Abstract
Background and Objective:
The aim of this study was to compare the cytotoxiceffects of local probiotic bacteria, including Lactobacillus paracasei, Lactobacillus brevis, while isolated from “Tarkhine” food and the induction of apoptosis in the HT–29 human colon adenocarcinoma cell line and normal fibroblasts.
Methods:
HT–29 and L–929 cell lines were treated with cell-bound exopolysaccharide extract (cb-EPS) from L. paracasei and L. brevis. The MTT assay was used to analyze cell viability. Cellular apoptosis was examined by flow cytometry and DNA fragmentation assay.
Results:
The cb-EPS from both probiotic bacteria prevented the proliferation of HT–29 colon cancer cells. In addi- tion, the cytotoxic and anti-proliferative effects of the exopolysaccharide extract from both bacteria in L–929 fibro- blasts were much lower than HT–29 cells. The induction of apoptosis in HT–29 cells was observed at 48h compared with 72h. It seems that the exopolysaccharides extracted from both bacteria have a greater effect on the induction of apoptosis at 48h. The cb-EPS of L. brevis showed more potent anti-proliferative and apoptotic properties than the cb- EPS of L. paracasei. The ladder pattern of DNA fragmentation confirmed the induction of apoptosis in cancer cells.
Conclusion:
The results of the MTT assay and apoptosis indicate that the induction of apoptosis by the exopolysac- charide from bacteria depends on the dose, time, and strain of bacteria. Further studies may contribute toward the understanding of using these probiotic bacteria as biological products to treat and prevent cancers.
Key Words: Lactobacillus, Probiotic, Apoptosis HT-29 Cells
Introduction
Probiotics are live microorganisms. They exert a positive effect on the host’s health when present sufficiently in the host body (1).
Certain strains of probiotics have a positive impact on host health that cannot be generalized to other strains. Lactobacillus strains have been shown to be benecial to the intestinal mucous membrane, which maintains and improves immunity level, and can reduce the movement of various bacteria through the intestinal mucosa. It also plays a key role in decreasing the rate of inflammatory bowel disease and irritable bowel syndrome (2). Several studies have mentioned the effect of probiotics on the digestive enzymes of animals and humans. The inhibition of the genotoxicity of carcinogens in vitro and in vivo, and suppression of carcinogen-induced lesions and tu- mors in experimental animals are evidence of the cancer-preventing properties of probiotics (3). Recent studies have indicated that bacterial cell extracts, exopolysaccharide extracts, cell walls, cellular metabolites, and even cellular DNA have cytotoxic, antitumor, and anti-cancer properties, and are safer compared to live cells (4-7). It is noteworthy to say that the factors mentioned above can be effective in the treatment of cancers without side effects (8).
Since colon cancer is one of the most common cancers in Iran, studies on the effects of local probiotic bacteria on the growth of cancer cells can play an effective role in protecting public health. These bacteria are also highly compatible with the gastrointestinal flora of Iranian people. By optimizing and enriching dairy and food products with these bacteria, successful steps can be taken to prevent intestinal cancer at a low cost. Studies on the cytotoxic properties of probiotics, including exopolysaccharide extract, are a new area of research. This study aims to evaluate the antiproliferative effect and induction of apoptosis of local probiotic bacteria, isolated from a traditional dish of “Tarkhine” on human colon cancer cell line HT–29.
Material and Methods
Microorganisms and Growth Conditions
In this study, two probiotic bacteria L. paracasia (TD3) and L. brevis (TD4) with the accession number IBRC_ M10784 and IBRC_M1079 were used. They were previously isolated by Dr. Tajabadi Ebrahimi and his col- leagues from the eponymous Iranian traditional food, recorded in the GenBank, the National Center for Biologi- cal Sciences and Genetic Resources.
The capacity of bile salt tolerance, cholesterol absorption, acidic resistance, and the ability to attach to the Caco–2 cell line of the bacteria have been established in a previous study (9). The bacteria were cultured in an MRS broth medium under anaerobic conditions and incubated for 48h at 37 °C for the subsequent steps.
EPS Production and Purification
Cell-bound exopolysaccharides (cb-EPS) were extracted separately according to Talon et al. Each isolate was grown in 10 ml of MRS broth. After inoculation, broth cultures were incubated at 37 ˚C for 48h under anaerobic condition. Then, the samples were centrifuged at 15000g for 15 min at 4°C. The pellets were applied to separate the cell-bound exopolysaccharide (cb-EPS) (10).
