Abstract
Thrombospondin 1 (TSP-1) is an extracellular matrix protein that interacts with a wide array of ligands including cell receptors, growth factors, cytokines and proteases to regulate various physiological and pathological processes. Constitutively expressed by certain ocular surface tissues (e.g. corneal and conjunctival epithelium), TSP-1 expression is modulated during ocular surface inflammation. TSP-1 is an important activator of latent TGF-β, serving to promote the immunomodulatory and wound healing functions of TGF-β. Mounting research has deepened our understanding of how TSP-1 expression (and lack thereof) contributes to ocular surface homeostasis and disease. Here, we review current knowledge of the function of TSP-1 in dry eye disease, ocular allergy, angiogenesis/lymphangiogenesis, corneal transplantation, corneal wound healing and infectious keratitis.
I. Introduction
The extracellular matrix is an intricate three-dimensional network of proteins that plays a critical role in the determination, differentiation, proliferation, polarity, migration and survival of cells [1]. Thrombospondins are a family of extracellular matrix proteins consisting of five members (TSP-1 to TSP-5) that interact with a variety of cell surface receptors, growth factors, cytokines and proteases to influence numerous in vivo phenomena including wound healing, angiogenesis and inflammation [2].
TSP-1 is a 450 kDa glycoprotein that was first identified as being released by platelets following thrombin treatment [3]. TSP-1 is expressed by an array of tissues including the cornea, lens, retinal pigment epithelium (RPE), activated endothelium, healing skin wounds and the spinal cord [2]. The contribution of TSP-1 to ocular immune, angiogenic and lymphangiogenic privilege has been an area of active investigation for the past 25 years [4]. This article reviews the progress that has been made in elucidating the contributions of this matricellular protein to ocular surface homeostasis and disease. The scope of this review is limited to the functions of TSP-1, and will not discuss the other thrombospondins TSP-2, −3, −4 and −5. While the majority of published reports have investigated the role of TSP-1, there is evidence that TSP-2 also influences numerous cellular and molecular phenomena in the eye [5–8]. First, an overview of the functions of TSP-1 is provided, as well as a description of TSP-1 expression by ocular tissues. We then address the role of TSP-1 in a variety of ophthalmic conditions; including dry eye disease (DED), ocular allergy, angiogenesis/lymphangiogenesis, wound healing, corneal transplantation and infectious keratitis.
II. Structure of TSP-1
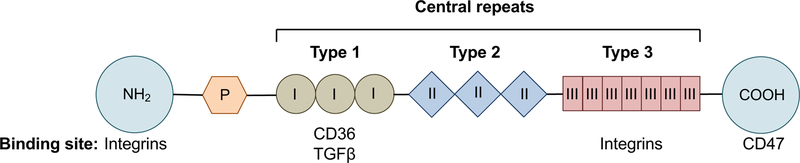
TSP-1 has a homotrimeric structure, with each monomer comprised of N- and C-terminal globular domains flanking a thin connecting strand consisting of a procollagen homology domain and three central repeats (Fig. 1) [9]. The N-terminal binds integrins that play an important role in chemotaxis and adhesion [10]. The procollagen homology domain promotes assembly of the trimer [11]. The three central repeats are defined as Type 1 (also known as thrombospondin structural homology repeats [TSRs]), Type 2 and Type 3 [12]. Type 1 repeats bind to CD36 and TGFβ, and Type 3 repeats interact with various integrins [2,13]. TSP-1 signaling through integrin-associated protein (CD47) occurs via the carboxy-terminal [14]. It is via these interactions that TSP-1 mediates its various immunoregulatory, anti-angiogenic and wound healing functions.
Figure 1. Structure of TSP-1.
TSP-1 is a 450 kDa homotrimeric molecule. Each monomer consists of a procollagen homology domain (P) and Type I, II, and III repeats flanked by globular amino (NH2) and carboxyl (COOH) terminals. CD36 and TGFβ bind to the Type 1 repeats. The binding sites for integrins are the N-terminal and the Type 3 repeats. CD47 binds the C-terminal domain.
III. Function of TSP-1
TSP-1 modulates cellular functions by interacting with multiple cellular receptors, each of which is expressed at various levels by different cell types [15]. TSP-1 is known to interact with integrins (including α3β1, αvβ3, and αIIbβ3), CD47, cellular glycosaminoglycans, CD36 and LDL receptor-related protein. Over recent years, much progress has been made in delineating key mechanisms by which TSP-1 contributes toward immune regulation, inhibits angiogenesis and promotes wound healing.
A. Immune regulation
The importance of the immunoregulatory role of TSP-1 has been highlighted by studies of TSP-1-null mice, which develop spontaneous pneumonia by 4 weeks of age with pulmonary lesions characterized by large numbers of neutrophils and macrophages [16]. TSP-1 is recognized as a major activator of the cytokine transforming growth factor-β 1 (TGF-β1) in vivo [17]. TGF-β1 is a multifunctional cytokine that modulates the immune response via numerous mechanisms (as shown in Fig. 2), in addition to regulating cellular proliferation, wound healing and extracellular matrix synthesis [18–20]. The histological abnormalities observed in multiple organ systems are very similar but relatively less intense in TSP-1-null mice as compared to TGF-β1-null mice, and treatment of TSP-1-null mice with a peptide derived from TSP-1 has been shown to increase levels of activated TGF-β1 and restore the phenotype to wild type (WT) [17]. The survival of TSP-1-null mice and their relatively lower intensity of overall inflammation as compared to that seen in TGF-β1-null mice supports the presence of TSP-1-independent mechanisms of TGF-β1 activation. Interestingly, while TSP-1 has been shown to be the primary activator of TGF-β1 in renal and lung fibrosis [21,22], there is evidence that TGF-β1 is not the major activator of TSP-1 in other compartments [23,24], leading to speculation that TGF-β1 may be regulated in a tissue-specific manner.
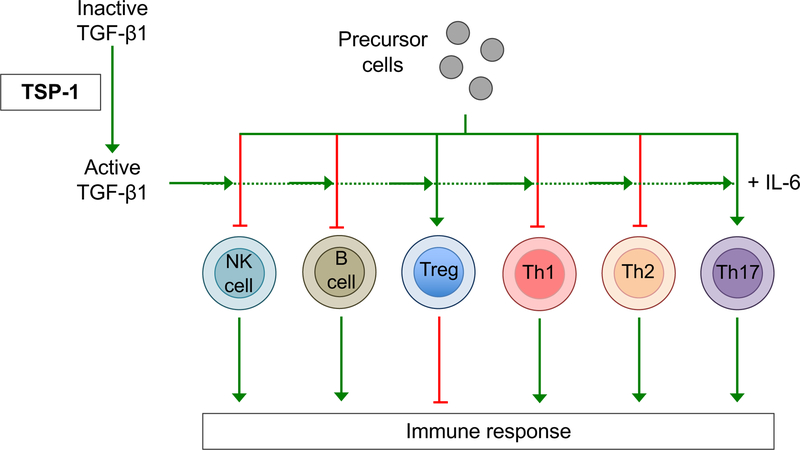
Figure 2. TSP-1 regulates TGF-β1-mediated modulation of the immune response.
TSP-1 promotes the activation of TGF-β1, which is required for the development of Foxp3+ Tregs, which suppress the pro-inflammatory response. TGFβ also limits the activity of NK cells, B cells, Th1 and Th2 cells thereby suppressing the immune response. However, in combination with IL-6, TGF-β1 supports the development of Th17 cells; pro-inflammatory cells that have been shown to play a critical role in the pathogenesis of various autoimmune inflammatory diseases.
TGF-β1 exists in its inactive form as a complex, with Latency Associated Peptide (LAP) and Latent TGF-β-Binding Protein (LTBP) [25]. In vivo, the mechanisms by which TGF-β1 is liberated from LAP and LTBP are not fully defined, but have been proposed to include activation via the integrin αvβ6 on epithelial cells, proteolytic activation by transglutaminase, as well as conformational change of LAP via physical interaction with TSP-1 [17,26–28]. Following activation, TGF-β1 promotes the generation of regulatory T cells (Tregs) by inducing Foxp3 expression [29]. Immunosuppressive cytokines such as TGF-β1 and Tregs are known to play a critical role in maintaining peripheral tolerance, preventing the initiation of deleterious immune responses to self or innocuous antigens, such as those in commensal bacteria [25]. In addition, and via a range of discrete mechanisms, TGF-β1 inhibits the pro-inflammatory immune response by limiting the clonal expansion and the functions of T helper 1 (Th1) and T helper 2 (Th2) effector cells, as well as B lymphocytes and natural killer cells [29,30]. By promoting the activation of TGF-β1, TSP-1 upregulates these various immunosuppressive mechanisms (as shown in Fig. 2) [31].
