Abstract
Background:
Some studies have investigated the prevalence of osteoporosis in patients with type 2 diabetes mellitus (T2DM) in China. However, the results were inconsistent. This review was performed to estimate the prevalence of osteoporosis in T2DM patients in the Chinese mainland and to characterize its epidemiology.
Methods:
A literature search was conducted utilizing PubMed, Scopus, Web of Science, Cochrane Library, CNKI, Wanfang database from their inception through June 2017. A total of 54 studies evaluating the prevalence rate of osteoporosis in T2DM patients were collected. Prevalence estimates from the individual study were combined utilizing random-effect models in Stata 12.0.
Results:
The pooled prevalence rate of osteoporosis in T2DM patients was 37.8%. Notably, osteoporosis was more frequent in females than in males (44.8% vs. 37.0%) and was increased with ageing (over 60: 40.1% vs. below 60: 26.5%). Osteoporosis prevalence was higher in less developed areas than in developed areas (41.0% vs. 32.7%) and almost the same between the southern and northern regions (37.6% vs. 38.2%). The prevalence rate between 2010 and 2017 decreased compared with the period between 2001 and 2009 (42.3% vs. 35.6%). Additionally, the meta-regression suggested that gender and age could significantly influence the estimation of prevalence rates respectively (P = 0.011, P = 0.022).
Conclusion:
Osteoporosis affects more than one-third of T2DM patients in China mainland. Females and older adults more likely require clinical prevention due to a higher prevalence. Further studies are needed to be conducted to incorporate and verify previous results.
Keywords: Type 2 diabetes mellitus, Osteoporosis, Prevalence, Meta-analysis, China
Introduction
With the aging population continuing to grow, there has been a significant rise in the prevalence of Type 2 diabetes mellitus (T2DM). It has been observed in practically all regions of the world, with 415 million people suffering from diabetes worldwide (1). In recent decades, people with diabetes in China have increased on a yearly basis. In 2007, there were about 40 million diabetics in China, and it has been estimated that diabetics in China will reach approximately 42.3 million by 2030 (2–4). By that time, China will replace India as the country with the most diabetes patients worldwide.
Diabetes influences the function of multiple organs in the human body, including the heart, brain, kidney, peripheral nerves, eyes, and feet. Additionally, there can also be more than 100 kinds of complications involved with the disease, and it is currently known as the disease with the most complications (5). Throughout recent years, studies on the correlation between diabetes and osteoporosis have been widely recognized by scholars. Both osteoporosis and diabetes are metabolic diseases, and they have a complicated relationship with each other. It is well recognized that type 1 diabetes mellitus can decrease bone mineral density (BMD) and increase the risk of bone fracture (6), while the correlation between T2DM and osteoporosis remains unclear. Therefore, it is necessary for both physicians and patients to be aware of the prevalence rate of osteoporosis in T2DM patients and to learn the relevant characteristics in the diagnosed populations for the sake of early prevention. At present, no systematic review and meta-analysis regarding the prevalence rate of osteoporosis in T2DM patients in China has been conducted.
The objective of this review was to acquire reference values for the prevalence of osteoporosis in T2DM patients utilizing the meta-analysis method. We also described the characteristics of T2DM patients with osteoporosis based on a large sample size, which is intended to provide evidence for physicians as well as the health supervision department.
Methods
Search Strategy
According to the PRISMA (2009) standard, we searched PubMed, Scopus, Web of science, Cochrane Library, CNKI, Wanfang data for relevant studies (updated until June 6, 2017). Subject retrieval words and keywords were as follows: type 2 diabetes mellitus, diabetes, T2DM, osteoporosis, prevalence, and epidemiological investigation. Literature languages are not limited.
Study Selection
Studies involved in this review met the following criteria: the participants were clinically diagnosed with T2DM in the Chinese Mainland; the study design was a cross-sectional study; the test equipment utilized was the BMD dual-energy X-ray absorptiometry and the parts that were tested consisted of the lumbar or the hip. Notably, the prevalence of osteoporosis in T2DM patients can be directly extracted from literature or indirectly calculated.
Data Collection and Assessment Processes
The retrieved studies were simultaneously and independently screened by two reviewers based on the previously formulated inclusion criteria. The extraction content mainly consisted of the first author, date of publication, diagnostic criteria, sample size, test instrument, test parts, and prevalence. When research reporting positive detection rate of osteoporosis in more than one part was observed, we chose the positive detection rate of the lumbar vertebra to be the standard. After disqualified and repetitive studies removing, the remaining studies were subjected to full-text reading and re-screening. If the information provided were unclear or disputable, the corresponding author of the study would be contacted for a thorough inquiry. Then, decisions of whether to keep the information or discard it would be made. Throughout the course of this process, all disagreements between reviewers were resolved through discussion.
Included studies were graded in 7 aspects according to the Combie evaluation tool which is as follows (7): the study design was scientific and rigorous; the data collection method was reasonable; the response rate of participants was reported; the total representativeness of samples were favorable; the research objective and methods were reasonable; the power of the test was reported; the statistical method was correct. “Yes”, “no” and “have no idea” were respectively utilized to evaluate each item, which was successively given 1 point, 0 points, and 0.5 points. The total score was 7.0 points (6.0∼7.0 points, 4.0∼5.5 points, and 0∼4.0 points were considered to an A, B and C level of quality respectively).