Cell-bound Exopolysaccharide Isolation
The pellet containing cells was washed in 5 ml of sterile physiological solution and then centrifuged at 15000 g for 15 min at 4°C. The viscous pellets were re-suspended in 5 ml of 0.05 M EDTA. The mixture was incubated under gentle agitation for 4 h at 4°C. Then, the cells were removed by centrifugation at 6000 rpm for 30 min at 4°C and the EPS was precipitated upon the addition of two volumes of cold 95% ethanol (Merck) followed by overnight incubation at 4°C. The EPS was recovered by centrifugation at 40°C at 6000 rpm for 30 min and dried in a laboratory environment. All tests were repeated three times.
Cb-EPS quantity assay
the precipitate was dissolved in 10 ml of distilled water and the total amount of carbohydrates in EPS was determined by phenol/sulfuric acid and glucose as the standard. Results were expressed in mg equivalent of glucose per liter of growth medium (10).
Preparation of Bacterial Extract Solutions
The stock solutions of exopolysaccharide extract of Probiotic L. paracasei and L. berevis were prepared in a complete medium (High-Glu DMEM (Gibco BRL, Scotland) supplemented with 10% fetal bovine serum (FBS- Gibco BRL, Scotland), 100 units/ml penicillin (Sigma), and 100µg/ml streptomycin (Sigma) with a concentra- tion of 400 mg/ml. Then, sterilized by filtration trough Millipore filter, 0.22 µm, and diluted by cell culture medium to various working concentrations (0.2-40 mg/ml).
Human Cell Culture
The HT-29 cells (Human colorectal adenocarcinoma, IBRC C10097) and L-929 cells (IBRC C10102) were purchased from the National Cell Bank in the Iranian Biological Resource Center. The HT-29 and L-929 cell lines were cultured in 25-sq cm culture flasks at 37 °C, with humidified 5% CO2 atmosphere, until they reached the desired population by High Glu DMEM medium supplemented with 10% heat inactivated fetal bovine serum (FBS-Gibco BRL, Scotland), 2 mM L_Glutamine (Gibco BRL, Scotland), 100 µg/ml streptomycin and 100 Iu/ ml penicillin (Sigma, USA). Cells were detached from the culture flask following 2 min incubation at 37 °C with a 0.05 solution of trypsin in phosphate buffered saline (PBS). The trypan blue dye exclusion staining is used for cell counting and cell viability analysis. Live cells were counted through a hemocitometer using phase contrast images.
In Vitro Cytotoxicity MTT Assay
The number of 5000 cells of HT-29 and L-929 cell lines per well, in a volume of 200 µl of medium, were grown in 96 well plates separately. After cell seeding, different filtered concentrations of exopolysaccharide extracts of both bacteria were added to cells and incubated for 4h at 37 °C in a humid atmosphere with 5 % CO2. Final concentrations of studied compounds in the treated wells were 0.2, 2, 20, and 40 mg/ml. Each concentration was experimented with at least four times on each cell line. In the control samples, only the complete medium was added to the cells. Then, the plates were incubated for 24, 48, and 72 h at 37° in a humidified incubator with a 5% CO2 environment.
The cell survival at different concentrations of each exopolysaccharide was measured by the MTT colorimetric assay based on tetrazolium dye reduction, as MTT turns into a purple formazan crystal by the activity of mito chondrial NAD(P)H-dependent oxidoreductase enzymes.
The cells were treated with 20 μL/well MTT (5 mg/mL in PBS solution) and incubated for another 4h. Super- natants were removed and 100µl of DMSO (as solvent) was added to each well to dissolve the purple formazan precipitates. Plates were placed on a Rotary shaker and agitated for 15–20 min, and then the plate reader mea- sured the OD in each of wells. The absorbance of formazan was read at 570 nm, with a 630 nm reference filter using an ELISA micro plate reader (TECAN-Sunrise, USA). The experiments were replicated more than four times and the reported data was the mean of 4-8 replicates. The percentage of cytotoxicity and cell viability was calculated using the following formulas:
Percent Cytotoxicity = 1- (mean absorbance of toxicant treated cells) / (mean absorbance of negative control) × 100.
Percent Viability = 100 – percent of Cytotoxicity.
IC50 (50% of growth inhibition) values of each exopolysaccharide in two different cell lines were determined in various incubation times.
Annexin V-FITC Assay for Apoptosis Analysis
Evaluation of apoptosis induction was performed in accordance with the Annexin V-FITC kit (eBioscience, USA Affymetrix,). For this purpose, the cells were treated with IC50 concentration of EPS extracts of both bacteria for 48 and 72h. Cellular analysis was performed using the flow cytometry device (Biocompare, USA).
