Abstract
Purpose:
The Advisory Committee on Immunization Practices recommended quadrivalent human papillomavirus vaccine (HPV4) for use in females in June 2006 and in males in October 2009. The objective of our study was to describe HPV4 uptake, single-dose coverage, and completion of the three-dose series among those 9–26 years of age, after the respective female and male vaccine licensures through June 2011.
Methods:
The study population included members of eight managed care organizations participating in the Vaccine Safety Datalink; we abstracted demographic and comprehensive vaccine information from electronic health records.
Results:
We found one-dose coverage increasing throughout the study period, to a high of 37.7% among females and 1.3% among males in June 2011. Among those receiving at least one HPV4 dose, three-dose series completion was 42% for females and 30.2% for males.
Conclusions:
Our results demonstrate low initiation and completion of the HPV4 series among those recommended to receive the vaccine. Although consistent with previous studies, these results highlight the continued need to develop, implement, and monitor strategies to increase HPV4 vaccine initiation and completion in younger adolescents to achieve maximum impact in reducing the burden of cervical cancer and other HPV-related diseases.
Keywords: Papillomavirus vaccines, Vaccination, HPV vaccines, Gardasil
Cervical cancer is the second most common cancer in women worldwide, and the majority of cases are associated with human papillomavirus (HPV) infection [1,2]. In the United States alone, the American Cancer Society estimates that 12,170 women were diagnosed with, and 4,290 women died from, cervical cancer in 2011 [3]. HPV has also been associated with anogenital and oropharyngeal cancers and is known to cause genital warts in both women and men [4–7]. Overall 33,369 HPV-associated cancers were diagnosed in the United States from 2004–2008, of which 26,000 are attributed directly to HPV [8]. The U.S. Food and Drug Administration licensed a quadrivalent HPV vaccine (HPV4) to protect against disease caused by HPV types 6, 11, 16, and 18 in females in June 2006 and in males in October 2009 [9,10]. The vaccine is administered as a three-dose series, with administration of the second and third doses recommended at 2 and 6 months, respectively, after the first dose. Currently, HPV4 is licensed for use in both females and males nine to 26 years of age, with routine immunization recommended for those 11–12 years of age [4,11].
Since the approval of HPV4 in females, studies of vaccine uptake, one-dose coverage, and three-dose series completion in this population in the United States have largely relied on self- or provider-reported vaccination data, or on single-site electronic health record (EHR)-based studies [12–19]. These previous studies are limited, however, in that: (1) self-reported data may not be as valid as EHR-based data; (2) geographic differences in vaccine coverage were not accurately captured by analyzing data from a single site; and/or (3) HPV4 data has not always been assessed across all ages. Further, due to the more recent licensure in males, very few estimates of coverage among males have been published, leading to a large knowledge gap in this area [20,21].
The goal of our study is to describe estimates of HPV4 uptake, coverage, and completion from July 2006 through June 2011 for females and from October 2009 through June 2011 for males. Our study will improve upon previous research by studying HPV4 uptake, coverage, and completion among males as well as females; incorporating data from multiple sites across the country; using EHR data; and including individuals of all ages recommended to receive HPV4. Describing initial uptake, coverage, and completion as accurately as possible is an important first step toward observing the effectiveness of vaccine recommendations and, through further research, identifying characteristics associated with vaccine coverage and completion. Through this work, better interventions can be developed to increase HPV4 vaccination rates further.
Methods
Data sources
We analyzed data obtained through the Vaccine Safety Datalink (VSD), a collaboration between the Centers for Disease Control and Prevention and 10 managed care organizations (MCOs) from across the United States [22]. Our analyses include data from eight VSD sites. We excluded two VSD sites because data needed for these analyses were not available. We extracted demographic and vaccine exposure information for study participants from the VSD data files maintained at each site. Institutional review boards at the participating MCOs approved this study.
