Abstract
Functional MRI connectivity has identified neurophysiology relevant to cognition and personality, motivating a search for relationships between brain architecture and emotional health and well-being. Two approaches were used to asses functional connectivity correlates of emotional health and well-being. The first approach used principal component analysis to evaluate resting-state functional magnetic resonance imaging data from the Human Connectome Project 1200 Subjects Data Release. Pairwise functional connectivity measurements were obtained from a 5 mm resolution parcellation of brain gray matter. Principal components were calculated for each individual and for group mean connectivity data and compared to obtain an estimate of typicality of functional connectivity for each component in each subject. Typicality scores were compared to reported emotional health metrics using a general linear model. The second approach calculated functional connectivity between each pair of networks from a 17-resting-state network cortical parcellation. Typicality of connectivity showed significant correlation across the population to emotional metrics corresponding to attitudes of anger and aggression in 3 of 10 principal components. Additionally, functional connectivity between the default and attentional networks was positively correlated with scores of attitudes of anger and aggression. These findings are consistent with a mechanism of impaired effortful control and decreased response inhibition of impulsivity.
Keywords: Emotional well-being, fMRI, principal component analysis, default mode network, attentional networks, effortful control
1. Introduction
The notion of a structure-function relationship between emotion, personality, or social function, and intrinsic brain networks or structures has long been hypothesized. Beginning with MacLean’s limbic system theory of emotion (Maclean, 1949, 1952), there has been extensive research to determine anatomical or neurosystem underpinnings of emotion. There are commonly established and well-studied associations with regards to regional activation during emotion induction or recall (Murphy et al., 2003; Phan et al., 2002). Some well-known associations, both within the context of healthy individuals and disease, include the role of the amygdala in fear induction and conditioning, sadness and stress associated with activity in the subcallosal cingulate (particularly in major depressive disorder), and a general role in emotional processing for the medial prefrontal cortex (Adolphs et al., 1995; Barad et al., 2006; Davis, 1992; Etkin et al., 2011; Fullana et al., 2018; Hamani et al., 2011; LaBar et al., 1998; Matsunaga et al., 2016; Phillips and LeDoux, 1992).
More than traditional regional activation in emotion processing, the strength of functional connectivity between brain regions may affect and modulate function. The relationship between functional connectivity and emotional traits has been explored most commonly in the context of mood, anxiety, psychotic and personality disorders, examining the role of the amygdala, frontal cortex, and anterior cingulate (Chechko et al., 2016; Green et al., 2015; Hermans et al., 2017; Khanna et al., 2017; Kim et al., 2011; Murrough et al., 2016; Nelson et al., 2015; Nicholson et al., 2017; Perlman et al., 2012; Smith et al., 2015; Williams et al., 2006). Functional connectivity correlates of emotional health and well-being in healthy controls has been identified for specific personality traits (Jiang et al., 2018; Williams et al., 2018), experience of negative emotion (Petrican et al., 2015), and anxiety (Takagi et al., 2018), motivating a data-driven search for additional relationships between emotional health and brain connectivity.
The Human Connectome Project (HCP) provides neuroimaging behavioral data in a large sample of healthy young adults in which to further probe functional connectivity correlates of emotional health and well-being (Glasser et al., 2013; Marcus et al., 2011; Smith et al., 2013; Van Essen et al., 2012). This dataset provides standardized behavioral measures which have the potential to covary across subjects in meaningful and interesting ways (Van Essen et al., 2013). The emotion metrics include measures of negative affect, psychological well-being, social relationships, and stress and self-efficacy, allowing a multifactorial search for relationships with brain connectivity (Babakhanyan et al., 2018; Gur et al., 2001; Gur et al., 2010).
Using the HCP 1200 Subjects Data Release, we studied functional connectivity correlates of emotional health and well-being. Within a healthy control population, measures of emotional health can be thought to lie on a distribution with the tails of the distribution representing atypical resilience or subclinical dysfunction that may be associated with atypical brain connectivity. Our hypothesis was that “typicality” of functional connectivity, defined as the similarity of connectivity measures to those of the population mean, may serve as a screening tool to identify specific brain networks and connections that could inform emotional health. An underlying assumption for this approach is that normative pressures in social relationships may predispose individuals who differ in brain connectivity from typical patterns to experience greater challenges in social integration and maintenance of mental health. Using typicality as a screening tool in a healthy population has several advantages: it allows a data-driven empirical approach that does not require a priori specification of hypothesized circuits that may underlie emotional health; typicality can be tested using multiscale frameworks ranging from individual circuits to whole brain paradigms mitigating statistical effects of multiple comparisons arising from other data-driven approaches; and it facilitates the identification of population variants that in more extreme cases may be influential in the pathophysiology of disorders of mood and personality. But a data-driven, multifactorial approach to comparing brain connectivity and emotional health involves many variables and may fail to identify important relationships with confidence given the resulting statistical limitations of making many comparisons.
We addressed this problem by comparing typicality of connectivity to reported behavioral covariates across the sample using a top-down approach. Initially, we examined very broad patterns of connectivity arising from many connections and then identified more and more specific patterns that may reliably predict emotional health. Such broad patterns of connectivity have traditionally been identified using techniques of spectral decomposition, including principal component analysis (PCA), singular value decompositions, and eigendecompositions. In the resting state functional connectivity literature these approaches have generally used PCA for dimensionality reduction of data, followed by independent component analysis (ICA) which removes higher order dependence among the components (Beckmann and Smith, 2005; Calhoun et al., 2001; Damoiseaux et al., 2006). The result is that ICA components are not weighted by importance, or how much they contribute to population variance, while principal components (PC) are ordered by their eigenvalues, accounting for progressively less and less contribution to population variance. Principal components exhibit related but different patterns from the more familiar independent components in resting state functional connectivity, with pieces of independent component networks distributed across principal components (Ferguson et al., 2017).
For typicality analysis, we elected to use PCA rather than ICA as a primary analysis method for three reasons. First, principal components are ordered by their contribution to population variance, so the method can capture the largest sources of variance among the population in the fewest possible number of components, facilitating a more compact, concise metric of typicality. Second, principal components do not change with selection of model order, removing an additional parameter that could affect results. Finally, we were unsure whether brain connectivity differences underlying emotional health would align to the boundaries of ICA-defined networks (for example within the default network or between sensorimotor and salience networks) and additionally wanted to explore the possibility that individual differences in brain connectivity related to emotional well-being might correspond to patterns that had been missed given how much of the resting state functional connectivity literature has been constrained within and between discrete ICA-derived boundaries. As a secondary analysis, we evaluated differences in traditional ICA-derived functional network connectivity for metrics of emotional health that showed covariation with typicality of functional connectivity.
