Abstract
Background:
Two functional polymorphisms in the matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) genes may contribute to periodontitis pathogenesis. However, the results were inconsistent and inconclusive. Therefore, to clarify precise associations of MMP-2-753 C>T and MMP-9-1562C>T polymorphisms with chronic (CP) and aggressive (AgP) periodontitis, we performed a systematic review and meta-analysis.
Methods:
A literature search was conducted using PubMed, Google Scholar, Embase, and Web of Science databases until 5 July 2017. The data were analyzed with CMA software, and risk estimates are expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs).
Results:
Nineteen case-control studies in ten publications with 2089 periodontitis cases and 2345 controls met the criteria. The pooled ORs indicated that MMP-2-753C>T and MMP-9-1562C>T polymorphisms were not significantly associated with risk of periodontitis in overall analysis. Stratified analyses by ethnicity and periodontitis type indicated that the MMP-9-1562C>T polymorphism showed a significant association with the risk of periodontitis among Caucasians and CP/AgP subgroup, whereas MMP-2-753C>T polymorphism was significantly associated with periodontitis risk only among Asians.
Conclusion:
MMP-2-753C>T and MMP-9-1562C>T polymorphisms may not be associated with risk of periodontitis in overall population. However, MMP-2-753C>T and MMP-9-1562C>T polymorphisms might have influence on the susceptibility of periodontitis by ethnicity.
Keywords: Periodontitis, MMP gene, Polymorphism, Meta-analysis
Introduction
Periodontitis is one of the most common causes of inflammatory bone loss in human (1–3). Periodontitis is a complex multifactorial disease that involves the interaction of environmental factors such as smoking and the patient’s related factors such as sex, age and systemic diseases (1,4). Majority of the population has experienced some level of gingival inflammation worldwide, and 5%–8% of the population suffering from severe forms of periodontitis (5). Both chronic and aggressive forms of periodontitis are characterized by inflammation (6). Periodontitis as most human diseases have a genetic component, which influences inflammatory and immune responses in this disease (7). Results from the twin and family studies, indicate a role of genetic component in development periodontitis (8,9). Many studies have shown a correlation between periodontitis and systemic disease involving genes such as Matrix Metalloproteinases (MMP) as a shared mechanism of inflammation (8,10,11).
Matrix metalloproteinases constitute a family of 25 zinc-dependent proteolytic enzymes, which are capable of degrading the extracellular matrix (ECM) (10–12). MMP-2 (Gelatinase-A) and -9 (Gelatinase-B) are two widely studied matrix metalloproteinases (13). MMP-2 gene (also known as 72-kDa type IV collagenase) is located on human chromosome 16q12.2 (14). MMP-2 gene encodes a protein that involved in the breakdown of ECM in normal physiological processes, such as embryonic development, reproduction and tissue remodeling (15). MMP-9 gene (also known as 92-kDa gelatinase or type V collagenase) is located on human chromosome 20q11.2–q13.1 (16). MMP-9 encodes a multidomain enzyme, a class of enzymes that belong to the zinc-metalloproteinases family involved in the degradation of the ECM (16,17). In the past decade, several epidemiologic studies have been investigated the association of MMP-9 -1562C>T (rs3918242) and MMP-2 -753C>T (rs2285053) polymorphisms with susceptibility to periodontitis (8,10,11). However, those studies results remain fairly inconsistent and inconclusive. A meta-analysis is a very powerful tool to obtain sufficient statistical power to detect the potential effect of these polymorphisms from individual studies with small size and the statistically low power.
Thus, we performed the current systematic and meta-analysis of all available case-control studies to provide more precise estimation of the association of MMP-2 -753C>T (rs2285053) and MMP-9 -1562C>T (rs3918242) polymorphisms with chronic/aggressive periodontitis susceptibility.
Materials and Methods
Search Strategy
A systematic search of eligible studies on the association of MMP-2 -753C>T (rs2285053) and MMP-9 -1562C>T (rs3918242) polymorphisms with periodontitis susceptibility was conducted in PubMed, ISI Web of Science, Google Scholar, and Embase databases up to July 15, 2017. The search strategies were based on combinations of the following keywords: (‘‘Matrix Metallopeptidase or ‘‘collagenase’’ or ‘’MMP’’ or ‘’MMP-2’’ or ‘’gelatinase A’’ or ‘’MMP-9’’ or ‘’gelatinase B’’ or ‘’MMP-9 -1562C>T’’ or ‘’MMP-2 -753C>T‘’ or ‘’ rs3918242’’ or ‘’ rs2285053’’) and (“periodontitis’’ or “periodontal disease” or ‘’chronic periodontitis’’ or ‘’CP’’ ‘’aggressive periodontitis’’ or ‘’AgP’’) and (‘‘gene’’ or ‘‘allele’’ or ‘‘genotype’’ or ‘‘mutation’’ or ‘‘variant’’ or ‘’single nucleotide polymorphisms’’ or ‘’SNPs’’ or ‘‘variation’’ or ‘‘polymorphism’’). The extracted publications were limited to English. Additionally, we have screened the references list of the retrieved original articles for more additional original articles. If there were multiple reports of the same study or overlapping data only the study with the largest sample sizes or the most recent one was included to the present meta-analysis.
Inclusion and Exclusion Criteria
Studies were included based on the following criteria: 1) only full-text and published studies; 2) studies with case-control or cohort design; 3) a study evaluated the association of MMP-9 -1562C>T (rs3918242) and MMP-2 -753C>T (rs2285053) polymorphisms with periodontitis (CP and/or AgP) susceptibility risk; 4) available genotypes frequencies of MMP-9 -1562C>T (rs3918242) and MMP-2 -753C>T (rs2285053) polymorphisms were provided to estimate the odds ratios (ORs) with 95% confidence intervals (CIs). The exclusion criteria were as follows: 1) the study was not conducted on periodontitis; 2) abstracts, case reports, and review articles; 3) studies with only case group (no control group); 4) studies on the other polymorphisms of the MMP-2 and MMP-9 genes; 5) studies without detail genotype frequencies, which were unable to calculate ORs; and 6) duplicate publications of data from the same study.