Study population and descriptive analyses
Our study population for the uptake and coverage analyses included individuals aged 9 to 26 years, for whom the vaccine was licensed, enrolled in any one of the participating sites. Our study period extended from June 2006 through June 2011 for females and October 2009 through June 2011 for males. Participants were considered eligible during a given month if they were enrolled in the MCO at any point in that month. Age was calculated at the end of each month for all participants. We defined vaccine uptake as the absolute number of HPV4 doses given to eligible participants and we defined single-dose vaccine coverage as the proportion of eligible participants who had ever received at least one HPV4 dose. We describe vaccine uptake by age and calendar quarter of vaccine administration and we describe vaccine coverage by age and participating study site. To account for potential off-label vaccine administration, we describe uptake of vaccine among females and males aged 27–39 years during the respective study periods and uptake of vaccine among males aged 9–39 years from June 2006 through September 2009. To highlight potential opportunities for increasing HPV4 vaccination, we describe the proportion of females and males vaccinated concomitantly (on the same day) with HPV4 and other vaccines, as well as the distribution of these other vaccine types.
Our study population for the HPV4 completion analysis included females aged 9–26 years who received their first HPV4 dose between July 1, 2006 and June 2010 and males aged 9–26 years who received their first HPV4 dose between October 2009 and June 2010, and who were continuously enrolled in their respective MCO for at least 1 year from the time of their first dose. We defined completion as the proportion of eligible participants receiving three HPV4 doses within 12 months of having received an initial dose. We describe vaccine completion by age of participant and calendar quarter of receipt of initial dose, and we describe the median time intervals between the first and second and second and third doses.
Results
Uptake
A total of 737,418 HPV4 doses were administered to females between July 2006 and June 2011 (Figure 1). Uptake rose from the beginning of the study period to the third quarter of 2007, when the greatest number of doses, 68,751, was administered. A decreasing trend followed, with lesser, cyclical peaks occurring in the third quarter of each year, to a nadir of 23,330 doses administered in the second quarter of 2010, after which point uptake began to increase again. The greatest number of doses was administered to females 15–16 years of age (145,279), followed by those 13–14 (139,796) and 11–12 (136,373) years of age. Nearly all (729,487 [98.9%]) doses were given as licensed to our study population; the remaining 7,931 doses were administered off-label to females aged 27–39 years.
Figure 1.
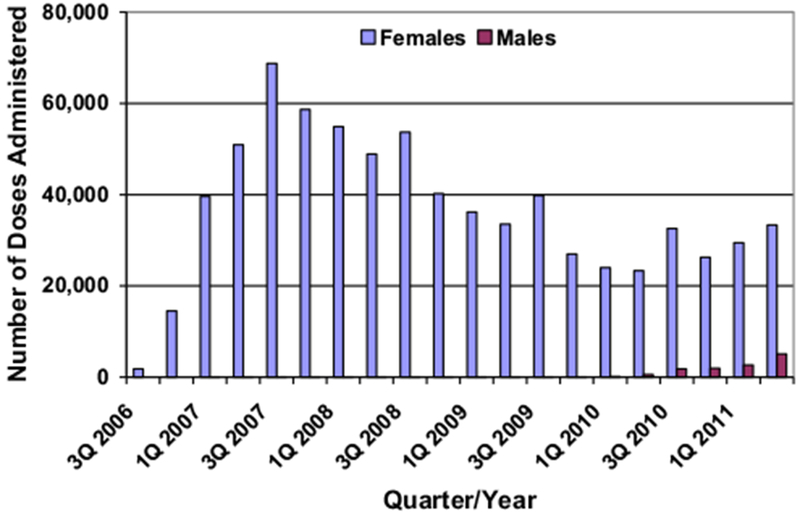
Number of HPV vaccine doses administered, by sex, July 2006–June 2011.
Among males, 12,162 doses were administered from October 2009 through June 2011, with uptake continually increasing throughout the study period (Figure 1). The greatest number of doses was administered to males 15–16 years of age (2,973), followed by those 13–14 (2,816) and 11–12 (2,545) years of age. Nearly all (12,029 [98.9%]) doses were given to our study population; the remaining 133 doses were administered to males aged 27–39. An additional 473 doses were administered off-label to males from June 2006 through September 2009, prior to HPV4 licensure.
