it is associated with a rapid drop in progesterone, followed by an increase in estrogens and concomitant increase in prolactin. Interestingly, if the females were hysterectomized and ovariectomized on pregnancy day 15, followed by exposure to pups 48 hours later, the onset of maternal behavior was significantly delayed and did not appear for another 2 or 3 days, suggesting the importance of high estradiol after hysterectomy (Siegel & Rosenblatt, 1975b). The respective inhibitory role of progesterone and facilitative function of estradiol were confirmed by concurrent injection of hormones following surgical procedures (Moltz et al., 1969; Bridges & Feder, 1978; Bridges et al., 1978b; Numan, 1978) or just before pup exposure (Stolzenberg et al., 2009). Similar observations were made with virgin females and males where a specific sequence of hormone exposure, with high prolactin (or placental lactogens) and high estradiol following progesterone withdrawal can stimulate maternal behavior and significantly reduce the onset of pup-directed aggressive behaviors (Moltz et al., 1969).
It should be noted that both male and virgin female rats, even after ovariectomy and hysterectomy, will respond to pups and show maternal behavior following several days of pup exposure, a process called sensitization (usually taking 4 to 9 days, (Rosenblatt, 1967; Fleming & Rosenblatt, 1974a; Stern, 1983; Brown, 1986; Brown & Douglas, 1991)). This suggests that the maternal behavior itself is not hormone-dependent, but the precise onset is: hormonal exposure allows the parturient female to immediately respond to the pups to provide appropriate care. Any disruption in the timing of onset of the behavior will significantly reduce the chance of survival for the offspring.
Mice are somewhat different from rats as they can show spontaneous maternal behavior such as pup retrieving, grooming and licking (Gandelman et al., 1970; Gandelman, 1973; Rosenson, 1975). The idea that maternal behavior is independent of hormones is further reinforced by studies in genetically modified mouse models. ERα knockout mice from a mixed background of C57BL/6J and 129 strains showed only mild impairment in maternal behaviors (Ogawa et al., 1998) although potential compensatory effects from ERβ activation was not investigated. In addition, C57BL/6J mice lacking aromatase, the key enzyme for synthesis of 17β-estradiol, still show maternal behavior (Stolzenberg & Rissman, 2011). It should however be noted that, even if maternal behavior is spontaneously shown by mice and can be triggered only by sensory input from pups, the quality of the behavior is usually inferior to parturient females, with slower pup retrieval and reduced amount of time crouching over pups (Stolzenberg & Rissman, 2011).
The results obtained from transgenic mice should be interpreted with caution due to the differences existing between strains (Numan, 2003). Indeed, strain differences were first documented more than 60 years ago (Thompson, 1953). For example, the widely used C57BL/6J strain is known to exhibit very poor maternal behavior, especially when primiparous, with a high level of infanticide at parturition, limited time spent on nest building or crouching over pups, contributing to a high mortality rate in pups (Broida & Svare, 1982; Brown et al., 1999). However, this same strain exhibits very short latency to pup retrieval in comparison to strains such as Balb/C or DBA/2 (Carlier et al., 1982). On the other side, DBA/2 exhibits a higher level of maternal care behavior, spending more time crouching over pups and nursing them (Brown et al., 1999).
In addition to the timing and quality of maternal behaviors, steroid hormones seem to play an important role for maternal motivation, at least in rats and mice. Indeed, the motivation of the parturient female rat or mouse to retrieve the pups is significantly higher than virgin females when placed in a novel or complex environment. For example, virgin rats and mice will show a significantly lower or absent maternal motivation when tested in a T-maze or when they have to press on a lever to obtain the pup (Gandelman et al., 1970; Hauser, 1985; Lee et al., 1999;Bridges et al., 1972). However, some maternal motivation can be induced in certain conditions, following extended pup exposure, even in animals devoid of circulating ovarian hormones (Scanlan et al., 2006; Seip & Morrell, 2008; Stolzenberg & Rissman, 2011).
Altogether, it is clear that hormones are required for the proper onset of maternal behavior in rodents. This hormonal shift during pregnancy, parturition and lactation is obviously vital to the survival of the offspring, but also insures that the mother will behave appropriately to sustain her own health to reproduce again. Interestingly, once maternal behavior has been established, the dependence on steroid hormones seems to be significantly reduced, if not absent. Indeed, circulating progesterone and estradiol level are basal and postpartum ovariectomy and hysterectomy do not affect maternal behavior. Furthermore, the presence of hormones that were required around parturition can become inhibitory during lactation. For example, some studies suggest that injection of estradiol in postpartum mice actually reduces maternal behavior (Kasper & Telegdy, 1975; Svare & Gandelman, 1975). Similarly, progesterone exposure just before parturition or during lactation significantly affects maternal displays, albeit differently depending on the timing of exposure (Moltz et al., 1969; Bridges & Feder, 1978; Bridges et al., 1978a; Numan, 1978; Grieb et al., 2017). The timing of hormone exposure is therefore fundamental to allow for adequate care of the offspring; potential disruption of this pattern is likely to result in adversity for the offspring, but also to the mother herself as we will discuss below. These hormones are essential to reset the neuronal network, allowing the female to mother.
While the effects of sex steroid hormones are, by far, the most investigated, stress hormones are also important in the display of parenting behaviors. The basal level of corticosterone is significantly elevated during the postpartum period compared to virgin females and corticosteroid binding globulin concentration is significantly reduced following parturition, probably due to a reduction in estradiol levels (Koch, 1969; Stern et al., 1973; Garland et al., 1987; Fischer et al., 1995; Pawluski et al., 2009b). However, the responsiveness of the hypothalamic-pituitary-adrenal axis is attenuated postpartum in the majority of species studied so far. Large numbers of studies, using various stressors, highlight the attenuation of ACTH secretion and corticosterone synthesis compared to control virgin animals (restraint: (da Costa et al., 2001); Noise: (Windle et al., 1997); Swimming: (Walker et al., 1995; Toufexis et al., 1998); Foot shock: (Stern et al., 1973); Ether evaporation: (Banky et al., 1994); LPS: (Shanks et al., 1999); Interleukin (Brunton et al., 2005)). Importantly, it should be noted that the presence of pups and relevance of the stressor for the safety of the pups modulates the maternal stress response (Deschamps et al., 2003). The hyporesponsiveness is linked to blunted activity of the opioidergic and noradrenergic systems during late pregnancy and lactation, via a modulation of steroid receptors (Douglas et al., 2003; Douglas et al., 2005; Slattery & Neumann, 2008; Brunton & Russell, 2011).
2.4.1. Direct exposure to EDCs in the adult female
As discussed above, the onset of maternal behaviors upon the birth of the pups requires very specific coordination of levels, ratios, and timing of several hormones. Disrupting the action of any one of these hormones during pregnancy and/or parturition is therefore likely to prevent the mother from appropriately responding to the pups to provide appropriate care. Endocrine disrupting chemicals (EDC) are one way that hormone action can be modified.
To date, most EDC studies that have examined the effects of compounds administered during pregnancy and/or lactation (e.g., directly to the adult female) have focused on chemicals that mimic estrogens, typically as estrogen receptor agonists. Ethinyl estradiol (EE2), a prototypical estrogen used in oral contraceptives, has produced conflicting effects on several aspects of maternal behavior. In the first study of EE2, exposure only during pregnancy limited the number of pups that were retrieved by treated rat dams, although other overt signs of toxicity due to the high dosage were also observed (Dugard et al., 2001). Yet a second study by the same group found exactly the opposite effect, where high dose EE2 exposures only during pregnancy to rats improved pup retrieval behaviors (Arabo et al., 2005). To address these inconsistent findings, another study examining lower, non-toxic doses of EE2 administered throughout pregnancy and the lactational period to mice found no significant changes to maternal behavior, but increased display of stereotypy behaviors (Catanese & Vandenberg, 2017b). This study might suggest that exposures to estrogen receptor agonists during pregnancy or lactation do not affect maternal behaviors, yet numerous studies of other xenoestrogens suggest otherwise. For example, exposures from the beginning of pregnancy through the entire lactational period to bisphenol A (BPA), a complex EDC with estrogen receptor agonist and androgen receptor antagonist activities among others (Vandenberg et al., 2009; Vandenberg et al., 2013), reduced licking and grooming of pups in rats (Della Seta et al., 2005); in mice, exposures during pregnancy also decreased licking and grooming of pups as well as the display of arched-back nursing, the posture associated with the greatest milk let-down (Kundakovic et al., 2013b) and decreased the time mothers spent on the nest (Palanza et al., 2002a). BPA was also shown to disrupt maternal behaviors in cynomolgus monkeys, with effects that were particularly pronounced in females with male offspring (Nakagami et al., 2009). Follow-up studies of bisphenol S (BPS), a BPA analogue often used in BPA-free consumer products, found that exposures during pregnancy and lactation increased the time mouse dams spent on the nest during the mid-to-late lactational period, and increased the latency to touch pups during retrieval assays (Catanese & Vandenberg, 2017a).
It is important to note that most of these compounds are known to modulate steroid receptor activity but little focus was paid to potential interactions of endocrine disrupter with steroid binding globulins. The concentration of sex hormone binding globulin (SHBG), a plasma protein secreted by the liver with a strong affinity for androgens and to a lesser extent to estrogens, increases significantly in women during gestation (Hammond, 2016). the precise function of this protein is not fully understood but its present would limit the amount of free, active, steroid. This protein is able to bind numerous well known endocrine disrupters such as bisphenol A, phthalate and nonyphenol, albeit in the micromolar range (Danzo, 1997; Déchaud et al., 1999; Hodgert Jury et al., 2000). The same low interaction between anthropogenic molecules and SHBG was observed in zebra fish, using QSAR modeling and in vitro competition assay (Thorsteinson et al., 2009; Miguel-Queralt and Hammond, 2008). To our knowledge, the physiological impact of this interaction is not known during pregnancy as the classical rodent models (rats and mice) do not express SHBG in adulthood (Sullivan et al., 1991). It should be noted that a humanized transgenic mouse line expressing human SHBG was developed and the potential impact of endocrine disrupter in the presence of plasma binding globulin could be investigated in a more physiologically relevant model (Jänne et al., 1998; Jänne et al., 1999).
Additional examples of disrupted maternal behaviors have been observed in female rodents exposed during pregnancy for a range of estrogenic EDCs including the phytoestrogen genistein (rat: Ball et al., 2010), industrial chemicals such as polychlorinated biphenyls (rat: Simmons et al., 2005; Krishnan et al., 2019), and the pesticides methoxychlor (mouse: Palanza et al., 2002b) and glyphosate (Dechartes et al., 2019). The anti-androgenic chemical chlorpyrifos, an organophosphate insecticide, increased pup grooming in female mice exposed during pregnancy (Venerosi et al., 2009). Finally, other known reproductive toxicants including the herbicides sulentrazone (rat: de Castro et al., 2007) and glyphosate (Dechartres et al. 2019), the insecticides carbaryl (meadow jumping mouse: Punzo, 2003) and fipronil (rat: Udo et al., 2014), and the herbicide 2,4-dichlorophenoxyacetic acid (rat: Sturtz et al., 2008) altered numerous aspects of maternal behavior including retrieval behaviors, infanticide, nest quality, and pup licking in adult females exposed during pregnancy. Albeit these last molecules seem to act as endocrine disrupter, their precise target(s) to alter maternal behavior is, to our knowledge, not defined.
Collectively, these studies of EDCs have shed light on the neuroendocrine mechanisms that play a role in the establishment and maintenance of maternal behaviors. Yet, these studies also offer the potential to evaluate the effects of dose, period of exposure and species/strain. For example, several studies revealed different effects of low doses than high doses; mouse dams exposed to a low dose of methoxychlor during pregnancy spent less time nursing and more time off the nest compared to controls, whereas higher doses had no effect on these outcomes (Palanza et al., 2002b). These kinds of non-monotonic dose responses are common for EDCs, and there are well-established mechanisms by which low doses can have effects that are not predicted by studies at higher doses (Vandenberg et al., 2012). The endocrine mechanisms for non-monotonic responses of maternal behaviors have not yet been elucidated, nor have they been explored for many other physiological and behavioral responses. It is however likely that the multiple molecular targets for EDCs, as confirmed for BPA (Rubin, 2011; Acconcia et al., 2015; MacKay & Abizaid, 2018), are one cause for non-linear responses.
To our knowledge, there is also no information available on the potential disrupting activity of compounds affecting progesterone, oxytocin, glucocorticoid or prolactin-sensitive pathways linked to maternal behavior. It should be mentioned that a number of pharmacological treatments, such as fluoxetine (Prozac®, (Gemmel et al., 2018) see Pawluski et al. this issue), haloperidol (Stern & Keer, 1999), diazepam (Yang et al., 2015), and bromocriptine (Bridges & Ronsheim, 1990; Price & Bridges, 2014), will also affect the neuro-endocrine system underlying maternal behavior; we will not discuss these examples in depth here, as these molecules were specifically developed to target the neuronal network and are used to alleviate mental health disorders, including those associated with maternal behavior.