2. Methods
2.1. Participants
From the Human Connectome Project 1200 Subjects Data Release, 1003 subjects of 1206 completed four 15-minute resting state acquisitions, and all of these subjects were used in this analysis (mean age = 28.7 years; SD = 3.7 years; age range: 22–37; 534 female subjects) providing 60 minutes per subject of FIX ICA cleaned Multiband BOLD resting state data (Griffanti et al., 2014; Moeller et al., 2010; Van Essen et al., 2013). The FIX ICA cleaned data was supplied for each subject with the 1200 subjects release of the Human Connectome Project (for example “rfMRI_REST1_LR_hp2000_clean.nii.gz”). We used these cleaned images without further preprocessing, and extracted time series from a parcellation of brain gray matter consisting of 6923 regions of interest covering cortical, subcortical, and cerebellar gray matter at 5 mm spatial resolution as previously described (Shah et al., 2016). The FIX ICA procedure obtains independent components from the preprocessed fMRI data and classifies components as likely representing BOLD signal or noise, and then regresses the time series associated with the noise components from the data, mitigating factors such as head motion, physiological artifacts and other sources of shared variance in the data unlikely to arise from neural activity, and has been shown to improve reproducibility of the resting state data as well as information related to brain-behavior relationships (Anderson et al., 2018; Griffanti et al., 2014; Shah et al., 2016).
Each of the 6923 regions of interest was within the brain for greater than 95% of the subjects (Shah et al., 2016), and for those cases where an ROI was outside the brain in a given subject, the values were treated as missing data. Additionally we extracted mean time series from a set of 17 brain networks derived from a prior study of the functional organization of the brain obtained from ICA decomposition in a large cohort of healthy control volunteers (Buckner et al., 2011; Yeo et al., 2011).
For each of four 15-minute scans in each subject, a 6923 × 6923 matrix of ROI-based functional connectivity was computed by calculating the Fisher-transformed Pearson correlation coefficient between each pair of ROIs’ time series. Similarly, a 17 × 17 matrix of network based functional connectivity was calculated by evaluating the correlation coefficient between mean time series from each pair of networks. Both 6923 × 6923 and 17 × 17 connectivity matrices were averaged across the four scans for each subject and used for subsequent analyses.
2.2. Measures of emotional health and well-being
Using a data-driven approach, we examined relationships between brain functional connectivity and 17 NIH Toolbox measures included with the Human Connectome Project dataset related to emotional health and well-being. These measures are briefly described with labels from the NIH Toolbox Brochure (http://www.healthmeasures.net/images/nihtoolbox/NIH_Toolbox_brochure_June_2017.pdf) below and with population summary values in Table 1:
Table 1.
Descriptive statistics of reported emotional well-being metrics
| (n = 1003) | ||
|---|---|---|
| n | % | |
| Female | 534 | 53.20 |
| M | SD | |
| Age (years) | 28.71 | 3.71 |
| Metric | ||
| Anger-Affect | 47.71 | 8.16 |
| Anger-Hostility | 50.33 | 8.55 |
| Anger-Aggression | 51.82 | 8.75 |
| Fear-Affect | 50.09 | 7.87 |
| Fear-Somatic Arousal | 51.83 | 8.13 |
| Sadness | 46.12 | 7.83 |
| General Life Satisfaction | 54.76 | 9.20 |
| Purpose and Meaning | 52.03 | 8.76 |
| Positive Affect | 50.22 | 7.86 |
| Friendship | 50.48 | 9.06 |
| Loneliness | 50.96 | 8.56 |
| Perceived Hostility | 48.60 | 8.47 |
| Perceived Rejection | 48.33 | 8.67 |
| Emotional Support | 51.47 | 9.46 |
| Instrumental Support | 48.03 | 9.02 |
| Perceived Stress | 48.12 | 9.06 |
| Self-Efficacy | 51.06 | 8.29 |
Note. This table describes the behavioral metrics corresponding to emotional well-being reported for the Human Connectome Project 1200 release.
Anger-Affect Survey: Attitudes associated with experiences of frustration related to affect.
Anger-Hostility Survey: Attitudes associated with experiences of hostility.
Anger-Aggression Survey: Attitudes associated with experiences related to physical aggression.
Fear-Affect Survey: Symptoms of anxiety related to unpleasant feelings or emotions.
Fear-Somatic Arousal Survey: Symptoms of anxiety that reflect autonomic arousal.
Sadness Survey: Unpleasant feelings or emotions of sadness.
General Life Satisfaction Survey: One’s cognitive evaluation of life experiences and whether people like their lives or not.
Meaning and Purpose Survey: The extent to which people feel their lives matter or make sense.
Positive Affect Survey: Feelings that reflect a level of pleasurable engagement with the environment, such as happiness, joy, excitement, enthusiasm, and contentment.
Friendship Survey: Perceptions of the availability of friends or companions with whom to interact or affiliate.
Loneliness Survey: Perceptions that one is alone, lonely or socially isolated from others.
Perceived Hostility Survey: How often people argue with me, yell at me, or criticize me.
Perceived Rejection Survey: How often people don’t listen when I ask for help, or don’t pay attention to me.
Emotional Support Survey: The perception that people in one’s social network are available to listen to one’s problems with empathy, caring and understanding.
Instrumental Support Survey: The perception that people in one’s social network are available to provide material or functional aid in completing daily tasks, if needed.
Perceived Stress Survey: Individual perceptions about the nature of events and their relationship to the values and coping resources of an individual.
Self-Efficacy Survey: A person’s belief in his/her capacity to manage functioning and have control over meaningful events.
2.3. Approach 1: Principal component analysis
Resting state functional connectivity data were analyzed using two separate approaches. The first approach was designed to identify common patterns of variation of functional connectivity across subjects and used principal components of resting-state functional connectivity (RSFC). We performed principal component analysis using singular value decomposition on 6923 × 6923 ROI-based correlation matrices. Principal components are the eigenvectors of RSFC matrices and identify covariance patterns in the functional brain data. Thus, the principal components from RSFC matrices represent a set of intrinsic brain networks which are hierarchically organized by the amount of signal variance within each component. If a subset of ROIs was not inside the brain for a given subject, the singular value decomposition was performed for the remaining matrix. For example, if 7 ROIs were not inside the brain for one subject, the singular value decomposition was performed using Matlab (“svds.m”) on a 6916 × 6916 functional connectivity matrix, and missing values were inserted as “NaN” in the corresponding locations to missing ROIs to produce a vector with 6923 elements for each component that aligned across subjects. The first 20 principal components were extracted for each subject, each a vector with 6923 elements (possibly with one or more “NaN” missing values).