Statistical Analysis
The primary analysis in this review was the pooled prevalence of osteoporosis in T2DM patients. Heterogeneity was evaluated using the Cochran Q statistic (P< 0.10 was considered to be statistically significant) and was quantified using the I2 index (where I2> 30% indicated moderate heterogeneity; I2> 50% substantial heterogeneity; and I2> 75% considerable heterogeneity). If the I2 test indicated a value > 50% which reflected significant heterogeneity, then a random-effect model was carried out. Conversely, a fixed-effect model was implemented. The subgroup analysis was conducted based on sex (male or female), average age (younger or older than 60 years old), region (south or north), economic level (developed or less developed) and date of publication (2001∼2009 or 2010∼2016). Besides, a meta-regression analysis was applied as a means to explore the source of heterogeneity among all the studies. Specifically, the concomitant variable includes the date of publication, the female proportion in subjects, region, sample size, economic level, and average age. Funnel plot with the proportion as the abscissa and the standard error as the ordinate was adopted to reflect the publication bias directly. Begg’s test and Egger’s test were both applied to create a qualitative judgment on publication bias. Additionally, the trim and filling method would be utilized to evaluate the stability of the obtained result if necessary. All the above analyses were conducted using the Stata 12.0 version, and all reported P values were two-sided with a statistical significance level of 0.05.
Results
Literature Search and Characteristics
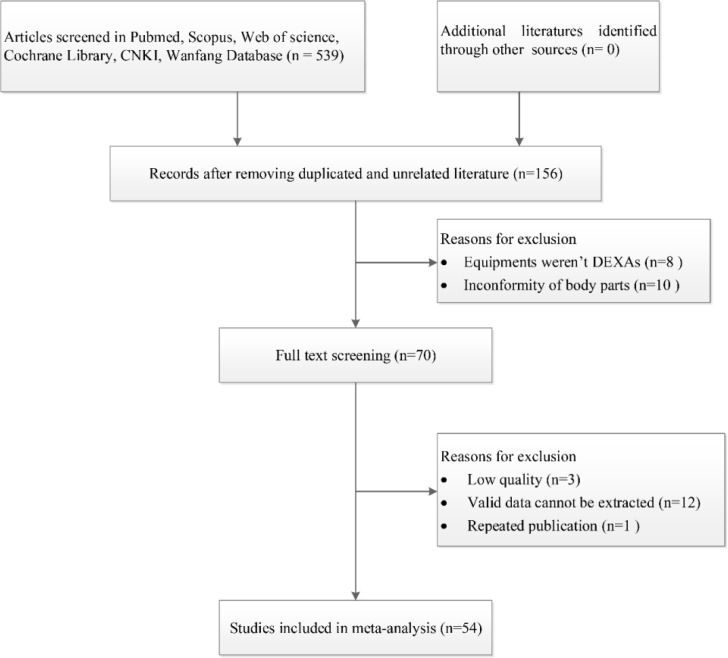
Figure 1 outlines the search strategy and the selection process. Of the 539 relevant studies initially identified, 383 duplicated records were removed, and 68 articles were subsequently excluded after reading titles and abstracts due to their irrelevant clinical question or incorrect publication type.
Fig. 1:
Flow diagram of included/excluded studies
After the full-text review of 70 studies, we further excluded 16 studies that did not meet the inclusion criteria. Finally, 54 studies with 13462 diabetic patients were included in the meta-analysis (8–61). The majority (79.6%) of the included articles scored greater than 4 points as assessed through the Combie evaluation tool, which indicated high and moderate quality literature. The main characteristics and details of each study are summarized in Table 1.
Table 1:
Characteristics of included studies
| No. | Area | Measuring instrument type | Measure parts | Sample size | M/F | Age (yr) | Quality |
|---|---|---|---|---|---|---|---|
| (1) | Hunan | USA Hologic-QDR-4500A | Lumbar, Left Hip | 1253 | 0/1253 | 59.8±8.61 | A |
| (2) | Liaoning | USA GE Lunar-Prodigy | Lumbar, Femoral Neck | 100 | 18/32 | 45∼75 | B |
| (3) | Jiangsu | France DMS CHALLENGER | Lumbar, Left Hip | 52 | 24/28 | 62.05±7.25 | B |
| (4) | Guang dong | NR | Lumbar, Left Hip | 165 | 85/80 | 60.91±8.91 | B |
| (5) | Zhejiang | USA GE Lunar-Prodigy | Lumbar | 72 | 33/39 | 55.9±3.3 | B |
| (6) | Jiangxi | France MEDILINK-OSTEOCORE2 | Lumbar, Femoral Neck | 100 | 52/48 | 63.9±6.5 | B |
| (7) | Hebei | France MEDILINK-OSTEOCORE2 | Lumbar, Hip | 105 | 59/46 | 62±11 | B |
| (8) | Hunan | USA Hologic-QDR-4500A | Lumbar, Hip | 168 | 0/168 | 60.95±12.16 | A |
| (9) | Shanghai | China DEXAUNT2000 | Lumbar, Hip | 70 | 29/41 | 68.26±7.35 | B |
| (10) | Zhejiang | France DMS CHALLENGER | Lumbar, Femoral Neck, Whole Body | 100 | NR | NR | C |
| (11) | Sichuan | NR | Lumbar, Hip | 230 | 100/130 | NR | C |