DNA Fragmentation Assay
HT–29 cells were treated with four different concentrations (0.2, 2.0, 20, and 40 mg/mL) of exopolysaccharide extract. After 24h, the cells were washed with PBS solution and the enzymatic disaggregation was carried out with Trypsin. The cell suspension was centrifuged at 4000 g and re-suspended in 600 μL of lysis buffer (mM Tris-HCl pH 7.5, 400 mM NaCl, 100 mM EDTA, 0.6% SDS), and 10 μL RNAase (4 mg/mL). The solution was incubated for 5 min at 37°C and then 200 μL of 6 M NaCl was added for protein precipitation. This prepared solution was incubated for 5 min on ice, and then centrifuged at 18000 g at room temperature for 10 min. The supernatant was collected and added to 600 μL of isopropanol and cooled for another 15 min on ice. Again, it was centrifuged at 18000 g for 20 min and the solution was washed with 600 μL of 70% ethanol, dried at room temperature and dissolved in 200 μL of buffer [10 mM Tris-HCl, Tris-EDTA, 1 mM EDTA (pH 8.0)].
Results
Cells Viability
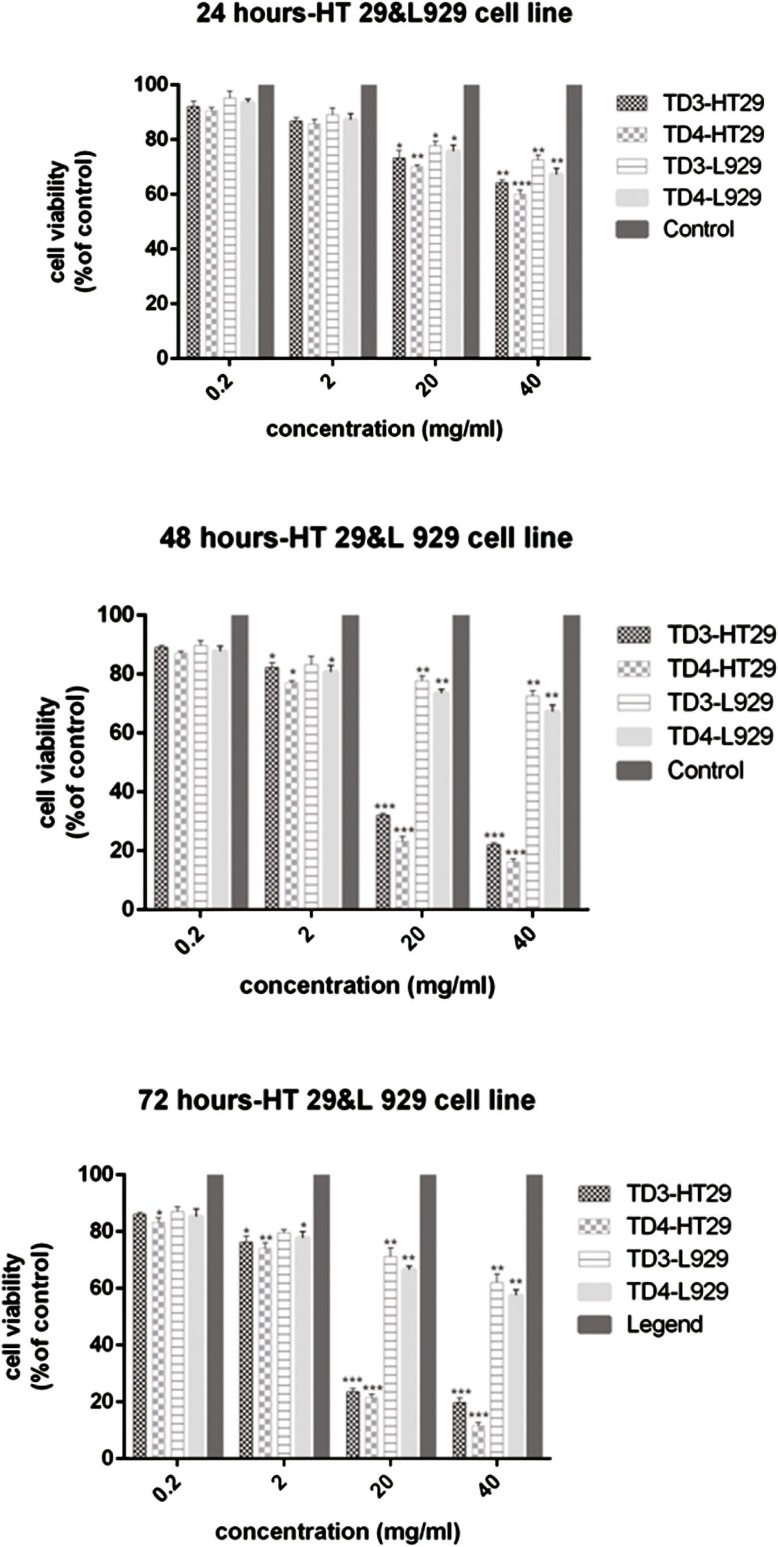
The percentage of viable HT–29 cells after treatment with different concentrations of exopolysaccharide ex- tract of L. paracasei and L. brevis bacteria is shown in Figure 1.