Interestingly, although predominantly understood as an immunoregulatory cytokine, TGF-β1 has additionally been shown to promote inflammation under certain conditions [29]. For example, TGF-β1 (in combination with IL-6) promotes the differentiation of IL-17-producing T-helper 17 (Th17) cells in an inflammatory microenvironment [32]. Th17 cells are known to play an important role in the pathogenesis of many autoimmune diseases; including rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, psoriasis and asthma [33]. In addition to regulating the differentiation and activity of lymphocytes, TGF-β1 controls the development and function of macrophages, dendritic cells and granulocytes [30]. Thus, solely through its activation of TGF-β1, TSP-1 has the potential to influence both pro-inflammatory and immunoregulatory responses. Notably, TSP-1 has also been reported to have immunosuppressive activity via other TGF-β isoforms. For example, TSP-1 has been demonstrated to activate the TGF-β2 isoform expressed by ocular antigen presenting cells (APCs), thereby promoting the induction of Tregs [34]. TSP-1 also has immunomodulatory effects that are independent of TGF-β. Indeed, TSP-1 has been shown to promote the extrathymic generation of Tregs directly via ligation of CD47, to suppress dendritic cell activation and to induce apoptosis of T cell effectors [35–37].
B. Angiogenesis
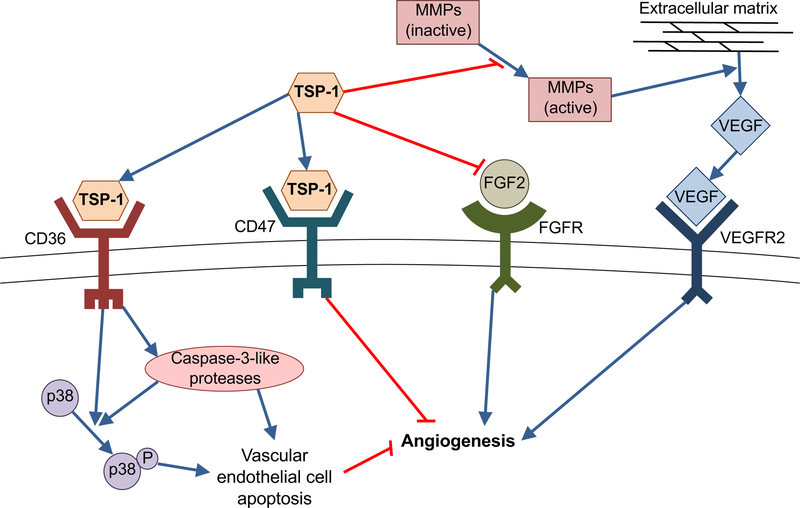
TSP-1 inhibits the growth of new blood vessels in a number of ways; including VEGF antagonism, induction of vascular endothelial cell apoptosis, and modulation of endothelial cell proliferation and migration [38]. Notably, TSP-1 was the first endogenous protein inhibitor of neovascularization to be recognized, having been identified by Good and colleagues in experiments employing a rat model of corneal angiogenesis [39]. TSP-1 antagonizes vascular endothelial growth factor (VEGF) function by multiple mechanisms. Firstly, TSP-1 limits the release of VEGF from the extracellular matrix, by inhibiting the activity of matrix metalloproteinases [40,41]. TSP-1 has also been demonstrated to directly bind VEGF, thereby promoting its clearance [42,43]. In addition, there is evidence that TSP-1 interrupts VEGF signal transduction, with inhibition of VEGF receptor 2 phosphorylation and reduced activation of the Akt pathway [44,45]. TSP-1 is also known to bind and sequester the angiogenic factor fibroblast growth factor-2, thereby suppressing its bioavailability and activity [46].
TSP-1 has been shown to limit neovascularization by inducing receptor-mediated apoptosis in vascular endothelial cells, a phenomenon that was shown to be dependent on the activation of CD36, caspase-3-like proteases and p38 mitogen-activated protein kinases [13,47]. TSP-1 has also been reported to inhibit the growth of new blood vessels in a CD36-independent manner by suppressing endothelial cell cycle progression through the Akt/MAPK pathway [48]. Another reported mechanism of TSP-1 antiangiogenic activity is via ligation of CD47; thereby acting to inhibit nitric oxide-stimulated vascular cell responses, including cell migration and proliferation [49]. Interaction of TSP-1 with CD47 has been shown to inhibit VEGFR2 phosphorylation, thereby impeding VEFR2 signaling [50]. Through this diverse array of mechanisms (detailed in Fig. 3), TSP-1 functions as a potent inhibitor of angiogenesis.
Figure 3. TSP-1 anti-angiogenic mechanisms.
TSP-1 binds CD36 to activate caspase-3 like proteases and p38 mitogen-activated protein kinases, resulting in vascular endothelial cell apoptosis. TSP-1 ligates CD47 to inhibit vascular endothelial cell migration and proliferation, via suppression of nitric oxide-stimulated vascular cell responses. TSP-1 binds and sequesters the pro-angiogenic molecular fibroblast growth factor-2 (FGF2). By suppressing the activation of matrix metalloproteinases (MMPs), TSP-1 inhibits the release of vascular endothelial growth factor (VEGF) from the extracellular matrix.
C. Wound healing
As noted above, TSP-1 upregulates TGF-β1 activity, which in addition to its immunoregulatory activity plays a critical role in wound repair and tissue regeneration [19]. The granulation tissue in TSP-1-null mice following wounding has been reported to be disorganized and less dense relative to wild-type control animals [51]. TSP-1-null mice exhibit delayed healing with persistent inflammation and late scab loss [51]. Further evidence for the importance of TSP-1 in wound healing is provided by the observation that TSP-1 induces fibroblast migration to a comparable degree to TGF-β1; indeed, in vivo implantation of collagen sponges soaked with TSP-1 into incisions in Wistar rats has been shown to result in levels of fibroblast infiltration comparable to sponges soaked in TGF-β1 [52]. Moreover, in vitro studies of wound healing using a collagen gel contraction model have shown that contraction is promoted by addition of TSP-1 (with a concomitant increase in TGF-β1) and inhibited by addition of anti-human TSP-1 antibody [52]. TSP-1 is also known to promote fibroblast development by stabilizing platelet-derived growth factor [53]. The contribution of TSP-1 to optimum wound healing is corroborated by studies employing antisense TSP-1 oligomers to treat wounds, in which treatment with antisense TSP-1 oligomers is observed to decrease macrophage expression of TSP-1 and delay both re-epithelialization and dermal reorganization [54].
In contrast with the studies described above, Streit and colleagues employed a transgenic mouse model with targeted overexpression of TSP-1 in the skin to demonstrate a marked delay in the healing of full-thickness skin wounds [55]. The investigators’ observed impaired fibroblast migration in vivo in the transgenic mice, an observation that was substantiated by in vitro data showing a TSP-1 dose-dependent decrease in dermal fibroblast migration toward collagen type I and toward fibronectin [55]. When contrasting these findings with the previously discussed reports of TSP-1 promoting fibrosis, it is important to note that the levels and duration of TSP-1 expression in the transgenic mice employed in this study exceed levels observed in wild-type mice following injury [56]. Thus, it is possible that the effect of TSP-1 in modulating fibrosis may be contingent upon its expression levels.
IV. Distribution of TSP-1 in ocular surface tissues
In quiet non-inflamed eyes, TSP-1 is constitutively expressed by the epithelium, Bowman’s layer, the posterior Descemet’s membrane and endothelium but is not detectable in the stroma (Table 1) [57–59]. In an investigation of the distribution of TSP-1 in human ocular surface epithelium, Sekiyama and colleagues demonstrated that TSP-1 was expressed by the corneal and limbal epithelia, but not by the conjunctival epithelium [57]. Furthermore, the investigators observed that TSP-1 transcripts were synthesized predominantly by basal corneal and limbal epithelial cells, and that the TSP-1 protein was present in, and just inferior to, these cells [57]. These observations were consistent with the earlier work of Hiscott and colleagues, who using light- and electron-microscopic immunohistochemical techniques observed TSP-1 in the corneal epithelial basement membrane as well as the cytoplasm of basal corneal epithelial cells [58].
Table 1.