Data Extraction
Two authors independently extracted the following data from each eligible study according to the inclusion criteria: the first author’s name, the year of publication, ethnicity, country of origin, total number of cases and controls, the frequencies of genotypes, minor allele frequencies (MAFs), and Hardy-Weinberg equilibrium test in control subjects. We have calculated the allele frequencies from corresponding genotype distributions using an online website. Any discrepancy between these two authors was resolved by reaching a consensus through discussion or the involvement of a third author who made the final decision through discussions.
Statistical Analysis
Odds ratio (OR) and 95% confidence intervals (CIs) were calculated to evaluate the strength of the associations of MMP-9 -1562C>T and MMP-2 -753C>T polymorphisms with risk of periodontitis. The significance of the pooled OR was determined by the Z-test. Pooled ORs were performed for both MMP-9 -1562C>T and MMP-2 -753C>T polymorphisms under the allele model (T vs. C), the heterozygote model (TC vs. CC), the homozygote model (TT vs. CC), the dominant model (TT+TC vs. CC), and the recessive model (TT vs. TC+CC). Heterogeneity (between-study inconsistency) was assessed by both the chi-square-based Q statistic (considered significant with P<0.10) and the I2 statistic. In the current meta-analysis the I2 values of 25, 50, and 75% meant a low, moderate, and high heterogeneity, respectively. When heterogeneity existed (P<0.10), a random-effects model weighted by the DerSimonian–Laird method was used to give a more conservative result; otherwise, a fixed-effects model weighted by the Mantel–Haenszel method would be applied. Sensitivity analyses were performed by omitting each particular study at a time. Hardy–Weinberg equilibrium (HWE) was evaluated for each study by Chi-square test in control groups, and P<0.05 was considered as a significant departure from HWE. Moreover, sensitivity analysis was also performed, excluding studies whose allele frequencies in controls exhibited significant deviation from the Hardy–Weinberg equilibrium (HWE), given that the deviation may denote bias. A meta-regression analysis was carried out to identify the major sources of between-studies variation in the results, using the log of the ORs from each study as ethnicity and types of periodontitis as the possible sources of heterogeneity. Moreover, the quality of selected studies was tested by the confirmation of HWE in control groups, and studies without the confirmation of HWE in controls were defined as low-quality studies, while studies with the confirmation of HWE in controls were defined as high-quality studies. Funnel plots and Egger’s test were used to examine publication bias (P<0.05). If publication bias existed, the Duval and Tweedie non-parametric ‘‘trim and fill’’ method was used to adjust for it. The statistical analysis for the current meta-analysis study was performed using the Comprehensive Meta-Analysis (CMA) software (version 2.2; Biostat, USA). In the current meta-analysis, all P-values were considered two-sided, and P=0.05 was set as the threshold value for statistical significance.
Results
Characteristics of the Included Studies
As shown in Fig. 1, the comprehensive search of literature under defined terms retrieved 197 articles. Of those 185 articles were excluded through duplicate screening and screening of titles and abstracts. Next, 45 studies were excluded because were reviews, case reports, irrelevance to the topic, not involving periodontitis research and lacking sufficient data. Finally, 19 case-control studies from ten publications (18–28) were identified in the meta-analysis. Basic characteristics of the selected articles are all listed in Table 1.
Fig. 1:
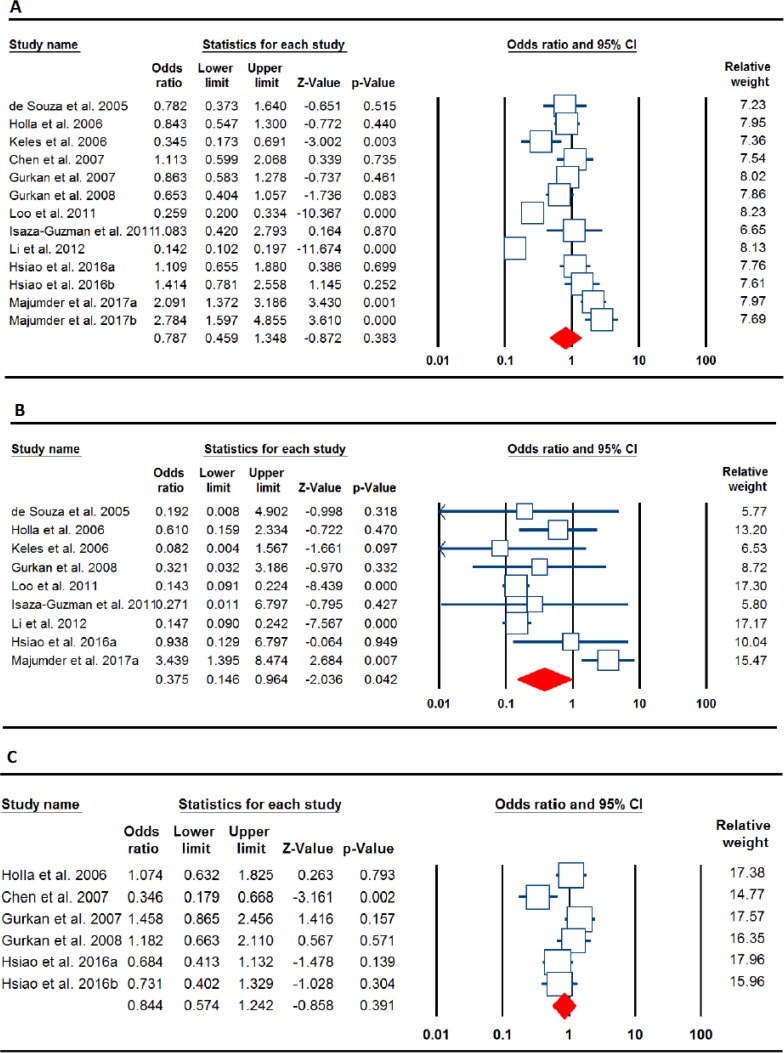
Forest plots for the association of the MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms with periodontitis risk. A: MMP-9 -1562C>T (allele model: T vs. C), B: MMP-9 -1562C>T (CP, homozygote model: TT vs. CC), and C: MMP-2 -753C>T (dominant model: TT+TC vs. CC)