Figure 1.
Comparison of the viability percentage of HT–29 cells and normal fibroblast cells L-929 treated with 0.2, 2, 20, and 40 mg/mL of cb-EPS extracts from L. paracasei (TD3) and L. brevis (TD4) at 72, 48, and 24h. The results are reported as survival percentages compared with the controls (P<0.05: *, P<0.01: **, P<0.001: ***).
The highest inhibitory effect on the proliferation of HT–29 cells was observed at concentrations of 20 mg/mL and 40 mg/mL after 24 h of treatment with the exopolysaccharide of L. paracasei at a statistically significant level in comparison to the control group (P<0.05, P<0.01, respectively). However, at concentrations of 0.2 mg/ mL and 2 mg/mL, there were no significant differences (P>0.05) in the reduction of cell survival percentage. The highest cytotoxic effect was observed at the concentrations of 20 and 40 mg/mL of both bacterial extracts after 48h (P<0.001). The L. paracasei extract showed less toxicity than the L. brevis extract for the same concentrations. The reduction of cell proliferation was also remarkable at a concentration of 2 mg/mL, and was significantly different from the control group (P<0.05). Whereas the concentration of 0.2 mg/mL of cb-EPS had the lowest lethal effect on both bacteria, and showed no statistically significant difference with the control group (P> 0.05). The results indicated that the 0.2 and 2 mg/mL concentrations of L. paracasei and L. brevis extracts have less toxic effects compared with the concentrations 20 and 40 mg/mL at 72 h. The decline of viability at
0.2 mg/mL of the L. paracasei extract did not differ significantly with the control group (P> 0.05). At a concentration of 2 mg/mL, a more toxic effect was observed on the HT–29 cell line, and the difference was statistically significant (P<0.05). The inhibition of cell proliferation at 0.2 and 2 mg/mL of cb-EPS from L. brevis indicated significant differences compared with the control group (P<0.05, P <0.001, respectively). At 20 mg/mL, cell proliferation was inhibited for both bacterial extracts (P=0.0001). The highest anti-proliferative activity was ob- served for both extracts at a concentration of 40 mg/mL of cb-EPS (P<0.001). The results showed that the IC50 value for the L. paracasei extract was 56.25 ±0.987 mg/mL after 24h, while the IC50 values for 48 and 72h were 12.5 ± 1.12 mg/mL and 11.5 ± 0.845 mg/mL, respectively, which were substantial in comparison with the con- trol group (P<0.001). The IC50 value for the L. brevis extract was 49.75 ±1.245 mg/mL at 24 h, and the values were reported to be 10.75 ± 1.034 mg/mL and 8.75 ± 0.956 mg/mL for 48 and 72h, respectively, which showed a significant variation at these three different time intervals in comparison with the control group (P <0.001).
Comparison of Cytotoxic Effect of Exopolysaccharide Extracts Obtained from L. paracasei and L. brevis
on L–929 Cell Line
The viability percentage of normal cells is shown in Figure 1. The 0.2 and 2 mg/mL concentrations of the ex- tracts of both bacteria did not have a significant toxic effect on fibroblast cells for 24h. The viability was about 90% for normal cells, and no significant difference was observed in comparison to the control group (P>0.05). Although the viability percentage at both 20 and 40 mg/mL concentrations, was lower than the control group in both bacteria (P<0.05, P<0.01), but due to 70% viability observed for fibroblast cells, we conclude that the extract from both bacteria has a less toxic effect on normal cells (Fig. 1). Furthermore, the reduction of viable cell percentages at 0.2 and 2 mg/mL concentrations from the extracts of both bacteria was not significant at all three observation intervals, in comparison with the control group (P>0.05). Similarly, comparing the cell survival percentage at 48 and 72h at different concentrations of the extract showed that the bacterial extract had lower toxicity and anti-proliferative properties in normal cells compared with cancerous cells. At the highest
concentration of extract (40 mg/mL) at 72h, the viability percentage was higher than 60% for normal cells (62%
of viability was as a result of treatment with L. paracasei extract and 60% viability was reported after the treat- ment with L. brevis at 72h).