| (12) | Liaoning | Korea OsteoSys-Dexxum T | Lumbar, Femoral Neck | 232 | 64/168 | 66.1±9.2 | B |
| (13) | Anhui | USA GE Lunar-Prodigy | Lumbar, Hip | 118 | 42/76 | 51.6±12.5 | C |
| (14) | Anhui | NR | Hip | 162 | 87/75 | 45∼73 | C |
| (15) | Jiangxi | France DMS CHALLENGER | Lumbar, Left Hip | 204 | 95/109 | 59.54±10.30 | B |
| (16) | Sichuan | NR | Lumbar, Hip | 223 | 135/88 | NR | C |
| (17) | Tianjin | USA NOLAND-XR-800 | Lumbar, Hip | 168 | 80/88 | 55.6±11.3 | B |
| (18) | Beijing | USA Hologic-QDR-4500A | Lumbar, Hip | 306 | 154/152 | 69.2±6.8 | C |
| (19) | Beijing | NR | Lumbar, Hip | 200 | 96/104 | 65.43±8.53 | B |
| (20) | Guizhou | Janpan DAS 600EX | Lumbar | 38 | 38/0 | 74.0±6.9 | C |
| (21) | Hebei | USA Hologic-QDR-4500A | Lumbar, Hip | 158 | 158/0 | 65.8±5.7 | B |
| (22) | Beijing | USA GE Lunar-Prodigy | Lumbar, Hip | 102 | 41/61 | 41.0∼94.4 | A |
| (23) | Heilongjiang | USA GE Lunar-Prodigy | Lumbar, Hip | 194 | 91/103 | 76.85±4.55 | C |
| (24) | Jilin | USA GE Lunar-Prodigy | Lumbar, Hip | 68 | 68/0 | 52.85±10.9 | B |
| (25) | Yunnan | NR | Lumbar | 137 | 56/81 | 59.96±11.73 | B |
| (26) | Sichuan | France MEDILINK-OSTEOCORE2 | Lumbar, Left Hip | 96 | 0/51 | 65.37±7.86 | A |
| (27) | Fujian | USA GE Lunar-Prodigy | Lumbar, Hip | 182 | 97/85 | 65.11±3.68 | B |
| (28) | Fujian | USA GE Lunar-Prodigy | Lumbar, Hip | 85 | 0/85 | NR | C |
| (29) | Jiangsu | USA GE Lunar-DPX-IQ | Lumbar, Hip | 70 | 70/0 | 78.5±16.7 | B |
| (30) | Hebei | France MEDILINK-OSTEOCORE3 | Lumbar, Hip | 150 | 0/150 | 62.5±13.6 | B |
| (31) | Henan | USA Hologic-QDR-4500A | Lumbar, Left Hip | 355 | 194/162 | NR | C |
| (32) | Jiangsu | USA Hologic-EXPLORER | Lumbar, Hip | 529 | 268/261 | 63.07±11.20 | B |
| (33) | Anhui | USA GE Lunar-Prodigy | Lumbar, Left Hip | 254 | 131/123 | 70.9±9.81 | A |
| (34) | Tianjin | USA GE Lunar-Prodigy | Lumbar, Hip | 125 | 125/0 | 55.8±11.4 | A |
| (35) | Yunnan | USA GE Lunar-Prodigy | Lumbar, Hip | 1218 | 679/602 | 63.79±10.33 | B |
| (36) | Hunan | USA Hologic-Delhpi A | Lumbar, Left Hip | 3110 | 0/3110 | 59.3±10.8 | A |
| (37) | Guangdong | USA NORLAND XR-36 | Lumbar, Hip | 61 | 25/36 | 41∼71 | B |
| (38) | Hunan | USA Hologic-QDR-4500A | Lumbar, Hip | 214 | 0/214 | 59±8 | A |
| (39) | Henan | USA Hologic-QDR-4500W | Lumbar | 52 | 21/31 | 69±7 | C |
| (40) | Guangdong | USA GE Lunar-DPX-L | Lumbar, Left Hip | 537 | 203/334 | 50∼79 | B |
| (41) | Guangdong | USA GE Lunar-DPX-IQ | Lumbar, Hip | 103 | 55/48 | 61±7 | B |
| (42) | Shanghai | USA GE Lunar-Prodigy | Lumbar, Hip | 70 | 29/41 | 68.16±7.35 | A |
| (43) | Hebei | USA GE Lunar-DPX-NT | Lumbar, Hip, Distal Radius | 50 | 0/50 | 60.02±4.13 | B |
| (44) | Hebei | France MEDILINK-OSTEOCORE3 | Lumbar, Hip | 46 | 17/29 | 60.58±9.18 | B |
| (45) | Beijing | USA NORLAND XR-36 | Lumbar, Hip | 52 | 52/0 | 66.0±9.8 | B |
| (46) | Liaoning | France DMS lexxos | Lumbar, Hip | 62 | 0/62 | 50∼82 | A |
| (47) | Chongqing | China DEXAUNT2000 | Lumbar, Hip | 78 | 31/47 | 71.25±9.46 | B |
| (48) | Hebei | France MEDILINK-OSTEOCORE3 | Lumbar, Hip | 150 | 0/150 | 62.98±12.12 | B |
| (49) | Anhui | USA GE Lunar-Prodigy | Lumbar, Left Hip | 302 | 114/188 | 50∼70 | B |
| (50) | Hunan | USA Hologic-QDR-4500A | Lumbar, Left Hip | 197 | 0/166 | 48∼81 | B |
| (51) | Beijing | USA GE Lunar-Prodigy | Lumbar, Hip, Whole Body | 104 | 67/37 | 59±12 | A |
| (52) | Hebei | France MEDILINK-OSTEOCORE3 | Lumbar, Hip, Whole Body | 68 | 0/68 | 64.79±8.29 | B |
| (53) | Hebei | USA GE Lunar-Prodigy | Lumbar, Hip, Whole Body | 106 | 544/52 | 59.89±9.60 | B |
| (54) | Hunan | USA Hologic-QDR-4500A | Lumbar, Hip | 248 | 98/150 | M: 59.86±11.38 F: 61.25±9.57 |