Table 1:
Main characteristics of studies included in this meta-analysis
| First Author | Country (Ethnicity) | Periodontitis Type | Case/Control | Cases | Controls | MAFs | HWE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes | Allele | Genotypes | Allele | ||||||||||||
| MMP-9 -1562C>T | CC | CT | TT | C | T | CC | CT | TT | C | T | |||||
| de Souza 2005 18 | Brazil(Mixed) | CP | 62/38 | 42 | 20 | 0 | 104 | 20 | 24 | 13 | 1 | 61 | 15 | 0.1974 | 0.623 |
| Holla 2006 19 | Czech(Caucasian) | CP | 169/135 | 122 | 43 | 4 | 287 | 51 | 93 | 37 | 5 | 223 | 47 | 0.1741 | 0.586 |
| Keles 2006 20 | Turkey(Caucasian) | CP | 70/70 | 57 | 13 | 0 | 127 | 13 | 42 | 24 | 4 | 108 | 32 | 0.2286 | 0.586 |
| Chen 2007 21 | China(Asian) | AgP | 79/128 | 62 | 15 | 2 | 139 | 19 | 101 | 26 | 1 | 228 | 28 | 0.1094 | 0.629 |
| Gurkan 2007 22 | Turkey(Caucasian) | AgP | 112/157 | 58 | 53 | 1 | 169 | 55 | 78 | 72 | 7 | 228 | 86 | 0.2739 | ≤0.001 |
| Gurkan 2008 23 | Turkey(Caucasian) | CP | 87/107 | 54 | 32 | 1 | 140 | 34 | 52 | 52 | 3 | 156 | 58 | 0.271 | 0.017 |
| Loo. 2011 24 | China(Asian) | CP | 280/250 | 143 | 73 | 64 | 359 | 201 | 43 | 72 | 135 | 158 | 342 | 0.684 | 0.001 |
| Isaza-Guzman 2011 25 | Colombia(Mixed) | CP | 69/54 | 58 | 11 | 0 | 127 | 11 | 47 | 6 | 1 | 100 | 8 | 0.0741 | 0.163 |
| Li 2012 26 | China(Asian) | CP | 122/532 | 68 | 26 | 28 | 162 | 54 | 99 | 156 | 277 | 354 | 710 | 0.6673 | 0.001 |
| Hsiao 2016a 27 | Taiwan(Asian) | CP | 129/117 | 96 | 31 | 2 | 223 | 35 | 90 | 28 | 2 | 205 | 29 | 0.1333 | 0.916 |
| Hsiao 2016b 27 | Taiwan(Asian) | AgP | 69/117 | 48 | 19 | 2 | 115 | 23 | |||||||
| Majumder 2017a 28 | India(Asian) | CP | 110/121 | 53 | 39 | 18 | 145 | 75 | 81 | 32 | 8 | 194 | 48 | 0.1983 | 0.063 |
| Majumder. 2017b 28 | India(Asian) | AgP | 38/121 | 17 | 11 | 10 | 45 | 31 | |||||||
| MMP-2 -753C>T | CC | CT | TT | C | T | CC | CT | TT | C | T | |||||
| Holla 2006 19 | Czech(Caucasian) | CP | 149/127 | 107 | 38 | 4 | 255 | 43 | 93 | 30 | 4 | 216 | 38 | 0.149 | 0.419 |
| Chen 2007 21 | China(Asian) | AgP | 167/128 | 63 | 15 | 1 | 298 | 36 | 98 | 28 | 2 | 224 | 32 | 0.125 | 1.000 |
| Gurkan. 2007 22 | Turkey(Caucasian) | AgP | 92/157 | 49 | 39 | 4 | 137 | 47 | 98 | 54 | 5 | 250 | 64 | 0.203 | 0.454 |
| Gurkan 2008 23 | Turkey(Caucasian) | CP | 87/107 | 51 | 32 | 4 | 134 | 40 | 67 | 37 | 3 | 171 | 43 | 0.200 | 0.426 |
| Hsiao 2016a 27 | Taiwan(Asian) | CP | 129/117 | 75 | 44 | 10 | 194 | 64 | 57 | 48 | 12 | 162 | 72 | 0.307 | 0.688 |
| Hsiao 2016b 27 | Taiwan(Asian) | AgP | 69/117 | 39 | 26 | 4 | 104 | 34 | 57 | 48 | 12 | ||||
CP: Chronic periodontitis; AgP: Aggressive periodontitis; Minor allelic frequency; HWE, Hardy-Weinberg equilibrium.
All studies were case-control in design. Of these case-control studies, for the MMP-9 -1562C>T polymorphism, 13 case-control studies in ten publications (18–28) were available, including 1396 cases and 1709 controls. For the MMP-2 -753C>T polymorphism, six case-control studies (19,21–23,27) involved a total of 693 cases and 636 controls. Among the 13 eligible studies for MMP-9 -1562C>T (rs3918242), four case-control studies (19,20,22,23) including 438 cases and 469 controls were undertaken in Caucasians (Czech and Turkey), seven case-control studies (21,24,26–28) containing 827 cases and 1144 controls were conducted in Asians (China, Taiwan, and India), and two case-control studies (18,25) with 131 cases and 92 controls was performed in Latinos populations (Brazil and Colombia). Of six case-control studies for MMP-2-753C>T (rs2285053), three case-control studies (19,22,23) including 328 cases and 391 controls were undertaken in Caucasians (Czech and Turkey) and three case-control studies (21,27) with 365 cases and 245 controls were performed in Asian (China and Taiwan) populations. The control populations of four studies deviated from Hardy–Weinberg equilibrium (HWE). Moreover, the minor allele frequencies (MAFs) for controls and genotype distributions for MMP-9 -1562C>T (rs3918242) and MMP-2 -753C>T (rs2285053) polymorphisms in different ethnicities are all listed in Table 1.