It is also important to note that the few EDC studies described above were dedicated to species that are mostly mono-parental (or at least rendered as such by the experimental paradigm). Only a handful of studies have investigated parental behavior in biparental species, including California mice (Peromyscus californicus) and to some extent avian species. These studies are important because an alteration of behavior of even one parent could lead to altered offspring outcomes (in addition to the chemical exposure experienced in ovo) but also could produce compensatory behaviors in the other parent. For example, work from Rosenfeld lab (Johnson et al., 2015) suggest that early exposure to BPA or ethynylestradiol of the male only will affect the maternal behavior of his partner non-exposed female. The parental behavior of the male himself was not affected by the exposure to these molecules and it was suggested that females were somehow able realize that the male partner was exposed to compound and, as a consequence, reduced their own parental investment in offspring. On the other hand, limited studies in birds suggest that males could be more sensitive to chemical exposures than females, likely due to the partial elimination of contaminants in the female by deposition into the eggs (Fisher et al., 2001; Verboven et al., 2009). These males were less likely to participate in the care of the nest, albeit survival of the offspring was not affected. It is however suggested that effects on offspring survival are likely to be masked by the short term behavioral adaptation of the compensating parent; in the long term, the extra energy burden required for this compensation is likely to result in reduced reproductive outcome during the next reproduction bout (see (Carere et al., 2010). These handful of studies clearly call for more comparative studies to understand the consequence of EDC exposure in biparental species.
2.4.2. Co-exposure to EDCs in the Mother and the offspring
As noted above, EDC exposures to the pregnant or lactating female are also likely to lead to exposures to the offspring, depending on the toxicokinetic properties of the compound being studied. Therefore, the effects of EDC exposures on the mother should be studied in light of maternal-pup interactions during the display of parental behaviors; abnormal maternal behaviors could be due to the mother’s response to abnormal pup development or behavior. For example, rat dams treated during pregnancy with one polychlorinated biphenyl, PCB77, spent more time on the nest and more time grooming their pups compared to untreated dams (Simmons et al., 2005). When a subsequent study used a cross-fostering design, it was determined that PCB77 exposure of both the offspring and the mother was required to disrupt nesting and grooming behaviors, highlighting the impact of EDC on the maternal-neonate dyad, but not on a single individual (Cummings et al., 2005).
In addition, it is important to note that rat mothers may discriminate the sex of their offspring and maternal behavior toward individual pups varies according to the sex of the pup, such that males usually receive more attention from their mother than females (licking and grooming, latency to retrieve (Moore & Morelli, 1979; Richmond & Sachs, 1984;; Deviterne & Desor, 1990). It should be noted that skewed litter composition (male only) in mice also lead to higher licking and grooming behaviors from the mother (Alleva et al., 1989; Musi et al., 1993). More importantly, hormone injections to the newborn, using progestins (Birke & Sadler, 1985) or androgens (Moore, 1982), modify maternal behaviors. These studies suggest that manipulations to the endocrine systems of pups are likely to affect maternal responsiveness and the pup-mother social interaction. To our knowledge, this aspect has not received the attention it deserves in studies of EDCs.
2.4.3. Effects of perinatal exposure on early organization of maternal behavior
As discussed above (section 2.4), EDC exposures in adulthood, during pregnancy and/or the lactational period, can disrupt the display of different aspects of maternal behavior. In this study design, not only is the mother exposed to the compound of interest, but the offspring are likely exposed via blood (in utero) or milk (during lactation). Other studies have examined whether developmental EDC exposures (typically during gestation, or gestation plus the perinatal period) can alter the display of maternal behaviors when the females reach adulthood. This work has mostly focused on evaluating the endocrine disrupting activities of estrogenic compounds including ethinyl estradiol (EE2), Bisphenol A and S (BPA, BPS), chlorpyrifos and lindane, an insecticide with both estrogenic and anti-estrogenic properties (Catanese et al., 2015). Low dose EE2 exposure only during perinatal development increased the time mice spent on self-care and nest building in adulthood, during the early postpartum period (Catanese & Vandenberg, 2018). Developmental BPA (Palanza et al., 2002a) and BPS exposure (Catanese & Vandenberg, 2017a) both decreased time spent on the nest once females reached adulthood; developmental BPS exposure also induced severe disruptions to maternal behavior including infanticide. Neonatal exposure of mice to chlorpyrifos increased the latency to build nests, decreased their latency to lick pups, and altered pup retrievals in adulthood (Venerosi et al., 2008). Finally, mice exposed to lindane during early development and throughout adulthood were less likely to retrieve their pups to the nest (Matsuura et al., 2005). Additional studies were performed in California mice and similarly to the common laboratory mice, exposure to BPA or EE2 during gestation and lactation affected the maternal behavior of the adult offspring, including a reduction of time spent in the nest and time nursing the pups (Johnson et al., 2015). Although the total number of studies examining the effects of early life EDC exposures on later-life display of maternal behaviors remains small, these studies collectively suggest that activation of estrogen signaling pathways during early development can have long lasting effects on pup-directed behaviors after parturition (see Johnson et al., 2018).
Activational effects of hormones during pregnancy, parturition and/or lactation are likely to be superimposed on organizational effects of sex steroid hormones from fetal, perinatal and pubertal periods of development. For example, females exposed to testosterone during the perinatal period have significantly reduced aspects of maternal behavior including less time spent grooming pups and poorer pup retrieval behaviors (Quadagno & Rockwell, 1972; Quadagno et al., 1973; Rosenberg & Herrenkohl, 1976). Bridges and colleagues (Bridges et al., 1973) found that perinatal treatment with androgens reduced maternal motivation to retrieve pups in a T maze while not affecting the onset of maternal behavior displays or the response in a simple environment such as the home cage. These studies suggest that, similar to what is known for the organization of the neurocircuitry underlying sexual behavior and the ovarian cycle, early modulation of hormone exposure around the time of birth is likely to affect later maternal behaviors. To our knowledge, little is known about the neuroendocrine changes occurring around birth that are necessary for appropriate maternal behavior later in life. However, the effects of early exposure to EDC on later parental behavior tend to confirm the existence of organizational effects of steroid on parental brain and behavior, albeit via unknown mechanisms.
3. Maternal brain
3.1. The core neurobiological circuit driving the expression of maternal behavior
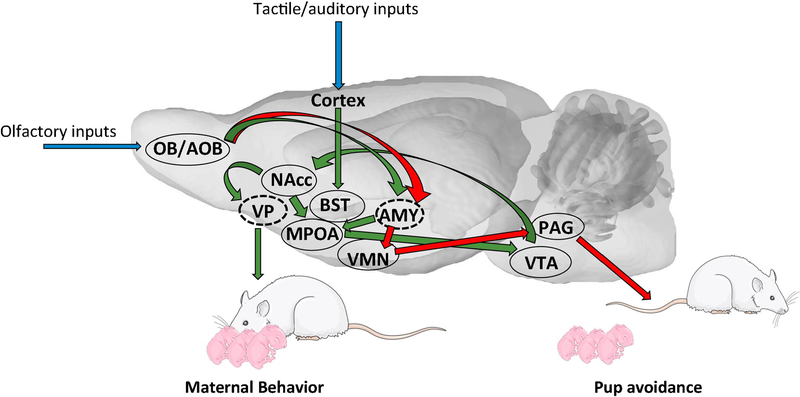
A neurobiological network model for the control of maternal behavior has been proposed in female rats (see figure 2). In this model, two opposite and competing pathways mediate the activation or inhibition of maternal behavior respectively (Sheehan, 2001). First, an aversive circuit, innervated by the vomeronasal organ, suppresses maternal behavior through amygdale (AMY)-dependent mechanisms. Indeed, experimental evidence demonstrates that the corticomedial amygdala exerts an inhibitory effect on the expression of maternal behavior. Lesions of this structure induce a facilitation of maternal behavior when tested in a sensitization paradigm in female rats (Fleming et al., 1980; Numan, 1993). In contrast, stimulation of the corticomedial amygdala delayed the sensitization response (Morgan, 1999). In addition to results showing that the corticomedial amygdala is involved in fear and anxiety processes (Luiten, 1985; Adamec, 1993) and the fact that anosmia facilitates maternal behavior in virgin female rats, the general view is that novel olfactory cues from the pups inhibit maternal responsiveness through fear arousing mechanisms in this structure.
Figure 2.
Schematic representation of the neural network in rat. Blue arrows indicate sensory inputs, green arrows represent the maternal network and red arrows represent neural network in non-maternal male and female rat to trigger pup avoidance. Nuclei in dashed lines are located more laterally. AMY: Amygdala; BST: Bed nucleus of the stria terminalis; MPOA: Medial preoptic area; NAcc: Nucleus Accumbens; OB/AOB: Olfactory bulb/Accessory olfactory bulb; PAG: Periaqueductal Gray; VP: Ventral pallidum; VMN: Ventromedian nucleus of the hypothalamus; VTA: Ventral Tegmental Area. Images of pups and adult rats were obtained from Servier Medical Art (https://smart.servier.com/)
By contrast, the medial preoptic area (MPOA) and the adjacent ventral part of the bed nucleus of the stria terminalis (vBST) have been shown to play a key role not only in the stimulation of the expression of maternal behavior at parturition but also for its maintenance throughout the lactational period (Numan, 2006; Numan & Stolzenberg, 2009; Olazabal et al., 2013). As a result of hormonal and sensory stimulation at the end of pregnancy and at parturition, this system becomes dominant over the aversion system, thus resulting in approach behavior and the expression of maternally responsive behaviors. Indeed, experiments have shown that disruption of the MPOA’s function, in a variety of species, produces a disturbance in maternal behavior. In rodents, electrical or excitotoxic lesions of the MPOA disrupt the onset of maternal behavior at parturition (Lee et al., 1999) or the sensitization of virgin females (Numan, 1977; Lee et al., 1999). A similar result was obtained when the dorsolateral efferent projections of the MPOA were disconnected (Numan, 1990b). Importantly, those lesions produced mainly deficits in the appetitive aspects of maternal behavior, as some nursing responses may remain in females. Finally, the MPOA is involved not only in the establishment of maternal behavior but also in its maintenance since lesion of this structure disrupts established maternal behaviors in lactating females (Numan, 1988).
The vBST is also involved in the onset of maternal behavior because lesion of this structure produces similar effects in lactating female rats (Numan, 1996). In other species such as sheep, the stimulatory role of the MPOA and BST has also been explored. Inactivation of the MPOA in primiparous mothers at parturition using the reversible anesthetic lidocaine severely disrupts the establishment of maternal behavior in ewes (Perrin et al., 2007). The same procedure was less effective when targeting the BST, but still produces deficits in maternal motivation to join the lamb (Perrin et al., 2007).
It is clear that these core structures act through a more extended neural circuit to ensure proper expression of maternal behaviors. Within this broader neural circuit, the projections from the MPOA to the ventral tegmental area (VTA) are posed to stimulate the mesolimbic dopaminergic projections to centers regulating reward such as the nucleus accumbens (NAcc, see below). Other MPOA/vBST projections to the periaqueductal gray (PAG) are involved in the reduction of pup avoidance. Finally, the paraventricular nucleus of the hypothalamus (PVN), which plays a key role in oxytocinergic signaling, is clearly involved in the establishment of maternal behavior.
Importantly, the brain regions described above are not only dedicated to maternal behavior but are also implicated in male and female sexual behavior and aggression. It is the specific hormone exposure pattern described above, during pregnancy as well as during the lactation period, that accurately rewires the brain circuitry and allow the female dams to respond appropriately to pup cues.
3.2. Motivation circuitry
While maternal behavior is primarily under hormonal control around the time of parturition, this control moves mainly to sensory control once the endocrine events of parturition have waned. The observation that the hormone sequence during pregnancy and at parturition promotes immediate maternal responsiveness toward pups, even in primiparous mother, has strong motivational implications. It suggests that hormonal action on the brain alters the function of defined neural circuits so that avoidance, rejection, and defensive circuits that would respond to novel stimuli in naïve virgins are inhibited, while neural circuits regulating approach, acceptance, and maternal responses are upregulated to respond strongly to newborn-related sensory cues.
In this context, it is not surprising that several studies showed that a higher estradiol/progesterone ratio at the end of pregnancy is linked to an increase in maternal motivation in a range of mammalian species (Poindron, 1980; Pryce, 1993; Gonzalez Mariscal, 1996; Numan, 2006). This role of gonadal hormones on maternal motivation is reflected in operant conditioning devices, for example through the amount of work mothers are willing to do to gain access to pup stimuli (Lee et al., 1999), as well as when using conditioned place preference (Fleming et al., 1994). Thus, the concomitant actions of the estradiol increase and progesterone withdrawal induces more pup-reinforced lever presses in parturient or hormonally treated female rodents, than in ovariectomized or virgins females (Hauser, 1985; Lee et al., 1999).
Then, as maternal motivation is less dependent on gonadal hormones during the postpartum period, the same neural systems that were modified by hormones at the end of pregnancy continue to be activated and strengthened by newborns during the postpartum period independent of hormone release. In this perspective, the dopaminergic (DA) system appears one of the main systems regulating maternal motivation. Injections of DA antagonists induce deficits in retrieval behavior in maternal rats or voles (Fleming et al., 1994; Lonstein & Fleming, 2002) (Stern & Keer, 1999; Pereira & Ferreira, 2006; Zhao & Li, 2010). When injected in this structure, DA antagonists of the D1 receptor induced a deficit in retrieval behavior, while D1 agonists reduced the latency to induce maternal behavior (Stolzenberg, 2007).
In conclusion, it is clear that DA in the nucleus NAcc is necessary for the proactive components of maternal motivation. It is important to note however, that exogenous steroid exposures (progesterone) during different stages of the postpartum period will affect maternal behavior (see for example (Herrenkohl, 1974; Herrenkohl & Reece, 1974; Grieb et al., 2017)), but this has not received much attention.