A |
Abbreviations: M, Male; F, Female; NR, Not Reported
Pooled Prevalence Rates of Osteoporosis in Diabetic Patients
Overall
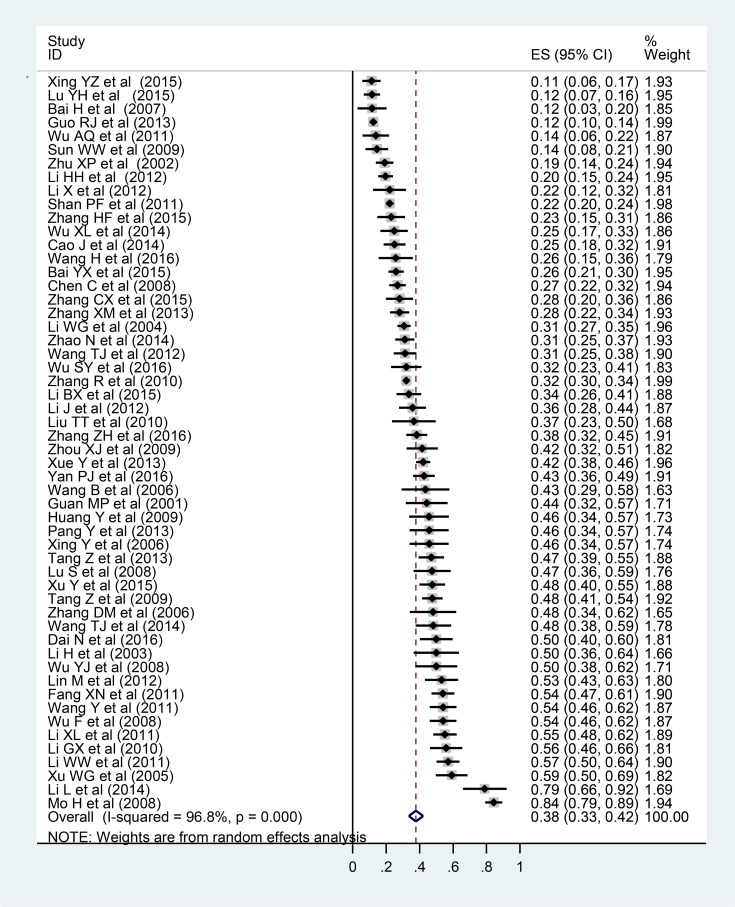
The pooled prevalence rate of osteoporosis in diabetic patients was 37.8% (95%CI: 33.5%, 42.1%, I2=96.8%, P<0.001) (Fig. 2). Subgroup analyses were applied based on sex, average age, region, economic level, and publication year in order to explore the source of heterogeneity.
Fig. 2:
Meta-analysis forest for the prevalence of osteoporosis in type 2 diabetes mellitus patients in Chinese mainland
Sex
Thirty-one studies and thirty-seven studies reported the prevalence rates of osteoporosis in male and female patients, respectively. The aggregated results indicated that the prevalence rates of osteoporosis in female and male were 44.8% (95%CI: 39.4, 50.2%) and 37.0% (95%CI: 27.5%, 49.8%) respectively (Table 2).
Table 2:
Prevalence of osteoporosis according to different items
| Category | Subgroup | NO. of studies | N (T2DM) | Events (OP) | Prevalence, 95%CI (%) | P (Q test) | I2(%) | Publication bias | |
|---|---|---|---|---|---|---|---|---|---|
| P (Begg’s) | P (Egger’s) | ||||||||
| Sex | Male | 31 | 3078 | 716 | 37.0 [27.5,49.8] | <0.001 | 90.4 | 0.905 | 0.711 |
| Female | 37 | 8477 | 2982 | 44.8 [39.4,50.2] | 96.1 | 0.143 | 0.001 | ||
| Average age | <60 | 12 | 5679 | 1649 | 26.5 [20.8,32.1] | <0.001 | 94.2 | 0.373 | 0.584 |
| ≧60 | 29 | 5267 | 1738 | 40.1 [33.3,46.9] | 96.9 | 0.511 | <0.001 | ||
| Area | Northern | 21 | 2901 | 1048 | 38.2 [31.2,45.1] | <0.001 | 94.3 | 0.147 | 0.007 |
| Southern | 33 | 10561 | 3332 | 37.6 [32.0,43.2] | 97.5 | 0.369 | 0.008 | ||
| Economic | Developed | 21 | 3371 | 1092 | 32.7 [26.2,39.1] | <0.001 | 94.6 | 0.147 | 0.155 |
| Less developed | 33 | 10091 | 3288 | 41.0 [35.2,46.8] | 97.5 | 0.193 | 0.001 | ||
| Publication year | 2001∼2009 | 18 | 2500 | 1006 | 42.3 [32.2,52.4] | <0.001 | 96.8 | 0.596 | 0.553 |
| 2010∼2016 | 36 | 10962 | 3374 | 35.6 [31.0,40.1] | 96.4 | 0.145 | 0.002 | ||
Abbreviations: T2DM, Type 2 Diabetes Mellitus; OP: Osteoporosis
Age
Twelve studies reported that patients average ages were younger than 60 years old, and the other twenty-nine studies reported 60 years old or older. The aggregated results revealed that the prevalence rates of osteoporosis in younger patients and older patients were 26.5% (95%CI: [20.8%, 32.1%]) and 40.1% (95%CI: [33.3%, 46.9%]) (Table 2).
Area
We geographically divided China into the southern and the northern regions with the Qinling Mountains-Huaihe River. Twenty-one studies and thirty-three studies were conducted in the southern and northern areas respectively. The aggregated results demonstrated that the prevalence rates of osteoporosis in patients living in the south (38.2%, 95%CI: [31.2%–45.1%]) and north (37.6%, 95%CI: [32.0%–43.2%]) reflected to be nearly the same (Table 2).
Economic Level
According to the China’s National Bureau of Statistics, developed regions with a GDP higher than 4 trillion were Beijing, Shanghai, Tianjin, Chongqing, Guangdong, Jiangsu, Shandong, Zhejiang, and Henan. Less developed regions with GDP lower than 4 trillion were Sichuan, Hubei, Hebei, Hunan, Fujian, Anhui, Liaoning, Shanxi, Inner Mongolia, Jiangxi, Guangxi, Heilongjiang, Jilin, Yunnan, Shanxi, Guizhou, Xinjiang, Gansu, Hainan, Ningxia, Qinghai, and Tibet. The pooled results illustrated that the prevalence rates of osteoporosis in patients living in the developed areas and less developed areas were 32.7% (95%CI:26.2%, 39.1%) and 41.0% (95%CI:35.2%, 46.8%) respectively (Table 2).