The Results of Apoptotic Induction in HT–29 Cells Treated with Exopolysaccharide Extracted from Probiotic Bacteria
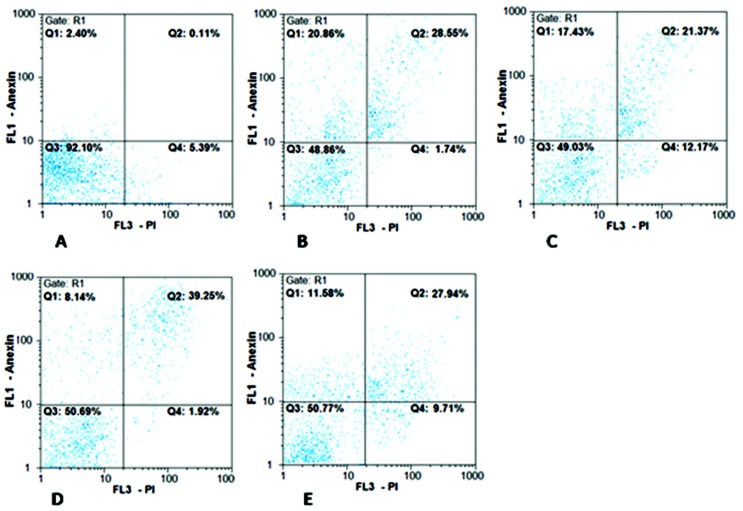
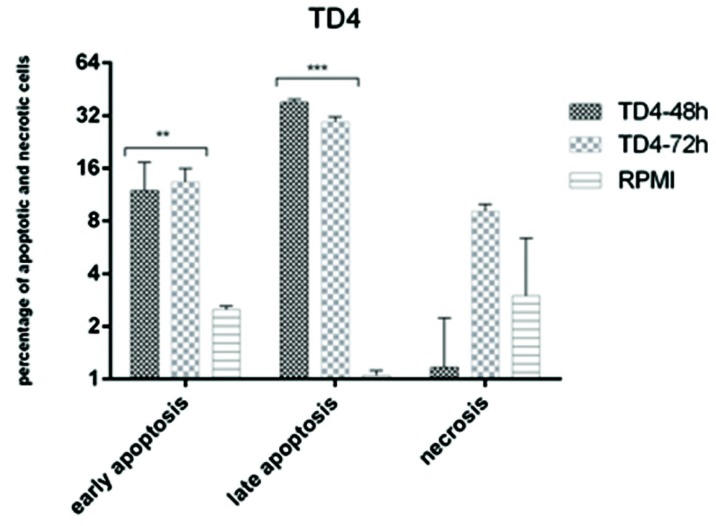
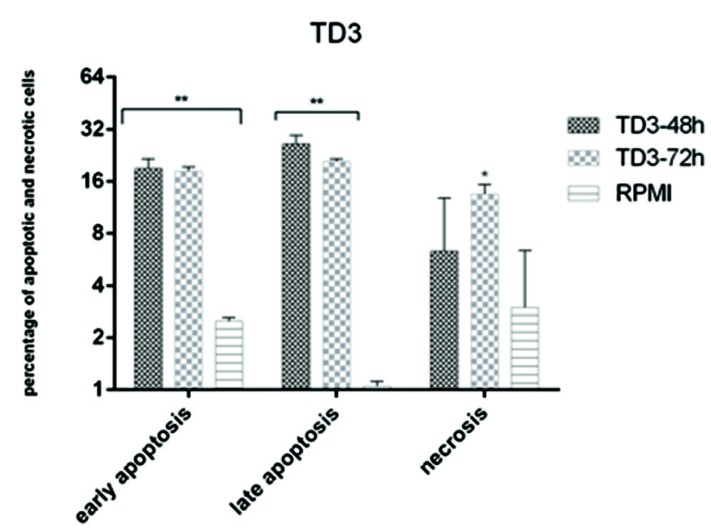
The results of apoptosis induction are shown in Figures 2-4. Both bacteria were able to induce apoptosis in the HT–29 cell line. The increase in apoptosis caused by the effect of the L. paracasei extract was significant both at 48 and 72h compared with the control group (P<0.01) (Fig. 3). The increase in the observed necrosis at 48h was not statistically significant in comparison with the control group (P>0.05). The extract of L. brevis bacteria was able to induce apoptosis at both 48 and 72h (P<0.001) (Figure 4). The comparison of the two bacteria showed that the bacterial extract of L. brevis is more potent than L. paracasei.
Figure 2.