3.3. Hormone receptor expression in maternal brain regions
The brain regions described above are directly targeted by endogenous steroids as well as exogenous EDCs. For example, the MPOA is central for both the onset and maintenance of maternal behavior in rodents. Importantly, numerous studies have now shown that hormones can specifically modulate the dam’s behavior toward pups via direct action on this brain region. Different receptors, including both estrogen receptor alpha and beta (Giordano et al., 1989; Giordano et al., 1990; Simerly et al., 1990; Yuan et al., 1995; Shughrue et al., 1997), progesterone receptor (Lauber et al., 1991; Guerra-Araiza et al., 2002; Guerra-Araiza et al., 2003; Maerkel et al., 2005; Quadros & Wagner, 2008) and prolactin receptors (Brown et al., 2010; Brown et al., 2017) are present within the MPOA. Other brain regions, directly or indirectly implicated in the control of maternal behavior, also strongly express these receptors, including the BnST, the septum, the VTA and the medial amygdala.
The involvement of these receptors in defined brain regions was investigated using hormone receptor agonists or antagonists implanted directly in the region of interest. For example, early studies with bilateral progesterone canula implanted in the MPOA, VMH, or VTA in hysterectomized-ovariectomized females treated peripherally with estradiol benzoate helped to decipher the mode of action and target of progesterone. Rather surprisingly, the implants failed to delay the onset of maternal care towards foster pups (Numan, 1978). It was hypothesized that progesterone might act at other sites or multiple sites concurrently to inhibit the rapid expression of maternal behavior in the rat. Similar methods were used to define the neural target of estradiol: direct bilateral implants of estradiol into the MPOA of either pregnancy-terminated primigravid rats (Numan, 1977), ovariectomized, virgin rats (Fahrbach & Pfaff, 1986) or progesterone- and estradiol-primed male rats (Rosenblatt & Ceus, 1998) produced shorter latencies to show maternal behaviors. Conversely, injection of the estrogen receptor antagonist tamoxifen into the MPOA reduced maternal behavior, while leaving intact female sexual behavior, demonstrating the specificity of action within this brain region in female (Ahdieh et al., 1987). Despite recent progress, hormone receptor expression in specific MPOA cell types and in other brain areas involved in parenting behavior remains incompletely described (See however (McHenry et al., 2017; Tsuneoka et al., 2017).
3.4. Neurobiological consequences of hormonal changes
Late pregnancy or pregnancy-mimicking exposures to estradiol were shown to have important effects on dopaminergic circuits. Indeed, a recent study indicates that estrogen receptor-positive neurons in the MPOA are necessary and sufficient to activate maternal behavior via an indirect activation of dopaminergic neurons in the VTA (Fang et al., 2018). A large number of estrogen receptor-positive cells are also GABAergic and project to non-dopaminergic cells that tonically inhibit surrounding dopaminergic cells. This disinhibition of dopaminergic cells following the activation of estrogen receptor-positive cells in the MPOA induces maternal behavior rapidly in mice. This confirms previous work of Numan and collaborators that the action of MPOA by estrogens leads to the release of dopamine in the NAcc to suppress inhibitory input to the ventral pallidum. The estrogen-sensitive release of dopamine not only affects the NAcc, but also the MPOA itself, likely to further modulate the ongoing behavioral pattern as well as the medial prefrontal cortex, the dorsal striatum, the septum, amygdala and hippocampus (Kalivas & Volkow, 2005; Numan et al., 2005). Interestingly, while estradiol activates dopamine release in the VTA to promote maternal behavior, it also exerts a direct inhibitory effect on tuberoinfundibular dopaminergic neurons (TIDA neurons, (Cramer et al., 1979; Blum et al., 1987; Morrell et al., 1989; Pasqualini et al., 1993). The role of this inhibition is to facilitate prolactin secretion by the pituitary gland, as prolactin release is repressed by the dopamine presence in the portal system (Jones & Naftolin, 1990; Arbogast & Voogt, 1993; 1994; DeMaria et al., 2000).
Changes in estrogens and/or progesterone during pregnancy and around parturition are also linked to major changes in various signalization pathways, including the oxytocinergic (OXT) system. The expression levels of estrogen receptors and oxytocin receptors in the MPOA and other regions such as the amygdala, are positively correlated with natural variations in maternal care (Champagne et al., 2001). It is well known that exposures to estrogens significantly increase oxytocin receptor expression in various brain regions directly involved in maternal behavior (Bale et al., 1995; Pedersen, 1997) but cortical regions are also impacted; oxytocin activity via oxytocin receptor expressing neurons in the auditory cortex and in the somatosensory cortex of mouse dams increases the salience of pup calls, facilitating pup retrieval (Sabihi et al., 2014; Marlin et al., 2015; Valtcheva & Froemke, 2018). While the OXT system is mostly activated by the social interaction with the pups, the onset of activation and fine tuning with early offspring-mother communication is modulated by steroid release during pregnancy. The release and action of numerous neuropeptides and neurotransmitters are also strongly modulated by estrogens and progesterone, in addition to the feedback received from the pups (see for example Prolactin (Tate-Ostroff & Bridges, 1985; Tate-Ostroff & Bridges, 1987; Grattan & Averill, 1990), GABA (Akbari et al., 2013; Gomora-Arrati et al., 2016), Neurotensin (Scotti et al., 2011; Tsuneoka et al., 2013), CRF (Keverne & Kendrick, 1991; Broad et al., 1995), and serotonin (Kendrick et al., 1992a; de Moura et al., 2015; Stamatakis et al., 2015)).
In addition to the major rewiring of existing cell groups, pregnancy is also associated with significant changes in neurogenesis. Neurogenesis is no longer thought to be limited to the fetal and neonatal brain, but is now understood to occur in mammals throughout life (Zhao et al., 2008; Ming & Song, 2011). Two brain regions, the sub-ventricular zone of the lateral ventricles and the sub-granular zone in the dentate gyrus of the hippocampus are known to produce new neurons throughout adulthood (Zhao et al., 2008; Migaud et al., 2010; Ming & Song, 2011; Lim & Alvarez-Buylla, 2014). Hippocampal cell proliferation is significantly altered throughout pregnancy and the postpartum period in rodents (Darnaudery et al., 2007; Leuner et al., 2007; Pawluski & Galea, 2007; Galea et al., 2014b; Hillerer et al., 2014; Pawluski et al., 2016) suggesting that the hormones of pregnancy can induce neurogenesis in this structure. Specific studies of estrogens revealed that these hormones induce neurogenesis in immature granule neurons in the adult female rat dentate gyrus (Galea, 2008).
Additional studies have shown a reduction in cell proliferation during late pregnancy and parturition (Galea, 2008; Pawluski et al., 2009a; Galea et al., 2013; Galea et al., 2014a), including in sheep (Brus et al., 2010; Brus et al., 2013). This decrease is transient and associated with maternal behavior; by weaning, cell proliferation is restored to the level observed in nulliparous rats and mice. The total number and density of immature neurons is significantly reduced in the dorsal and ventral rat hippocampus (Leuner et al., 2007; Pawluski & Galea, 2007; Workman et al., 2015) although see (Hillerer et al., 2014). On the other hand, it is interesting to note that neurogenesis is upregulated in the subventricular zone during these same periods. It should be mentioned that neurogenesis is also observed in the hypothalamus in numerous species, including mouse (Kokoeva et al., 2005), rat (Pencea et al., 2001; Xu et al., 2005; Matsuzaki et al., 2009; Perez-Martin et al., 2010), hamster (Huang et al., 1998), sheep (Hazlerigg et al., 2013; Migaud et al., 2015) and human (Pellegrino et al., 2018). A very few studies suggest that the newly generated hypothalamic neurons may be involved in metabolism, energy balance, and weight regulation (Bolborea & Dale, 2013; Lee & Blackshaw, 2014) but to our knowledge, no study has really investigated the involvement of these new neurons in maternal behavior. It is however important to note that gestation and lactation is linked to major metabolic changes (Newbern & Freemark, 2011; Mouzon & Lassance, 2015). The changes that have been reported include alterations in dendritic architecture, as well as the number and density of spines in different brain regions within the maternal network including the hippocampus, the olfactory bulb, the medial prefrontal cortex, the NAcc and the medial amygdala in addition to what is observed in the hypothalamus (Langle et al., 2002; Theodosis et al., 2006; Oliet & Bonfardin, 2010; Hillerer et al., 2014).
Plasticity in the maternal brain is obviously not limited to neurons, and gestation and motherhood were shown to be associated with major remodeling of astrocytes (Featherstone et al., 2000; Salmaso et al., 2005; Salmaso and Woodside, 2006; Salmaso et al., 2011), oligodendrocytes (Gregg et al., 2007; Maheu et al., 2009) and microglial cells (Haim et al., 2017; Eid et al., 2019). More specifically, astrocytes within the supraoptic and paraventricular nuclei of the hypothalamus show significant morphological changes correlated with the release of oxytocin that occurs during lactation (Theodosis and Poulain, 1984a; Theodosis and Poulain 1984b; Theodosis et al., 1986; Montagnese et al., 1988). Also in the MPOA, the number of astrocytes of multiparous rats recently exposed to pups was significantly higher compared to non-pup exposed multiparous females (Featherstone et al., 2000). These changes were observed not only in the hypothalamus but also in various brain regions, including the cingulate cortex (Salmaso and Woodside, 2006; Salmaso et al., 2011). In addition, the total number of microglia in the hippocampus, prefrontal cortex, amygdala, and nucleus accumbens was found to be significantly decreased during late pregnancy and early postpartum period (Haim et al., 2017) and the morphology of these cells and more specifically the size of the process was also altered in the hippocampus, during the early postpartum period (Eid et al., 2019).
It should also be noted that several studies have highlighted the modulatory role of reproductive experience as multiparity significantly affects hormone release and hormone sensitivity, the neurocircuitry and maternal behavior (Lévy, 1995; Fleming & Korsmit, 1996; Pawluski et al., 2006; Pawluski & Galea, 2007; Pawluski et al., 2009b).
3.5. Disruptions to the maternal brain: lessons from EDCs
There is a large literature describing the effects of EDCs on various brain regions and/or behaviors in mice and rats: for example, perinatal exposure to estrogenic chemicals are known to alter expression of hormone receptors and hormone-sensitive proteins in brain regions such as the hypothalamus and the midbrain [see for example (Maerkel et al., 2007; Rebuli et al., 2014; Walker et al., 2014; Arambula et al., 2016; Johnson et al., 2017)]. However, the vast majority of these studies were conducted in non-pregnant and non-lactating females, making it difficult to extrapolate these findings to the maternal brain and maternal behavior.
Only a small number of studies have examined the effects of estrogenic EDCs on the MPOA during the actual display of maternal behaviors (e.g., during the lactational period), either in females exposed during adulthood (pregnancy and lactation) or in females exposed during perinatal development. In a study that evaluated the effects of adult BPA exposure in rats, administered during pregnancy and lactation, estrogen receptor expression was examined in the MPOA, arcuate nucleus, and ventromedial nucleus (Aloisi et al., 2001). High dose BPA (mg/kg) decreased estrogen receptor expression in the arcuate nucleus but not the MPOA. Interestingly, studies of adult mouse dams exposed to low doses of BPS (µg/kg) during pregnancy and lactation found increased expression of estrogen receptor in the MPOA during lactation (Catanese & Vandenberg, 2017a). In contrast to these findings, low doses of EE2 during pregnancy and lactation did not alter estrogen receptor expression in the MPOA (Catanese & Vandenberg, 2017b), emphasizing the need for more studies to understand the mechanisms by which estrogenic EDCs disrupt sensitive brain regions in the maternal brain.
Along the same lines, EDCs such as BPA are also known to affect motivational and reward systems, but this work has been conducted in non-maternal models. For example, in rats, gestational and/or juvenile exposure to BPA led to an alteration of dopamine related genes, involved in dopamine metabolism and transport in the prefrontal cortex (Castro et al., 2015a,b) as well as in the hippocampus, amygdale and several midbrain nuclei including the ventral tegmental area (Matsuda et al., 2010; 2012; Tanida et al., 2009 ). To our knowledge, only one study investigated monoamine concentrations in BPA-exposed dams and found that 4 or 40 mg/kg/day treatment from pregnancy day 6 to lactational day 20 did not lead to change in dopamine in the hypothalamus, medulla oblongata nor in the cerebellum when investigated 3 weeks after delivery (Honma et al., 2006). To our knowledge, there is no other study investigating the direct impact of EDCs on the motivation system in pregnant or lactating models but current recognition that dopamine as well as neurotransmitters/neuromodulator systems can be affected by environmental chemicals strongly suggest that maternal motivation might also be altered, warranting more detail investigations.
A small number of studies suggest that the estrogenic EDC BPA and its analogue BPS can interfere with neurogenesis, although no studies have examined neurogenesis specifically during pregnancy, parturition, or the lactational period. BPA exposure in utero disrupts neurogenesis in the fetal mouse neocortex (Komada et al., 2012) and prepubertal BPA exposure alters neurogenesis in the hippocampus (Kim et al., 2011). A recent study in developing zebrafish revealed that exposure to low doses of either BPA or BPS increased neurogenesis in the hypothalamus (Kinch et al., 2015), although the relevance of these findings to maternal behavior is unclear. Future studies examining the effects of EDCs on neuronal cell proliferation in known neurogenic zones such as hippocampus, the subventricular zone and the hypothalamus, as well as other regions known to be responsible for maternal behaviors, in species that display maternal behaviors, are needed.
Additional studies are also needed to examine a greater number of EDCs, including compounds with broader mechanisms of action. EDC studies should benefit from the rich literature evaluating the effects of steroid hormones on the maternal brain, including numerous studies that have evaluated the effects of hormones administered to the adult female including exposures during pregnancy (Sheehan & Numan, 2002; Numan & Stolzenberg, 2009).