Publication Year
Eighteen studies were published between the 2000 and 2009 period, and 36 studies were published between 2010 and 2016. The merged results demonstrated that the prevalence rates of osteoporosis in diabetic patients from 2010 to 2016 and from 2001 to 2009 were 35.6% (95%CI: [31.0%, 40.1%]) and 42.3% (95%CI: [32.2%, 52.4%]) respectively (Table 2).
Regression Analysis and Publication Bias
Pre-specified meta-regression designated that publication year, areas, sample size, economic level, and quality scores did not affect the merged prevalence. However, sex (P=0.011) and average age (P=0.022) in participants remarkably influenced the overall outcome. Significant asymmetry existed in the funnel plot (Fig. 3). The results of the Begg’s test (P = 0.058) and the Egger’s test (P < 0.001) lacked consistency, which hinted that publication bias existed (Table 3). Therefore, we adopted the trim and fill method to examine the publication bias. The number of trimming and filling study was one after three times of iteration. The P value presented no reversal result before and after trimming and filling (P<0.001 and P<0.001) as it designated that the results were comparatively stable even though the publication bias existed.
Fig. 3:
Funnel plot for publication bias
Table 3:
Results of Meta-regression for the prevalence of osteoporosis in type 2 diabetes mellitus
| Covariate | Meta-regression coefficient | 95 % Confidence interval | P value |
|---|---|---|---|
| Year of publication | −0.008 | −0.020 to 0.003 | 0.164 |
| Female proportion (%) | 0.189 | 0.045 to 0.333 | 0.011 |
| Area (northern vs southern) | −0.006 | −0.099 to 0.087 | 0.899 |
| Sample size | −0.00006 | −0.0002 to 0.00003 | 0.185 |
| Economic level (developed vs not developed) | −0.082 | −0.173 to 0.008 | 0.072 |
| Average age | 0.010 | 0.002 to 0.018 | 0.022 |
| Quality score | −0.013 | −0.051 to 0.024 | 0.482 |
Discussion
In this systematic review, we estimated that in Chinese mainland, (a) the pooled prevalence rate of osteoporosis in diabetic patients is 37.8%, (b) old age and being of the female sex are both factors which correlate with a higher prevalence of osteoporosis and (c) economic level has a potential impact on the prevalence of osteoporosis in T2DM patients. To our knowledge, we are the first to systematically collect and analyze the studies utilizing DXA in T2DM patients.
According to the current results, the merged prevalence rate of osteoporosis in T2DM patients was much higher than the prevalence rate of primary osteoporosis in the Chinese Mainland reported by Chen (62). Notably, Chen has suggested that long-term high blood glucose might increase the risk for osteoporosis in diabetic patients largely. Additionally, some pathological mechanisms were related to this problem, such as deficiency or disorder of insulin, obesity, sexual hormone disturbance, and diabetic complications (63). In the early stage, Sosa and Wakasugi supposed that diabetes would not significantly impair bone metabolic status (64, 65). Recently, Athulya et al. also reported a negative difference in BMD between diabetic and non-diabetic subjects in India (66). Nevertheless, they noticed that the prevalence of osteoporosis was higher in the T2DM group than that in the control group.
Based on the results of the regression analysis, gender was a significant risk factor of osteoporosis in T2DM patients. We observed that the prevalence rate of osteoporosis in female T2DM patients (44.8%) was much higher than that in males (37.0%), and that they were higher than the results of primary osteoporosis reported by Chen respectively (62). Furthermore, age was another significant risk factor of osteoporosis in T2DM patients. The prevalence rate of osteoporosis (40.1%) in patients with an average age above 60 was higher than that in patients under 60 (26.5%). This result was consistent with the generally accepted concept that human bone mass would decline with age after reaching a peak.
In the subgroup analysis of the region, we did not detect an apparent difference between the prevalence rates of osteoporosis in T2DM patients residing in the southern and northern regions of China (37.6%, 38.2%). However, these two rates were higher than that of primary osteoporosis in the south and north China in Chen’s study (south: 23.17%, north: 20.13%) (62). Climates and eating habits, which were regarded as the influential factors of primary osteoporosis, appeared to have little impact on the prevalence rate of osteoporosis in T2DM patients in the south and north of China (67–70). The subgroup analysis of the economic level manifested that the prevalence rate of osteoporosis (32.7%) in diabetic patients from developed areas was significantly lower than that in patients from less developed regions (41%). Due to the in-equal distribution of medical resources, diabetic patients from developed areas are prone to receive better medical care and afford long-term medical expenses, which overall contributes to a more stable condition of diabetes.
On the contrary, a shortage of medical resources and a lower income level may aggravate the condition of diabetes, causing a high prevalence rate of osteoporosis. Notably, economic growth may be one of the primary reasons for the decline in osteoporosis prevalence in T2DM patients after the 2010 period (35.6%) compared with that in patients from 2001 to 2009 (42.3%). Therefore, we inferred that economic development was beneficial in establishing a stable T2DM condition, which is also crucial in preventing diabetic complications such as osteoporosis (71).
Study Limitations
There were some limitations to this review. Firstly, we included 54 studies, but the number of involved participants still proves to be insufficient. Secondly, all patient information was obtained from hospitals and more women than men were involved in this study, which possibly leads to a higher pooled prevalence rate. Thirdly, a majority of included studies lacked the diabetes course information. Consequently, we were unable to analyze the associations between the diabetes course and the prevalence of osteoporosis in the subgroup analyses.