Quantitative Synthesis
MMP-9 -1562C>T Polymorphism
The main results of MMP-9 -1562C>T polymorphism meta-analysis are shown in Table 2. The pooled results based on all included studies not showed a significant association between MMP-9 -1562C>T and periodontitis risk under all genetic models (allele model: T vs. C, OR=0.787, 95% C=0.459–1.348, P=0.383 (Fig. 2A); heterozygote model: TC vs. CC, OR=0.795, 95% CI=0.546–1.156, P=0.229; homozygote model: TT vs. CC, OR=0.600, 95% CI=0.237–1.517, P=0.280 (Fig. 2B); dominant model: TT+TC vs. CC, OR=0.767, 95% CI=0.463–1.269, P=0.301; and recessive model: TT vs. TC+CC, OR=0.691, 95% CI=0.330–1.444, P=0.326). In the subgroup analyses by the disease type, there was a significant association between MMP-9 -1562C>T polymorphism and periodontitis risk under the homozygote model (TT vs. CC: OR=0.375, 95% C=0.146–0.964, P=0.042) and the recessive model (TT vs. TC+CC: OR=0.473, 95% C=0.235–0.954, P=0.036) in the CP group. In addition, there was a significant association between MMP-9 -1562C>T polymorphism and periodontitis under the recessive model (TT vs. TC+CC: OR=2.585, 95% C=1.177–5.678, P=0.018) in the AgP group. In the subgroup analyses by ethnicity, there was a significant association between MMP-9 -1562C>T polymorphism and periodontitis risk under all genetic models (allele model: T vs. C, OR=0.723, 95% C=0.572–0.914, P=0.007; heterozygote model: TC vs. CC, OR=0.753, 95% CI=0.568–1.000, P=0.050; homozygote model: TT vs. CC, OR=0.347, 95% CI 0.133–0.909, P=0.031; dominant model: TT+TC vs. CC, OR=0.709, 95% CI=0.538–0.936, P=0.015; and recessive model: TT vs. TC+CC, OR=0.378, 95% CI=0.145–0.983, P=0.046) in the Caucasians, but not in Asian and Latinos populations. Moreover, when stratifying the studies by HWE status, a significant association between MMP-9 -1562C>T polymorphism and periodontitis risk was observed only under recessive model (TT vs. TC+CC, OR=1.873, 95% CI=1.123–3.125, P=0.016) (Table 2).
Table 2:
The results of meta-analysis for association of MMP-9 -1562C>T polymorphism with periodontitis risk
| Polymorphism | Genetic Model | Type of Model | Heterogeneity | Odds Ratio | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I2(%) | PH | OR | 95% CI | Ztest | POR | PBeggs | PEggers | |||
| Overall | T vs. C | Random | 94.15 | ≤0.001 | 0.787 | 0.459–1.348 | −0.872 | 0.383 | 0.854 | 0.033 |
| TC vs. CC | Random | 78.19 | ≤0.001 | 0.795 | 0.546–1.156 | −1.202 | 0.229 | 0.427 | 0.180 | |
| TT vs. CC | Random | 86.17 | ≤0.001 | 0.600 | 0.237–1.517 | −1.080 | 0.280 | 0.854 | 0.156 | |
| TT+TC vs. CC | Random | 89.69 | ≤0.001 | 0.767 | 0.463–1.269 | −1.034 | 0.301 | 0.582 | 0.050 | |
| TT vs. TC+CC | Random | 79.48 | ≤0.001 | 0.691 | 0.330–1.444 | −0.983 | 0.326 | 0.854 | 0.206 | |
| Periodontitis Type | ||||||||||
| CP | T vs. C | Random | 94.45 | ≤0.001 | 0.611 | 0.321–1.163 | −1.500 | 0.134 | 0.754 | 0.140 |
| TC vs. CC | Random | 81.95 | ≤0.001 | 0.676 | 0.416–1.101 | −1.573 | 0.116 | 0.465 | 0.326 | |
| TT vs. CC | Random | 82.78 | ≤0.001 | 0.375 | 0.146–0.964 | −2.036 | 0.042 | 0.916 | 0.357 | |
| TT+TC vs. CC | Random | 90.85 | ≤0.001 | 0.607 | 0.324–1.137 | −1.558 | 0.119 | 0.602 | 0.175 | |
| TT vs. TC+CC | Random | 71.17 | 0.001 | 0.473 | 0.235–0.954 | −2.092 | 0.036 | 1.000 | 0.452 | |
| AgP | T vs. C | Random | 74.32 | 0.009 | 1.373 | 0.812–2.320 | 1.183 | 0.237 | 1.000 | 0.405 |
| TC vs. CC | Fixed | 0.00 | 0.716 | 1.112 | 0.806–1.534 | 0.644 | 0.519 | 0.308 | 0.283 | |
| TT vs. CC | Random | 63.47 | 0.042 | 1.849 | 0.407–8.396 | 0.796 | 0.426 | 0.734 | 0.307 | |