4. EDCs: What is next for maternal studies?
The number of studies investigating the potential endocrine disrupting activities in offspring following EDC exposure during the perinatal period (gestation, or gestation + lactation) is rapidly increasing. These experimental paradigms involve, in a large number of cases, the direct exposure of the dam and indirect (via placenta and/or milk) exposure of the offspring. Unfortunately, the mothers are often considered only as a way to treat the offspring, and it is the offspring who are typically the target of toxicological studies; thus, potential effects on the dam’s brain and behavior are rarely investigated, ignoring the importance of the mother-offspring dyad for offspring development. Equally important, few studies have evaluated the long term consequences of EDC exposures for the well-being of the mother, and her potential to reproduce again and perform appropriate maternal behaviors. The investigation of potential disrupting activities of chemicals in mothers is rendered difficult by several parameters that we would like to highlight here:
As stated above, the fundamental understanding of the maternal brain and its remodeling by the endocrine system, both at the spatial and temporal level, is far from being complete. Without this basic knowledge, it is currently impossible to fully grasp the potential impact of environmental chemicals such as EDCs.
There are strain-specific responses and phenotypic differences in response to EDC exposures. Numerous factors including the administered dose, timing and duration of exposure (perinatal vs. adult, during gestation) are likely to affect the responses observed (Spearow et al., 2001; Kendziorski et al., 2012) as has already been shown in studies investigating the perinatal effects of EDCs. In addition, differences in animal husbandry (composition of the cages, including material used for bedding and water bottles, phytoestrogen free diet, etc.) are likely to cause differences in response to exposure. Normalized experimental conditions should be defined and used to provide better foundations to explore the effects of EDCs.
The offspring influences, through direct tactile contact, olfactory cues, and ultrasonic vocalizations, the amount of parental care given by the mother. In rodents, the male is more likely to initiate contact with the mother and in turn, licking and grooming from the dam toward their male offspring is usually more important and fundamental for the male for develop appropriate socio-sexual interaction later in life (Moore, 1984; Della Seta et al., 2005; Porrini et al., 2005). Early EDC exposures that disrupt these features may alter the offspring’s ability to stimulate maternal care, yet few studies have examined the effects of EDCs on olfaction or ultrasonic vocalizations (see however Johnson et al., 2018; Harris et al., 2018; Krishnan et al., 2019). Cross fostering studies seem to confirm this hypothesis as the behavioral outcome (social response and anxiety) of juvenile male and female mice was not only affected by BPA exposure but also by early interaction with the biological or foster non exposed dam (Cox et al., 2010). This remains an important research need.
While most studies assume that changes in behavior are a consequence of the direct alteration of the central nervous system physiology by anthropogenic molecules, several studies showed that some molecules could also affect the periphery, such as the nipple and mammary gland, resulting in the modulation of nursing behavior, as observed for BPS (LaPlante et al., 2017). Numerous compounds are known to affect mammary gland development (for example Ventura et al., 2016; Perrot-Applanat et al., 2018; Mandrup et al., 2015; LaPlante et al., 2018) but the outcome on maternal behavior was, to our knowledge not investigated. In the same line of idea, some EDC seem to act on the maternal brain and behavior partly by modulating the dam’s microbiota, as suggested for BPA (Javurek et al., 2016) and for glyphosate (Dechartres et al., 2019). This point to the importance of looking at maternal behavior, like any other behavior, as an output from a whole organism/individual and not only as a consequence of a few changes in neuronal activation.
The comparative perspective is currently missing and does not allow one to draw general conclusions on the impact of EDC exposure, especially because most of the experimental studies performed so far have concentrated on altricial rodents. Even though there are many benefits to using rodents (ease of manipulation of rodents, short duration of gestation, well-established genetic mutants, etc.), our investigation of EDCs disrupting activities is non-existent in species with alternative maternal strategies.
In conclusion, EDCs can induce adverse consequences for progeny, even if disruptions to maternal behavior are mild. Although childhood abuse is associated with increased vulnerability to several psychopathologies (Kaffman & Meaney, 2007; Gilbert et al., 2009) and other diseases including cancer and heart disease (Felitti et al., 1998), even modest alterations to parental behavior can affect the development and health of the offspring.
Highlights.
Hormone secretion organizes the adult female brain to trigger maternal behaviors
Alterations to these precise patterns of hormone release affect maternal behavior
Exposures to EDCs during vulnerable periods can alter maternal behaviors
Although EDCs are known to affect the brain, the maternal brain remains poorly studied
Hormone secretion organizes the adult female brain to trigger maternal behaviors
Alterations to these precise patterns of hormone release affect maternal behavior
Exposures to EDCs during vulnerable periods can alter maternal behaviors
Although EDCs are known to affect the brain, the maternal brain remains poorly studied
Acknowledgements
MK is a CNRS permanent research fellow and is supported by the French National Research Agency (ANR09-CESA-006, 2009–2013) and ANSES (2012-2-077, 2013-2016). LNV was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health [Award Number K22ES025811]. TDC was supported by La Région Bretagne (SAD), Rennes Métropoles and the University of Rennes 1 (Défis Emergents 2018). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and other funding agencies. The funders played no role in the writing of the report or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: LNV has received travel reimbursement from Universities, Governments, NGOs and Industry, to speak about endocrine-disrupting chemicals.
References
- Acconcia F, Pallottini V & Marino M (2015) Molecular Mechanisms of Action of BPA. Dose-response : a publication of International Hormesis Society, 13, 1559325815610582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec RE, McKay D (1993) Amygdala, kindling, anxierty, and corticotrophin releasing factor (CRF). Physiology and Behavior, 54, 423–431. [DOI] [PubMed] [Google Scholar]
- Adewale HB, Todd KL, Mickens JA & Patisaul HB (2011) The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology, 32, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahdieh HB, Mayer AD & Rosenblatt JS (1987) Effects of brain antiestrogen implants on maternal behavior and on postpartum estrus in pregnant rats. Neuroendocrinology, 46, 522–531. [DOI] [PubMed] [Google Scholar]
- Akbari EM, Shams S, Belay HT, Kaiguo M, Razak Z, Kent CF, Westwood T, Sokolowski MB & Fleming AS (2013) The effects of parity and maternal behavior on gene expression in the medial preoptic area and the medial amygdala in postpartum and virgin female rats: A microarray study. Behavioral neuroscience, 127, 913–922. [DOI] [PubMed] [Google Scholar]
- Alleva E, Caprioli A & Laviola G (1989) LITTER GENDER COMPOSITION AFFECTS MATERNAL-BEHAVIOR OF THE PRIMIPAROUS MOUSE DAM (MUS-MUSCULUS). Journal of Comparative Psychology, 103, 83–87. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Della Seta D, Ceccarelli I & Farabollini F (2001) Bisphenol-A differently affects estrogen receptors-alpha in estrous-cycling and lactating female rats. Neurosci Lett, 310, 49–52. [DOI] [PubMed] [Google Scholar]
- Arabo A, Lefebvre M, Fermanel M & Caston J (2005) Administration of 17alpha-ethinylestradiol during pregnancy elicits modifications of maternal behavior and emotional alteration of the offspring in the rat. Brain Res Dev Brain Res, 156, 93–103. [DOI] [PubMed] [Google Scholar]
- Arambula SE, Belcher SM, Planchart A, Turner SD & Patisaul HB (2016) Impact of Low Dose Oral Exposure to Bisphenol A (BPA) on the Neonatal Rat Hypothalamic and Hippocampal Transcriptome: A CLARITY-BPA Consortium Study. Endocrinology, 157, 3856–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast LA & Voogt JL (1993) Progesterone reverses the estradiol-induced decrease in tyrosine hydroxylase mRNA levels in the arcuate nucleus. Neuroendocrinology, 58, 501–510. [DOI] [PubMed] [Google Scholar]
- Arbogast LA & Voogt JL (1994) Progesterone suppresses tyrosine hydroxylase messenger ribonucleic acid levels in the arcuate nucleus on proestrus. Endocrinology, 135, 343–350. [DOI] [PubMed] [Google Scholar]
- Baez-Mendoza R & Schultz W (2013) The role of the striatum in social behavior. Front Neurosci, 7, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Pedersen CA & Dorsa DM (1995) CNS oxytocin receptor mRNA expression and regulation by gonadal steroids. Advances in experimental medicine and biology, 395, 269–280. [PubMed] [Google Scholar]
- Ball ER, Caniglia MK, Wilcox JL, Overton KA, Burr MJ, Wolfe BD, Sanders BJ, Wisniewski AB & Wrenn CC (2010) Effects of genistein in the maternal diet on reproductive development and spatial learning in male rats. Horm Behav, 57, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banky Z, Nagy GM & Halasz B (1994) Analysis of pituitary prolactin and adrenocortical response to ether, formalin or restraint in lactating rats: rise in corticosterone, but no increase in plasma prolactin levels after exposure to stress. Neuroendocrinology, 59, 63–71. [DOI] [PubMed] [Google Scholar]
- Birke LI & Sadler D (1985) Maternal behavior in rats and the effects of neonatal progestins given to the pups. Developmental psychobiology, 18, 467–475. [DOI] [PubMed] [Google Scholar]
- Blum M, McEwen BS & Roberts JL (1987) Transcriptional analysis of tyrosine hydroxylase gene expression in the tuberoinfundibular dopaminergic neurons of the rat arcuate nucleus after estrogen treatment. The Journal of biological chemistry, 262, 817–821. [PubMed] [Google Scholar]
- Bolborea M & Dale N (2013) Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends in neurosciences, 36, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R, Zarrow MX, Gandelman R & Denenberg VH (1972) Differences in maternal responsiveness between lactating and sensitized rats. Developmental psychobiology, 5, 123–127. [DOI] [PubMed] [Google Scholar]
- Bridges RS (1984) A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology, 114, 930–940. [DOI] [PubMed] [Google Scholar]
- Bridges RS (2015) Neuroendocrine regulation of maternal behavior. Frontiers in neuroendocrinology, 36, 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS & Feder HH (1978) Inhibitory effects of various progestins and deoxycorticosterone on the rapid onset of maternal behavior induced by ovariectomy-hysterectomy during late pregnancy in rats. Hormones and behavior, 10, 30–39. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RP, Friesen HG, Stuer AM & Mann PE (1996) Endocrine communication between conceptus and mother: placental lactogen stimulation of maternal behavior. Neuroendocrinology, 64, 57–64. [DOI] [PubMed] [Google Scholar]
- Bridges RS & Ronsheim PM (1990) Prolactin (PRL) regulation of maternal behavior in rats: bromocriptine treatment delays and PRL promotes the rapid onset of behavior. Endocrinology, 126, 837–848. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Rosenblatt JS & Feder HH (1978a) Serum progesterone concentrations and maternal behavior in rats after pregnancy termination: behavioral stimulation after progesterone withdrawal and inhibition by progesterone maintenance. Endocrinology, 102, 258–267. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Rosenblatt JS & Feder HH (1978b) Stimulation of maternal responsiveness after pregnancy termination in rats: effect of time of onset of behavioral testing. Horm Behav, 10, 235–245. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Zarrow MX & Denenberg VH (1973) The role of neonatal androgen in the expression of hormonally induced maternal responsiveness in the adult rat. Hormones and behavior, 4, 315–322. [Google Scholar]
- Broad KD, Keverne EB & Kendrick KM (1995) Corticotrophin releasing factor mRNA expression in the sheep brain during pregnancy, parturition and lactation and following exogenous progesterone and oestrogen treatment. Brain research. Molecular brain research, 29, 310–316. [DOI] [PubMed] [Google Scholar]
- Brown RE (1986) Social and hormonal factors influencing infanticide and its suppression in adult male Long-Evans rats (Rattus norvegicus). J Comp Psychol, 100, 155–161. [PubMed] [Google Scholar]
- Brown RE & Douglas S (1991) The behaviour of adult male Long-Evans rats Rattus norvegicus toward pups of different ages. Behavioural processes, 23, 89–102. [DOI] [PubMed] [Google Scholar]
- Brown RS, Kokay IC, Herbison AE & Grattan DR (2010) Distribution of prolactin-responsive neurons in the mouse forebrain. The Journal of comparative neurology, 518, 92–102. [DOI] [PubMed] [Google Scholar]
- Brown RSE, Aoki M, Ladyman SR, Phillipps HR, Wyatt A, Boehm U & Grattan DR (2017) Prolactin action in the medial preoptic area is necessary for postpartum maternal nursing behavior. Proceedings of the National Academy of Sciences of the United States of America, 114, 10779–10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ & Russell JA (2005) Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. The Journal of neuroscience : the official journal of the Society for Neuroscience, 25, 5117–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ & Russell JA (2011) Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat. Stress (Amsterdam, Netherlands), 14, 6–12. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA (2008) The expectant brain: adapting for motherhood. Nature Reviews Neuroscience, 9, 11–25. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, russell JA (2010) Endocrine induced changes in brain function during pregnancy. Brain research, 1364, 198–215. [DOI] [PubMed] [Google Scholar]