Constitutive expression of TSP-1 by non-inflamed ocular surface tissues.
| Location | Reports of TSP-1 expression |
| Corneal epithelium | Sekiyama et al. [57], Hiscott et al. [58], Matsuba [59] |
| Bowman’s layer | Sekiyama et al. [57] |
| Descemet’s membrane | Hiscott et al. [58] |
| Corneal endothelium | Hiscott et al. [58], Matsuba [59], Uno et al. [60], Blanco-Mezquita [61] |
| Limbal epithelium | Sekiyama et al. [57] |
The expression of TSP-1 is regulated by ocular surface inflammation. For example, following corneal injury, TSP-1 expression is observed in the stroma (where it is usually undetectable) underlying the wounded epithelium at 24 hours post-injury, and reaches peak expression after approximately one week [59]. In experimental DED, exposure to a desiccating environment increases corneal epithelial expression of TSP-1 [62]. Interestingly, Soriano- Romani and colleagues have reported that the expression of TSP-1 by conjunctival fibroblasts is decreased after in vitro stimulation with IL-1β in serum-free culture conditions, although this effect was not observed in serum-supplemented cultures [63]. Further work is required to shed light on precisely how TSP-1 expression by different ocular surface tissues is modulated during inflammation.
V. Dry eye disease
DED is a multifactorial inflammatory disorder of the ocular surface affecting millions of individuals [64–66]. DED is characterized by tear film instability and hyperosmolarity, ocular surface inflammation and neurosensory abnormalities that result in symptoms of discomfort and visual disturbance [67]. While the pathogenesis of DED has not been fully deciphered, there is evidence that immune-mediated inflammation contributes to both disease initiation and exacerbation [68,69]. Indeed, it has previously been shown that adoptive transfer of CD4+ T cells derived from mice with experimental DED (after depletion of CD25+ T cells) to healthy nude mice results in recipient mice developing corneal epitheliopathy with inflammation of ocular surface and lacrimal gland tissues [70]. The current paradigm for understanding DED immunopathogenesis is that desiccating stress causes tear hyperosmolarity, which in turn promotes the activation of intracellular signaling pathways that trigger the production of proinflammatory cytokines (e.g. tumor necrosis factor α [TNFα], interleukin 1 [IL-1]) [68]. This inflammatory milieu induces APC maturation [68]. Mature APCs migrate via afferent lymphatics across chemokine gradients to the draining lymph nodes, where they promote the differentiation of helper T cell 1 (Th1) and Th17 cells [68,71]. These effector T cells acquire chemokine receptors (e.g. CCR5 and CXCR3) that promote their migration via efferent blood vessels to ocular surface tissues [72], where they secrete proinflammatory cytokines including interferon γ (IFNγ) and IL-17, as well as matrix metalloproteinases, cell adhesion molecules and prolymphangiogenic factors [68]. These pro-inflammatory mechanisms are constrained by immunoregulatory cellular and molecular mediators, most notably CD4+CD25+Foxp3+ regulatory T cells (Tregs). Tregs suppress effector T cells by cytolysis, by the release of immunoregulatory cytokines (e.g. IL-10, IL-35 and TGF-β), by competing for IL-2 and by modulating dendritic cell function [73]. Despite their opposing functional properties, Th17 cells and Tregs share a similar developmental pathway, with IL-6 inducing polarization toward a Th17 (rather than Treg) phenotype [74]. Moreover, both Th17 cells and Tregs exhibit a high grade of plasticity, and undergo functional adaptations according to signals from their microenvironment [33].
A. DED studies utilizing TSP-1-null mice
The contribution of TSP-1 to the generation of ocular surface Th17 immunity in experimental DED has been investigated using TSP-1-null mice [75]. To induce DED, Gandhi and colleagues exposed TSP-1-null mice and WT mice to desiccating stress, in combination with regular subcutaneous injections of scopolamine hydrobromide. The investigators observed decreased corneal epitheliopathy and reduced CD4+ T cell infiltration in the conjunctiva of TSP-1-null mice relative to WT [75]. In both the cornea and conjunctiva, and in contrast to WT, TSP-1-null mice did not demonstrate upregulated expression of IL-17A in response to desiccation. Furthermore, adoptive transfer of CD4+ T cells from TSP-1-null mice after exposure to desiccating stress into RAG-1-null mice (deficient in mature T and B cells) resulted in milder ocular surface disease relative to adoptive transfer of CD4+ T cells sourced from WT control [75]. These data suggest that TSP-1 is critical for the generation of pathogenic CD4+ T cells in DED. As discussed previously, TSP-1 is a major activator of TGF-β, a cytokine that is crucial in the developmental pathway of both Th17 and Treg cells. It is interesting to note that, using WT mice with local neutralization of TGF-β, Gandhi and colleagues were able to mimic the TSP-1-null DED phenotype, suggesting that the observed phenomena in TSP-1-null mice may largely be due to decreased TGF-β activation. The investigators did not report Treg frequencies and function in TSP-1-null mice, but other studies have demonstrated that TSP-1 is also essential for Treg induction in ocular tissues [76,77].
In contrast to the pro-inflammatory role of TSP-1 described by Gandhi and colleagues, TSP-1 has been reported in multiple other studies of experimental DED to have an immunoregulatory function. Turpie and colleagues have reported that TSP-1-null mice spontaneously develop chronic ocular surface disease, characterized by lacrimal gland apoptosis and concomitant dysfunction, CD4+ T cell infiltration of the lacrimal gland, corneal epitheliopathy, corneal edema and reduced conjunctival goblet cell frequencies [77]. The investigators also describe increased Th17 frequencies in the lacrimal gland and periphery of TSP-1-null mice, and decreased Tregs in the periphery [77]. Real-time PCR on RNA isolated from lacrimal gland tissue demonstrated higher IL-6 expression in TSP-1-null mice relative to WT, which may favor development of Th17 cells in TSP-1-deficient glands [77]. Increased expression of the Th17-associated cytokines IL-6 and IL-17A have also been reported in the conjunctiva and the draining lymph nodes of TSP-1-null mice, in addition to increased expression of the Th1-associated cytokines IFN-γ and TNF-α [78]. Moreover, the transcription factors related to these pro-inflammatory cell types, RORγt and Tbet respectively, have been reported to be upregulated in TSP-1-null mice in the conjunctiva and lymph nodes [78]. In contrast, Foxp3 expression was significantly decreased in the lymph nodes of TSP-1-null mice [78]. Thus there are some apparent contradictions in the published reports on the role of TSP-1 in DED; on the one hand TSP-1-null animals have been reported to be resistant to developing DED in response to desiccating stress with decreased generation of pathogenic CD4+ T cells [75], yet on the other TSP-1-null animals have been reported to develop a form of DED spontaneously with enhanced Th1 and Th17 cell immunity [77,78]. In attempting to reconcile these reports, it is important to note that Gandhi and colleagues used Oregon Green Dextran 488 (70,000 molecular weight) to assess corneal epitheliopathy, in comparison with the sodium fluorescein (376 molecular weight) employed by Turpie and colleagues [75,77,78]. It may be that the >180 fold difference in the molecular weight of the fluorescent dye used resulted in different test sensitivity for the detection of ocular surface damage. Furthermore, it is interesting to note that in Gandhi and colleagues’ report, prior to desiccating stress TSP-1-null mice were observed to have approximately 3-fold higher frequencies of CD4+ T cells in conjunctival epithelium relative to naïve, consistent with the reports of spontaneous ocular surface disease in these animals [75,77,78]. The relatively high frequencies of CD4+ T cells observed in the conjunctival epithelium of TSP-1-null mice may contribute to a ceiling effect, whereby further increases in CD4+ T cells after exposure to desiccating stress are not observed due to pre- existing high numbers [75].
B. Contribution of neuronal abnormalities to DED in TSP-1-null mice
Recent studies have investigated whether neuronal abnormalities in the lacrimal glands and ocular surface tissues of TSP-1-null mice may contribute to the spontaneous disruption of ocular surface homeostasis observed in these animals [79,80]. Under physiological conditions, afferent sensory nerves detect noxious stimuli at the ocular surface, and act via a neural reflex arc to trigger the release of neurotransmitters from efferent nerves [81]. Bhattacharya and colleagues report that, compared to WT mice, TSP-1-null mice exhibit decreased neural innervation and increased vascularity of the lacrimal glands [79]. The authors used a variety of evaluations of lacrimal gland function (high KCl-evoked secretion, α1D-adrenergic agonist-stimulated secretion and ATP and MeSATP-stimulated peak [Ca2+] responses) to demonstrate impaired glandular function in TSP-1-null mice [79]. Notably, these observations were made before spontaneous mononuclear infiltrates in these animals were apparent (i.e. at 24 weeks of age [77]). Disruption of cellular signaling pathways in TSP-1-null mice may interfere with the secretory function of the lacrimal gland, thereby disturbing ocular surface homeostasis and triggering the development of dry eye disease [79].