| TT+TC vs. CC | Fixed | 41.65 | 0.162 | 1.199 | 0.880–1.632 | 1.151 | 0.250 | 0.308 | 0.238 | |
| TT vs. TC+CC | Fixed | 60.89 | 0.053 | 2.585 | 1.177–5.678 | 2.367 | 0.018 | 0.734 | 0.327 | |
| By ethnicity | ||||||||||
| Caucasian | T vs. C | Fixed | 48.15 | 0.122 | 0.723 | 0.572–0.914 | −2.717 | 0.007 | 0.089 | 0.013 |
| TC vs. CC | Fixed | 36.74 | 0.192 | 0.753 | 0.568–1.000 | −1.962 | 0.050 | 0.089 | 0.044 | |
| TT vs. CC | Fixed | 0.00 | 0.594 | 0.347 | 0.133–0.909 | −2.155 | 0.031 | 0.308 | 0.058 | |
| TT+TC vs. CC | Fixed | 46.13 | 0.135 | 0.709 | 0.538–0.936 | −2.429 | 0.015 | 0.089 | 0.015 | |
| TT vs. TC+CC | Fixed | 0.00 | 0.640 | 0.378 | 0.145–0.983 | −1.994 | 0.046 | 0.308 | 0.107 | |
| Asian | T vs. C | Random | 96.84 | ≤0.001 | 0.856 | 0.345–2.121 | −0.336 | 0.737 | 0.763 | 0.035 |
| TC vs. CC | Random | 87.48 | ≤0.001 | 0.812 | 0.424–1.555 | −0.628 | 0.530 | 0.367 | 0.073 | |
| TT vs. CC | Random | 92.92 | ≤0.001 | 1.017 | 0.279–3.710 | 0.026 | 0.979 | 0.229 | 0.067 | |
| TT+TC vs. CC | Random | 94.36 | ≤0.001 | 0.806 | 0.339–1.919 | −0.487 | 0.626 | 0.367 | 0.015 | |
| TT vs. TC+CC | Random | 89.37 | ≤0.001 | 1.092 | 0.404–2.946 | 0.173 | 0.863 | 0.229 | 0.061 | |
| Mixed | T vs. C | Fixed | 0.00 | 0.596 | 0.885 | 0.494–1.585 | −0.412 | 0.681 | NA | NA |
| TC vs. CC | Fixed | 0.00 | 0.453 | 1.081 | 0.553–2.111 | 0.228 | 0.820 | NA | NA | |
| TT vs. CC | Fixed | 0.00 | 0.883 | 0.228 | 0.023–2.242 | −1.267 | 0.205 | NA | NA | |
| TT+TC vs. CC | Fixed | 0.00 | 0.512 | 0.978 | 0.509–1.879 | −0.066 | 0.947 | NA | NA | |
| TT vs. TC+CC | Fixed | 0.00 | 0.915 | 0.227 | 0.023–2.213 | −1.277 | 0.202 | NA | NA | |
| By HWE | ||||||||||
| T vs. C | Random | 75.10 | ≤0.001 | 1.138 | 0.771–1.679 | 0.652 | 0.514 | 0.602 | 0.337 | |
| TC vs. CC | Fixed | 33.17 | 0.152 | 1.067 | 0.848–1.342 | 0.554 | 0.579 | 0.916 | 0.921 | |
| TT vs. CC | Random | 53.28 | 0.029 | 1.366 | 0.570–3.273 | 0.699 | 0.485 | 0.175 | 0.026 | |
| TT+TC vs. CC | Random | 63.18 | 0.005 | 1.102 | 0.761–1.597 | 0.516 | 0.606 | 0.916 | 0.696 | |
| TT vs. TC+CC | Fixed | 45.56 | 0.065 | 1.873 | 1.123–3.125 | 2.402 | 0.016 | 0.175 | 0.028 | |
CP: Chronic periodontitis; AgP: Aggressive periodontitis; NA: not applicable
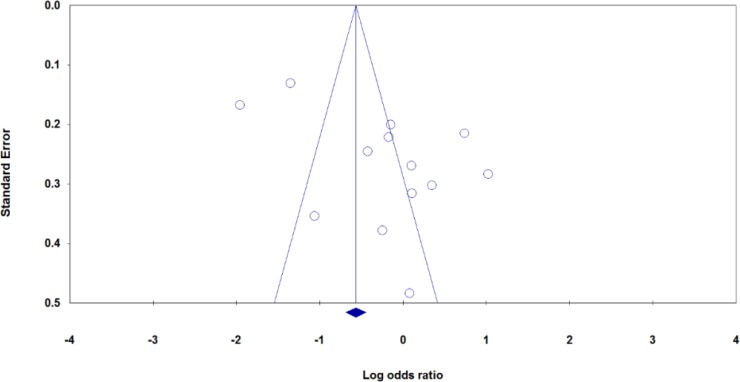
Fig. 2:
Begg’s funnel plots of the MMP-9-1562C>T polymorphism and periodontitis risk for publication bias test under allele model (T vs. C)
MMP-2-753C>T Polymorphism
The main results of MMP-2 -753C>T polymorphism meta-analysis are shown in Table 3. The overall analyses suggested no significant association between the MMP-2 -753C>T polymorphism and periodontitis susceptibility in all genetic models (allele model: T vs. C, OR=0.940, 95% C=0.780–1.132, P=0.513; heterozygote model: TC vs. CC, OR=0.985, 95% CI=0.776–1.249, P=0.898; homozygote model: TT vs. CC, OR=0.827, 95% CI=0.486–1.406, P=0.482; dominant model: TT+TC vs. CC, OR=0.844, 95% CI=0.574–1.242, P=0.391 (Fig. 2C); and recessive model: TT vs. TC+CC, OR=0.828, 95% CI=0.492–1.394, P=0.477). In the subgroup analyses, there was not a significant association between MMP-2 -753C>T polymorphism and periodontitis risk under all five genetic models in the CP and AgP groups. We then performed stratified analysis by ethnicity and found a significant association between the MMP-2 -753C>T polymorphism and periodontitis risk in the Asians under the allele model (T vs. C, OR=0.766, 95% C=0.590–0.996, P=0.046) and the recessive mode (TT vs. TC+CC, OR=0.587, 95% CI=0.320–1.237, P=0.002), but not in Caucasian populations.