- Brus M, Keller M & Levy F (2013) Temporal features of adult neurogenesis: differences and similarities across mammalian species. Frontiers in neuroscience, 7, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Franceschini I, Keller M & Levy F (2010) Evidence for cell proliferation in the sheep brain and its down-regulation by parturition and interactions with the young. Hormones and behavior, 58, 737–746. [DOI] [PubMed] [Google Scholar]
- Carere C, Costantini D, Sorace A, Santucci D & Alleva E (2010) Bird populations as sentinels of endocrine disrupting chemicals. Annali Dell Istituto Superiore Di Sanita, 46, 81–88. [DOI] [PubMed] [Google Scholar]
- Carlier M, Roubertoux P & Cohen-Salmon C (1982) Differences in patterns of pup care in Mus musculus domesticus l-Comparisons between eleven inbred strains. Behavioral and neural biology, 35, 205–210. [DOI] [PubMed] [Google Scholar]
- Carretero MI, Segovia S, Gomez F, Del Cerro MC (2003) Bicuculline infusion into the accessory olfactory bulb facilitates the induction of maternal behavior in rats. Scandinavian Journal of Psychology, 44, 273–277. [DOI] [PubMed] [Google Scholar]
- Castro B, Sánchez P, Torres JM, Ortega E. (2015a) Bisphenol A, bisphenol F and bisphenol S affect differently 5α-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats. Environ Res 142:281–7. [DOI] [PubMed] [Google Scholar]
- Castro B, Sánchez P, Miranda MT, Torres JM, Ortega E.(2015b) Identification of dopamine- and serotonin-related genes modulated by bisphenol A in the prefrontal cortex of male rats. Chemosphere 139:235–9. [DOI] [PubMed] [Google Scholar]
- Catanese MC, Suvorov A & Vandenberg LN (2015) Beyond a means of exposure: a new view of the mother in toxicology research. Toxicol Res, 4, 592–612. [Google Scholar]
- Catanese MC & Vandenberg LN (2017a) Bisphenol S (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology, 158, 516–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanese MC & Vandenberg LN (2017b) Low doses of 17 ethinyl estradiol alter the maternal brain and induce stereotypies in CD-1 mice exposed during pregnancy and lactation. Reprod Toxicol, 73, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanese MC & Vandenberg LN (2018) Developmental estrogen exposures and disruptions to maternal behavior and brain: effects of ethinyl estradiol, a common positive control. Horm Behav, 101, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S & Meaney MJ (2001) Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A, 98, 12736–12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Rothschild G, Mizrahi A (2011) Multisensory integration of natural odors and sounds in the auditory cortex. Neuron, 72, 357–369. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C & Rissman EF (2010) Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Hormones and Behavior, 58, 754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer OM, Parker CR Jr. & Porter JC (1979) Estrogen inhibition of dopamine release into hypophysial portal blood. Endocrinology, 104, 419–422. [DOI] [PubMed] [Google Scholar]
- Crowley WR (2015) Neuroendocrine regulation of lactation and milk production. Compr Physiol, 5, 255–291. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Nunez AA & Clemens LG (2005) A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol Behav, 85, 83–91. [DOI] [PubMed] [Google Scholar]
- da Costa AP, Ma X, Ingram CD, Lightman SL & Aguilera G (2001) Hypothalamic and amygdaloid corticotropin-releasing hormone (CRH) and CRH receptor-1 mRNA expression in the stress-hyporesponsive late pregnant and early lactating rat. Brain research. Molecular brain research, 91, 119–130. [DOI] [PubMed] [Google Scholar]
- Danzo BJ (1997) Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environmental Health Perspective, 105, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudery M, Perez-Martin M, Del Favero F, Gomez-Roldan C, Garcia-Segura LM & Maccari S (2007) Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology, 32, 803–812. [DOI] [PubMed] [Google Scholar]
- de Castro VL, Destefani CR, Diniz C & Poli P (2007) Evaluation of neurodevelopmental effects on rats exposed prenatally to sulfentrazone. Neurotoxicology, 28, 1249–1259. [DOI] [PubMed] [Google Scholar]
- Dechartres J, Pawluski JL, Gueguen M-M,Jablaoui A, Maguin E, Rhimi M, Charlier TD (2019) Glyphosate and Glyphosate-based herbicide exposure during the peripartum period affects maternal brain plasticity, maternal behavior and microbiome. Journal of Neuroendocrinology, in press [DOI] [PubMed]
- Déchaud H, Ravard C, Claustrat F, de la Perrière AB, Pugeat M. (1999) Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG). Steroids, 64, 328–34. [DOI] [PubMed] [Google Scholar]
- de Moura AC, Lazzari VM, Becker RO, Gil MS, Ruthschilling CA, Agnes G, Almeida S, da Veiga AB, Lucion AB & Giovenardi M (2015) Gene expression in the CNS of lactating rats with different patterns of maternal behavior. Neuroscience research, 99, 8–15. [DOI] [PubMed] [Google Scholar]
- Della Seta D, Minder I, Dessi-Fulgheri F & Farabollini F (2005) Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res Bull, 65, 255–260. [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD & Freeman ME (2000) Ovarian steroids influence the activity of neuroendocrine dopaminergic neurons. Brain research, 879, 139–147. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B & Walker CD (2003) Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. Journal of neuroendocrinology, 15, 486–497. [DOI] [PubMed] [Google Scholar]
- Deviterne D & Desor D (1990) SELECTIVE PUP RETRIEVING BY MOTHER RATS - SEX AND EARLY DEVELOPMENT CHARACTERISTICS AS DISCRIMINATION FACTORS. Developmental psychobiology, 23, 361–368. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Brunton PJ, Bosch OJ, Russell JA & Neumann ID (2003) Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology, 144, 5268–5276. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Meddle SL, Toschi N, Bosch OJ & Neumann ID (2005) Reduced activity of the noradrenergic system in the paraventricular nucleus at the end of pregnancy: implications for stress hyporesponsiveness. Journal of neuroendocrinology, 17, 40–48. [DOI] [PubMed] [Google Scholar]
- Dragunow M & Faull R (1989) The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods, 29, 261–265. [DOI] [PubMed] [Google Scholar]
- Dugard ML, Tremblay-Leveau H, Mellier D & Caston J (2001) Prenatal exposure to ethinylestradiol elicits behavioral abnormalities in the rat. Brain Res Dev Brain Res, 129, 189–199. [DOI] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA & Wu Z (2014) Neural control of maternal and paternal behaviors. Science, 345, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach SE & Pfaff DW (1986) Effect of preoptic region implants of dilute estradiol on the maternal behavior of ovariectomized, nulliparous rats. Horm Behav, 20, 354–363. [DOI] [PubMed] [Google Scholar]
- Fang YY, Yamaguchi T, Song SC, Tritsch NX & Lin D (2018) A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron, 98, 192–207.e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M (2011) A bold view of the lactating brain: functional magnetic resonance imaging studies of suckling in awake dams. Journal of neuroendocrinology, 23, 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Fleming AS & Ivy GO (2000) Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behavioral Neuroscience, 114, 158–172. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP & Marks JS (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med, 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Fischer D, Patchev VK, Hellbach S, Hassan AH & Almeida OF (1995) Lactation as a model for naturally reversible hypercorticalism plasticity in the mechanisms governing hypothalamo-pituitary- adrenocortical activity in rats. The Journal of clinical investigation, 96, 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Bortolotti GR, Fernie KJ, Smits JE, Marchant TA, Drouillard KG & Bird DM (2001) Courtship behavior of captive American kestrels (Falco sparverius) exposed to polychlorinated biphenyls. Archives of Environmental Contamination and Toxicology, 41, 215–220. [DOI] [PubMed] [Google Scholar]
- Fleming AS & Korsmit M (1996) Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci, 110, 567–582. [DOI] [PubMed] [Google Scholar]
- Fleming AS & Rosenblatt JS (1974a) Maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol, 86, 957–972. [DOI] [PubMed] [Google Scholar]
- Fleming AS & Rosenblatt JS (1974b) Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol, 86, 221–232. [DOI] [PubMed] [Google Scholar]
- Fleming AS & Rosenblatt JS (1974c) Olfactory regulation of maternal behavior in rats. II. Effects of peripherally induced anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J Comp Physiol Psychol, 86, 233–246. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M & Rusak B (1994) Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav Neurosci, 108, 724–734. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Vaccarino F & Luebke C (1980) Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav, 25, 731–743. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Funabashi T, Kawaguchi M, Mitsushima D & Kimura F (2007) Bisphenol A induces transforming growth factor-beta3 mRNA in the preoptic area: a cDNA expression array and Northern blot study. Neurosci Lett, 411, 81–85. [DOI] [PubMed] [Google Scholar]
- Gaffori O & Le Moal M (1979) Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiol Behav, 23, 317–323. [DOI] [PubMed] [Google Scholar]
- Galea LA (2008) Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev, 57, 332–341. [DOI] [PubMed] [Google Scholar]
- Galea LA, Leuner B & Slattery DA (2014a) Hippocampal plasticity during the peripartum period: Influence of sex steroids, stress and ageing. J Neuroendocrinol [DOI] [PMC free article] [PubMed]
- Galea LA, Leuner B & Slattery DA (2014b) Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. Journal of neuroendocrinology, 26, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C & Hamson DK (2013) Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol, 25, 1039–1061. [DOI] [PubMed] [Google Scholar]
- Gandelman R (1973) Induction of maternal nest building in virgin female mice by the presentation of young. Horm Behav, 4, 191–197. [DOI] [PubMed] [Google Scholar]
- Gandelman R, Zarrow MX & Denenberg VH (1970) Maternal behavior: differences between mother and virgin mice as a function of the testing procedure. Developmental psychobiology, 3, 207–214. [DOI] [PubMed] [Google Scholar]
- Garland HO, Atherton JC, Baylis C, Morgan MR & Milne CM (1987) Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. The Journal of endocrinology, 113, 435–444. [DOI] [PubMed] [Google Scholar]
- Gemmel M, Harmeyer D, Bogi E, Fillet M, Hill LA, Hammond GL, Charlier TD & Pawluski JL (2018) Perinatal fluoxetine increases hippocampal neurogenesis and reverses the lasting effects of pre-gestational stress on serum corticosterone, but not on maternal behavior, in the rat dam. Behavioural brain research, 339, 222–231. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E & Janson S (2009) Burden and consequences of child maltreatment in high-income countries. Lancet, 373, 68–81. [DOI] [PubMed] [Google Scholar]
- Giordano AL, Ahdieh HB, Mayer AD, Siegel HI & Rosenblatt JS (1990) Cytosol and nuclear estrogen receptor binding in the preoptic area and hypothalamus of female rats during pregnancy and ovariectomized, nulliparous rats after steroid priming: correlation with maternal behavior. Horm Behav, 24, 232–255. [DOI] [PubMed] [Google Scholar]
- Giordano AL, Siegel HI & Rosenblatt JS (1989) Nuclear estrogen receptor binding in the preoptic area and hypothalamus of pregnancy-terminated rats: correlation with the onset of maternal behavior. Neuroendocrinology, 50, 248–258. [DOI] [PubMed] [Google Scholar]
- Gomora-Arrati P, Dominguez G & Agmo A (2016) GABA Receptors in the Medial Preoptic Area Modulate the Onset of Oestradiol-Induced Maternal Behaviour in Hysterectomised-Ovariectomised, Pregnant Rats. Journal of neuroendocrinology, 28. [DOI] [PubMed] [Google Scholar]
- Gonzalez Mariscal G, Melo AI, Jimenez P, Beyer C, Rosenblatt JS (1996) Estradiol, progesterone, and prolactin regulate maternal nest-building in rabbits. Journal of neuroendocrinology, 8, 901–907. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J & Zoeller RT (2015) EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev, 36, E1–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan DR & Averill RL (1990) Effect of ovarian steroids on a nocturnal surge of prolactin secretion that precedes parturition in the rat. Endocrinology, 126, 1199–1205. [DOI] [PubMed] [Google Scholar]
- Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW & Weiss S (2007) White matter plasticity and enhanced remyelination in the maternal CNS. Journal of Neuroscience, 27, 1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb ZA, Tierney SM & Lonstein JS (2017) Postpartum inhibition of ovarian steroid action increases aspects of maternal caregiving and reduces medial preoptic area progesterone receptor expression in female rats. Hormones and behavior, 96, 31–41. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Coyoy-Salgado A & Camacho-Arroyo I (2002) Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain research bulletin, 59, 105–109. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Villamar-Cruz O, Gonzalez-Arenas A, Chavira R & Camacho-Arroyo I (2003) Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. Journal of neuroendocrinology, 15, 984–990. [DOI] [PubMed] [Google Scholar]
- Hammond GL (2016) Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action Journal of Endocrinology 230, R13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EP, Allardice HA, Schenk AK & Rissman EF (2018) Effects of maternal or paternal bisphenol A exposure on offspring behavior. Hormones & Behavior, 101, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H, Gandelman R (1985) Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Hormones and behavior, 19, 454–468. [DOI] [PubMed] [Google Scholar]
- Hazlerigg DG, Wyse CA, Dardente H, Hanon EA & Lincoln GA (2013) Photoperiodic variation in CD45-positive cells and cell proliferation in the mediobasal hypothalamus of the Soay sheep. Chronobiology international, 30, 548–558. [DOI] [PubMed] [Google Scholar]