Using the same animal model, Tatematsu and colleagues have evaluated spontaneous structural and functional changes to the corneal nerves in young (4 weeks of age) and older (12 weeks of age) TSP-1-null vs. age-matched WT mice [80]. In vivo confocal microscopy studies demonstrated decreased corneal nerve density in older TSP-1-null compared to older WT mice, with no difference between the young groups. However, when nerve density was assessed using in vitro immunofluorescence microscopy with staining for anti-Class β-tubulin antibody, decreased nerve density was detected in both young and older TSP-1-null mice compared to age-matched controls [80]. The investigators conducted double-labeling immunofluorescence studies using anti-calcitonin gene-related peptide (CGRP) and anti-Substance P antibodies with anti-Class III β-tubulin antibody, and demonstrated that CGRP-containing corneal nerves, but not Substance P-containing nerves, were decreased with age in TSP-1-null mice [80]. This observation correlates TSP-1 deficiency, and the spontaneous ocular surface disease observed in TSP-1-null mice, with an age-related decrease in CGRP-containing corneal nerves. In doing so, these data suggest that a deficiency of CGRP-positive corneal nerves may be implicated in the pathogenesis of the spontaneous corneal epitheliopathy observed in TSP-1-null mice, and furthermore that TSP-1 may contribute toward either the development or repair of CGRP-positive corneal nerves. Interestingly, the differential regulation of CGRP- and Substance P-containing corneal nerves is consistent with reported data for tear neuropeptide levels following LASIK, in which tear CGRP concentrations (but not Substance P) were found to be elevated relative to healthy control participants [82]. However, more research is required to determine the mechanisms by which TSP-1 modulates corneal nerve growth, repair and function, and in turn how this may influence the neural reflex arc in the setting of DED.
C. DED studies employing topical treatment to block/augment TSP-1
Further evidence for the immunoregulatory role of thrombospondin-1 in dry eye disease is provided by Tan and colleagues, who demonstrate that topical application of recombinant TSP-1 eye drops to WT C57BL/6 mice reduces DC maturation, decreases local expression of inflammatory cytokines (including IL1β, IL-6, IL-23 and IL-17A), inhibits generation of Th17 cells in the draining lymph nodes and ameliorates DED severity relative to human serum albumin-treated controls [62]. This study suggests that topical application of recombinant TSP-1 may be a viable therapeutic approach to reducing DED immunopathology. In agreement with conjunctival data from a previous report [63], the investigators show that expression of TSP-1 by the corneal epithelium is increased in response to desiccating stress. Furthermore, the investigators demonstrate that corneal epithelial cells derived from mice exposed to desiccating stress have a greater capacity to suppress DC maturation relative to corneal epithelial cells from WT mice, an effect which is enhanced by recombinant TSP-1 and abrogated by TSP-1 blockade [62]. These data provide evidence for the role of corneal epithelium-derived TSP-1 in regulating the immune response to desiccating stress. It is interesting to note that the spontaneous ocular surface disease observed in TSP-1-null mice has been shown to be ameliorated by topical treatment with a TSP-derived CD47-binding peptide (4N1K) [83] as well as by a TGFβ-activating peptide (KRFK) [84]. Similar to the study by Tan and colleagues [62], the authors report that treatment with 4N1K or KRFK inhibits peripheral pathogenic Th17 generation and decreases corneal epitheliopathy [83,84]. Moreover, the investigators show enhanced peripheral Treg frequencies and Foxp3 expression [83,84].
Other work has demonstrated the critical role played by TSP-1 expressed by conjunctival epithelial cells in converting their endogenously expressed latent TGFβ2 to its active form, via binding to cell surface receptor CD36, thereby supporting an immature phenotype of dendritic cells [85]. Reduced CD36 immunostaining in combination with increased detection of TSP-1 in the conjunctiva of mice exposed to desiccating stress further suggests the lack of biologically active TGFβ in peripheral tissue, thereby contributing to the disruption of mucosal homeostasis, similar to that observed in TSP-1-null mice [63]. Together these studies provide strong evidence for the role of TSP-1 in modulating the balance between pro-inflammatory Th17 cells and immunosuppressive Tregs, the dysregulation of which is a core feature of DED immunopathogenesis [62,68,69,83].
D. TSP-1 single-nucleotide polymorphisms in DED
Work by Contreras-Ruiz and colleagues in human patients with post-refractive surgery chronic keratoconjunctivitis also support the role of TSP-1 in modulating ocular surface immune homeostasis [86]. In a study of active US Army soldiers, the investigators demonstrated that patients with single-nucleotide polymorphisms (SNPs) in the encoding gene for TSP-1 (resulting in reduced TSP-1 expression at the ocular surface) exhibit higher susceptibility to chronic keratoconjunctivitis following refractive surgery [86].
VI. Ocular allergy
The immunoregulatory role of TSP-1 at the ocular surface is further supported by investigations employing a model of allergic eye disease [87]. Indeed, Smith and colleagues have demonstrated that a deficiency in DC expression of TSP-1 results in amplified secondary T cell responses and increased clinical disease [87]. In this model, mice are immunized with OVA and adjuvant, and then 14 days later are challenged with topical ocular OVA administration once/day for 7 days. Notably, the investigators used 8-week old mice TSP-1-null mice in their study, prior to the development of spontaneous ocular surface disease reported in these animals at 12-weeks of age [77,83]. Thus, Smith and colleagues conclude that their observations are independent of spontaneous autoimmune phenomena. It is interesting that only marginal differences in clinical allergic eye disease were observed in OVA-immunized TSP-1 null mice compared to WT controls, and that much larger differences were observed when an adoptive transfer model of allergy induction was employed, in which T cells from OVA-sensitized WT mice are adoptively transferred into naive WT or TSP-1-null recipient mice [87]. By utilizing this model, the authors diminish the potential confounding effects of global TSP-1 deficiency on other immune cells. Similar to the experimental DED study referenced previously [83], the authors show that treatment with the CD47-binding TSP-1-derived peptide 4N1K ameliorates disease severity [87]. The findings of this study corroborate the importance of CD47-mediated negative regulation of DC and T cell functions by TSP-1 [88].
VII. Angiogenesis/lymphangiogenesis
Corneal transparency is required for optimal vision. Thus, the normal cornea is free of both blood and lymphatic vessels [89]. However, inflammatory conditions at the ocular surface (such as infectious keratitis [90]) promote the ingrowth of both blood and lymphatic vessels, impairing the optical properties of the cornea. There are a host of anatomical, cellular and molecular factors that work in concert to inhibit angiogenesis and preserve corneal clarity [91]. TSP-1 is one of these potent endogenous antiangiogenic factors.
Cursiefen and colleagues have provided evidence for the inhibition of corneal angiogenesis by endogenous TSP-1 [7]. Using a model of suture-induced corneal angiogenesis, the investigators report that TSP-1-null mice exhibit significantly greater corneal angiogenesis at 7 days post-suturing than WT control mice. Interestingly, while TSP-1-null mice did not develop spontaneous corneal angiogenesis, they developed significantly increased iris vessel density compared to WT controls [7]. Furthermore, although young TSP-1-null mice do not develop spontaneous corneal neovascularization, spontaneous corneal lymphangiogenesis has been reported in older TSP-1-null mice [92]. In this second paper by Cursiefen and colleagues, increased lymphangiogenesis was observed in TSP-1-null mice at 6 months of age relative to WT control mice. Moreover, corneal suture-induced inflammation resulted in significantly greater lymphangiogenesis in 8-week old TSP-1-null mice relative to age-matched WT control animals, an observation that was abrogated by topical treatment with recombinant human TSP-1 [92]. The investigators demonstrate that TSP-1 binds CD36 on monocytic cells to regulate the expression of vascular endothelial growth factor C (VEGF-C), thereby modulating lymphangiogenesis [92]. Note that VEGF-C is known to induce lymphangiogenesis by binding to its high-affinity receptor VEGFR3 on lymphatic endothelial cells [93]. Interestingly, treatment with TSP-1 fails to suppress basic fibroblast growth factor-induced corneal neovascularization in mice deficient in CD36 [94], offering further evidence for the importance of the TSP-1/CD36 axis in regulating the development of blood and lymphatic vessels. Although not demonstrated at the cornea, there are other critical signaling pathways by which TSP-1 exerts its antiangiogenic function [38]. For example, by binding CD47, TSP-1 blocks the interaction between CD47 and VEGFR2, thereby inhibiting VEGFR2 phosphorylation and its downstream signaling [95].