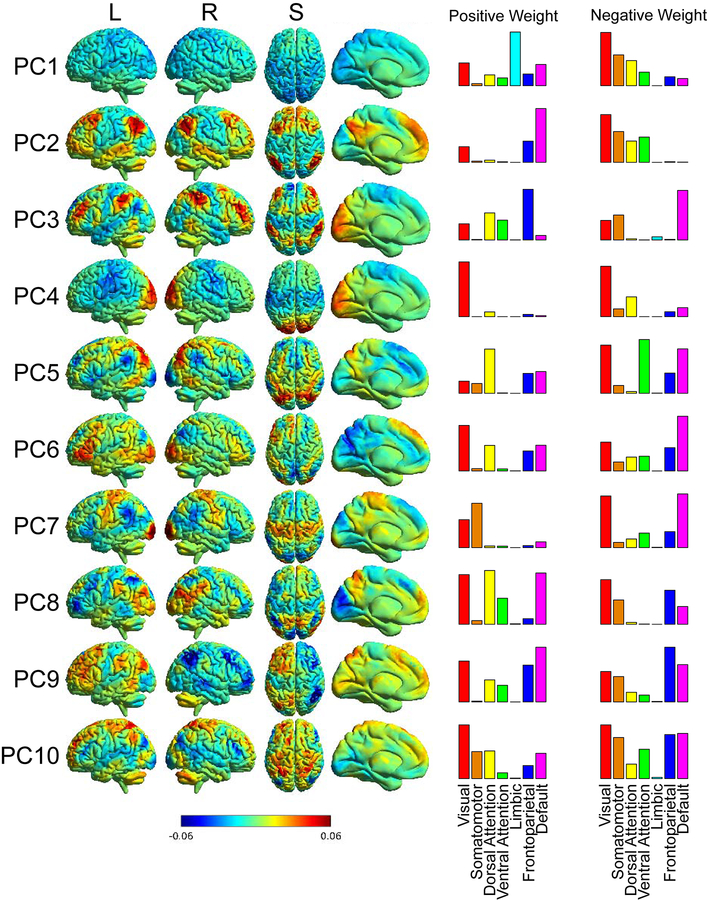
Functional connectivity matrices for 6923 × 6923 ROIs were also averaged across all 1003 subjects to obtain group mean functional connectivity, and principal components from the group averaged data were also obtained. The networks for the first 10 principal components of the group mean were back-projected onto anatomical space in order to visualize the networks that contribute strongly to signal variance within RSFC (Figure 1).
Figure 1:
Principal components back-projected into anatomical space. The color bar represents a unitless weighting factor where red indicates a positive weighting, and blue indicates a negative weighting. The columns to the right show histograms of ROIs showing positive or negative weight (1 standard deviation above and below the mean for each component) that are represented by 7 canonical ICA-derived functional networks.
For individual subjects, principal components showed similarity to the group-averaged principal components, but occasional differences were seen in the order of components for a given subject, as described for an analysis using PCA in a smaller cohort of HCP subjects to evaluate correlations to fluid intelligence (Ferguson et al., 2017). The first 20 principal components of each subject were compared to the first 10 principal components of the group averaged data using Pearson correlation coefficient. For each group level component, the individual component exhibiting the highest absolute value of correlation was selected as the ‘best match’ for that group level component in that individual and the absolute value of correlation was recorded. The correlation between group component and corresponding individual subject component produced a measure of typicality of an individual’s functional connectivity pattern for each component to the population averaged connectivity: specifically, how correlated an individual’s principal components were to population-averaged principal components.
2.4. Approach 2: Functional connectivity across a resting state 17-network parcellation
The second approach calculated functional connectivity between each pair of networks for each subject from a 17 network cortical parcellation of the supratentorial brain and cerebellum (Buckner et al., 2011; Yeo et al., 2011). This approach was designed to evaluate the spatial distribution of functional connectivity differences for metrics of emotional well-being correlated with atypical functional connectivity from the first approach. Average time series were extracted from each of the 17 distributed brain networks and each network was treated as a single ROI. Correlation coefficients were estimated for each ROI pair. These results were Fisher transformed to improve normality and a matrix consisting of the correlation coefficients for the group mean were reported. These correlation coefficients are representative of functional connectivity between different networks.
2.5. Functional connectivity correlation to metrics of emotional well-being
In order to assess the correlative nature of principal component analysis to emotional well-being and health, comparison of metrics of emotional well-being to the measure of correlation to the group mean (typicality) was performed for each principal component. We used the HCP behavioral measures related to emotion for this analysis. The measures in this analysis included 17 NIH Toolbox measures. For further information, please see Table 1. Typicality of functional connectivity (correlation between individual and group principal components for each of the first 10 group-averaged principal components) was correlated with subject-level scores for each metric using a nonparametric model (Spearman partial correlation) that included age, sex, and head motion as covariates. These results were then corrected for multiple comparison using false discovery rate (q(FDR) <0.05) across all 10 components and all 17 metrics of emotional well-being. To perform multiple comparison correction, the FDR criterion was applied simultaneously to all 170 p-values and not for each component or behavioral metric separately. For metrics showing significant correlation to functional connectivity typicality, Spearman correlations were also performed between those metrics and functional connectivity between 136 pairs of the 17 intrinsic connectivity networks for the Yeo et al. parcellation(Buckner et al., 2011; Yeo et al., 2011), also corrected for multiple comparisons using false discovery rate.
3. Results
We used principal component analysis to analyze how typicality of connectivity may align with emotional health and well-being. Figure 1 shows the first 10 principal components of the group-averaged RSFC data mapped onto anatomical space. As can be seen, each principal component corresponds to patterns from more familiar intrinsic connectivity networks obtained from independent component analysis. To compare principal components with canonical ICA-derived networks, we assigned each of the 6923 ROIs to one of 7 canonical networks by overlaying the ROI onto Yeo2011_7Networks_MNI152_FreeSurferConformed1mm_LiberalMask.nii.gz obtained from http://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011. Each ROI was assigned to one of the 7 networks that comprised the mode of voxels within the ROI on the parcellation image. Then, for each principal component, we identified all ROIs which were greater than or less than one standard deviation from the mean for that component (either positively or negatively weighted) and demonstrated which canonical networks comprised the extreme values for each principal component with a histogram in the two right columns of Figure 1.
Many of the principal components show patterns associated with multiple intrinsic connectivity networks, for example in principal component 5 where regions in the dorsal attention network have positive weight, while regions in the ventral attention network have negative weight. Principal component 9 contains information on brain lateralization. Principal component 2 shows strong resemblance to the canonical default network among positively weighted regions, with negatively weighted regions distributed among visual, somatomotor, dorsal attention, and ventral attention networks. Principal component 1 shows relatively weak loading of primarily sensory brain regions (negative weight) and limbic network (positive weight) and may be related to the level of global connectivity in a subject, or may be associated with periods of drowsiness in the scanner where sensory stimulation is lower. There is no accepted naming convention for principal components in the literature and we have simply referred to them by the group PC number (e.g. “PC 5”) to avoid conflating them with traditional ICA-defined networks (such as “default network”).
Given the reported associations in the literature between amygdalar function and emotional health, we evaluated whether the amygdala showed higher weighting for any particular component and found none of the 10 group principal components showing specific weighting of the amygdala by visual inspection. We identified literature-derived MNI coordinates for the amygdala using www.neurosynth.org (x=+/− 22, y=−4, z=−18) and evaluated the weighting of the amygdala in each component. The weighting was within one-half of one standard deviation for weighting values across the brain for 9 of 10 components, and was near the mode in the 10th component (PC 1, which showed a skewed distribution).