Flow cytometry chart for evaluating apoptosis induced by cb-EPS extracts of L. paracasei (TD3) and L. brevis (TD4) at 48 and 72h. A: control cells (untreated cells); B, C: IC50 concentration of L. paracasei at 48 and 72 h, respectively. D, E: IC50 concentration of L. brevis at 48 and 72h, respectively. Q1 represents early apoptotic cells with Annexin-FITC + and PI¯ staining index, Q2 represents late apoptotic cells with Annexin-FITC + and PI +, Q3 represents healthy cells with Annexin- FITC¯ and PI¯ staining index and Q4 represents necrotic cells with Annexin-FITC¯ and PI + staining index
Figure 4.
Comparison between apoptosis and necrosis induced by cb-EPS extract of L. brevis (TD4) in HT-29 cell line
Figure 3.
Comparison between apoptosis and necrosis induced by cb-EPS extracts of L. paracasei (TD3) in HT-29 cell line
DNA Fragmentation Test Results
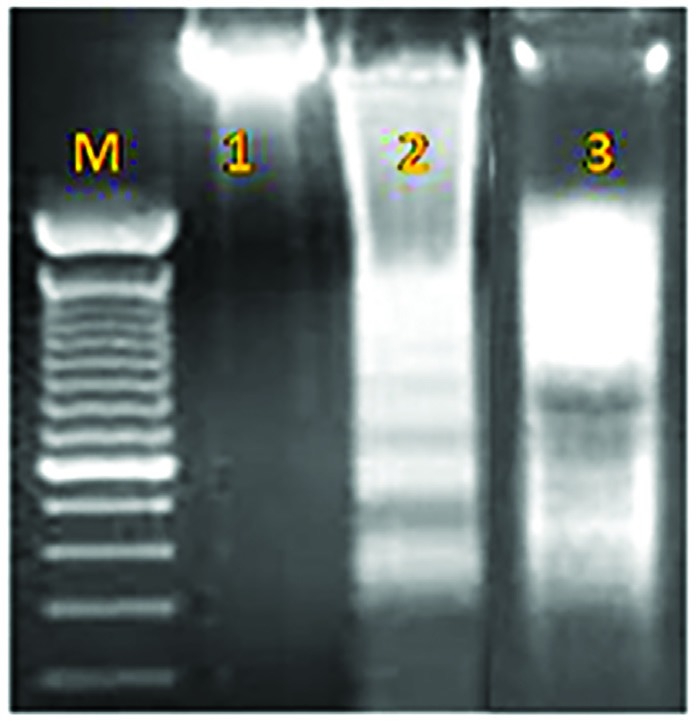
The ladder pattern of DNA fragmentation in cells treated with cb-EPS from both bacteria at 48h confirmed the induction of apoptosis in the HT–29 cell line (Figure. 5).
Figure 5.
DNA Fragmentation Assay. M: ladder, 1: untreated cells, 2: HT-29 cells treated by cb-EPS extracts of L. brevis, 3: HT-29 cells treated by cb-EPS extracts of L. paracasei
Discussion
Nowadays, cancer is one of the most important health problems around the world, and colorectal cancer is one of the most dangerous types of cancers. It is also one of the most common types of gastrointestinal cancer in Iran, ranked third among Iranian men and fourth in women (11). Hence, considering the effect of intestinal microflora on reducing the progression of intestinal cancer, the production of new types of products that prevent cancer without any harmful effects on healthy cells, seems to be important. Therefore, probiotics are being used for this purpose. Probiotics play an important role in preventing colon cancer by stimulating the immune sys- tem, destroying destructive and carcinogenic enzymes such as secondary bile acids and mutagenic substances produced by intestinal bacteria (12).
Probiotics include live microbial supplements that have beneficial effects on host cells in different ways in cluding the production of inhibiting compounds, competing with pathogens for chemicals and connecting sites, stimulating and regulating immune system activity, and improving the microbial balance of the intestine (13,14).