VIII. Corneal transplantation
Corneal allografts are the most common type of solid tissue transplantation, with over 45,000 performed in the US each year [96,97]. Grafts placed in uninflamed and nonvascularized host beds (‘low risk’ transplantation) enjoy high rates of graft survival (>90%) without the need for systemic long-term immunosuppression or human leukocyte antigen tissue matching [96,98]. However, when the relatively immune quiescent environment of the cornea is disrupted by inflammation and neovascularization (‘high risk’ transplantation) then rejection rates exceed 50% despite maximal immunosuppression [96,99].
Corneal inflammation and neovascularization are known to upregulate both the afferent and efferent arms of the alloimmune response. A pro-inflammatory microenvironment promotes maturation of both donor-derived APCs from the graft (direct pathway of allosensitization) and host-derived APCs from the peripheral cornea (indirect pathway) [98]. Interestingly, previously it was thought that the indirect pathway was the exclusive mechanism by which graft antigens were recognized by the host immune system [100], but subsequent work revealed the important contribution of donor-derived APCs [101,102]. APCs egress the cornea via lymphatic vessels to present alloantigens to naïve T cells, resulting in clonal expansion and differentiation into IFN-γ–secreting CD4+ Th1 cells [98]. Corneal neovascularization supports the migration of alloreactive Th1 cells across a chemokine gradient through blood vessels to the graft, where they induce tissue opacification and graft rejection. Given the capacity of TSP-1 to modulate both APC function and angiogenesis, studies have been conducted that employ a murine model of corneal transplantation to investigate the mechanisms by which TSP-1 influences graft outcome.
Increased transplant rejection has been reported in BALB/c recipients of grafts sourced from C57BL/6 TSP-1-null donors, relative to WT C57BL/6 donors [103]. These data imply that graft expression of TSP-1 is a critical determinant of transplant survival. In their report, Saban and colleagues demonstrate that direct allosensitization (i.e. resulting from donor-derived APCs) is inhibited by graft-derived TSP-1, but not indirect allosensitization [103]. Moreover, the investigators show that APC-derived TSP-1 regulates the capacity of APCs to allosensitize T cells, demonstrating that TSP-1 renders APCs resistant to maturation [103]. In addition to TSP-1 negatively regulating APC maturation, TSP-1 was observed to inhibit CCR7 expression by APCs, thus impairing their capacity to migrate to lymph nodes via CCL19/21 gradients [103,104]. Interestingly, no difference in corneal angiogenesis following transplantation was observed between TSP-1-null allografts and WT allografts, suggesting that the predominant factor determining graft survival was immune modulation rather than inhibition of angiogenesis. The effect of TSP-1 on lymphangiogenesis was not evaluated. There is evidence that the presence of lymphatic vessels has a relatively greater influence on corneal graft survival as compared to blood vessels [105]. The crucial importance of the lymphatic system in determining allograft survival is further evidenced by the fact that cervical lymphadenectomy (surgical removal of the draining lymph nodes) results in 100% corneal transplant survival [106]. Corneal transplantation is known to induce lymphangiogenesis, the inhibition of which results in prolonged graft survival [107]. It is notable therefore that the effect of TSP-1 on the regulation of lymphangiogenesis in corneal transplantation has not been systematically investigated, and the capacity of TSP-1 to influence this aspect of the afferent alloimmune response remains unknown.
The importance of TSP-1 in determining the outcome of corneal transplantation is further evidenced by data demonstrating that SNPs of TSP-1 are associated with an increased risk of allograft rejection [108]. In a study of 378 corneal transplant recipients with risk factors for graft failure, it was shown that the TSP-1 SNP rs1478604 A was associated with increased corneal allograft rejection (odds ratio 1.58), with other SNPs also demonstrating a trend toward increased rejection [108]. Larger genetic studies are required to corroborate this work, yet it provides compelling evidence that TSP-1-mediated modulation of the alloimmune response and/or regulation of blood/lymphatic vessel growth critically influences the long-term survival of corneal transplants.
IX. Corneal wound healing
As previously discussed, TSP-1 is a major activator of the cytokine TGF-β1, which in addition to its immunomodulatory properties plays a critical role in wound repair and scarring [17,109]. Irrespective of the degree to which TSP-1’s wound healing activities are mediated via TGF-β1, multiple studies provide evidence that TSP-1 is an important factor promoting corneal wound repair [59–61,110]. However, given the variable distribution of TSP-1 in the normal cornea (expressed by epithelium, Bowman’s layer, Descemet’s membrane and endothelium but not stroma [57–59]) and the array of injury models utilized by these studies, it is important to interpret their data in the context of the type of model employed.
Using a model of corneal epithelial debridement (i.e. leaving Descemet’s membrane and stroma intact), Uno and colleagues used immunohistochemical analyses to demonstrate TSP-1 expression at the surface of the wounded cornea from 30 minutes post-injury until re-epithelialization was complete [60]. Data from organ culture experiments show that exogenous TSP-1 accelerates the re-epithelialization process, in contrast to anti-TSP-1 antibody that inhibits re-epithelialization [60]. Subsequently, the same group reported that vitamin A-deficient mice fail to express TSP-1 on the wounded corneal surface following epithelial debridement, and noted that this correlates with delayed re-epithelialization [111]. Thus, the authors propose that upregulated TSP-1 expression may serve to promote epithelial migration and adhesion following corneal epithelial debridement. Although the mechanism for this phenomenon is not known, it is possible that it is partly TGF-β1-mediated, given that TGF-β1 induces lamellar differentiation of corneal epithelial cells [112]. In addition to corneal injury, these data demonstrating the role of TSP-1 in corneal re-epithelialization are highly relevant to epithelial defects in the setting of diabetes. Diabetic keratopathy results in epithelial fragility, recurrent erosions and impaired wound healing [113]. Reduced TSP-1 levels have been reported in aqueous and vitreous humor samples from diabetic humans and animals [114,115]. Given the importance of TSP-1 in the repair of corneal nerves ([80] see Section IV.B of review), and the central role of peripheral neuropathy in diabetic keratopathy pathogenesis [116,117], reduced levels of ocular TSP-1 in diabetes may compound the epitheliopathy observed in this condition.
Matsuba and colleagues, using an epithelial debridement model, have reported increased TSP-1 expression in the basement membrane zone following injury but without penetration of TSP-1 expression into the stroma [59]. Interestingly, when these investigators employed a different keratectomy model of corneal injury, in which the corneal basement membrane and the anterior stroma were removed, TSP-1 expression was observed in the stroma subjacent to the wounded epithelium within 24 hours of injury [59]. Lagging approximately 3 days behind TSP-1 expression, increased α-smooth muscle actin (a marker of myofibroblasts) was observed in the same region of the stroma [59]. Based on the colocalization of TSP-1 and α-smooth muscle actin expression, the authors propose that stromal TSP-1 expression may prompt the transformation of keratocytes into myofibroblasts [59]. Consistent with this hypothesis is the observation that corneal expression of α-smooth muscle actin and development of corneal haze are dramatically reduced in TSP-1-null mice using the same keratectomy model [5].
Blanco-Mezquita and colleagues have conducted further studies employing TSP-1-null mice and a model of full-thickness penetrating corneal incisions to investigate the role of TSP-1 in wound healing when all corneal layers were damaged [61]. The investigators showed that corneal transparency was restored by 1 month in WT mice, with evidence of coalescence of both the endothelium and Descemet’s membrane. In contrast, deficient corneal wound healing with persistent gaping and edema over 30 days of follow-up was observed in TSP-1-null mice [61]. Interestingly, the temporal pattern of TSP-1 expression was of initial expression by the corneal epithelium (at 24 hours post-injury), followed by gradual extension into the stroma (peaking at 14 days post-injury). The authors did not observe expression of intracellular TSP-1 by myofibroblasts or keratocytes, and suggest that corneal epithelium is the primary source of TSP-1 in this model. A noteworthy finding was the endothelial expression of TSP-1 following injury, coupled with the persistent corneal edema and non-healing disruption to the endothelial layer observed in TSP-1-null mice [61]. Taken together, these findings indicate that TSP-1 may play an important role in endothelial repair following injury. Such a conclusion is consistent with in vitro data showing impaired adhesive and migratory properties of corneal endothelial cells derived from TSP-1-null mice relative to WT mice [118]. Despite much progress in our understanding of the anatomical and temporal patterns of TSP-1 expression following corneal injury, and its association with α-smooth muscle actin expression, the precise mechanisms by which TSP-1 might promote the generation of myofibroblasts (i.e. predominantly directly or via TGF-β1) remain unknown.