One-dose coverage
A monthly average of 419,478 eligible females and 610,250 eligible males were included in the study populations for the coverage analyses. The proportion of females targeted for vaccination receiving at least one HPV4 dose increased to a high of 37.7% in June 2011. By the end of the study period, one-dose coverage was highest among girls 17–18 years of age (60.6%), followed by those 15–16 (59.2%) and 19–20 years of age (53%) (Figure 2). One-dose coverage was lowest among girls 9–10 years of age (.6%). One-dose coverage among adolescent females 13–18 years of age was 56.2%. Although the overall trend of increasing coverage appeared at all participating sites, coverage varied from 25.7% to 48.6% among sites (Table 1).
Figure 2.
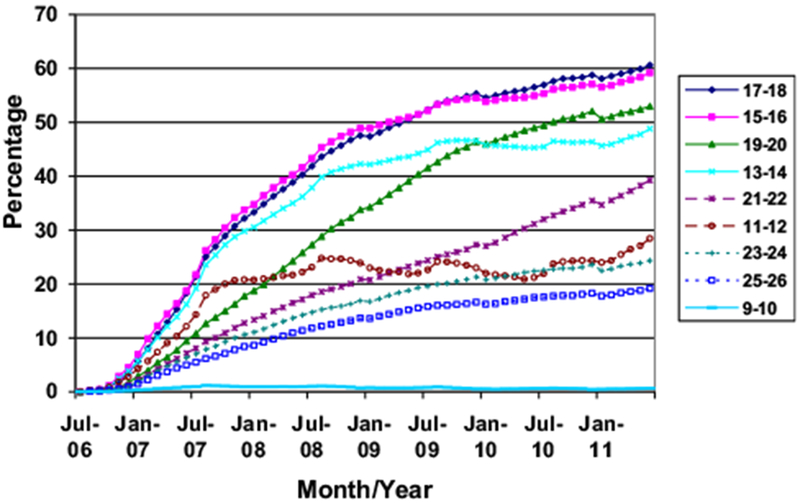
HPV vaccine one-dose coverage among females, by age and date, July 2006–June 2011.
Table 1.
HPV vaccine one-dose coverage, by sex and participating managed care organization (MCO), June 2011
| Region | Managed care organization | Females (%) | Males (%) |
|---|---|---|---|
| South | 1 | 25.7 | 2.9 |
| Midwest | 2 | 41.5 | .8 |
| 3 | 38.1 | 1.3 | |
| West | 4 | 48.6 | 1.4 |
| 5 | 45.4 | 1.2 | |
| 6 | 44.4 | 1.6 | |
| 7 | 38.1 | .3 | |
| 8 | 34.8 | 1.3 |
The proportion of males targeted for vaccination receiving at least one HPV4 dose increased to 1.3% in June 2011. The highest coverage was seen in males 15–16 years of age (2.7%), followed by those 13–14 (2.6%) and 17–18 (2.3%) years of age; the lowest coverage was seen in males 9–10 years of age (.2%). One-dose coverage among adolescent males 13–18 years of age was 2.5%. At the end of the study period, coverage among males varied from .3% to 2.9% across sites (Table 1).
Three-dose completion
A total of 311,213 females received at least one HPV4 dose from July 2006 through June 2010 and were continuously enrolled for the subsequent 12-month period. Of them, 100,988 (32.5%) received only one HPV4 dose, 79,587 (25.6%) received two, and 130,638 (42%) completed the three-dose series within a year. The mean and median times between the first and second doses were 183.7 and 83 days, respectively; those between the second and third doses were 213 and 140 days, respectively. The proportion of females completing the series within a year varied from 46.1% among those 17–18 years of age to 50.8% among those 25–26 years of age, with no discernible trend, by age. Series completion was highest (51.4%) among females receiving their index shot during the first quarter of 2007 and lowest (31.9%) among those receiving their index shot during the fourth quarter of 2009 (Figure 3).
Figure 3.