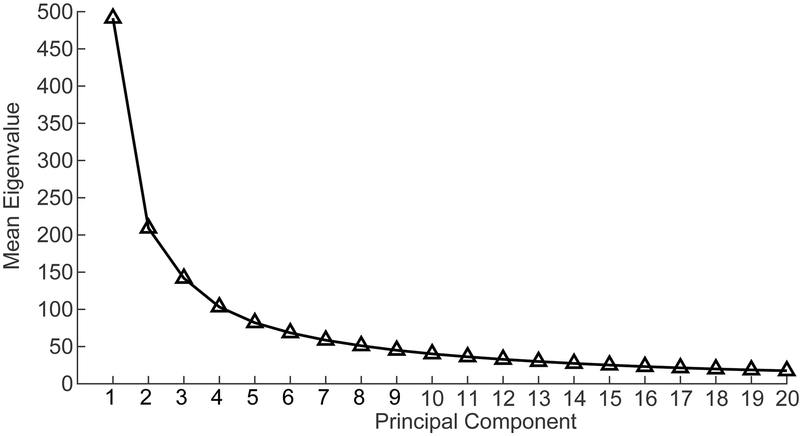
Figure 2 shows the connection between the first 20 principal components and their respective eigenvalues. These eigenvalues decrease steadily as the principal components approach 20. While the number of principal components to include is to some extent arbitrary, it has been previously demonstrated that individual variability of the order and architecture of principal components becomes large beyond about the first 10 components, and consistent individual-level patterns are less reliable (Ferguson et al., 2017). For this reason, we limited analysis to the first 10 principal components as defined by group-averaged functional connectivity.
Figure 2:
First 20 principal components as a function of their respective eigenvalues. Note the decrease in eigenvalue and, by extension, the decrease in variance accounted for as the principal components progress from 1 to 20.
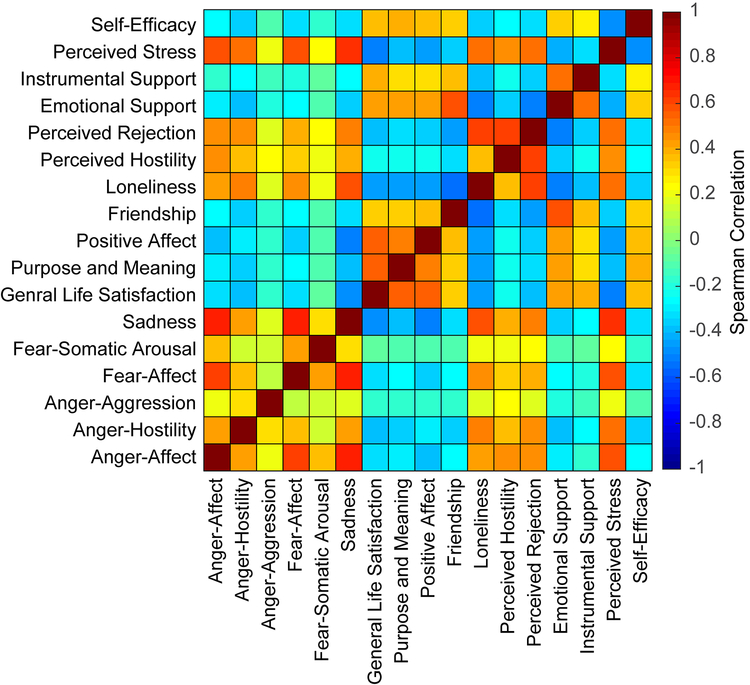
We assessed how metrics corresponding to emotional well-being and health covaried across the population prior to assessing correlation to group mean principal components. Metrics associated with anger, fear, and sadness show relatively high correlation to each other and to metrics corresponding to loneliness and stress (which are also correlated to each other). Psychological metrics (psychological well-being and life-outlook) show similar distribution across subjects and exhibit an association with metrics corresponding to perception of social interactions and perception of self, which also exhibit a correlation to one another (see Figure 3).
Figure 3:
Emotional well-being metric correlation across subjects. Negative affect metrics, such as attitudes associated with anger and fear are associated with one another, as well as with negative aspects of social relationships and stress. Positive affect metrics, such as life satisfaction and psychological well-being are associated with one another and positive aspects of social relationships and perceived self-efficacy.
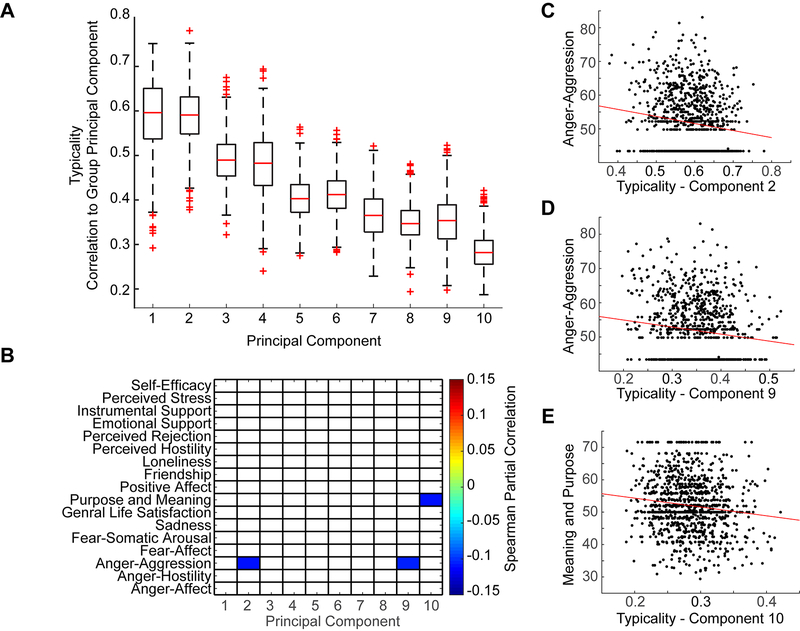
After establishing typicality of connectivity (i.e. how correlated individuals’ principal components were to the group mean principal components), we assessed correlation between typicality of connectivity and metrics of emotional health and well-being, including age, sex, and mean head motion as covariates (Figure 4).
Figure 4:
A. Range of typicality across subjects for each of the 10 principal components derived from group averaged data. Range is shown as a boxplot where the box represents 25 to 75 percentile of the data. Extreme values are shown as “+”. B. Typicality in principal components 1 through 10 correlated with emotional well-being metrics, thresholded q(FDR < .05). Note that there is a negative association between anger-aggression and typicality in principal components 2 and 9. There is also a negative association between attitudes of life purpose and meaning and principal component 10. C. Scatter plot shows scores of typicality for principal component 2 compared to Anger-aggression metric scores. Red line shows best linear fit. D. Scatter plot shows scores of typicality for principal component 9 compared to Anger-aggression metric scores. Red line shows best linear fit. E. Scatter plot shows scores of typicality for principal component 10 compared to Purpose and Meaning metric scores. Red line shows best linear fit.