X. Infectious keratitis
Herpes simplex virus (HSV) keratitis is the leading cause of infectious corneal blindness in developed countries, and the prevalence of ocular HSV infection in the US has been estimated to be 500,000 [119,120]. Due to recurrences of HSV keratitis causing stromal scarring with concomitant visual impairment, HSV keratitis is a common indication for corneal transplantation [121]. Yet the risk of graft failure is high in this setting, since inflammation and stromal neovascularization associated with HSV keratitis abolish the immune quiescence normally enjoyed by the cornea [90,122].
Choudhary and colleagues have investigated the effect of HSV1 infection on TSP-1 expression by human keratocytes in vitro, hypothesizing that HSV1 inhibits the synthesis of TSP-1 by keratocytes, thus abrogating its anti-angiogenic properties and promoting stromal neovascularization [123]. The authors observed a 50% reduction in TSP-1 protein expression by keratocytes 8 hours after infection with HSV1, with no TSP-1 expression detected at 24 hours after infection. Given these findings, and considering the known immunomodulatory and anti-angiogenic properties of TSP-1, treatment with recombinant TSP-1 or TSP-1-derived peptides may be a viable approach to reduce HSV keratitis disease severity, limit stromal neovascularization and enhance the survival of subsequent corneal allografts. However, currently there is a shortage of in vivo data validating this approach.
XI. Conclusion
There is substantial evidence that TSP-1 is critical for the preservation of ocular surface health as indicated, for example, by development of spontaneous dry eye disease, increased corneal angiogenesis and delayed corneal wound healing observed in TSP-1-null mice [61,77,83,92]. Furthermore, TSP-1 polymorphisms have been associated with increased risk of chronic ocular surface inflammation and corneal allograft rejection [86,108]. The diverse immunomodulatory and tissue reparative functions of TSP-1 underscore the translational potential of TSP-1-derived therapeutics. Despite considerable progress in our understanding of the activities of TSP-1, however, numerous questions remain unanswered. For example, there is a relative lack of mechanistic data detailing the ligands to which TSP-1 is binding to induce the phenomena observed at the ocular surface. Furthermore, the extent to which TSP-1 is mediating its effects via TGF-β1 activation or independently of TGF-β1 has not been comprehensively delineated in corneal inflammatory disease. Investigation of these questions will permit the development of more targeted approaches to realize the therapeutic potential of TSP-1 at the ocular surface.
Acknowledgments
This work was supported by the National Institute of Health (EY012963 and EY020889) to R.D.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adams JC, Lawler J. The Thrombospondins. Cold Spring Harb Perspect Biol 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lawler JW, Slayter HS, Coligan JE. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem 1978;253:8609–16. [PubMed] [Google Scholar]
- [4].Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol 2003;74:179–85. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- [5].Masli S, Sheibani N, Cursiefen C, Zieske J. Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation. Curr Eye Res 2014;39:759–74. doi: 10.3109/02713683.2013.877936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haddadin RI, Oh D-J, Kang MH, Villarreal G, Kang J-H, Jin R, et al. Thrombospondin-1 (TSP1)–Null and TSP2-Null Mice Exhibit Lower Intraocular Pressures. Investig Opthalmology Vis Sci 2012;53:6708. doi: 10.1167/iovs.11-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, et al. Roles of thrombospondin-1 and −2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci 2004;45:1117–24. [DOI] [PubMed] [Google Scholar]
- [8].Hiscott P, Paraoan L, Choudhary A, Ordonez JL, Al-Khaier A, Armstrong DJ. Thrombospondin 1, thrombospondin 2 and the eye. Prog Retin Eye Res 2006;25:1–18. doi: 10.1016/J.PRETEYERES.2005.05.001. [DOI] [PubMed] [Google Scholar]
- [9].Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm 2011;2011:296069. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, et al. Recognition of the N-terminal Modules of Thrombospondin-1 and Thrombospondin-2 by α6β1 Integrin. J Biol Chem 2003;278:40679–87. doi: 10.1074/jbc.M302014200. [DOI] [PubMed] [Google Scholar]
- [11].Sid B, Sartelet H, Bellon G, El Btaouri H, Rath G, Delorme N, et al. Thrombospondin 1: a multifunctional protein implicated in the regulation of tumor growth. Crit Rev Oncol Hematol 2004;49:245–58. doi: 10.1016/j.critrevonc.2003.09.009. [DOI] [PubMed] [Google Scholar]
- [12].Tan K, Duquette M, Liu J, Dong Y, Zhang R, Joachimiak A, et al. Crystal structure of the TSP-1 type 1 repeats: a novel layered fold and its biological implication. J Cell Biol 2002;159:373–82. doi: 10.1083/jcb.200206062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jiménez B, Volpert OV., Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 2000;6:41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- [14].Floquet N, Dedieu S, Martiny L, Dauchez M, Perahia D. Human thrombospondin’s (TSP-1) C-terminal domain opens to interact with the CD-47 receptor: A molecular modeling study. Arch Biochem Biophys 2008;478:103–9. doi: 10.1016/j.abb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- [15].Silverstein RL. The face of TSR revealed: an extracellular signaling domain is exposed. J Cell Biol 2002;159:203–6. doi: 10.1083/jcb.200209138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 1998;101:982–92. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 1998;93:1159–70. [DOI] [PubMed] [Google Scholar]
- [18].Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β – an excellent servant but a bad master. J Transl Med 2012;10:183. doi: 10.1186/1479-5876-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- [20].Hinz B The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol 2015;47:54–65. doi: 10.1016/J.MATBIO.2015.05.006. [DOI] [PubMed] [Google Scholar]
- [21].Chen Y, Wang X, Weng D, Tian L, Lv L, Tao S, et al. A TSP-1 synthetic peptide inhibits bleomycin-induced lung fibrosis in mice. Exp Toxicol Pathol 2009;61:59–65. doi: 10.1016/j.etp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- [22].Daniel C, Wiede J, Krutzsch HC, Ribeiro SMF, Roberts DD, Murphy-Ullrich JE, et al. Thrombospondin-1 is a major activator of TGF-β in fibrotic renal disease in the rat in vivo. Kidney Int 2004;65:459–68. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- [23].Evrard S, Bluteau O, Tulliez M, Rameau P, Gonin P, Zetterberg E, et al. Thrombospondin-1 is not the major activator of TGF-β1 in thrombopoietin-induced myelofibrosis. Blood 2011;117:246–9. doi: 10.1182/blood-2010-07-294447. [DOI] [PubMed] [Google Scholar]
- [24].Abdelouahed M, Ludlow A, Brunner G, Lawler J. Activation of Platelet-transforming Growth Factor β−1 in the Absence of Thrombospondin-1. J Biol Chem 2000;275:17933–6. doi: 10.1074/jbc.275.24.17933. [DOI] [PubMed] [Google Scholar]
- [25].Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA. Transforming Growth Factor-β Regulation of Immune Responses. Annu Rev Immunol 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- [26].Yehualaeshet T, O’Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, et al. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol 1999;155:841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–28. [DOI] [PubMed] [Google Scholar]
- [28].Nunes I, Gleizes PE, Metz CN, Rifkin DB. Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta. J Cell Biol 1997;136:1151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wan YY, Flavell RA. “Yin-Yang” functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sanjabi S, Oh SA, Li MO. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harb Perspect Biol 2017;9:a022236. doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev n.d;11:59–69. [DOI] [PubMed] [Google Scholar]
- [32].Basu R, Hatton RD, Weaver CT. The Th17 family: flexibility follows function. Immunol Rev 2013;252:89–103. doi: 10.1111/imr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 2014;13:668–77. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- [34].Mir FA, Contreras-Ruiz L, Masli S. Thrombospondin-1-dependent immune regulation by transforming growth factor-β2-exposed antigen-presenting cells. Immunology 2015;146:547–56. doi: 10.1111/imm.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Doyen V, Rubio M, Braun D, Nakajima T, Abe J, Saito H, et al. Thrombospondin 1 Is an Autocrine Negative Regulator of Human Dendritic Cell Activation. J Exp Med 2003;198:1277–83. doi: 10.1084/jem.20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, et al. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol 2006;177:3534–41. [DOI] [PubMed] [Google Scholar]