Conclusion
This review demonstrates that osteoporosis is common in T2DM patients. Old age and being of the female sex proved to reflect a higher osteoporosis prevalence. Notably, economic development may favorably decrease the prevalence of osteoporosis in diabetic patients. However, it is necessary to conduct further studies so as to incorporate previous results, into clinical prevention for osteoporosis in T2DM patients.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This study was supported by the Natural Science Foundation of China (NSFC) (81473692); Jiangsu Province Advantage Disciplines Foundation for School of Nursing of Nanjing University of Chinese Medicine (Decree No. 37 issued by People’s Government of Jiangsu Province Office in 2014).
Footnotes
Conflicts of interest
The authors indicated no conflicts of interest.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. (2017). IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract, 128:40–50. [DOI] [PubMed] [Google Scholar]
- 2.Shen X, Vaidya A, Wu S, Gao X. (2016). The Diabetes Epidemic in China: An Integrated Review of National Surveys. Endocr Pract, 22:1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rathmann W, Giani G, Wild SH, Roglic G, Green A, Sicree R, King H. (2004). Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030 [8] (multiple letter). Diabetes Care, 27:2568–2569. [DOI] [PubMed] [Google Scholar]
- 4.Xiang HD. (2003). Chronic diabetic complications and related macro-vascular diseases of in-patients with diabetes in mainland of China—A national retrospective analysis in recent 10 years. ZhongGuo Tang Niao Bing Za Zhi, 4:5–10. [Google Scholar]
- 5.Mijnhout GS, Scheltens P, Diamant M, Biessels GJ, Wessels AM, Simsek S, Snoek FJ, Heine RJ. (2006). Diabetic encephalopathy: A concept in need of a definition. Diabetologia, 49:1447–1448. [DOI] [PubMed] [Google Scholar]
- 6.Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL. (2017). Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol, 13:208–219. [DOI] [PubMed] [Google Scholar]
- 7.E C (1996). Pocket guide to critical appraisal. John Wiley&Sons, London. [Google Scholar]
- 8.Shan PF, Wu XP, Zhang H, Cao XZ, Yuan LQ, Liao EY. (2011). Age-related bone mineral density, osteoporosis rate and risk of vertebral fracture in mainland Chinese women with type 2 diabetes mellitus. J Endocrinol Invest, 34:190–196. [DOI] [PubMed] [Google Scholar]
- 9.Dai N, Yan P, Zhang C, Zhang H. (2016). Analysis of related risk factors of diabetes combined with osteoporosis. ZhongGuo Yi Yao Dao Bao, 13: 69–71+ 84. [Google Scholar]
- 10.Bai H, Yin SN, Jing DQ. (2007). Analysis of bone mineral density change in type 2 diabetic elderly men. Gan Ran Yan Zheng Xiu Fu:107–109.
- 11.Bai Y, Wu J, Wang G. (2015). Risk Factors of Type 2 Diabetes Mellitus Complicated With Osteoporosis. Zhong Wai Yi Liao, 34:20–21. [Google Scholar]
- 12.Cao J, Jiang X. (2014). Analysis of the Level and Influencing Factors of Bone Mineral Density in Patients with Type 2 Diabetes. Yi Xue Zong Shu, 20:2078–2080. [Google Scholar]
- 13.Chen C, Xing X, Chen R, Xing C, Ren A. (2008). Bone density changes and its risk factors in patients with type 2 diabetes. Lin Chuang Nei Ke Za Zhi:489–490.
- 14.Guan M, Xue Y, Zou H. (2001). Anaysis of bone mineral density changes in type 2 diabetic patients. Di Yi Jun Yi Da Xue Xue Bao:684–685.
- 15.Guo R. (2013). Relationship between prevalence of osteoporosis and age in elderly female patients with type 2 diabetes mellitus. Shi Yong Tang Niao Bing Za Zhi, 9:29–31. [Google Scholar]
- 16.Huang Y. (2009). Preliminary study of the relationship between body composition and bone mineral density in postmenopausal patients with type 2 diabetes. MD. Hebei Medical Unversity. [Google Scholar]
- 17.Li B, Zhou X, Liang X, Chang W, Zhao S, Zhang Y. (2015). The alternation of bone metabolism index and bone mineral density of elderly male type 2 diabetes mellitus combined with depression patients. ZhongGuo Yi Xue Qian Yan Za Zhi, 7:17–20. [Google Scholar]
- 18.Li H, Guo Y. (2003). BMD examination of elderly patients with type 2 diabetes complicatedwith osteoporosis. ZhongGuo Lin Chuang Kang Fu:2868–2869.
- 19.Li H, Jiang T. (2012). Bone density level and its risk factors in patients with type 2 diabetic nephropathy. ZhongGuo Lao Nian Xue Za Zhi, 32:2711–2713. [Google Scholar]
- 20.Plantinga L, Rao MN, Schillinger D. (2012). Prevalence of self-reported sleep problems among people with diabetes in the United States, 2005–2008. Prev Chronic Dis, 9: E76. [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Xia S. (2014). Analysis of Bone Mineral Density in Elderly Male Inpatients with Type 2 Diabetes in Guizhou Provincial Organ. Gui Yang Zhong Yi Xue Yuan Xue Bao, 36:66–68. [Google Scholar]
- 22.Li W, Mai K, Lin W, Zhong Q. (2004). Clinical analysis of osteoporosis prevalence in the elderly with type 2 diabetics. Zhonghua Lao Nian Bing Yan Jiu Za Zhi:23–25.