The NIH Toolbox measure for attitudes of anger corresponding to physical aggression showed a significantly negative correlation to typicality of functional connectivity for principal component 2 (p = 0.00046, rho = −0.11) and component 9 (p = 0.00079, rho = −0.11). When typicality scores for all 10 components were averaged together to obtain one “typicality score” for each subject, the typicality result was not significantly correlated with scores of anger associated with aggression (p = 0.18, rho = −0.04). These data indicate that in healthy populations, individuals exhibiting connectivity patterns similar to population mean patterns for these principal components (2 and 9) are less likely to report aggression. Principal component 10 showed a negative correlation with the metric assessing an individual’s perceived life purpose (p=0.00038, rho = −0.11).
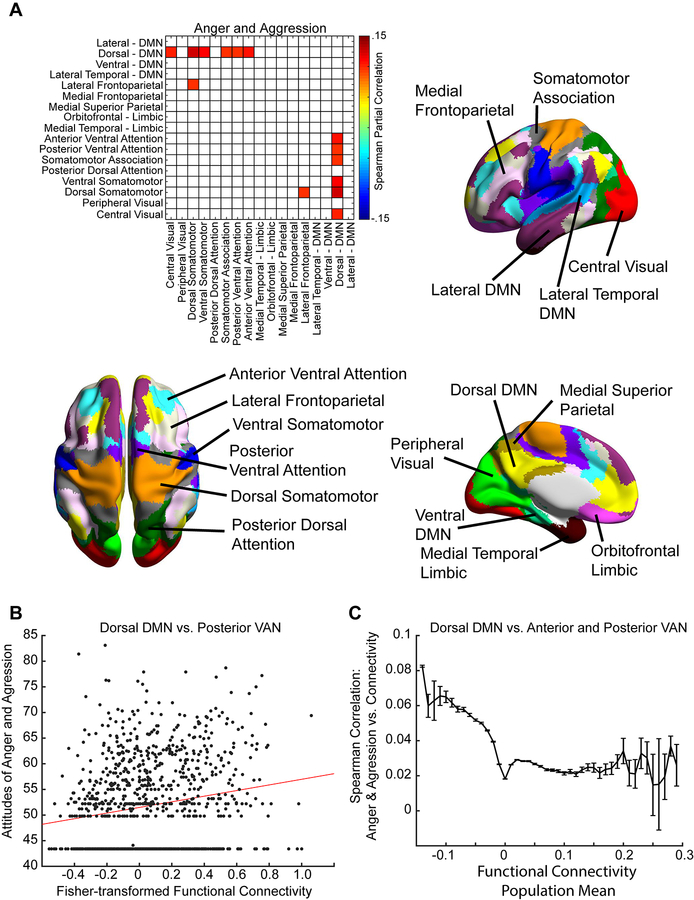
To further evaluate this result, we assessed functional connectivity between 17 pairs of intrinsic connectivity networks for correlation across subjects with scores on the anger-aggression metric (see Figure 5A). Significant associations were thresholded for q(FDR) < .05 across all network pairs. Positive correlations were noted between functional connectivity primarily between the default network and sensory and attention networks (dorsal default mode network (DMN) to central visual: p = 0.0012, rho = 0.10; dorsal DMN to dorsal somatomotor: p = 0.000093, rho = 0.12; dorsal DMN to ventral somatomotor: p = 0.00051, rho = 0.11; dorsal DMN to somatomotor attention: p = 0.0025, rho = 0.10; dorsal DMN to posterior ventral attention: p = 0.0019, rho = 0.10; dorsal DMN to anterior ventral attention: p = 0.000078, rho = 0.11), as well as between the lateral frontoparietal and dorsal somatomotor network (p = 0.0022, rho = 0.097). No significant correlates were found after multiple comparison correction for connectivity between the 17 network pairs and scores of perceived life purpose.
Figure 5:
A. Spearman correlation between functional connectivity across 17 functional networks and anger-aggression scores, thresholded for q(FDR<.05). There is overconnectivity observed between default network and sensory networks, as well as between default network and attentional networks. Each of the 17 networks is illustrated on a labeled, color-coded parcellation of the brain shown to the right and below. B. Scatter plot shows functional connectivity for each subject between the dorsal DMN and posterior ventral attention network compared to anger-aggression metric scores. Red line shows best linear fit. C. Individual connections from each ROI within the dorsal DMN to each ROI within the anterior and posterior ventral attention network were grouped into bins based on population mean functional connectivity for each ROI to ROI connection. The error bars show standard error of the mean for correlation between anger-aggression scores and functional connectivity for the connections within each bin.
Figure 5B shows a representative scatter plot for the association between attitudes of anger and aggression and functional connectivity of one of these network pairs: the dorsal DMN and posterior ventral attention network. As with relationships seen with principal components, the effect size is small, but significant relating attitudes of anger and aggression and functional connectivity between the default and ventral attention network.
Increased connectivity between regions in these two networks could either represent decreased anticorrelation between the networks or increased positive correlation between the networks, as previous work has shown gradients of connectivity ranging from anticorrelated to positively correlated between these networks depending on which subregions of the network are examined. To distinguish between these possibilities, we looked at individual connections from ROIs within the dorsal DMN (507 ROIs) to either the posterior ventral attention network (403 ROIs) or anterior ventral attention network (344 ROIs). ROIs (from among the 6923 ROIs covering the gray matter described above) were assigned to one of the 17 networks by intersecting the ROI with a parcellation of these 17 networks: Yeo2011_17Networks_MNI152_FreeSurferConformed1mm_LiberalMask.nii.gz obtained from http://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011. Each ROI was assigned to one of the 17 networks that comprised the mode of voxels within the ROI on the parcellation image. We grouped the connections between the dorsal DMN and ventral attention network (507 × 747 connections) into bins based on the population mean connectivity averaged across all 1003 subjects for each connection. Bin widths were assigned from −0.15 to 0.3 at 0.01 increments of Fisher-transformed population mean connectivity. Figure 5C shows the results, with increased correlation between attitudes of anger and aggression only for bins that were anticorrelated between the dorsal DMN and ventral attention network.
4. Discussion
To assess functional connectivity correlates for emotional health and wellbeing, we examined typicality of functional connectivity across the Human Connectome Project 1200 Subjects Data Release. We found that typicality of functional connectivity in principal components 2 and 9 was negatively correlated to the NIH Toolbox assessment of anger pertaining to aggression. Typicality of functional connectivity in principal component 10 was negatively correlated with reported life purpose. In order to better understand functional connectivity underpinnings for these associations observed, we then assessed correlation of functional connectivity and anger-aggression across 17 resting state networks. We found a positive association between high scores of anger-aggression and functional connectivity between default mode and attentional and sensory networks. At least for default vs. ventral attention network, this increased connectivity for subjects reporting higher scores of attitudes of anger-aggression primarily represented decreased anticorrelation between subregions of the default and ventral attention network.