Table 3:
The results of meta-analysis for association of MMP-2 -753C>T polymorphism with periodontitis risk
| Polymorphism | Genetic Model | Type of Model | Heterogeneity | Odds Ratio | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I2(%) | PH | OR | 95% CI | Ztest | POR | PBeggs | PEggers | |||
| Overall | T vs. C | Fixed | 17.36 | 0.301 | 0.940 | 0.780–1.132 | −0.654 | 0.513 | 1.000 | 0.832 |
| TC vs. CC | Fixed | 0.00 | 0.463 | 0.985 | 0.776–1.249 | −0.128 | 0.898 | 1.000 | 0.571 | |
| TT vs. CC | Fixed | 0.00 | 0.715 | 0.827 | 0.486–1.406 | −0.703 | 0.482 | 0.707 | 0.384 | |
| TT+TC vs. CC | Random | 64.75 | 0.014 | 0.844 | 0.574–1.242 | −0.858 | 0.391 | 0.452 | 0.212 | |
| TT vs. TC+CC | Fixed | 0.00 | 0.797 | 0.828 | 0.492–1.394 | −0.711 | 0.477 | 0.707 | 0.899 | |
| Periodontitis Type CP | T vs. C | Fixed | 9.48 | 0.331 | 0.914 | 0.706–1.182 | −0.689 | 0.491 | 0.296 | 0.192 |
| TC vs. CC | Fixed | 0.00 | 0.386 | 0.940 | 0.680–1.299 | −0.375 | 0.708 | 0.296 | 0.440 | |
| TT vs. CC | Fixed | 0.00 | 0.536 | 0.834 | 0.421–1.652 | −0.522 | 0.602 | 0.296 | 0.306 | |
| TT+TC vs. CC | Fixed | 15.62 | 0.306 | 0.931 | 0.684–1.269 | −0.451 | 0.652 | 0.296 | 0.330 | |
| TT vs. TC+CC | Fixed | 0.00 | 0.657 | 0.890 | 0.455–1.739 | −0.341 | 0.733 | 0.296 | 0.403 | |
| AgP | T vs. C | Fixed | 46.58 | 0.154 | 0.969 | 0.740–1.270 | −0.225 | 0.822 | 1.000 | 0.372 |
| TC vs. CC | Fixed | 21.62 | 0.279 | 1.040 | 0.731–1.480 | 0.219 | 0.826 | 1.000 | 0.314 | |
| TT vs. CC | Fixed | 0.00 | 0.438 | 0.816 | 0.351–1.898 | −0.472 | 0.637 | 1.000 | 0.898 | |
| TT+TC vs. CC | Random | 82.45 | 0.003 | 0.731 | 0.324–1.650 | −0.754 | 0.451 | 0.296 | 0.059 | |
| TT vs. TC+CC | Fixed | 0.00 | 0.494 | 0.741 | 0.323–1.697 | −0.709 | 0.478 | 1.000 | 0.805 | |
| By ethnicity Caucasian | T vs. C | Fixed | 0.00 | 0.587 | 1.162 | 0.890–1.516 | 1.104 | 0.269 | 1.000 | 0.551 |
| TC vs. CC | Fixed | 0.00 | 0.753 | 1.227 | 0.888–1.692 | 1.238 | 0.216 | 1.000 | 0.549 | |
| TT vs. CC | Fixed | 0.00 | 0.763 | 1.333 | 0.583–3.046 | 0.681 | 0.496 | 1.000 | 0.823 | |
| TT+TC vs. CC | Fixed | 0.00 | 0.713 | 1.233 | 0.902–1.686 | 1.311 | 0.190 | 1.000 | 0.768 | |
| TT vs. TC+CC | Fixed | 0.00 | 0.798 | 1.237 | 0.546–2.805 | 0.510 | 0.610 | 1.000 | 0.766 | |
| Asian | T vs. C | Fixed | 0.00 | 0.906 | 0.766 | 0.590–0.996 | −1.998 | 0.046 | 0.296 | 0.536 |
| TC vs. CC | Fixed | 0.00 | 0.912 | 0.759 | 0.533–1.079 | −1.537 | 0.124 | 0.296 | 0.108 | |
| TT vs. CC | Fixed | 0.00 | 0.918 | 0.590 | 0.295–1.181 | −1.489 | 0.136 | 1.000 | 0.834 | |
| TT+TC vs. CC | Fixed | 40.21 | 0.188 | 0.587 | 0.421–0.818 | −3.144 | 0.002 | 1.000 | 0.488 | |
| TT vs. TC+CC | Fixed | 0.00 | 0.836 | 0.630 | 0.320–1.237 | −1.343 | 0.179 | 0.296 | 0.274 | |
CP: Chronic periodontitis; AgP: Aggressive periodontitis.
Minor Allele Frequencies (MAFs)
The allele and genotype distributions of MMP-9 -1562C>T and MMP-2 -753C>T polymorphisms by ethnicity are presented in Table 1. The minor allele frequencies of the MMP-9 -1562C>T and MMP-2 -753C>T polymorphisms exhibited ethnic variations. The MMP-9 -1562T allele frequencies in the Asians, Caucasians and Latinos populations were 39.65% (10.9%–68.4%), 22.35% (17.4%–27.3%) and 13.55% (7.4%–19.7%), respectively. The MMP-2 -753T allele frequencies in the Asians and Caucasians were 21.6% (12.5%–30.7%) and 17.6% (14.9%–20.3%), respectively. Therefore, the frequencies of the MMP-9 -1562T and MMP-2 -753T alleles in Caucasians were less than Asians.
Sensitivity Analysis
We have performed sensitivity analysis by omitting individual studies to assess the effect of each publication on the overall results. However, the significance of pooled ORs not influenced by omitting those studies, indicating that the results were stable. Additionally, we have performed sensitivity analysis by omitting six studies in which the genotype distributions of MMP-9 -1562C>T polymorphism in the healthy controls significantly deviated from the HWE. The results showed a significant association between MMP-9 -1562C>T polymorphism and periodontitis risk under recessive model (TT vs. TC+CC, OR=1.873, 95% CI=1.123–3.125, P=0.016), which suggests that the results of our meta-analysis are affected by HWE status.
Publication Bias
We have performed Begg’s funnel plot and Egger’s test to detect the publication bias of included studies. The shape of the funnel plot did not reveal any evidence of obvious asymmetry for MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms under all genetic models. However, the results of Egger’s test showed evidence of publication bias for MMP-9 -1562C>T under allele model (T vs. C: PBegg’s=0.854, PEgger’s=0.033). Then, we have used the Duval and Tweedie non-parametric ‘‘trim and fill’’ method to adjust the publication bias. However, meta-analysis with and without ‘‘trim and fill’’ did not draw different conclusion, indicating that our findings were statistically robust.