- 23.Li W. (2011) Relevance study of distribution characteristics of type 2 diabetes combined with osteoporosis and its TCM syndrome. MD. Beijing University of Chinese Medicine. [Google Scholar]
- 24.Li X, Liu Q, Guo W. (2012). Analysis of the Bone Mineral Density and its Influencing Factors in Male Patients with Type 2 Diabetes. ZhongGuo Lao Nian Xue Za Zhi, 32:921–923. [Google Scholar]
- 25.Lin H, Huang X, Xiu Q, Cheng G. (2012). The Effect of Type 2 Diabetes on Bone Mineral Density of Postmenopausal Women. ZhongGuo Lao Nian Xue Za Zhi, 32:3172–3173. [Google Scholar]
- 26.Lu S, WAng C, Huang R. (2008). Change of bone mineral density in type 2 diabetes and its influential factors. Chongqing Yi Xue:1200–1202.
- 27.Mo H, Liu S, Zhou Z, Li J, Chen J. (2008). Risk factors of BMD in postmenopausal patients and the prevalence of osteoporosis. ZhongGuo Lao Nian Xue Za Zhi:1921–1924.
- 28.Pang Y. (2013). Analysis of bone mineral density in patients with type 2 diabetes. Shanghai Yi Xue, 34:20–23. [Google Scholar]
- 29.Tang Z. (2009) Clinical study of bone mineral density in patients with type 2 diabetes and the related factors of complicated osteoporosis. MD. Centeral South University. [Google Scholar]
- 30.Tang Z, Qin A, Zhao X, Li W, Li L, Chen K. (2013). Relationship between Chronic Complications and Osteoporosis among Postmenopausal Women with Type 2 Diabetes Mellitus. Xian Dai Sheng Wu Yi Xue Jin Zhan, 13:2955–2958. [Google Scholar]
- 31.Wang B. (2006) The preliminary investigation of the relationship between bone mineral density and the function of islet cell in type 2 diabetic patients. MD. Hebei Medical Unversity. [Google Scholar]
- 32.Wang H, Chang X, Zhang H. (2010). Analysis of bone mineral density in eldly male type 2 diabetic patient. Xian Dai Yi Xue, 38:474–476. [Google Scholar]
- 33.Wang T, Wang Z, Chen C. (2014). Correlation Between Metabolism Syndrome and Osteoporosis in Elderly Female Patients with Type 2 Diabetes. ZhongGuo Lao Nian Xue Za Zhi, 34:1760–1762. [Google Scholar]
- 34.Wang Y, Liu Y, Zhang W, Li B, Li Y. (2011). Factors of Bone mass and bone turnover characteristics in postmenopausal women with type 2 diabetes mellitus. ZhongGuo Lao Nian Xue Za Zhi, 31:1506–1508. [Google Scholar]
- 35.Wu A, Zheng W, Zheng X, Xu C, Lin C. (2011). Comparative Study on Bone Mineral Density of Lumbar Vertebrae Between Type 2 Diabetic Patients and Healthy People. Yi Xue Yan Jiu Za Zhi, 40:111–113. [Google Scholar]
- 36.Wu F. (2008) Diversification of bone mineral density and TRACP-5b, CTX-I, BAP in postmenopausal diabetic women and related factors analyzed. MD. Hebei Medical Unversity. [Google Scholar]
- 37.Wu S. (2016). Relevance study of bone density and vitamin D level in elderly patients with type 2 diabetes. ZhongGuo Xian Dai Yi Sheng, 54:89–91. [Google Scholar]
- 38.Wu X. (2014) The relationship between serum Sclerostin, Dkk1 levels and bone mineral density in T2DM patients. MD. Hebei Medical Unversity. [Google Scholar]
- 39.Xing Y, Zhao J, Zhang L, Che W. (2006). Relationship between osteoporosis and non-insulin dependent diabetes mellitus in elderly. ZhongGuo Gu Zhi Shu Song Za Zhi:482–484+ 454.
- 40.Xing Y, Li C, Zhang Q, Yu Q, Yu D. (2015). Relationship between serum bilirubin levels and osteoporosis in middle and old aged male patients with type 2 diabetes mellitus. Tianjin Yi Ke Da Xue Xue Bao, 21:426–429. [Google Scholar]
- 41.Xu Y, Lv G, Zhang S. (2015). Analysis of risk factors of diabetes combined with osteoporosis. Huaihai Yi Yao, 33:464–466. [Google Scholar]
- 42.Xu W, Yi Z. (2005). Changes of bone mineral density and metabolism in type 2 diabetes mellitus of different traditional Chinese medicine syndrome types. ZhongGuo Lin Chuang Kang Fu:182–184.
- 43.Xue Y, Hu J, Lin Y, Cheng J. (2013). Analysis of Risk Factors of Type 2 Diabetes Mellitus Complicated With Osteoporosis. Shi Yong Lin Chuang Yi Yao Za Zhi, 17:155–156. [Google Scholar]
- 44.Yan P, Ouyang F, Ma H, He J, Li J. (2016). Relationship of serum cystatin-C levels with osteoporosis in patients with type 2 diabetes mellitus. Shanxi Yi Ke Da Xue Xue Bao, 47: 59–63+ 96. [Google Scholar]
- 45.Zhang H, Li C. (2015). Relationship between bone density and fasting insulin level in elderly patients with type 2 diabetes. ZhongGuo Lao Nian Xue Za Zhi, 35:4541–4543. [Google Scholar]
- 46.Zhang R. (2010) The prevalence of osteoporosis and BMD in nonselected female population. MD. Centeral South University. [Google Scholar]
- 47.Zhang X, Wu Y, Liu Y, Zhang J, Fu C, Jin L. (2013). Correlation analysis of osteoporosis-related risk factors in patients with type 2 diabetes in Dandong area. ZhongGuo Gu Zhi Shu Song Za Zhi, 19:10–13. [Google Scholar]
- 48.Zhang Z, Yan P, Zhong H, Li H, He X. (2016). Correlation Between Vibrating Perception Threshold and Osteoporosis in Patients with Type 2 Diabetes Peripheral Neuropathy. Chengdu Yi Xue Yuan Xue Bao, 11: 182–185+ 190. [Google Scholar]
- 49.Zhang D, Lv X, He X, Liu J. (2006). Relationship between bone mineral density and serum insulin-like growth factor-I in elder women with type 2 diabetes mellitus. ZhongGuo Gu Zhi Shu Song Za Zhi:142–144.