- Herrenkohl LR (1974) Differential effects of progesterone on lactation and nursing behavior in late pregnant and postparturient rats. Physiology & behavior, 13, 495–499. [DOI] [PubMed] [Google Scholar]
- Herrenkohl LR & Reece RP (1974) Effects of progesterone injections administered during late pregnancy on lactation and nursing behavior in the rat. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 145, 1047–1049. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Jacobs VR, Fischer T & Aigner L (2014) The maternal brain: an organ with peripartal plasticity. Neural Plast, 2014, 574159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgert Jury H, Zacharewski TR & Hammond GL (2000) Interactions between human plasma sex hormone-binding globulin and xenobiotic ligands. Journal of Steroid Biochemistry and Molecular Biology, 75, 167–76. [DOI] [PubMed] [Google Scholar]
- Honma T 1, Miyagawa M, Suda M, Wang RS, Kobayashi K, Sekiguchi S. (2006) Effects of perinatal exposure to bisphenol A on brain neurotransmitters in female rat offspring. Ind Health 44:510–24. [DOI] [PubMed] [Google Scholar]
- Huang L, DeVries GJ & Bittman EL (1998) Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. Journal of neurobiology, 36, 410–420. [DOI] [PubMed] [Google Scholar]
- Jacobson CD, Terkel J, Gorski RA & Sawyer CH (1980) Effects of small medial preoptic area lesions on maternal behavior: retrieving and nest building in the rat. Brain Res, 194, 471–478. [DOI] [PubMed] [Google Scholar]
- Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA & Rosenfeld CS (2016) Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes, 7, 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski M & Terkel J (1985) Incidence of pup killing and parental behavior in virgin female and male rats (Rattus norvegicus): differences between Wistar and Sprague-Dawley stocks. J Comp Psychol, 99, 93–97. [PubMed] [Google Scholar]
- Jänne M, Deol HK, Power SG, Yee SP & Hammond GL (1998) Human sex hormone-binding globulin gene expression in transgenic mice. Molecular Endocrinology, 12, 123–36 [DOI] [PubMed] [Google Scholar]
- Jänne M, Hogeveen KN, Deol HK & Hammond GL (1999) Expression and regulation of human sex hormone-binding globulin transgenes in mice during development. Endocrinology, 140, 4166–74. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Javurek AB, Painter MS, Peritore MP, Ellersieck MR, Roberts RM & Rosenfeld CS (2015) Disruption of parenting behaviors in california mice, a monogamous rodent species, by endocrine disrupting chemicals. PLoS One, 10:e0126284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Spollen WG, Manshack LK, Bivens NJ, Givan SA & Rosenfeld CS (2017) Hypothalamic transcriptomic alterations in male and female California mice (Peromyscus californicus) developmentally exposed to bisphenol A or ethinyl estradiol. Physiological reports, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Ellersieck MR & Rosenfeld CS (2018) Hypothalamic gene expression changes in F1 California mice (Peromyscus californicus) parents developmentally exposed to bisphenol A or ethinyl estradiol. Heliyon, 4, e00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Farrington MJ, Murphy CR, Caldo PD, McAllister LA, Kaur S, Chun C, Ortega MT, Marshall BL, Hoffmann F, Ellersieck MR, Schenk AK & Rosenfeld CS (2018). Multigenerational effects of bisphenol A or ethinyl estradiol exposure on F2 California mice (Peromyscus californicus) pup vocalizations. PLoS One 13, e0199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EE & Naftolin F (1990) Estrogen effects on the tuberoinfundibular dopaminergic system in the female rat brain. Brain research, 510, 84–91. [DOI] [PubMed] [Google Scholar]
- Kaffman A & Meaney MJ (2007) Neurodevelopmental sequelae of postnatal maternal care in rodents: Clinical and research implications of molecular insights. J Child Psychol Psychiatry, 48, 224–244. [DOI] [PubMed] [Google Scholar]
- Kalivas PW & Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. The American journal of psychiatry, 162, 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kasper P & Telegdy G (1975) The effect of oestrogen and progesterone on maternal behaviour in rats. Acta physiologica Academiae Scientiarum Hungaricae, 46, 257–265. [PubMed] [Google Scholar]
- Keller M, Meurisse M, Poindron P, Nowak R, Ferreira G, Shayit M & Levy F (2003) Maternal experience influences the establishment of visual/auditory, but not olfactory recognition of the newborn lamb by ewes at parturition. Developmental psychobiology, 43, 167–176. [DOI] [PubMed] [Google Scholar]
- Keller M, Meurisse M, Poindron P, Nowak R, Ferreira G, Shayit M, Lévy F (2003) Maternal experience influences the estbalisment of visual/auditory, but not olfactory recognition of the newborn lamb by ewes at parturition. Developmentl Psychobiology, 43, 167–176. [DOI] [PubMed] [Google Scholar]
- Keller M, Meurisse M, Lévy F (2004) Mapping the neural substrates involved in the formation of olfactory offspring memory in sheep. Behavioral neuroscience, 20, 3433–3441. [DOI] [PubMed] [Google Scholar]
- Kendrick KM (1994) Neurobiological correlates of visual and olfactory recognition in sheep. Behavioral Processes, 33, 89–111. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA (1987) Intracerebroventricular oxytocin stimulates maternal behavior in the sheep. Neuroendocrinology, 46, 56–61. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Hinton MR & Goode JA (1992a) Oxytocin, amino acid and monoamine release in the region of the medial preoptic area and bed nucleus of the stria terminalis of the sheep during parturition and suckling. Brain research, 569, 199–209. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Levy F & Keverne EB (1992b) Changes in the sensory processing of olfactory signals induced by birth in sheep. Science, 256, 833–836. [DOI] [PubMed] [Google Scholar]
- Kendziorski JA, Kendig EL, Gear RB & Belcher SM (2012) Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17alpha-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod Toxicol, 34, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB & Kendrick KM (1991) Morphine and corticotrophin-releasing factor potentiate maternal acceptance in multiparous ewes after vaginocervical stimulation. Brain research, 540, 55–62. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Levy F, Poindron P & Lindsay DR (1983) Vaginal stimulation: an important determinant of maternal bonding in sheep. Science, 219, 81–83. [DOI] [PubMed] [Google Scholar]
- Kim ME, Park HR, Gong EJ, Choi SY, Kim HS & Lee J (2011) Exposure to bisphenol A appears to impair hippocampal neurogenesis and spatial learning and memory. Food Chem Toxicol, 49, 3383–3389. [DOI] [PubMed] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong JH, Habibi HR & Kurrasch DM (2015) Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A, 112, 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Bridges RS (1990) Morphine treatment and reproductive condition alter olfactory preferences for pup and adult male. Developmental psychobiology, 23, 331–347. [DOI] [PubMed] [Google Scholar]
- Kleinman DG & Malcom JR (1981) The evolution of male parental investment in mammals. In Gubernick D, Klopfer P (eds) Parental care in mammals Plenum Publishing Corporation, pp. 347–387. [Google Scholar]
- Koch B (1969) [Binding of corticosterone to plasma proteins and regulation of the level of free steroid during pregnancy and lactation in the rat]. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme, 1, 129–135. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H & Flier JS (2005) Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science, 310, 679–683. [DOI] [PubMed] [Google Scholar]
- Komada M, Asai Y, Morii M, Matsuki M, Sato M & Nagao T (2012) Maternal bisphenol A oral dosing relates to the acceleration of neurogenesis in the developing neocortex of mouse fetuses. Toxicology, 295, 31–38. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Rahman S, Hasbum A, Morales D, Thompson LM, Crews D & Gore AC (2019) Maternal care modulates transgenerational effects of endocrine-disrupting chemicals on offspring pup vocalizations and adult behaviors. Horm Behav 107:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal MB (1980) Placentophagia: a biobehavioral enigma (or De gustibus non disputandum est). Neuroscience and biobehavioral reviews, 4, 141–150. [DOI] [PubMed] [Google Scholar]
- Kristal MB, Graber GC (1976) Placentophagia in nonpregnant rats: influence of estrous cycle stage and birthplace. Physiology and Behavior, 17, 599–605. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP & Champagne FA (2013a) Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proceedings of the National Academy of Sciences of the United States of America, 110, 9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP & Champagne FA (2013b) Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A [DOI] [PMC free article] [PubMed]
- Langle SL, Poulain DA & Theodosis DT (2002) Neuronal-glial remodeling: a structural basis for neuronal-glial interactions in the adult hypothalamus. Journal of physiology, Paris, 96, 169–175. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ & Pfaff DW (1991) Sex difference in estradiol regulation of progestin receptor mRNA in rat mediobasal hypothalamus as demonstrated by in situ hybridization. Neuroendocrinology, 53, 608–613. [DOI] [PubMed] [Google Scholar]
- LaPlante CD, Catanese MC, Bansal R & Vandenberg LN (2017). Bisphenol S Alters the Lactating Mammary Gland and Nursing Behaviors in Mice Exposed During Pregnancy and Lactation. Endocrinology, 158, 3448–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlante CD, Bansal R, Dunphy KA, Jerry DJ & Vandenberg LN (2018) Oxybenzone Alters Mammary Gland Morphology in Mice Exposed During Pregnancy and Lactation. The Journal of the Endocrine Society, 2, 903–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Clancy S & Fleming AS (1999) Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res, 100, 15–31. [DOI] [PubMed] [Google Scholar]
- Lee DA & Blackshaw S (2014) Feed your head: neurodevelopmental control of feeding and metabolism. Annual review of physiology, 76, 197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mirescu C, Noiman L & Gould E (2007) Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus, 17, 434–442. [DOI] [PubMed] [Google Scholar]
- Lévy F, Keller M (2009) Olfactory mediation of maternal behavior in selected mammalian species. Behavioral Brain Research, 200, 336–345. [DOI] [PubMed] [Google Scholar]
- Lévy F, Keller M, Cornilleau F, Moussu C, Ferreira G (2010) Vaginocervical stimulation of ewes induces the rapid formation of a new bond with an alien young without interfering with a previous bond. Developmental psychobiology, 52, 537–544. [DOI] [PubMed] [Google Scholar]
- Lévy F, Keller M, Poindron P (2004) Olfactory regulation of maternal behavior in mammals. Hormones and behavior, 46, 284–302. [DOI] [PubMed] [Google Scholar]
- Lévy F, Kendrick KM, Keverne EB, Piketty V, Poindron P (1992) Intracerebral oxytocin is important for the onset of maternal behavior in inexperienced ewes delivered under peridural anesthesia. Behavioral neuroscience, 106, 427–432. [DOI] [PubMed] [Google Scholar]
- Lévy F, Locatelli A, Piketty V, Tillet Y, Poindonr P (1995) Involvement of the main but not the accessory olfactory system in maternal behavior of primiparous and multiparous ewes. Physiology and Behavior, 57, 97–104. [DOI] [PubMed] [Google Scholar]
- Lévy F, Poindron P, Le Neindre P (1983) Attraction and repulsion by amniotic fluids and their olfactory control in the ewe around parturition. Physiology and Behavior, 31, 687–692. [DOI] [PubMed] [Google Scholar]
- Lévy F, Keller M (2008) Olfactory mediation of maternal behavior in selected mammalian species. Advances in the Study of Behavior, 200, 336–345. [DOI] [PubMed] [Google Scholar]
- Lim DA & Alvarez-Buylla A (2014) Adult neural stem cells stake their ground. Trends Neurosci, 37, 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC & Schreiner CE (2007) Auditory cortical detection and discrimination correlates with communicative significance. PLoS biology, 5, e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS & Fleming AS (2002) Parental behaviors in rats and mice. Curr Protoc Neurosci, Chapter 8, Unit 8 15. [DOI] [PubMed]
- Lonstein JS, Greco B, De Vries GJ, Stern JM & Blaustein JD (2000) Maternal behavior stimulates c-fos activity within estrogen receptor alpha-containing neurons in lactating rats. Neuroendocrinology, 72, 91–101. [DOI] [PubMed] [Google Scholar]
- Luft CD, Pereda E, Banissy MJ & Bhattacharya J (2014) Best of both worlds: promise of combining brain stimulation and brain connectome. Front Syst Neurosci, 8, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiten PG, Koolhaas JM, de Boer S, Koopman SJ (1985) The cortico-medial amygdala in the central nervous system organization of agonistic behavior. Brain research, 332, 283–297. [DOI] [PubMed] [Google Scholar]
- MacKay H & Abizaid A (2018) A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA). Hormones and behavior, 101, 59–67. [DOI] [PubMed] [Google Scholar]
- Maerkel K, Durrer S, Henseler M, Schlumpf M & Lichtensteiger W (2007) Sexually dimorphic gene regulation in brain as a target for endocrine disrupters: developmental exposure of rats to 4-methylbenzylidene camphor. Toxicol Appl Pharmacol, 218, 152–165. [DOI] [PubMed] [Google Scholar]
- Maerkel K, Lichtensteiger W, Durrer S, Conscience M & Schlumpf M (2005) Sex- and region-specific alterations of progesterone receptor mRNA levels and estrogen sensitivity in rat brain following developmental exposure to the estrogenic UV filter 4-methylbenzylidene camphor. Environmental toxicology and pharmacology, 19, 761–765. [DOI] [PubMed] [Google Scholar]
- Maheu ME, Akbari EM & Fleming AS (2009) Callosal oligodendrocyte number in postpartum Sprague-Dawley rats. Brain Research, 1267,18–24. [DOI] [PubMed] [Google Scholar]