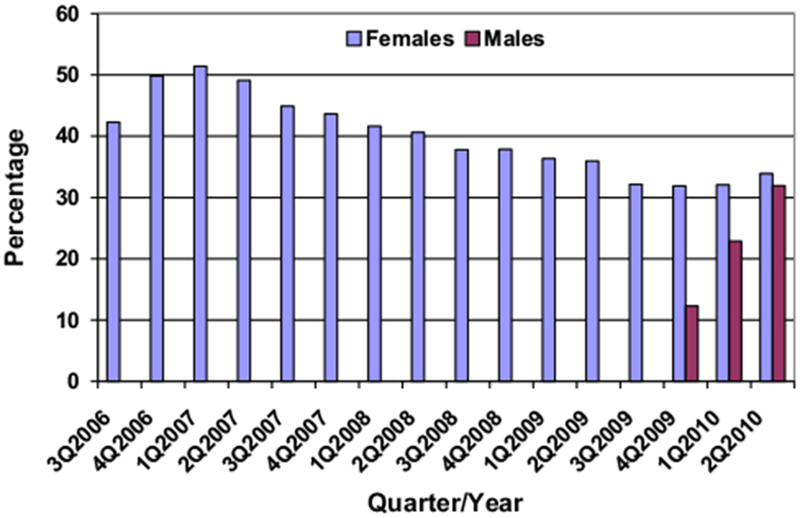
Completion of the three-dose HPV series in one year, by sex and quarter of index dose, July 2006–June 2011.
A total of 888 males received at least one HPV4 dose from October 2009 through June 2010 and were continuously enrolled for the subsequent 12-month period. Of them, 378 (42.6%) received only one dose, 242 (27.3%) received two, and 268 (30.2%) completed the three-dose series within a year. The mean and median times between the first and second doses were 132.6 and 89 days, respectively; those between the second and third doses were 154.6 and 136 days, respectively.
Concomitant vaccination
Of the 729,487 HPV4 doses administered to our female study population, 300,883 (41.2%) were given on the same day as at least one additional vaccine type. Concomitant vaccination was highest among those 11–12 years of age (59%); it was also highest among 63.4% of all females receiving their first HPV4 dose, 23.5% receiving their second, and 23.9% receiving their third. Females vaccinated concomitantly received a mean of 1.76 and median of 2 other vaccine doses simultaneously, with a range of 1 to 10. Of the 12,029 HPV4 doses administered to our male study population, 5,667 (47.1%) were given on the same day as at least one additional vaccine type. Concomitant vaccination among males was highest among those 11–12 years of age (64.4%) and occurred among 59.7% of males receiving their first HPV4 dose, 20.5% receiving their second, and 18.8% receiving their third. Males vaccinated concomitantly received a mean of 1.8 and median of 2 other vaccine doses simultaneously, with a range of 1 to 8. The most frequent vaccine types administered along with HPV4 for females and males are shown in Table 2.
Table 2.
Vaccine types most frequently administereda concomitantly with HPV vaccine
| Vaccine | Females N (%) | Males N (%) |
|---|---|---|
| MCV4b | 150,379 (28.4) | 3,060 (29.4) |
| Tdapc | 139,391 (26.3) | 2,768 (26.6) |
| Hepatitis Ad | 75,726 (14.3) | 1,404 (13.5) |
| Varicella | 70,531 (13.3) | 1,149(11.1) |
| Influenzae | 70,272 (13.3) | 1,529 (14.7) |
| Total vaccines administered concomitantly | 529,961 | 10,397 |
≥5% of all vaccine types administered.
Quadrivalent meningococcal conjugate vaccine.
Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed.
Includes all hepatitis A antigen-containing vaccines.
Includes seasonal, trivalent, and novel H1N1; live and inactivated influenza vaccines.