Anger-aggression was negatively correlated with typicality of functional connectivity in principal components 2 and 9. This association indicates that brain regions represented in these principal components may disproportionately contribute to regulation or processing of anger related to physical aggression. In principal component 2, the default network is disproportionately represented in this component. The default network is composed of several core subregions (Greicius et al., 2003; Greicius and Menon, 2004; Morris et al., 2000; Ongur et al., 2003; Vogt et al., 1995), which in turn are commonly attributed to key functional hubs corresponding to different aspects of internal thought processes (Andrews-Hanna et al., 2014). These hubs include the ventral medial and dorsal prefrontal cortex and posterior cingulate clearly seen in principal component 2. Principal component 9 is characterized by lateralization of the brain’s association cortex.
4.1. Emotion and brain imaging
Lindquist et al. (2012) found that discrete emotions could not be limited to distinct brain regional localization, but rather a set of interacting brain regions or networks mediating emotional experience and processing. In the same way, it is unlikely that a single principal component can explain the relationship between anger-aggression scores and principal components 2 and 9. This is further supported by a positive correlation between anger-aggression scores and functional connectivity between the default and attention networks shown in Figure 5. Connectivity between these two networks has been described as anticorrelated (Fox et al., 2005; Fransson, 2005), and individuals exhibiting high scores on the anger-aggression metric may therefore exhibit decreased anticorrelation between default and attentional networks. More specifically, there are subregions of the default network that are anticorrelated to specific subregions of brain attentional networks, with gradients of connectivity ranging from anticorrelated connections to positively correlated connections across the networks (Anderson et al., 2011) that are differentially expressed anatomically in distinct hubs of brain networks (Uddin et al., 2009), and develop during childhood and adolescence (Chai et al., 2014).
We find that the relationship between attitudes of anger-aggression and functional connectivity is primarily driven by decreased anticorrelation between the two networks, a trait that has been associated with impaired performance in working memory (Hampson et al., 2010; Keller et al., 2015), response inhibition (Kelly et al., 2008), cognitive control (Dwyer et al., 2014), and attention (Rohr et al., 2016).
The default network is activated during spontaneous, unconstrained events such as mind-wandering, imagining ones future, recollecting personal past, or self-reference (Christoff et al., 2009; D’Argembeau et al., 2005; Gusnard et al., 2001; Mason et al., 2007; Schacter et al., 2007; Schacter et al., 2012; Spreng et al., 2009; Spreng et al., 2010). The attention network is actively engaged with directed attention and working memory (Corbetta and Shulman, 2002; Fox et al., 2006). These two networks are considered to be anti-correlates of one another, and the mediation of this relationship has been attributed to the frontal parietal cortex (Gao and Lin, 2012; Uddin et al., 2008). What this may mean is that there is discrimination between how an individual takes in signals from the environment, and how an individual thinks about those signals in reference to self.
4.2. Effortful control and aggression
Negative emotions corresponding to sadness, fear, and anger both along normal and extreme continuums of these emotions can be measured based on specific attitudes and experiences. Anger is traditionally associated with hostile and cynical attitudes and can be measured in terms of behavior (aggression) or emotion/attitude (hostility and anger affect) (Gershon et al., 2013). Aggression, therefore, is not an emotion but a manifestation and behavior of anger. Aggression can be described using two distinctive patterns, reactive and proactive; reactive aggression is most commonly associated with response to stimuli causing anger or involving threat, while proactive aggression is seen more to be a learned trait resulting in personal gain in exchange for aggression (Vitaro and Brendgen, 2005).
If aggression is essentially the physical manifestation of anger, then the question arises: How is aggression regulated? Certainly anger can be experienced without outward physical aggression, so there must be a mechanism whereby aggression is controlled and exhibited. A strong candidate for this regulation is effortful control, which is the suppression of instinctive reactions to environmental stimuli (Gazzaley and D’Esposito, 2008; MacDonald, 2008). Effortful control recruits frontal cortex areas to constrain reactive, reflexive emotional responses to stimuli (such as an amygdala-triggered fear response to a strange noise) (Anders et al., 2004; Angrilli et al., 1996). Critical regions within frontal cortex that are associated with effortful control are prefrontal cortex and orbitofrontal cortex (Posner and Rothbart, 1998; Rothbart, 2005).
Two aspects of effortful control are useful for the current discussion: the suppression of impulsivity and the regulation of negative emotions. Davidson, Putman, and Larson (2000b) found that the orbitofrontal cortex and anterior cingulate cortex, through connection to the amygdala, are implicated in the ability to inhibit impulsivity. Essentially, activation results in inhibition of emotional behavior, and deficits in this connection may result in increased likelihood of impulsive aggression (Davidson et al., 2000a; Davidson et al., 2000b). Amygdala-orbitofrontal cortex coupling seems to be critically important in the suppression of impulsive aggression. In individuals with intermittent explosive disorder, there is weak amygdala-orbitofrontal cortex coupling in response to presented angry faces (Coccaro et al., 2007). Prefrontal cortex, particularly orbitofrontal cortex, exhibits connections to ventral anterior cingulate cortex and amygdala and plays a key role in the regulation of negative emotion (Banks et al., 2007; Bechara et al., 2000). Activity within ventral anterior cingulate cortex is associated with regulation of anger when imagining anger-evoking scripts, and is shown to be more active during tasks involving focused attention for individuals with increased social insight, or better control in social situations (Allman et al., 2001; Bush et al., 2000; Dougherty et al., 1999). Prefrontal cortex and amygdala are both recruited for up and down regulation of negative emotion, but orbitofrontal is recruited primarily in the down regulation of negative emotions (Ochsner et al., 2004); this finding has been supported using surface EEG and suppression tests (Davidson et al., 2000b; Jackson et al., 2000).
4.3. Default and attentional brain networks overconnectivity, aggression, and effortful control
Studies examining the default network have found reduction of activity during effortful control (Gusnard et al., 2001; Shulman et al., 1997). In fact, Knyazev et al. (2017) showed that as children develop, there is increased discrimination between the default network and networks closely associated with processing and acting on external signals (namely executive control and salience networks). They examined cohorts of school children at three different developmental stages using EEG at rest, in regions found in fMRI, and compared this to adults under the same conditions. It was found that for higher discrimination between default and attentional/executive networks, parents reported higher effortful control scores for the children (Knyazev et al., 2017). The current study found an increase in connectivity between the default and attentional networks corresponding to increased scores on aggressiveness self-report metrics. This finding is further supported by the lack of activity for orbitofrontal cortex seen in the principal components of interest (principal components 2 and 9). Our results may be consistent with weaker suppression of impulsivity and aberrant regulation of negative emotions. It is possible that the over-connectivity between the default and attention networks is representative of aberrant regulation of negative emotions while the lack of orbitofrontal cortex involvement in brain principal components relative to aggressiveness may be indicative of both the lack of regulation of negative emotions and of the lack of suppression of impulsivity.