- Mandrup KR, Johansson HK, Boberg J, Pedersen AS, Mortensen MS, Jørgensen JS, Vinggaard AM & Hass U (2015) Mixtures of environmentally relevant endocrine disrupting chemicals affect mammary gland development in female and male rats. Reproductive Toxicology, 5G4, 47–57 [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’Amour J A, Chao MV & Froemke RC (2015) Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature, 520, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques DM (1979) Roles of the main and vomeronasal systems in the response of the female hamster to young. Behavioral and neural biology, 26, 311–329. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Saika S, Amano K, Shimizu E, Sajiki J (2010) Changes in brain monoamine levels in neonatal rats exposed to bisphenol A at low doses. Chemosphere 78:894–906. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sutoh C, Nakazawa K, Amano K, Sajiki J, Shimizu E (2012) Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain. Prog Neuropsychopharmacol Biol Psychiatry 39:273–9. d [DOI] [PubMed] [Google Scholar]
- Matsuura I, Saitoh T, Tani E, Wako Y, Iwata H, Toyota N, Ishizuka Y, Namiki M, Hoshino N, Tsuchitani M & Ikeda Y (2005) Evaluation of a two-generation reproduction toxicity study adding endpoints to detect endocrine disrupting activity using lindane. J Toxicol Sci, 30 Spec No, 135–161. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Katakura M, Hara T, Li G, Hashimoto M & Shido O (2009) Proliferation of neuronal progenitor cells and neuronal differentiation in the hypothalamus are enhanced in heat-acclimated rats. Pflugers Archiv : European journal of physiology, 458, 661–673. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR & Stuber GD (2017) Hormonal gain control of a medial preoptic area social reward circuit. Nature neuroscience, 20, 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA & Moltz H (1989) Pheromonal emission by pregnant rats protects against infanticide by nulliparous conspecifics. Physiology & behavior, 46, 591–595. [DOI] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I & Pillon D (2010) Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci, 32, 2042–2052. [DOI] [PubMed] [Google Scholar]
- Migaud M, Butrille L & Batailler M (2015) Seasonal regulation of structural plasticity and neurogenesis in the adult mammalian brain: focus on the sheep hypothalamus. Frontiers in neuroendocrinology, 37, 146–157. [DOI] [PubMed] [Google Scholar]
- Ming GL & Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron, 70, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Queralt S & Hammond GL (2008) Sex hormone-binding globulin in fish gills is a portal for sex steroids breached by xenobiotics. Endocrinology, 149, 4269–75. [DOI] [PubMed] [Google Scholar]
- Moltz H, Levin R & Leon M (1969) Differential effects of progesterone on the maternal behavior of primiparous and multiparous rats. Journal of comparative and physiological psychology, 67, 36–40. [DOI] [PubMed] [Google Scholar]
- Montagnese C, Poulain DA, Vincent JD & Theodosis DT (1988) Synaptic and neuronal-glial plasticity in the adult oxytocinergic system in response to physiological stimuli. Brain Research Bulletin, 20, 681–692. [DOI] [PubMed] [Google Scholar]
- Moore CL (1982) Maternal behavior of rats is affected by hormonal condition of pups. Journal of comparative and physiological psychology, 96, 123–129. [DOI] [PubMed] [Google Scholar]
- Moore CL (1984) Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol, 17, 347–356. [DOI] [PubMed] [Google Scholar]
- Moore CL & Morelli GA (1979) Mother rats interact differently with male and female offspring. Journal of comparative and physiological psychology, 93, 677–684. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Watchus JA, Milgram NW, Fleming AS (1999) The long lasting effects of electrical stimulation of th emedial preoptic area and medial amygdala on maternal behavior in female rats. Behavioural brain research, 99, 61–73. [DOI] [PubMed] [Google Scholar]
- Morishige WK, Pepe GJ & Rothchild I (1973) Serum luteinizing hormone, prolactin and progesterone levels during pregnancy in the rat. Endocrinology, 92, 1527–1530. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Rosenthal MF, McCabe JT, Harrington CA, Chikaraishi DM & Pfaff DW (1989) Tyrosine hydroxylase mRNA in the neurons of the tuberoinfundibular region and zona incerta examined after gonadal steroid hormone treatment. Molecular endocrinology (Baltimore, Md.), 3, 1426–1433. [DOI] [PubMed] [Google Scholar]
- Mouzon SH & Lassance L (2015) Endocrine and metabolic adaptations to pregnancy; impact of obesity. Hormone molecular biology and clinical investigation, 24, 65–72. [DOI] [PubMed] [Google Scholar]
- Musi B, Deacetis L & Alleva E (1993) INFLUENCE OF LITTER GENDER COMPOSITION ON SUBSEQUENT MATERNAL-BEHAVIOR AND MATERNAL AGGRESSION IN FEMALE HOUSE MICE. Ethology, 95, 43–53. [Google Scholar]
- Nakagami A, Negishi T, Kawasaki K, Imai N, Nishida Y, Ihara T, Kuroda Y, Yoshikawa Y & Koyama T (2009) Alterations in male infant behaviors towards its mother by prenatal exposure to bisphenol A in cynomolgus monkeys (Macaca fascicularis) during early suckling period. Psychoneuroendocrinology, 34, 1189–1197. [DOI] [PubMed] [Google Scholar]
- Newbern D & Freemark M (2011) Placental hormones and the control of maternal metabolism and fetal growth. Current opinion in endocrinology, diabetes, and obesity, 18, 409–416. [DOI] [PubMed] [Google Scholar]
- Nowak R, Keller M, Lévy F (2011) Mother-young relationships in sheep: a model for a multidisciplinary apporach of the study of attcahment in mammals. Journal of neuroendocrinology, 23, 1042–1053. [DOI] [PubMed] [Google Scholar]
- Nowak R, Porter RH, Lévy F, Orgeur P, Schaal B (2000) Role of mother-young interactions in the survival of the offspring in domestic mammals. Reviews in Reproduction, 5, 153–163. [DOI] [PubMed] [Google Scholar]
- Numan M (1974) Medial preoptic area and maternal behavior in the female rat. J Comp Physiol Psychol, 87, 746–759. [DOI] [PubMed] [Google Scholar]
- Numan M (1978) Progesterone inhibition of maternal behavior in the rat. Horm Behav, 11, 209–231. [DOI] [PubMed] [Google Scholar]
- Numan M (1990a) Long-term effects of preoptic area knife cuts on the maternal behavior of postpartum rats. Behav Neural Biol, 53, 284–290. [DOI] [PubMed] [Google Scholar]
- Numan M (2006) Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev, 5, 163–190. [DOI] [PubMed] [Google Scholar]
- Numan M, Corodimas KP, Numan MJ, Factor EM & Piers WD (1988) Axon-sparing lesions of the preoptic region and substantia innominata disrupt maternal behavior in rats. Behav Neurosci, 102, 381–396. [DOI] [PubMed] [Google Scholar]
- Numan M, Corodimas KP, Numan MJ, Factor EM, Piers WD (1988) Axon-sparing lesions of the preoptic region and substantia innominata disrupt maternal behavior in rats. Behavioral neuroscience, 102, 381–396. [DOI] [PubMed] [Google Scholar]
- Numan M & Insel T (2003) The neurobiology of parental behavior Springer Verlag, New York, NY. [Google Scholar]
- Numan M, McSparren J, Numan MJ (1990b) Dorsolateral connections of th emedial preoptic area and maternal behavior in rats. Behavioral neuroscience, 104, 694–679. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF & Smith CD (2005) Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res, 158, 53–68. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ (1996) A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Developmental psychobiology, 29, 23–51. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, English JB (1993) Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Hormones and behavior, 27, 56–81. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisarul BR (1977) Medial preoptic area and onset of maternal behavior in rat. Journal of comparative and physiological psychology, 91, 146–164. [DOI] [PubMed] [Google Scholar]
- Numan M & Smith HG (1984) Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci, 98, 712–727. [DOI] [PubMed] [Google Scholar]
- Numan M & Stolzenberg DS (2009) Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol, 30, 46–64. [DOI] [PubMed] [Google Scholar]
- Numan M.i.T. (2003) The neurobiology of parental behavior Springer. [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS & Pfaff DW (1998) Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology, 139, 5070–5081. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, Gonzalez-Mariscal G, Levy F, Lucion AB, Morrell JI, Numan M & Uriarte N (2013) Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neuroscience and biobehavioral reviews, 37, 1875–1892. [DOI] [PubMed] [Google Scholar]
- Oliet SH & Bonfardin VD (2010) Morphological plasticity of the rat supraoptic nucleus--cellular consequences. The European journal of neuroscience, 32, 1989–1994. [DOI] [PubMed] [Google Scholar]
- Palanza P, Howdeshell KL, Parmigiani S & vom Saal FS (2002a) Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect, 110, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Morellini F, Parmigiani S & vom Saal FS (2002b) Ethological methods to study the effects of maternal exposure to estrogenic endocrine disrupters: a study with methoxychlor. Neurotoxicol Teratol, 24, 55–69. [DOI] [PubMed] [Google Scholar]
- Pasqualini C, Guibert B & Leviel V (1993) Short-term inhibitory effect of estradiol on tyrosine hydroxylase activity in tuberoinfundibular dopaminergic neurons in vitro. Journal of neurochemistry, 60, 1707–1713. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM & Galea LA (2009a) Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Frontiers in neuroendocrinology, 30, 343–357. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Charlier TD, Lieblich SE, Hammond GL & Galea LA (2009b) Reproductive experience alters corticosterone and CBG levels in the rat dam. Physiology & behavior, 96, 108–114. [DOI] [PubMed] [Google Scholar]
- Pawluski JL & Galea LA (2007) Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience, 149, 53–67. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Lambert KG & Kinsley CH (2016) Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Hormones and behavior, 77, 86–97. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Vanderbyl BL, Ragan K & Galea LA (2006) First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behavioural brain research, 175, 157–165. [DOI] [PubMed] [Google Scholar]
- Peake GT, Buckman MT, Davis LE & Standefer J (1983) Pituitary and placentally derived hormones in cerebrospinal fluid during normal human pregnancy. The Journal of clinical endocrinology and metabolism, 56, 46–52. [DOI] [PubMed] [Google Scholar]
- Pedersen CA (1997) Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Annals of the New York Academy of Sciences, 807, 126–145. [DOI] [PubMed] [Google Scholar]
- Pellegrino G, Trubert C, Terrien J, Pifferi F, Leroy D, Loyens A, Migaud M, Baroncini M, Maurage CA, Fontaine C, Prevot V & Sharif A (2018) A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus). The Journal of comparative neurology, 526, 1419–1443. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ & Luskin MB (2001) Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci, 21, 6706–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M & Ferreira A (2006) Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res, 175, 139–148. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM & Fernandez-Llebrez P (2010) IGF-I stimulates neurogenesis in the hypothalamus of adult rats. The European journal of neuroscience, 31, 1533–1548. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M, Kolf-Clauw M, Michel C & Beausoleil C (2018) Alteration of mammary gland development by bisphenol a and evidence of a mode of action mediated through endocrine disruption. Molecular and Cellular Endocrinology, 475, 29–53. [DOI] [PubMed] [Google Scholar]
- Perrin G, Meurisse M & Levy F (2007) Inactivation of the medial preoptic area or the bed nucleus of the stria terminalis differentially disrupts maternal behavior in sheep. Hormones and behavior, 52, 461–473. [DOI] [PubMed] [Google Scholar]
- Poindron P, Le Neindre P (1980) Endocrine and sensory regulationof maternal behavior in the ewe. Advances in the Study of Behavior, 2, 75–119. [Google Scholar]
- Poindron P, Lévy F, Krehbiel D (1988) Genital, olfactory and endocrine interactions in the development of maternal behaviout in the parturient ewe. Psychoneuroendocrinology, 13, 9–125. [DOI] [PubMed] [Google Scholar]
- Porrini S, Belloni V, Della Seta D, Farabollini F, Giannelli G & Dessi-Fulgheri F (2005) Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull, 65, 261–266. [DOI] [PubMed] [Google Scholar]
- Price AK & Bridges RS (2014) The effects of bromocriptine treatment during early pregnancy on postpartum maternal behaviors in rats. Developmental psychobiology, 56, 1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Dobeli M, Martin RD (1993) Effects of sex steroids on maternal motivation in the common marmoset development and application of an operant system with maternal reinforcement. J Comp Psychol, 107, 99–115. [DOI] [PubMed] [Google Scholar]
- Punzo F (2003) Effects of carbaryl-treated bait on maternal behavior and sprint performance in the meadow jumping mouse, Zapus hudsonius. Bull Environ Contam Toxicol, 71, 37–41. [DOI] [PubMed] [Google Scholar]
- Quadagno DM, McCullough J, Ho GK & Spevak AM (1973) Neonatal gonadal hormones: effect on maternal and sexual behavior in the female rat. Physiology & behavior, 11, 251–254. [DOI] [PubMed] [Google Scholar]
- Quadagno DM & Rockwell J (1972) The effect of gonadal hormones in infancy on maternal behavior in the adult rat. Hormones and behavior, 3, 55–62. [DOI] [PubMed] [Google Scholar]
- Quadros PS & Wagner CK (2008) Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology, 149, 3054–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Cao J, Sluzas E, Delclos KB, Camacho L, Lewis SM, Vanlandingham MM & Patisaul HB (2014) Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol Sci, 140, 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond G & Sachs BD (1984) Maternal discrimination of pup sex in rats. Developmental psychobiology, 17, 87–89. [DOI] [PubMed] [Google Scholar]
- Robertson MC & Friesen HG (1981) Two forms of rat placental lactogen revealed by radioimmunoassay. Endocrinology, 108, 2388–2390. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA & Herrenkohl LR (1976) Maternal behavior in male rats: critical times for the suppressive action of androgens. Physiology & behavior, 16, 293–297. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS (1967) Nonhormonal basis of maternal behavior in the rat. Science, 156, 1512–1514. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS & Ceus K (1998) Estrogen implants in the medial preoptic area stimulate maternal behavior in male rats. Horm Behav, 33, 23–30. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS & Siegel HI (1975) Hysterectomy-induced maternal behavior during pregnancy in the rat. J Comp Physiol Psychol, 89, 685–700. [DOI] [PubMed] [Google Scholar]