Discussion
Our results of one-dose HPV4 coverage, especially among females, are largely consistent with previously published estimates with regard to coverage rates and geographic variation [4,12,16,19,23]. For other estimates provided during the same time frame in 2011, our study results indicate a similar one-dose coverage proportion among adolescent females (56%) and a lower one-dose coverage proportion among adolescent males (2.5%) than published (53% and 8%, respectively) [21]. Our coverage-estimate variations by site are also consistent with previous results showing substantial variation in HPV4 coverage rates across different areas of the country, largely explained by differences in state statutes recommending HPV4 vaccination and clinical counseling to receive HPV4 [24–26]. Pertaining to the latter, differences in practices, office procedures to increase vaccination, valuing HPV4 vaccine, and belief in HPV4 vaccination have all been associated with higher rates of physician counseling to receive HPV4; and, having a physician specifically recommend HPV4 vaccination has been associated with a higher proportion of adolescent females initiating and completing the HPV4 series [26,27].
Unfortunately, after the initial postlicensure period during which HPV4 uptake and coverage increased dramatically, we observed a decreasing trend from the third quarter of 2007 through the second quarter of 2010 leading to a lower rate of HPV4 initiation among females, relative to other vaccines targeting adolescent populations—a finding also consistent with previous literature [28]. Given that our data were gathered from insured populations among MCOs that provide HPV4 at no additional cost, our low coverage rate is unlikely to be due to a lack of insurance coverage and the high cost of HPV4 (approximately $130 per dose), contributory factors noted in previous studies [15,19,29,30]. We postulate this decreasing trend was due to barriers specific to HPV4 vaccination, including a lack of knowledge about HPV-related disease (other than cervical cancer), a reluctance on the part of parents and providers to talk about HPV4 vaccination, a low estimation of HPV-related disease risk, concerns about potential side effects, and concerns about the relationship between HPV4 vaccination and initiation of sexual activity [16,19,29,31–33]. A lack of institutional interventions to increase HPV4 vaccination among the participating study sites likely also played a part in this decreasing trend of HPV4 uptake.
Our results, showing only 42% females and 30% of males completed the three-dose series within a year of receiving their index shot, are also concerning, because these individuals maintained continuous insurance enrollment over the course of the study period and have overcome other barriers to HPV4 series initiation. This completion rate among females continues to be similar to those reported in other studies (35%–56%), although a completion rate as high as 83% was reported in Ontario, Canada. Completion rates among males have not been assessed previously [19,21,34,35]. Low completion rates for both males and females highlights the need for continued efforts to improve completion of the three-dose HPV4 series for all adolescents.
One possible limitation of this study is that records of vaccines administered to individuals outside of the participating MCOs may not have been included in our analyses, leading to underestimates of both one-dose coverage and three-dose completion. However, because the vaccine is a covered benefit within the participating MCOs and some MCOs incorporate state immunization registry data into VSD files, this effect is likely to be negligible. Therefore, we are confident that our data are valid in indicating low rates of HPV4 one-dose coverage and three-dose completion.
HPV4 has demonstrated high efficacy at preventing persistent HPV infection and HPV-associated lesions and, therefore, has the potential to prevent significant morbidity and mortality in those who receive it prior to HPV exposure as well as protecting their future sexual partners [11]. Strategies to increase HPV vaccine uptake and completion should be developed and encouraged; parents, healthcare providers, MCOs, and immunization programs all have a role in increasing HPV vaccination rates and reducing barriers to vaccination [36,37]. For instance, our results demonstrating higher uptake of vaccine in the third quarter of each calendar year, relative to the surrounding time period, are likely due to adolescent health visits prior to the start of the school year to receive other required vaccinations, at which point HPV4 was also given. Yet, that a high proportion of our study participants received no other vaccines concomitantly—including roughly 35% of males and 40% of females 11–12 years of age—suggests an opportunity for further improvement in this area. Other strategies include the addition of an HPV4 coverage target within Healthy People 2020 and a 2012 Healthcare Effectiveness Data and Information Set measure to track HPV4 vaccination rates within health plans [38,39]. All of these strategies are expected to increase HPV4 vaccination coverage and completion, particularly for 11- and 12-year-old girls and boys, who are the least likely to be sexually active and, consequently, among whom vaccine effectiveness may be the highest.