The default network exhibits negatively correlated connections with brain attentional networks which may facilitate a division of cognitive resources between networks processing stimulus-independent cognition and internal narrative from those processing attention to external stimuli (Andrews-Hanna et al., 2014; Corbetta and Shulman, 2002; Fox et al., 2005; Mason et al., 2007). Coactivation of these two broad networks may represent either intrusive stimuli interrupting introspection or difficulty silencing internal narrative when directing attention to external stimuli. This may contribute to abnormalities in response inhibition (Miyake et al., 2000). This is consistent with our finding that coactivation of these two networks is associated with physical aggression via an inability to suppress impulsivity.
4.4. Limitations
While typicality at the principal component level provided a useful threshold for assessing general emotional health and well-being, it is a screening assessment and may miss important results. Here we observed that typicality of functional connectivity, or correlation of individual principal components to group mean principal components, was negatively associated with physical aggression, a measure of trait anger, indicating that individuals most like the group mean were less likely to exhibit physical aggression. This was the only meaningful result of typicality screening, but it is likely that other emotional metrics are associated with more specific, localized brain activation patterns, as opposed to large scale patterns shown in principal component analysis. Future work may assess the difference between broad range measurements such as principal component analysis and more acute measures of brain activation across the population. A second limitation of this work is that we did not have a direct measure of effortful control in these metrics. Measuring baseline aggressiveness is an indirect measure of lack of effortful control in this study, but future work could include standardized measures of effortful control (Rothbart, 2005). A third limitation of this work is the small effect size observed. There are over 1000 subjects in this study and the correlation values are relatively small.
4.5. Conclusions
Typicality of functional connectivity is negatively associated with physical aggression in principal components resembling key brain networks, primarily, default and attentional networks. When examining functional connectivity for high scores of anger-aggression, we found a positive correlation between these scores and coactivation of default and attentional networks. A relationship is hypothesized with aberrant regulation of negative emotion and impulsivity. Future work might consider whether similar findings are observed in pathological cohorts such as antisocial personality disorder or individuals with a history of violence.
5. References
- Adolphs R, Tranel D, Damasio H, Damasio AR, 1995. Fear and the human amygdala. J. Neurosci 15, 5879–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P, 2001. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann. N. Y. Acad. Sci 935, 107–117. [PubMed] [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N, 2004. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Hum Brain Mapp 23, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D, 2011. Connectivity Gradients Between the Default Mode and Attention Control Networks. Brain Connectivity 1, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Anderson JS, Palande S, Wang B, 2018. Topological data analysis of functional MRI connectivity in time and space domains. Lecture Notes in Computer Science 11083, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN, 2014. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrilli A, Mauri A, Palomba D, Flor H, Birbaumer N, Sartori G, di Paola F, 1996. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain 119 (Pt 6), 1991–2000. [DOI] [PubMed] [Google Scholar]
- Babakhanyan I, McKenna BS, Casaletto KB, Nowinski CJ, Heaton RK, 2018. National Institutes of Health Toolbox Emotion Battery for English- and Spanish-speaking adults: normative data and factor-based summary scores. Patient Relat. Outcome Meas 9, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL, 2007. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect Neurosci 2, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Gean PW, Lutz B, 2006. The role of the amygdala in the extinction of conditioned fear. Biol. Psychiatry 60, 322–328. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, 2000. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 10, 295–307. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM, 2005. Tensorial extensions of independent component analysis for multisubject FMRI analysis. NeuroImage 25, 294–311. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT, 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol 106, 2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci 4, 215–222. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ, 2001. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp 14, 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S, 2014. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci 26, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechko N, Kellermann T, Augustin M, Zvyagintsev M, Schneider F, Habel U, 2016. Disorder-specific characteristics of borderline personality disorder with co-occurring depression and its comparison with major depression: An fMRI study with emotional interference task. NeuroImage: Clinical 12, 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW, 2009. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U S A 106, 8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL, 2007. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol. Psychiatry 62, 168–178. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci 3, 201–215. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E, 2005. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage 25, 616–624. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF, 2006. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U S A 103, 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH, 2000a. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychological bulletin 126, 890–909. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL, 2000b. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science 289, 591–594. [DOI] [PubMed] [Google Scholar]
- Davis M, 1992. The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15, 353–375. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, Macklin ML, Fischman AJ, Rauch SL, 1999. Anger in healthy men: a PET study using script-driven imagery. Biol. Psychiatry 46, 466–472. [DOI] [PubMed] [Google Scholar]
- Dwyer DB, Harrison BJ, Yucel M, Whittle S, Zalesky A, Pantelis C, Allen NB, Fornito A, 2014. Large-scale brain network dynamics supporting adolescent cognitive control. J. Neurosci 34, 14096–14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MA, Anderson JS, Spreng RN, 2017. Fluid and flexible minds: Intelligence reflects synchrony in the brain’s intrinsic network architecture. Netw. Neurosci 1, 192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME, 2006. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U S A 103, 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME, 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U S A 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, 2005. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp 26, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullana MA, Albajes-Eizagirre A, Soriano-Mas C, Vervliet B, Cardoner N, Benet O, Radua J, Harrison BJ, 2018. Fear extinction in the human brain: A meta-analysis of fMRI studies in healthy participants. Neurosci. Biobehav. Rev 88, 16–25. [DOI] [PubMed] [Google Scholar]
- Gao W, Lin W, 2012. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum. Brain Mapp 33, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley AH, D’Esposito MT, 2008. Unifying prefrontal cortex function, in: Miller BL, Cummings JL (Eds.), The Human Frontal Lobes Guilford, New York, pp. 187–206. [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ, 2013. NIH toolbox for assessment of neurological and behavioral function. Neurology 80, S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, 2013. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, Lee J, 2015. Social cognition in schizophrenia. Nat. Rev. Neurosci 16, 620–631. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V, 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U S A 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V, 2004. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cogn. Neurosci 16, 1484–1492. [DOI] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM, 2014. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage 95, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE, 2001. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 25, 766–776. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE, 2010. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J. Neurosci. Methods 187, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME, 2001. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U S A 98, 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM, 2011. The subcallosal cingulate gyrus in the context of major depression. Biol. Psychiatry 69, 301–308. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT, 2010. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn. Reson. Imaging 28, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Kanen JW, Tambini A, Fernandez G, Davachi L, Phelps EA, 2017. Persistence of Amygdala-Hippocampal Connectivity and Multi-Voxel Correlation Structures During Awake Rest After Fear Learning Predicts Long-Term Expression of Fear. Cereb. Cortex 27, 3028–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ, 2000. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology 37, 515–522. [PubMed] [Google Scholar]