- Rosenson LM (1975) Responses of virgin mice, and of maternal females, to normal and caesarian-section delivered pups. Journal of comparative and physiological psychology, 88, 670–677. [DOI] [PubMed] [Google Scholar]
- Routledge EJ, White R, Parker MG & Sumpter JP (2000) Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ER beta. J Biol Chem, 275, 35986–35993. [DOI] [PubMed] [Google Scholar]
- Royle NJ, Russell AF, Wilson AJ (2014) The evolution of flexible parenting. Science, 345, 776–781. [DOI] [PubMed] [Google Scholar]
- Rubin BS (2011) Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. The Journal of steroid biochemistry and molecular biology, 127, 27–34. [DOI] [PubMed] [Google Scholar]
- Sabihi S, Dong SM, Durosko NE & Leuner B (2014) Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Frontiers in behavioral neuroscience, 8, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaso N, Popeski N, Peronace LA & Woodside B (2005) Differential effects of reproductive and hormonal state on basic fibroblast growth factor and glial fibrillary acid protein immunoreactivity in the hypothalamus and cingulate cortex of female rats. Neuroscience, 134, 1431–40. [DOI] [PubMed] [Google Scholar]
- Salmaso N & Woodside B (2006) Upregulation of astrocytic basic fibroblast growth factor in the cingulate cortex of lactating rats: time course and role of suckling stimulation. Hormones & Behavior, 50, 448–53. [DOI] [PubMed] [Google Scholar]
- Salmaso N, Cossette MP & Woodside B (2011) Pregnancy and maternal behavior induce changes in glia, glutamate and its metabolism within the cingulate cortex. PLoS One, 6, e23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan VF, Byrnes EM & Bridges RS (2006) Reproductive experience and activation of maternal memory. Behavioral neuroscience, 120, 676–686. [DOI] [PubMed] [Google Scholar]
- Scotti MA, Stevenson SA & Gammie SC (2011) Changes in CNS response to neurotensin accompany the postpartum period in mice. Hormones and behavior, 60, 177–184. [DOI] [PubMed] [Google Scholar]
- Sèbe F, Aubon T, Boué A, Poindron P (2008) Mother-young vocal communication and acoustic recognition promote preferential nuersing in sheep. Journal of Experimental Biology, 211, 3554–3562. [DOI] [PubMed] [Google Scholar]
- Seip KM & Morrell JI (2008) Exposure to pups influences the strength of maternal motivation in virgin female rats. Physiology & behavior, 95, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks P, Wood S, Ingram CD & Lightman SL (1999) The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. Journal of neuroendocrinology, 11, 857–865. [DOI] [PubMed] [Google Scholar]
- Sheehan T & Numan M (2002) Estrogen, progesterone, and pregnancy termination alter neural activity in brain regions that control maternal behavior in rats. Neuroendocrinology, 75, 12–23. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Paul M, Amaral E, Numan MJ, Numan M (2001) Evidence that the medial amygdala projects to the anterior/ventromedial hypothalamic nuclei to inhibit maternal behavior in rats. Neuroscience, 106, 341–356. [DOI] [PubMed] [Google Scholar]
- Shoji H & Kato K (2006) Maternal behavior of primiparous females in inbred strains of mice: a detailed descriptive analysis. Physiol Behav, 89, 320–328. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV & Merchenthaler I (1997) Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol, 388, 507–525. [DOI] [PubMed] [Google Scholar]
- Siegel HI & Rosenblatt JS (1975a) Estrogen-induced maternal behavior in hysterectomized-overiectomized virgin rats. Physiol Behav, 14, 465–471. [DOI] [PubMed] [Google Scholar]
- Siegel HI & Rosenblatt JS (1975b) Hormonal basis of hysterectomy-induced maternal behavior during pregnancy in the rat. Horm Behav, 6, 211–222. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M & Swanson LW (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. The Journal of comparative neurology, 294, 76–95. [DOI] [PubMed] [Google Scholar]
- Simmons SL, Cummings JA, Clemens LG & Nunez AA (2005) Exposure to PCB 77 affects the maternal behavior of rats. Physiol Behav, 84, 81–86. [DOI] [PubMed] [Google Scholar]
- Slattery DA & Neumann ID (2008) No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. The Journal of physiology, 586, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA (1974) Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behavioral Biology, 12, 55–66. [DOI] [PubMed] [Google Scholar]
- Spearow JL, O’Henley P, Doemeny P, Sera R, Leffler R, Sofos T & Barkley M (2001) Genetic variation in physiological sensitivity to estrogen in mice. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica, 109, 356–364. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Kalpachidou T, Raftogianni A, Zografou E, Tzanou A, Pondiki S & Stylianopoulou F (2015) Rat dams exposed repeatedly to a daily brief separation from the pups exhibit increased maternal behavior, decreased anxiety and altered levels of receptors for estrogens (ERalpha, ERbeta), oxytocin and serotonin (5-HT1A) in their brain. Psychoneuroendocrinology, 52, 212–228. [DOI] [PubMed] [Google Scholar]
- Stern JM (1983) Maternal behavior priming in virgin and caesarean-delivered Long-Evans rats: effects of brief contact or continuous exteroceptive pup stimulation. Physiology & behavior, 31, 757–763. [DOI] [PubMed] [Google Scholar]
- Stern JM, Goldman L & Levine S (1973) Pituitary-adrenal responsiveness during lactation in rats. Neuroendocrinology, 12, 179–191. [DOI] [PubMed] [Google Scholar]
- Stern JM & Keer SE (1999) Maternal motivation of lactating rats is disrupted by low dosages of haloperidol. Behavioural brain research, 99, 231–239. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ & Numan M (2007) Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behavioral neuroscience, 121, 907–919. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS & Rissman EF (2011) Oestrogen-independent, experience-induced maternal behaviour in female mice. J Neuroendocrinol, 23, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Zhang KY, Luskin K, Ranker L, Balkema J, Bress J & Numan M (2009) A single injection of 17beta-estradiol at the time of pup presentation promotes the onset of maternal behavior in pregnancy-terminated rats. Horm Behav, 56, 121–127. [DOI] [PubMed] [Google Scholar]
- Sturtz N, Deis RP, Jahn GA, Duffard R & Evangelista de Duffard AM (2008) Effect of 2,4-dichlorophenoxyacetic acid on rat maternal behavior. Toxicology, 247, 73–79. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Petrusz P, Szpirer C, Joseph DR (1991) Alternative processing of androgen-binding protein RNA transcripts in fetal liver. Journal of Biological Chemistry, 266, 143–154 [PubMed] [Google Scholar]
- Svare B & Gandelman R (1975) Postpartum aggression in mice: inhibitory effect of estrogen. Physiology and Behavior, 14, 31–35. [DOI] [PubMed] [Google Scholar]
- Tanida T 1, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, Tabuchi Y, Nanmori T, Qi WM, Inamoto T, Yokoyama T, Kitagawa H& Hoshi N (2009) Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicoogyl Letters, 189:40–7. [DOI] [PubMed] [Google Scholar]
- Tate-Ostroff B & Bridges RS (1985) Plasma prolactin levels in parental male rats: effects of increased pup stimuli. Hormones and behavior, 19, 220–226. [DOI] [PubMed] [Google Scholar]
- Tate-Ostroff BA & Bridges RS (1987) Regulation of prolactin secretion in parental rats: roles of steroid priming and pituitary responsiveness. Psychoneuroendocrinology, 12, 385–391. [DOI] [PubMed] [Google Scholar]
- Terkel J & Rosenblatt JS (1968) Maternal behavior induced by maternal blood plasma injected into virgin rats. J Comp Physiol Psychol, 65, 479–482. [DOI] [PubMed] [Google Scholar]
- Terkel J & Rosenblatt JS (1972) Humoral factors underlying maternal behavior at parturition: corss transfusion between freely moving rats. J Comp Physiol Psychol, 80, 365–371. [DOI] [PubMed] [Google Scholar]
- Theodosis DT & Poulain DA (1984) Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience, 11, 183–193. [DOI] [PubMed] [Google Scholar]
- Theodosis DT & Poulain DA (1984) Evidence that oxytocin-secreting neurones are involved in the ultrastructural reorganisation of the rat supraoptic nucleus apparent at lactation. Cell Tissue Research, 235, 217–219. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Montagnese C, Rodriguez F, Vincent JD & Poulain DA (1986) Oxytocin induces morphological plasticity in the adult hypothalamo-neurohypophysial system. Nature, 322, 738–740. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Koksma JJ, Trailin A, Langle SL, Piet R, Lodder JC, Timmerman J, Mansvelder H, Poulain DA, Oliet SH & Brussaard AB (2006) Oxytocin and estrogen promote rapid formation of functional GABA synapses in the adult supraoptic nucleus. Molecular and cellular neurosciences, 31, 785–794. [DOI] [PubMed] [Google Scholar]
- Thorsteinson N, Ban F, Santos-Filho O, Tabaei SM, Miguel-Queralt S, Underhill C, Cherkasov A & Hammond GL (2009) In silico identification of anthropogenic chemicals as ligands of zebrafish sex hormone binding globulin. Toxicology and Applied Pharmacology, 234, 47–57. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Thrivikraman KV, Plotsky PM, Morilak DA, Huang N & Walker CD (1998) Reduced noradrenergic tone to the hypothalamic paraventricular nucleus contributes to the stress hyporesponsiveness of lactation. Journal of neuroendocrinology, 10, 417–427. [DOI] [PubMed] [Google Scholar]
- Tsuneoka Y, Maruyama T, Yoshida S, Nishimori K, Kato T, Numan M & Kuroda KO (2013) Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. J Comp Neurol, 521, 1633–1663. [DOI] [PubMed] [Google Scholar]
- Tsuneoka Y, Yoshida S, Takase K, Oda S, Kuroda M & Funato H (2017) Neurotransmitters and neuropeptides in gonadal steroid receptor-expressing cells in medial preoptic area subregions of the male mouse. Scientific reports, 7, 9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo MS, Sandini TM, Reis TM, Bernardi MM & Spinosa HS (2014) Prenatal exposure to a low fipronil dose disturbs maternal behavior and reflex development in rats. Neurotoxicol Teratol, 45c, 27–33. [DOI] [PubMed] [Google Scholar]
- Valtcheva S & Froemke RC (2018) Neuromodulation of maternal circuits by oxytocin. Cell and tissue research [DOI] [PMC free article] [PubMed]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT & Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev, 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, Hunt PA, Newbold RR, Rubin BS, Saili KS, Soto AM, Wang HS & Vom Saal FS (2013) Low dose effects of Bisphenol A: An integrated review of in vitro, laboratory animal and epidemiology studies. Endocrine Disruptors, 1, e25078. [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS & Soto AM (2009) Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocrine Reviews, 30, 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Cutuli D, Colonnello V, Cardona D, Ricceri L & Calamandrei G (2008) Neonatal exposure to chlorpyrifos affects maternal responses and maternal aggression of female mice in adulthood. Neurotoxicol Teratol, 30, 468–474. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Scattoni ML & Calamandrei G (2009) Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in CD-1 mouse pups. Environmental Health, 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura C, Nieto MR, Bourguignon N, Lux-Lantos V, Rodriguez H, Cao G, Randi A, Cocca C & Núñez M (2016) Pesticide chlorpyrifos acts as an endocrine disruptor in adult rats causing changes in mammary gland and hormonal balance. Journal of Steroid Biochemistry and Molecular Biology, 156, 1–9. [DOI] [PubMed] [Google Scholar]
- Verboven N, Verreault J, Letcher RJ, Gabrielsen GW & Evans NP (2009) Nest temperature and parental behaviour of Arctic-breeding glaucous gulls exposed to persistent organic pollutants. Animal Behaviour, 77, 411–418. [Google Scholar]
- Walker CD, Trottier G, Rochford J & Lavallee D (1995) Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. Journal of neuroendocrinology, 7, 615–622. [DOI] [PubMed] [Google Scholar]
- Walker DM, Goetz BM & Gore AC (2014) Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol Endocrinol, 28, 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM & Gore AC (2011) Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol, 7, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner BP & Sheard NM (1933) Maternal behavior in rats, pp. 245.
- Windle RJ, Wood S, Shanks N, Perks P, Conde GL, da Costa AP, Ingram CD & Lightman SL (1997) Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. Journal of neuroendocrinology, 9, 407–414. [DOI] [PubMed] [Google Scholar]
- Workman JL, Raineki C, Weinberg J & Galea LAM (2015) Alcohol and pregnancy: Effects on maternal care, HPA axis function, and hippocampal neurogenesis in adult females. Psychoneuroendocrinology, 57, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM (1994) Alterations of the cortical representation of the rat ventrum induced by nursing behavior. Journal of Neuroscience, 14, 1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M & Ide C (2005) Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Experimental neurology, 192, 251–264. [DOI] [PubMed] [Google Scholar]
- Yang Y, Qin J, Chen W, Sui N, Chen H & Li M (2015) Behavioral and pharmacological investigation of anxiety and maternal responsiveness of postpartum female rats in a pup elevated plus maze. Behavioural brain research, 292, 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Bowlby DA, Brown TJ, Hochberg RB & MacLusky NJ (1995) Distribution of occupied and unoccupied estrogen receptors in the rat brain: effects of physiological gonadal steroid exposure. Endocrinology, 136, 96–105. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W & Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell, 132, 645–660. [DOI] [PubMed] [Google Scholar]
- Zhao C & Li M (2010) c-Fos identification of neuroanatomical sites associated with haloperidol and clozapine disruption of maternal behavior in the rat. Neuroscience, 166, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]