Using a robust design, our study provides strong estimates of HPV4 uptake, one-dose coverage, and three-dose completion for the first five years postlicensure for females and for almost two full years postlicensure for males. For the latter group, it provides one of the first published estimates of these metrics and the first using EHRs data sources. These results provide a baseline for comparing vaccination rates in insured populations over time and can be used for further research in this area, such as monitoring the success of the noted strategies implemented to raise HPV4 vaccination rates.
IMPLICATIONS AND CONTRIBUTION.
Our findings highlight the continued need to increase HPV vaccination, particularly among younger girls and boys prior to the onset of sexual activity. Our study focuses on a different female study population than in previously published studies and provides one of the first estimates of HPV vaccine uptake and coverage in males.
Acknowledgments
Financial support for this study was provided in full by the Centers for Disease Control and Prevention (200-2002-00732), through America’s Health Insurance Plans. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Additional VSD investigators and project staff who contributed to this study include Eric Weintraub, Natalie McCarthy (Centers for Disease Control and Prevention, Atlanta, Georgia); Tracey Lieu, Maya Dutta-Lin, Rich Fox (Harvard Vanguard Medical Associates and Harvard Pilgrim Health Care, Boston, Massachusetts); Lisa Jackson, Patti Benson (Group Health, Seattle, Washington); Edward Belongia, James Donahue (Marshfield Clinic Research Foundation, Marshfield, Wisconsin); James Nordin, Leslie Kuckler (Health Partners Research Foundation, Minneapolis, Minnesota); JoAnn Shoup (Kaiser Permanente Colorado, Denver, Colorado); Roger Baxter (Kaiser Permanente Vaccine Study Center, Oakland, California); Jill Mesa, Michelle Henninger, Karen Riedlinger, Lois Drew (Kaiser Permanente Northwest, Portland, Oregon).
References
- [1].Lowy DR, Solomon D, Hildesheim A, et al. Human papillomavirus infection and the primary and secondary prevention of cervical cancer. Cancer 2008; 113(Suppl. 7):1980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Subramanya D, Grivas PD. HPV and cervical cancer: Updates on an established relationship. Postgrad Med 2008;120:7–13. [DOI] [PubMed] [Google Scholar]
- [3].American Cancer Society. Cancer facts & figures 2011. Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- [4].Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60:1705–8. [PubMed] [Google Scholar]
- [5].Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the U.S. Cancer 2008;113(Suppl. 10):2892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer 2008;113(Suppl. 10):3036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chaturvedi AK. Beyond cervical cancer: Burden of other HPV-related cancers among men and women. J Adolesc Health 2010;46(Suppl. 4): S20–6. [DOI] [PubMed] [Google Scholar]
- [8].Centers for Disease Control and Prevention. Human papillomavirus-associated cancers—United States, 2004–2008. MMWR Morb Mortal Wkly Rep 2012;61:258–61. [PubMed] [Google Scholar]
- [9].Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890–907. [DOI] [PubMed] [Google Scholar]
- [10].Food and Drug Administration. Product approval-prescribing information [package insert]. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant]. Merck & Co, Inc.; 2009. [Google Scholar]
- [11].Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine–Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56:1–24. [PubMed] [Google Scholar]
- [12].Caskey R, Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: Results of a national survey. J Adolesc Health 2009;45:453–62. [DOI] [PubMed] [Google Scholar]
- [13].Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010;59:630–2. [PubMed] [Google Scholar]
- [14].Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am J Epidemiol 2010;171:357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Conroy K, Rosenthal SL, Zimet GD, et al. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Womens Health (Larchmt) 2009;18:1679–86. [DOI] [PubMed] [Google Scholar]