- Jiang R, Calhoun VD, Zuo N, Lin D, Li J, Fan L, Qi S, Sun H, Fu Z, Song M, Jiang T, Sui J, 2018. Connectome-based individualized prediction of temperament trait scores. NeuroImage 183, 366–374. [DOI] [PubMed] [Google Scholar]
- Keller JB, Hedden T, Thompson TW, Anteraper SA, Gabrieli JD, Whitfield-Gabrieli S, 2015. Resting-state anticorrelations between medial and lateral prefrontal cortex: association with working memory, aging, and individual differences. Cortex 64, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP, 2008. Competition between functional brain networks mediates behavioral variability. NeuroImage 39, 527–537. [DOI] [PubMed] [Google Scholar]
- Khanna MM, Badura-Brack AS, McDermott TJ, Embury CM, Wiesman AI, Shepherd A, Ryan TJ, Heinrichs-Graham E, Wilson TW, 2017. Veterans with post-traumatic stress disorder exhibit altered emotional processing and attentional control during an emotional Stroop task. Psychol. Med 47, 2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ, 2011. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural brain research 223, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev GG, Savostyanov AN, Bocharov AV, Slobodskaya HR, Bairova NB, Tamozhnikov SS, Stepanova VV, 2017. Effortful control and resting state networks: A longitudinal EEG study. Neuroscience 346, 365–381. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA, 1998. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20, 937–945. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF, 2012. The brain basis of emotion: a meta-analytic review. Behav. Brain. Sci 35, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KB, 2008. Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychol. Rev 115, 1012–1031. [DOI] [PubMed] [Google Scholar]
- Maclean PD, 1949. Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosomatic medicine 11, 338–353. [DOI] [PubMed] [Google Scholar]
- Maclean PD, 1952. Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalogr Clin. Neurophysiol 4, 407–418. [DOI] [PubMed] [Google Scholar]
- Marcus DS, Harwell J, Olsen T, Hodge M, Glasser MF, Prior F, Jenkinson M, Laumann T, Curtiss SW, Van Essen DC, 2011. Informatics and data mining tools and strategies for the human connectome project. Front. Neuroinform 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN, 2007. Wandering minds: the default network and stimulus-independent thought. Science 315, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga M, Kawamichi H, Koike T, Yoshihara K, Yoshida Y, Takahashi HK, Nakagawa E, Sadato N, 2016. Structural and functional associations of the rostral anterior cingulate cortex with subjective happiness. NeuroImage 134, 132–141. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD, 2000. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn. Psychol 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K, 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med 63, 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R, Paxinos G, Petrides M, 2000. Architectonic analysis of the human retrosplenial cortex. J. Comp. Neurol 421, 14–28. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD, 2003. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective & Behavioral Neuroscience 3, 207–233. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA, Schwartz J, DeWilde KE, Averill C, Jia-Wei Yang G, Wong E, Tang CY, Krystal JH, Iosifescu DV, Charney DS, 2016. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum. Brain Mapp 37, 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Bjorkquist OA, Olsen EK, Herbener ES, 2015. Schizophrenia symptom and functional correlates of anterior cingulate cortex activation to emotion stimuli: An fMRI investigation. Psychiatry Res 234, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Rabellino D, Densmore M, Frewen PA, Paret C, Kluetsch R, Schmahl C, Theberge J, Neufeld RW, McKinnon MC, Reiss J, Jetly R, Lanius RA, 2017. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp 38, 541–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ, 2004. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL, 2003. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol 460, 425–449. [DOI] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, Paulus MP, Brown GG, Frank GK, Campbell-Sills L, Yang TT, 2012. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. J. Affect. Disord 139, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrican R, Saverino C, Shayna Rosenbaum R, Grady C, 2015. Inter-individual differences in the experience of negative emotion predict variations in functional brain architecture. NeuroImage 123, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I, 2002. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage 16, 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE, 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience 106, 274–285. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, 1998. Attention, self-regulation and consciousness. Philos. Trans. R. Soc. Lond. B Biol. Sci 353, 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr CS, Vinette SA, Parsons KA, Cho IY, Dimond D, Benischek A, Lebel C, Dewey D, Bray S, 2016. Functional Connectivity of the Dorsal Attention Network Predicts Selective Attention in 4–7 year-old Girls. Cereb. Cortex 27,4350–4360. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, 2005. The development of effortful control, in: Mayr U, Awh E, Keele S (Eds.), Developing individuality in the human brain: A tribute to Michael Posner American Psychological Association, Washington D.C., pp. 167–188. [Google Scholar]
- Schacter DL, Addis DR, Buckner RL, 2007. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci 8, 657–661. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK, 2012. The future of memory: remembering, imagining, and the brain. Neuron 76, 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah L, Cramer JA, Ferguson MA, Birn RM, Anderson JS, 2016. Reliability and reproducibility of individual differences in functional connectivity acquired during task and resting state. Brain and Behavior 6, e00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE, 1997. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J. Cogn. Neurosci 9, 648–663. [DOI] [PubMed] [Google Scholar]
- Smith R, Allen JJ, Thayer JF, Lane RD, 2015. Altered functional connectivity between medial prefrontal cortex and the inferior brainstem in major depression during appraisal of subjective emotional responses: A preliminary study. Biological psychology 108, 13–24. [DOI] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MP, Kelly M, Laumann T, Miller KL, Moeller S, Petersen S, Power J, Salimi-Khorshidi G, Snyder AZ, Vu AT, Woolrich MW, Xu J, Yacoub E, Ugurbil K, Van Essen DC, Glasser MF, 2013. Resting-state fMRI in the Human Connectome Project. NeuroImage 80, 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS, 2009. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci 21, 489–510. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL, 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 53, 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Sakai Y, Abe Y, Nishida S, Harrison BJ, Martinez-Zalacain I, Soriano-Mas C, Narumoto J, Tanaka SC, 2018. A common brain network among state, trait, and pathological anxiety from whole-brain functional connectivity. NeuroImage 172, 506–516. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Clare Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP, 2008. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum. Brain Mapp 30,625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, Consortium WU-MH, 2013. The WU-Minn Human Connectome Project: an overview. NeuroImage 80, 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, Della Penna S, Feinberg D, Glasser MF, Harel N, Heath AC, Larson-Prior L, Marcus D, Michalareas G, Moeller S, Oostenveld R, Petersen SE, Prior F, Schlaggar BL, Smith SM, Snyder AZ, Xu J, Yacoub E, 2012. The Human Connectome Project: a data acquisition perspective. NeuroImage 62, 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaro F, Brendgen M, 2005. Proactive and Reactive Aggression, in: Tremblay RE, Hartup WW, Archer J (Eds.), Developmental Origins of Aggression The Guilford Press, New York, pp. 178–201. [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR, 1995. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J. Comp. Neurol 359, 490–506. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E, 2006. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J. Neurosci 26, 9264–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PG, Johnson KT, Curtis BJ, King JB, Anderson JS, 2018. Individual differences in aesthetic engagement are reflected in resting-state fMRI connectivity: Implications for stress resilience. NeuroImage 179, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]