Discussion
In general, several studies revealed the relation between MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms and susceptibility of periodontitis, however; the main findings from the different case-control studies did not reach the same conclusion. This inconsistency may result from the small sample size and the different experimental methods such as genotyping methods, ethnicity background, and subject’s gender. The present study showed that the MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms were not associated with the susceptibility of periodontitis in overall analysis. However, there is still a need for further research and screening of etiological relations of the MMP-2 and 9 genes functional polymorphisms with the susceptibility of periodontitis. The limited statistical results may be reasonable due to the differences in the ethnic background, because different populations have different frequencies of alleles, and different genetic backgrounds may affect periodontitis risk. Therefore, we have performed subgroup analyses by ethnicity and disease type. The stratified analyses by ethnicity and periodontitis type revealed that the MMP-9 -1562C>T polymorphism showed a significant association with the risk of periodontitis among Caucasians and CP/AgP subgroup, whereas MMP-2 -753C>T polymorphism was significantly associated with periodontitis risk only among Asians.
We found that the presence of T allele in MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms is not significantly associated with an increased risk of periodontitis. Recently, two meta-analyses estimated the association between MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms and periodontitis risk, which was basically in accordance with our results that MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms may not contribute to the susceptibility of periodontitis in overall analysis (8,11). Moreover, at least four case-control studies (in two publications) (27,28) have not included in the meta-analysis (8). Additionally, Li et al. have included only two studies for MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms (11). Thus, the ongoing uncertainty still exists and the conclusion by might be biased by not inclusion of new published studies (8,11).
In our meta-analysis, we accurately assessed the association between these MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms and the risk of chronic/aggressive periodontitis by taking into account the effects of new published studies. Moreover, a significant association had not found between MMP-2 -753C>T polymorphism and periodontitis according to disease type and ethnicity (8), while we have found that MMP-2 -753C>T polymorphism was significantly associated with periodontitis risk only among Asians. Therefore, both MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms might have influence on the susceptibility of periodontitis by ethnicity background. Additionally, in the present meta-analysis we have provided the actually numbers of minor allele frequencies (MAFs) in the controls. The conclusion by our meta-analysis was more credible.
Between-study heterogeneity is very common and expected in the genetic association studies of meta-analysis (29,30). Therefore, it is necessary to evaluate the magnitude of heterogeneity in a meta-analysis for determining the strengths of pooled results (31,32). We found relatively a high heterogeneity (>70%) for MMP-9 -1562C>T in overall analysis in all genetic models, but not for MMP-2 -753C>T polymorphism. We suggested that several factors including genetic backgrounds for cases and controls, diverse genotype distribution of MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms in the included ethnicity groups, types of periodontitis (CP/AgP), different genotyping methods, sample size of included studies, and uneven selection criteria for the cases and controls in different studies, responsible for such heterogeneity in our meta-analysis. Moreover, we have performed subgroup analysis by ethnicity, periodontitis type and HWE status to finding source of heterogeneity. The heterogeneity was reduced in the AgP group and also disappeared in Caucasians and Latinos populations, but not in the Asians and by HWE status. In addition, we have found that that the heterogeneity was significantly reduced in the small sample size group in all genetic models, suggesting that the total sample size, ethnicity background, periodontitis type and HWE status were the sources of heterogeneity.
Although we performed a comprehensive meta-analysis, some limitations should be acknowledged. First, the number of published studies for MMP-2 -753C>T polymorphism was not sufficiently large for a comprehensive analysis, especially for stratified analyses by ethnicity. Second, the majority of the included studies was Asians or Caucasians, because of limited available data for MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms from another ethnicity such as Africans, our results should be interpreted with caution. Larger studies are needed to clarify whether these two polymorphisms could truly affect the risk of periodontitis in other ethnicities. Third, our results were based on single-factor estimates without adjustment for other risk factors, therefore; our results may be affected by additional confounding factors, such as age, gender, smoking status, another chronic disease such as diabetes, caused serious confounding bias. If we had been able to acquire more detailed data, we would have achieved estimations that are more precise by adjusting for other potential covariates, but most of the included studies either did not report the data or aggregated them in different ways, making it impossible to include the data in the current meta-analysis. Therefore, studies with good design are needed in the future, and ORs adjusted for other confounding factors need reporting. Finally, it is also possible that language bias will exist, as in the present meta-analysis we have only included articles published in English. Finally, we have not addressed the effect of gene-gene and gene-environment interactions.
Conclusion
MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms may not be associated with periodontitis risk in overall analysis. However, both MMP-2 -753C>T and MMP-9 -1562C>T polymorphisms might have influence on the susceptibility of periodontitis by ethnicity background.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
No grants were involved in supporting this work.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Taba M, Jr, Souza SL, Mariguela VC. (2012). Periodontal disease: a genetic perspective. Braz Oral Res, 26 Suppl 1:32–8. [DOI] [PubMed] [Google Scholar]