- [16].Kahn JA, Rosenthal SL, Jin Y, et al. Rates of human papillomavirus vaccination, attitudes about vaccination, and human papillomavirus prevalence in young women. Obstet Gynecol 2008;111:1103–10. [DOI] [PubMed] [Google Scholar]
- [17].Taylor LD, Hariri S, Sternberg M, et al. Human papillomavirus vaccine coverage in the United States, National Health and Nutrition Examination Survey, 2007–2008. Prev Med 2011;52:398–400. [DOI] [PubMed] [Google Scholar]
- [18].Stokley S, Cohn A, Dorell C, et al. Adolescent vaccination-coverage levels in the United States: 2006–2009. Pediatrics 2011;128:1078–86. [DOI] [PubMed] [Google Scholar]
- [19].Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–9. [DOI] [PubMed] [Google Scholar]
- [20].Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011;60:1117–23. [PubMed] [Google Scholar]
- [21].Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years–United States, 2011. MMWR Morb Mortal Wkly Rep 2012;61:671–7. [PubMed] [Google Scholar]
- [22].Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: A model for monitoring immunization safety. Pediatrics 2011;127(Suppl. 1): S45–53. [DOI] [PubMed] [Google Scholar]
- [23].Rosenthal SL, Rupp R, Zimet GD, et al. Uptake of HPV vaccine: Demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolesc Health 2008;43:239–45. [DOI] [PubMed] [Google Scholar]
- [24].Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13–17 years—United States, MMWR Morb Mortal Wkly Rep 2009;58:997–1001. [PubMed] [Google Scholar]
- [25].National Conference of State Legislatures. HPV Vaccine: State legislation and statutes. 2012. July .
- [26].Kramer MR, Dunlop AL. Inter-state variation in human papilloma virus vaccine coverage among adolescent girls in the 50 U.S. states, 2007. Matern Child Health J 2012;16(Suppl. 1):S102–10. [DOI] [PubMed] [Google Scholar]
- [27].Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: A statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009;18: 2325–32. [DOI] [PubMed] [Google Scholar]
- [28].Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13–17 years—United States, MMWR Morb Mortal Wkly Rep 2010;59:1018–23. [PubMed] [Google Scholar]
- [29].Daley MF, Crane LA, Markowitz LE, et al. Human papillomavirus vaccination practices: A survey of U.S. physicians 18 months after licensure. Pediatrics 2010;126:425–33. [DOI] [PubMed] [Google Scholar]
- [30].Kharbanda EO, Lee GM, Koenigs L. Financing vaccines for adolescents: A position paper of the society for adolescent health and medicine. J Adolesc Health 2011;48:320–1. [DOI] [PubMed] [Google Scholar]
- [31].deVisser R, McDonnell E. Correlates of parents’ reports of acceptability of human papilloma virus vaccination for their school-aged children. Sex Health 2008;5:331–8. [DOI] [PubMed] [Google Scholar]
- [32].Klug SJ, Hukelmann M, Blettner M. Knowledge about infection with human papillomavirus: A systematic review. Prev Med 2008;46:87–98. [DOI] [PubMed] [Google Scholar]
- [33].Zimet GD, Weiss TW, Rosenthal SL, et al. Reasons for non-vaccination against HPV and future vaccination intentions among 19–26-year-old women. BMC Womens Health 2010;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Niccolai LM, Mehta NR, Hadler JL. Racial/ethnic and poverty disparities in human papillomavirus vaccination completion. Am J Prev Med 2011;41: 428–33. [DOI] [PubMed] [Google Scholar]
- [35].Smith LM, Brassard P, Kwong JC, et al. Factors associated with initiation and completion of the quadrivalent human papillomavirus vaccine series in an Ontario cohort of grade 8 girls. BMC Public Health 2011;11:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Herzog TJ, Huh WK, Einstein MH. How does public policy impact cervical screening and vaccination strategies? Gynecol Oncol 2010;119:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McRee AL, Reiter PL, Brewer NT. Vaccinating adolescent girls against human papillomavirus—Who decides? Prev Med 2010;50:213–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].National Committee for Quality Assurance. National Committee for Quality Assurance. Available at: http://www.ncqa.org/LinkClick.aspx?fileticket=O-31v4G27sU%3d&tabid=1415; 2011.
- [39].U.S. Department of Health and Human Services. Healthy people 2020. Objective IID-11: Increase routine vaccination coverage levels for adolescents. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23; 2011.