In 2011, Taverniti et al. suggested the term “Parabiotic” as a new term besides Probiotic. This term indicates that non-living microbes can have a positive health impact on humans, as many therapeutic and beneficial properties of probiotic bacteria are independent of their survival (15). The ability of live bacteria to elicit anti-tumor properties and health effects is well-established. However, there have always been concerns from the FAO and WHO about the use of live probiotic bacteria. Furthermore, the risk of the use of these bacteria and the occurrence of local or systemic inflammatory response in various individuals, especially those with immunodeficiency (patients with weakened immune systems or auto-immune diseases), and children have been reported (16). Therefore, in recent years, the tendency to use non-live bacteria and their fractions for prevention and treatment has increased, and various studies have been conducted to test the therapeutic properties and the effectiveness of non-live bacteria, including the specific metabolites of these bacteria, such as exopolysaccharide extract, peptidoglycan, and cellular DNA. Several studies have shown that these compounds can also elicit cytotoxic, anti- tumor, and therapeutic properties. In addition, they are safer than live cells (4-6, 8). Various studies have been conducted to confirm the therapeutic effects and efficiency of these bacterial products on reducing food aller- gies, gastrointestinal diseases (including diarrhea), immune diseases, cancer treatment, especially colon cancer and the reduction of side effects from the administration of live bacteria. The potential risks, including septi- cemia, bacteremia, antibiotic resistance, and the transmission of antibiotic resistance genes from these bacteria suggest the use of alternatives to probiotics. The results of various studies have shown that non-live bacteria and their metabolites confer a health benefit to the user, and some scientific pieces of evidence have demonstrated the high efficacy of these non-live bacteria and their metabolites for the treatment and prevention of diseases (15, 16). Interestingly, in recent years, various studies have been carried out on non-live probiotic bacteria and the evaluation of their characteristics has indicated interesting results. It is noteworthy that these characteristics are completely dependent on the strain and are different from one strain to another.
The present study showed that exopolysaccharide extracts of L. paracasei and L. brevis isolated from Tarkhine have an anti-proliferative effect on HT–29 cancer cells. Therefore, the comparison of the anti-proliferative activity and cell growth in the presence of the L. paracasei extract at the highest concentration of 40 mg/mL for 24 and 72h represents an increase of anti-proliferative activity and cell death from 36% to 80% (about 40% increase of cell death). A similar result was observed for anti-proliferative and cytotoxic activity as a result of the treat- ment with the L. brevis extract, as it increased from 40% to 90% (an approximate 50% increase in cell death). On the other hand, the anti-proliferative and inhibitory effects on normal cells were much lower than cancerous cells. According to the results, both bacteria were able to induce higher apoptosis in 48h than 72h in the HT–29 cell line. It seems that the bacteria appear to be more effective in inducing apoptosis within 48h.
By comparing the potency of the two bacterial extracts, we concluded that the cb-EPS produced by L. brevis has more cytotoxic, antiproliferative, and induced effects than the cb-EPS of L. paracasei. Therefore, with regard to the results of MTT and apoptosis, it can be concluded that the induction of apoptosis by cb-EPS is strain, dose and time-dependent.
There are limited articles and reports regarding the effect of exopolysaccharide extracted from probiotic bacteria on cancer cells. Kim et al. showed that both free exopolysaccharide and those linked to the cell surface inhibit the growth of PANC–1 and HT–29 cancer cells within 72h in vitro. Conversely, these two exopolysaccharides did not affect normal cells. This is in agreement with the findings of our study. The treatment of cancer cells using exopolysaccharide could increase the expression of Caspase–3 in comparison with control cells, which might lead to the inhibition of cancer cell growth by apoptotic induction (17).
The soluble polysaccharides isolated from L. acidophilus 606 and L. casei displayed anti-proliferative and anti- cancer activity on HT–29 cells. L. acidophilus 606 showed stronger activity and had a less toxic effect on normal fibroblast cells and could prevent the growth of fibroblast cells by only 20%. The polysaccharide product of L. acidophilus 606 was observed to induce apoptosis in HT–29 cells by DNA fragmentation (18).
Seockmo et al. (2009) reported that the polysaccharide extracted from B. bifidum BGN4 has inhibitory effects on several cancer cell lines, such as HT–29 and HCT–116, but is not effective on normal cells (19).
In another study by Kim et al (2010), the inhibition of HT–29 cell line proliferation by cb-EPS was demonstrated. Considering the increase of Beclin, GRP78, Bax, Bak expressions, and the decline of Bcl2 expression, it was suggested that cb-EPS was able to inhibit cell growth through both autophagy and cell apoptosis (6).
The results of this study are in line with other published reports that have been mentioned. The inhibition of HT–29 cell growth as a consequence of the effect of exopolysaccharide extracted from L. paracasei and L. brevis was confirmed in a dose, time, and strain-dependent manner. The signaling pathways by which the exopolysaccharides inhibit and prevent the growth of cancer cells are not fully understood, but it seems that it can activate the pathways for inducing apoptosis.
Various studies have suggested different mechanisms to induce apoptosis by probiotics. For example, the effect of short-chain fatty acids, such as butyrate, has been shown to inhibit growth and induce apoptosis in colon cancer cell lines (20).
Butyrate is also produced by the breakdown of lactate and acetate (21,22). An increase in lactate and acetate productions through bacteria such as Bifidobacteria and Lactobacillus can indirectly contribute to the production of butyrate and the induction of apoptosis (23). Butyrate seems to enhance P21 activity and can inhibit the activity of cdk, which causes an increase in caspase–3 protein activity. Eventually, this process increases Bax activity and decreases Bcl–2 activity and, consequently, leads to the induction of apoptosis (23).