- 2.AlJehani YA. (2014). Risk factors of periodontal disease: review of the literature. Int J Dent, 2014:182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Mashhadiabbas F, Neamatzadeh H, Nasiri R, et al. (2018). Association of vitamin D receptor BsmI, TaqI, FokI, and ApaI polymorphisms with susceptibility of chronic periodontitis: A systematic review and meta-analysis based on 38 case–control studies. Dent Res J (Isfahan), 15(3):155–165. [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Amar S. (2006). Periodontal disease and systemic conditions: a bidirectional relationship. Odontology, 94(1):10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hugoson A, Norderyd O. (2008). Has the prevalence of periodontitis changed during the last 30 years? J Clin Periodontol, 35(8 Suppl):338–45. [DOI] [PubMed] [Google Scholar]
- 6.Highfield J. (2009). Diagnosis and classification of periodontal disease. Aust Dent J, 54 Suppl 1:S11–26. [DOI] [PubMed] [Google Scholar]
- 7.Tarannum F, Faizuddin M. (2012). Effect of gene polymorphisms on periodontal diseases. Indian J Hum Genet, 18(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng H, Yan Y, Jin YH, et al. (2016). Matrix metalloproteinase gene polymorphisms and periodontitis susceptibility: a meta-analysis involving 6,162 individuals. Sci Rep, 6:24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laine ML, Loos BG, Crielaard W. (2010). Gene polymorphisms in chronic periodontitis. Int J Dent, 2010:324719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song GG, Kim JH, Lee YH. (2013). Toll-like receptor (TLR) and matrix metalloproteinase (MMP) polymorphisms and periodontitis susceptibility: a meta-analysis. Mol Biol Rep, 40(8):5129–41. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Zhu Y, Singh P, Ajmera DH, et al. (2016). Association of Common Variants in MMPs with Periodontitis Risk. Dis Markers, 2016: 1545974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikhpour E, Noorbakhsh P, Foroughi E, et al. (2018). A Survey on the Role of Interleukin-10 in Breast Cancer: A Narrative. Rep Biochem Mol Biol, 7(1):30–37. [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimi Z, Lotfi S, Ahmadi A, et al. (2018). Matrix metalloproteinase-2 C-735T and its interaction with matrix metalloproteinase-7 A-181G polymorphism are associated with the risk of preeclampsia: influence on total antioxidant capacity and blood pressure. J Obstet Gynaecol, 38(3):327–32. [DOI] [PubMed] [Google Scholar]
- 14.Klein T, Bischoff R. (2011). Physiology and pathophysiology of matrix metalloproteases. Amino Acids, 41(2):271–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu P, Takai K, Weaver VM, et al. (2011). Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb Perspect Biol, 3(12): a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maral S, Acar M, Balcik OS, et al. (2015). Matrix Metalloproteinases 2 and 9 Polymorphism in Patients With Myeloproliferative Diseases: A STROBE-Compliant Observational Study. Medicine (Baltimore), 94(16):e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rybakowski JK. (2009). Matrix Metalloproteinase-9 (MMP9)-A Mediating Enzyme in Cardiovascular Disease, Cancer, and Neuropsychiatric Disorders. Cardiovasc Psychiatry Neurol, 2009:904836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Souza AP, Trevilatto PC, Scarel-Caminaga RM, et al. (2005). Analysis of the MMP-9 (C-1562 T) and TIMP-2 (G-418C) gene promoter polymorphisms in patients with chronic periodontitis. J Clin Periodontol, 32(2):207–11. [DOI] [PubMed] [Google Scholar]
- 19.Holla LI, Fassmann A, Mužík J, et al. (2006). Functional Polymorphisms in the Matrix Metalloproteinase-9 Gene in Relation to Severity of Chronic Periodontitis. J Periodontol, 77(11):1850–5. [DOI] [PubMed] [Google Scholar]
- 20.Keles GC, Gunes S, Sumer AP, et al. (2006). Association of Matrix Metalloproteinase-9 Promoter Gene Polymorphism With Chronic Periodontitis. J Periodontol, 77(9):1510–4. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Wang Q, Ma ZW, et al. (2007). MMP-2, MMP-9 and TIMP-2 gene polymorphisms in Chinese patients with generalized aggressive periodontitis. J Clin Periodontol, 34(5):384–9. [DOI] [PubMed] [Google Scholar]
- 22.Gürkan A, Emingil G, Saygan BH, et al. (2008). Gene polymorphisms of matrix metalloproteinase-2, -9 and -12 in periodontal health and severe chronic periodontitis. Arch Oral Biol, 53(4):337–45. [DOI] [PubMed] [Google Scholar]
- 23.Gürkan A, Emingil G, Saygan BH, et al. (2007). Matrix Metalloproteinase-2, -9, and -12 Gene Polymorphisms in Generalized Aggressive Periodontitis. J Periodontol, 78(12):2338–47. [DOI] [PubMed] [Google Scholar]
- 24.Loo WTY, Wang M, Jin LJ, et al. (2011). Association of matrix metalloproteinase (MMP-1, MMP-3 and MMP-9) and cyclooxygenase-2 gene polymorphisms and their proteins with chronic periodontitis. Arch Oral Biol, 56(10):1081–90. [DOI] [PubMed] [Google Scholar]
- 25.Isaza-Guzmán DM, Arias-Osorio C, Martínez-Pabón MC, et al. (2011). Salivary levels of matrix metalloproteinase (MMP)-9 and tissue inhibitor of matrix metalloproteinase (TIMP)-1: a pilot study about the relationship with periodontal status and MMP-9(-1562C/T) gene promoter polymorphism. Arch Oral Biol, 56(4):401–11. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Yue Y, Tian Y, Li J, et al. (2012). Association of matrix metalloproteinase (MMP)-1, 3, 9, interleukin (IL)-2, 8 and cyclooxygenase (COX)-2 gene polymorphisms with chronic periodontitis in a Chinese population. Cytokine, 60(2):552–60. [DOI] [PubMed] [Google Scholar]
- 27.Hsiao YF, Yang LC, Chou YS, et al. (2016). Matrix metalloproteinase-2, -9, and tissue inhibitor of MMP-2 gene polymorphisms in Taiwanese periodontitis patients. J Dent Sci, 11(4):411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majumder P, Singh SJ, Nair V, et al. (2017). Alliance of matrix metalloproteinase-9 (MMP-9) promoter gene polymorphism with chronic and aggressive periodontitis in Indian population. Meta Gene, 12:88–93. [Google Scholar]
- 29.Gohari M, Neámatzadeh H, Jafari MA, et al. (2016). Association between the p53 codon 72 polymorphism and primary open-angle glaucoma risk: Meta-analysis based on 11 case-control studies. Indian J Ophthalmol, 64(10):756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobhan MR, Mahdinezhad-Yazdi M, Aghili K, et al. (2018). Association of TNF-α-308 G>T; A and −238 G>T; A polymorphisms with knee osteoarthritis risk: A case-control study and meta-analysis. J Orthop, 15(3):747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeghiyeh T, Hosseini Biouki F, Mazaheri M, et al. (2017). Association between Catechol-O-Methyltransferase Val158Met (158G/A) Polymorphism and Suicide Susceptibility: A Meta-analysis. J Res Health Sci, 17(2):e00383. [PubMed] [Google Scholar]
- 32.Abedinzadeh M, Zare-Shehneh M, Neamatzadeh H, et al. (2015). Association between MTHFR C677T polymorphism and risk of prostate cancer: Evidence from 22 studies with 10,832 cases and 11,993 controls. Asian Pac J Cancer Prev, 16(11):4525–30 [DOI] [PubMed] [Google Scholar]