- [37].Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol 2007;178:5930–9. [DOI] [PubMed] [Google Scholar]
- [38].Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and −2. Cold Spring Harb Perspect Med 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A 1990;87:6624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bein K, Simons M. Thrombospondin Type 1 Repeats Interact with Matrix Metalloproteinase 2. J Biol Chem 2000;275:32167–73. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]
- [41].Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci 2001;98:12485–90. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis 1999;3:147–58. [DOI] [PubMed] [Google Scholar]
- [43].Greenaway J, Lawler J, Moorehead R, Bornstein P, LaMarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J Cell Physiol 2007;210:807–18. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sun J, Hopkins BD, Tsujikawa K, Perruzzi C, Adini I, Swerlick R, et al. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am J Physiol Circ Physiol 2009;296:H1344–51. doi: 10.1152/ajpheart.01246.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang X, Kazerounian S, Duquette M, Perruzzi C, Nagy JA, Dvorak HF, et al. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J 2009;23:3368–76. doi: 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Colombo G, Margosio B, Ragona L, Neves M, Bonifacio S, Annis DS, et al. Non-peptidic thrombospondin-1 mimics as fibroblast growth factor-2 inhibitors: an integrated strategy for the development of new antiangiogenic compounds. J Biol Chem 2010;285:8733–42. doi: 10.1074/jbc.M109.085605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dawson DW, Volpert O V, Pearce SF, Schneider AJ, Silverstein RL, Henkin J, et al. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol 1999;55:332–8. [DOI] [PubMed] [Google Scholar]
- [48].Oganesian A, Armstrong LC, Migliorini MM, Strickland DK, Bornstein P. Thrombospondins Use the VLDL Receptor and a Nonapoptotic Pathway to Inhibit Cell Division in Microvascular Endothelial Cells. Mol Biol Cell 2008;19:563–71. doi: 10.1091/mbc.e07-07-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 2006;281:26069–80. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- [50].Kaur S, Roberts DD. CD47 applies the brakes to angiogenesis via vascular endothelial growth factor receptor-2. Cell Cycle 2011;10:10–2. doi: 10.4161/cc.10.1.14324. [DOI] [PubMed] [Google Scholar]
- [51].Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol 2002;161:831–9. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sakai K, Sumi Y, Muramatsu H, Hata K, Muramatsu T, Ueda M. Thrombospondin-1 promotes fibroblast-mediated collagen gel contraction caused by activation of latent transforming growth factor beta-1. J Dermatol Sci 2003;31:99–109. [DOI] [PubMed] [Google Scholar]
- [53].Krishnaswami S, Ly QP, Rothman VL, Tuszynski GP. Thrombospondin-1 promotes proliferative healing through stabilization of PDGF. J Surg Res 2002;107:124–30. [DOI] [PubMed] [Google Scholar]
- [54].DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol 1996;148:1851–60. [PMC free article] [PubMed] [Google Scholar]
- [55].Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J 2000;19:3272–82. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal 2009;3:215–25. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sekiyama E, Nakamura T, Cooper LJ, Kawasaki S, Hamuro J, Fullwood NJ, et al. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest Ophthalmol Vis Sci 2006;47:1352–8. doi: 10.1167/iovs.05-1305. [DOI] [PubMed] [Google Scholar]
- [58].Hiscott P, Seitz B, Schlötzer-Schrehardt U, Naumann GO. Immunolocalisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell Tissue Res 1997;289:307–10. [DOI] [PubMed] [Google Scholar]
- [59].Matsuba M, Hutcheon AEK, Zieske JD. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res 2011;93:534–40. doi: 10.1016/j.exer.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Uno K, Hayashi H, Kuroki M, Uchida H, Yamauchi Y, Kuroki M, et al. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun 2004;315:928–34. doi: 10.1016/j.bbrc.2004.01.146. [DOI] [PubMed] [Google Scholar]
- [61].Blanco-Mezquita JT, Hutcheon AEK, Zieske JD. Role of thrombospondin-1 in repair of penetrating corneal wounds. Invest Ophthalmol Vis Sci 2013;54:6262–8. doi: 10.1167/iovs.13-11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tan X, Chen Y, Foulsham W, Amouzegar A, Inomata T, Liu Y, et al. The immunoregulatory role of corneal epithelium-derived thrombospondin-1 in dry eye disease. Ocul Surf 2018;16:470–7. doi: 10.1016/j.jtos.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Soriano-Romaní L, Contreras-Ruiz L, García-Posadas L, López-García A, Masli S, Diebold Y. Inflammatory Cytokine-Mediated Regulation of Thrombospondin-1 and CD36 in Conjunctival Cells. J Ocul Pharmacol Ther 2015;31:419–28. doi: 10.1089/jop.2015.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol 2009;2:375–6. doi: 10.1038/mi.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 2009;182:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol 2017. doi: 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- [67].Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II Report Executive Summary. Ocul Surf 2017;15:802–12. doi: 10.1016/j.jtos.2017.08.003. [DOI] [PubMed] [Google Scholar]
- [68].Stevenson W, Chauhan SK, Dana R. Dry Eye Disease. Arch Ophthalmol 2012;130:90. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res 2012;31:271–85. doi: 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Niederkorn JY, Stern ME, Pflugfelder SC, De Paiva CS, Corrales RM, Gao J, et al. Desiccating Stress Induces T Cell-Mediated Sjögren’s Syndrome-Like Lacrimal Keratoconjunctivitis. J Immunol 2006;176. [DOI] [PubMed] [Google Scholar]
- [71].Kodati S, Chauhan SK, Chen Y, Dohlman TH, Karimian P, Saban D, et al. CCR7 is critical for the induction and maintenance of Th17 immunity in dry eye disease. Invest Ophthalmol Vis Sci 2014;55:5871–7. doi: 10.1167/iovs.14-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci 2009;50:3802–7. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Foulsham W, Marmalidou A, Amouzegar A, Coco G, Chen Y, Dana R. Review: The function of regulatory T cells at the ocular surface. Ocul Surf 2017;15:652–9. doi: 10.1016/j.jtos.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- [75].Gandhi NB, Su Z, Zhang X, Volpe EA, Pelegrino FSA, Rahman SA, et al. Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress. J Leukoc Biol 2013;94:1293–301. doi: 10.1189/jlb.1012524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Futagami Y, Sugita S, Vega J, Ishida K, Takase H, Maruyama K, et al. Role of Thrombospondin-1 in T Cell Response to Ocular Pigment Epithelial Cells. J Immunol 2007;178:6994–7005. doi: 10.4049/JIMMUNOL.178.11.6994. [DOI] [PubMed] [Google Scholar]
- [77].Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjögren’s syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol 2009;175:1136–47. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Contreras-Ruiz L, Regenfuss B, Mir FA, Kearns J, Masli S. Conjunctival Inflammation in Thrombospondin-1 Deficient Mouse Model of Sjögren’s Syndrome. PLoS One 2013;8:e75937. doi: 10.1371/journal.pone.0075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bhattacharya S, García-Posadas L, Hodges RR, Makarenkova HP, Masli S, Dartt DA. Alteration in nerves and neurotransmitter stimulation of lacrimal gland secretion in the TSP-1−/− mouse model of aqueous deficiency dry eye. Mucosal Immunol 2018;11:1138–48. doi: 10.1038/s41385-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tatematsu Y, Khan Q, Blanco T, Bair JA, Hodges RR, Masli S, et al. Thrombospondin-1 Is Necessary for the Development and Repair of Corneal Nerves. Int J Mol Sci 2018;19. doi: 10.3390/ijms19103191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Meng ID, Kurose M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res 2013;117:79–87. doi: 10.1016/j.exer.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chao C, Golebiowski B, Zhao X, Chen S, Zhou S, Stapleton F. Long-term Effects of LASIK on Corneal Innervation and Tear Neuropeptides and the Associations With Dry Eye. J Refract Surg 2016;32:518–24. doi: 10.3928/1081597X-20160603-01. [DOI] [PubMed] [Google Scholar]