- 50.Zhao N, Li S, Fang X, Li C. (2014). Study of the relationship between bone mineral density and serum uric acid in senile diabetic patients. ZhongGuo Gu Zhi Shu Song Za Zhi, 20:784–788. [Google Scholar]
- 51.Fang X, Lai X, Zheng G. (2011). Bone density level and its risk factors in patients with type 2 diabetes. Shandong Yi Yao, 51:41–42. [Google Scholar]
- 52.Li G. (2010). Effect of type 2 diabetes mellitus and analysis of osteoporosis-related risk factors. ZhongGuo Gu Zhi Shu Song Za Zhi, 16:344–346. [Google Scholar]
- 53.Li X, Zhu X, Yu W. (2011). Preliminary study of the effect of type 2 diabetes mellitus on bone metabolism in the elderly. ZhongGuo Gu Zhi Shu Song Za Zhi, 17:11–14. [Google Scholar]
- 54.Lu Y, Zhang Y, Zhang Z. (2015). Bone mineral density analysis of incipient type 2 diabetes. Jilin Yi Xue:3783–3784, 3785.
- 55.Sun W, Wang L. (2009). Correlation between Bone Mineral Density and Insulin Level in Type 2 Diabetic Patients. ZhongGuo Quan Ke Yi Xue, 12: 2119–2120, 2123. [Google Scholar]
- 56.Wang T, Wang Z, Chen C. (2012). Correlation Between CYS-C in Type 2 Diabetes and Osteoporosis. Hebei Yi Ke Da Xue Xue Bao, 33:1290–1293. [Google Scholar]
- 57.Wu Y, Zhao F. (2008). Analysis of bone mineral density in female type 2 diabetes patients. ZhongGuo Gu Zhi Shu Song Za Zhi, 14: 335–336, 340. [Google Scholar]
- 58.Zhang C, Xie Y, Zhang G. (2015). Relevance study of bone density level in patients with type 2 diabetes. Zhong Wai Yi Xue Yan Jiu: 78–79.
- 59.Zhou X, Zheng L, Jin X. (2009). Clinical observation of bone mineral density changes in 106 type 2 diabetes patients. Chongqing Yi Xue, 38:2717–2718. [Google Scholar]
- 60.Zhu X, Wu X, Zhou Z, Huang G, Wang W. (2002). Bone mineral density comparison of female type 2 diabetes mellitus with healthy women. ZhongGuo Tang Niao Bing Za Zhi, 10:216–218. [Google Scholar]
- 61.Liu T. (2010) Effect of type 2 diabetes mellitus on osteoporosis incidence and analysis of osteoporosis-related risk factors. MD. Jilin University. [Google Scholar]
- 62.Chen P, Li Z, Hu Y. (2016). Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health, 16: 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo GT, Giandalia A, Romeo EL, Nunziata M, Muscianisi M, Ruffo MC, Catalano A, Cucinotta D. (2016). Fracture Risk in Type 2 Diabetes: Current Perspectives and Gender Differences. Int J Endocrinol, 2016: 1615735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sosa M, Dominguez M, Navarro MC, Segarra MC, Hernández D, De Pablos P, Betancor P. (1996). Bone mineral metabolism is normal in non-insulin-dependent diabetes mellitus. J Diabetes Complications, 10:201–205. [DOI] [PubMed] [Google Scholar]
- 65.Wakasugi M, Wakao R, Tawata M, Gan N, Koizumi K, Onaya T. (1993). Bone mineral density measured by dual energy X-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus. Bone, 14:29–33. [DOI] [PubMed] [Google Scholar]
- 66.Asokan AG, Jaganathan J, Philip R, Soman RR, Sebastian ST, Pullishery F. (2017). Evaluation of bone mineral density among type 2 diabetes mellitus patients in South Karnataka. J Nat Sci Biol Med, 8:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maier GS, Jakobs P, Roth KE, Kurth AA, Maus U. (2013). Is there an epidemic vitamin D deficiency in German orthopaedic patients? Clin Orthop Relat Res, 471:3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakavachara P, Viprakasit V. (2013). Children with hemoglobin E/β-thalassemia have a high risk of being vitamin D deficient even if they get abundant sun exposure: A study from Thailand. Pediatr Blood Cancer, 60:1683–1688. [DOI] [PubMed] [Google Scholar]
- 69.Zeng Q, Huang S, Chen R. (1997). 10-year epidemiological study on rheumatic diseases in Shantou area. Zhonghua Nei Ke Za Zhi, 36: 193–197 [Article in Chinese]. [PubMed] [Google Scholar]
- 70.Luo WD, Zhao G, Shu J, Lao HC, Lin F, Guo LM, He SX, Yuan Y. (2017). Prevalence and Correlation Factors of Osteoporosis in Middle-aged and Elderly People of Zhuang Nationality in Yunnan Based on A Survey. ZhongGuo Quan Ke Yi Xue, 20:912–917. [Google Scholar]
- 71.Yan P, Zhang Z, Feng J, Li H, Gao C, Yang J, Zhong H, Wan Q, Xu Y. (2017). Association between serum total bilirubin levels, bone mineral density, and prevalence of osteoporosis in Chinese patients with type 2 diabetes. Int J Clin Exp Pathol, 10:5784–5798. [Google Scholar]