- [83].Contreras Ruiz L, Mir FA, Turpie B, Masli S. Thrombospondin-derived peptide attenuates Sjögren’s syndrome-associated ocular surface inflammation in mice. Clin Exp Immunol 2017;188:86–95. doi: 10.1111/cei.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Soriano-Romaní L, Contreras-Ruiz L, López-García A, Diebold Y, Masli S. Topical Application of TGF-β-Activating Peptide, KRFK, Prevents Inflammatory Manifestations in the TSP-1-Deficient Mouse Model of Chronic Ocular Inflammation. Int J Mol Sci 2018;20:9. doi: 10.3390/ijms20010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One 2015;10:e0120284. doi: 10.1371/journal.pone.0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Contreras-Ruiz L, Ryan DS, Sia RK, Bower KS, Dartt DA, Masli S. Polymorphism in THBS1 Gene Is Associated with Post-Refractive Surgery Chronic Ocular Surface Inflammation. Ophthalmology 2014;121:1389–97. doi: 10.1016/j.ophtha.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Smith RE, Reyes NJ, Khandelwal P, Schlereth SL, Lee HS, Masli S, et al. Secondary allergic T cell responses are regulated by dendritic cell-derived thrombospondin-1 in the setting of allergic eye disease. J Leukoc Biol 2016;100:371–80. doi: 10.1189/jlb.3A0815-357RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sarfati M, Fortin G, Raymond M, Susin S. CD47 in the immune response: role of thrombospondin and SIRP-alpha reverse signaling. Curr Drug Targets 2008;9:842–50. [DOI] [PubMed] [Google Scholar]
- [89].Cursiefen C Immune Privilege and Angiogenic Privilege of the Cornea. Immune Response Eye, vol. 92, Basel: KARGER; 2007, p. 50–7. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- [90].Giménez F, Suryawanshi A, Rouse BT. Pathogenesis of herpes stromal keratitis--a focus on corneal neovascularization. Prog Retin Eye Res 2013;33:1–9. doi: 10.1016/j.preteyeres.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Foulsham W, Coco G, Amouzegar A, Chauhan SK, Dana R. When Clarity Is Crucial: Regulating Ocular Surface Immunity. Trends Immunol 2018;39:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med 2011;208:1083–92. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tammela T, Alitalo K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell 2010;140:460–76. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- [94].Jiménez B, Volpert OV., Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularizationby thrombospondin-1. Nat Med 2000;6:41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- [95].Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 Inhibits VEGF Receptor-2 Signaling by Disrupting Its Association with CD47. J Biol Chem 2010;285:38923–32. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 2000;19:625–43. [DOI] [PubMed] [Google Scholar]
- [97].Eye Bank Associtaion of America. 2017. Eye banking statistical report Available at: Https://Restoresight.Org/What-We-Do/Publications/Statistical-Report/: n.d.
- [98].Amouzegar A, Chauhan SK, Dana R. Alloimmunity and Tolerance in Corneal Transplantation. J Immunol 2016;196:3983–91. doi: 10.4049/jimmunol.1600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].The Collaborative Corneal Transplantation Studies Research Group. The collaborative corneal transplantation studies (CCTS): effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol 1992;110:1392. [PubMed] [Google Scholar]
- [100].Sano Y, Streilein JW, Ksander BR. Detection of minor alloantigen-specific cytotoxic T cells after rejection of murine orthotopic corneal allografts: evidence that graft antigens are recognized exclusively via the indirect pathway. Transplantation 1999;68:963–70. [DOI] [PubMed] [Google Scholar]
- [101].Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed micronenvironment. J Immunol 2004;173:4464–9. [DOI] [PubMed] [Google Scholar]
- [102].Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci 2003;44:581–9. [DOI] [PubMed] [Google Scholar]
- [103].Saban DR, Bock F, Chauhan SK, Masli S, Dana R. Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J Immunol 2010;185:4691–7. doi: 10.4049/jimmunol.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Jin Y, Shen L, Chong E-M, Hamrah P, Zhang Q, Chen L, et al. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol Vis 2007;13:626–34. [PMC free article] [PubMed] [Google Scholar]
- [105].Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, et al. Cutting Edge: Lymphatic Vessels, Not Blood Vessels, Primarily Mediate Immune Rejections After Transplantation. J Immunol 2010;184:535–9. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci 2001;42:1293–8. [PubMed] [Google Scholar]
- [107].Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, et al. Inhibition of Hemangiogenesis and Lymphangiogenesis after Normal-Risk Corneal Transplantation by Neutralizing VEGF Promotes Graft Survival. Investig Opthalmology Vis Sci 2004;45:2666. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- [108].Winton HL, Bidwell JL, Armitage WJ. Thrombospondin-1 Polymorphisms Influence Risk of Corneal Allograft Rejection. Investig Opthalmology Vis Sci 2014;55:2115. doi: 10.1167/iovs.13-13681. [DOI] [PubMed] [Google Scholar]
- [109].Yang L, Qiu CX, Ludlow A, Ferguson MW, Brunner G. Active transforming growth factor-beta in wound repair: determination using a new assay. Am J Pathol 1999;154:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hiscott P, Armstrong D, Batterbury M, Kaye S. Repair in avascular tissues: fibrosis in the transparent structures of the eye and thrombospondin 1. Histol Histopathol 1999;14:1309–20. doi: 10.14670/HH-14.1309. [DOI] [PubMed] [Google Scholar]
- [111].Uno K, Kuroki M, Hayashi H, Uchida H, Kuroki M, Oshima K. Impairment of thrombospondin-1 expression during epithelial wound healing in corneas of vitamin A-deficient mice. Histol Histopathol 2005;20:493–9. doi: 10.14670/HH-20.493. [DOI] [PubMed] [Google Scholar]
- [112].Nishimura T, Toda S, Mitsumoto T, Oono S, Sugihara H. Effects of hepatocyte growth factor, transforming growth factor-beta1 and epidermal growth factor on bovine corneal epithelial cells under epithelial-keratocyte interaction in reconstruction culture. Exp Eye Res 1998;66:105–16. doi: 10.1006/exer.1997.0419. [DOI] [PubMed] [Google Scholar]
- [113].Ljubimov AV Diabetic complications in the cornea. Vision Res 2017;139:138–52. doi: 10.1016/j.visres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sheibani N, Sorenson CM, Cornelius LA, Frazier WA. Thrombospondin-1, a Natural Inhibitor of Angiogenesis, Is Present in Vitreous and Aqueous Humor and Is Modulated by Hyperglycemia. Biochem Biophys Res Commun 2000;267:257–61. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- [115].Wang S, Gottlieb JL, Sorenson CM, Sheibani N. Modulation of Thrombospondin 1 and Pigment Epithelium–Derived Factor Levels in Vitreous Fluid of Patients With Diabetes. Arch Ophthalmol 2009;127:507. doi: 10.1001/archophthalmol.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Schultz RO, Peters MA, Sobocinski K, Nassif K, Schultz KJ. Diabetic keratopathy as a manifestation of peripheral neuropathy. Am J Ophthalmol 1983;96:368–71. [DOI] [PubMed] [Google Scholar]
- [117].Bikbova G, Oshitari T, Baba T, Yamamoto S. Neuronal Changes in the Diabetic Cornea: Perspectives for Neuroprotection. Biomed Res Int 2016;2016:1–8. doi: 10.1155/2016/5140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Scheef EA, Huang Q, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of corneal endothelial cells from wild type and thrombospondin-1 deficient mice. Mol Vis 2007;13:1483–95. [PubMed] [Google Scholar]
- [119].Azher TN, Yin X-T, Tajfirouz D, Huang AJ, Stuart PM. Herpes simplex keratitis: challenges in diagnosis and clinical management. Clin Ophthalmol 2017;11:185–91. doi: 10.2147/OPTH.S80475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Farooq A V, Shukla D Herpes Simplex Epithelial and Stromal Keratitis: An Epidemiologic Update. Surv Ophthalmol 2012;57:448–62. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Larkin DF. Corneal transplantation for herpes simplex keratitis. Br J Ophthalmol 1998;82:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kuffova L, Knickelbein JE, Yu T, Medina C, Amescua G, Rowe AM, et al. High-Risk Corneal Graft Rejection in the Setting of Previous Corneal Herpes Simplex Virus (HSV)-1 Infection. Invest Ophthalmol Vis Sci 2016;57:1578–87. doi: 10.1167/iovs.15-17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Choudhary A, Hiscott P, Hart CA, Kaye SB, Batterbury M, Grierson I. Suppression of thrombospondin 1 and 2 production by herpes simplex virus 1 infection in cultured keratocytes. Mol Vis 2005;11:163–8. [PubMed] [Google Scholar]