Lan et al. (2007) indicated that the effects of short-chain fatty acids, such as butyrate, are effective in inducing apoptosis and the shift between apoptosis and necrosis depends on pH changes inside the cell (23).
The differences between L. paracasei and L. brevis may be due to the induction of apoptosis associated with short-chain fatty acids and changes in pH, which have not been examined in the present study.
Conclusion
Both cb-EPS produced by L. paracasei and L. brevis caused the death of HT–29 cancer cells. Considering the cell survival percentages with a similar concentration of the exopolysaccharide extract and time of observation, cb-EPS produced from L. paracasei and L. brevis showed less toxic effects on the fibroblasts compared with the HT–29 cells line, indicating a distinction between normal cells and cancerous cells by cb-EPS. The EPS pro- duced from L. brevis showed more anti-proliferative activity, apoptotic induction and anti-cancer activity than the L. paracasei bacteria. The L. paracasei extract also showed less anti-proliferative and cytotoxic effects on fibroblasts compared with L. brevis.
The results of this study indicated that the induction of apoptosis strongly depends on cb-EPS dose, bacterial strain, and treatment time.
Acknowledgements
The authors would like to acknowledge Islamic Azad University and Takgene Company, for contribution in this research.
Conflict of Interest
The authors declare that there is no conflict of interest in the publication of this paper.
References
- 1.Hoffman FA, Heimbach JT, Sanders ME, Hibberd PL. Executive summary: scientific and regulatory challenges of de- velopment of probiotics as foods and drugs. Clin Infect Dis. 2008;46(suppl 2):53–7. doi: 10.1086/523342. [DOI] [PubMed] [Google Scholar]
- 2.Lee B, Bak Y. Irritable bowel syndrome, gut microbiota and probiotics. J Neuro Gastro Enterol Motil. 2011;17(3):252–66. doi: 10.5056/jnm.2011.17.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns AJ, Rowland IR. Anti-carcinogenicity of probiotics and prebiotics. Curr Issues Intest Microbiol . 2000;1(1):13–24. [PubMed] [Google Scholar]
- 4.Manjunath N, Ranganathan B. A cytotoxic substance produced by a wild culture of Lactobacillus casei D-34 against tumor cells. Indian J Exp Biol. 1989;27:141–145. [PubMed] [Google Scholar]
- 5.Kim JY, Woo HJ, Kim YS, Kim KH, Lee HJ. Cell cycle dysregulation induced by cytoplasm of Lactococcus lactis ssp lactis in SNUC2A, a colon cancer cell line. Nutr Cancer. 2003;46:197–201. doi: 10.1207/S15327914NC4602_13. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y, Oh S, Yun HS, Oh S, Kim SH. Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett Appl Microbiol. 2010;51(2):123–130. doi: 10.1111/j.1472-765X.2010.02859.x. [DOI] [PubMed] [Google Scholar]
- 7.Fichera GA, Giese G. Non-immunologically-mediated cytotoxicity of Lactobacillus casei and its derivative pepti- doglycan against tumor cell lines. Cancer Lett. 1994;85(1):93–103. doi: 10.1016/0304-3835(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 8.Lahtinen SJ. Probiotic viability - does it matter? Microb Ecol Health Dis. 2012;18 doi: 10.3402/mehd.v23i0.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajabady Ebrahimi M, Bahrami H, Ziary Z. Tarkhineh source of probiotic lactic acid bacteria. The Quarterly Jour- nal of Biological Sciences, Islamic Azad University Zanjan. 2011;4(12):1–9. [Google Scholar]
- 10.Tallon R, Bressollier P, Urdaci MC. Isolation and Characterization of Two Exopolysaccharides Produced by Lac- tobacillus plantarum EP56. Res Microbiol. 2003;154:705–712. doi: 10.1016/j.resmic.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Fateh S, Amini M. An epidemiologic study of colorectal cancer in Arak during 1994-2004. Iran J Surgery. 2008;2(16):11–17. [Google Scholar]
- 12.Singh J, Rivenson A, Tomita M, Shimamura S, Ishibashi N, Reddy BS. Bifidobacterium longum, a lactic acid-pro- ducing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesis. Carcinogenesis. 1997;18(4):833–41. doi: 10.1093/carcin/18.4.833. [DOI] [PubMed] [Google Scholar]
- 13.Tukmechi A, Bandboni M. The effect of yeast supplementation on the growth and immune system in rainbow trout (Oncorhynchus mykiss) Journal of Veterinary Research. 2013;68(1):69–78. [Google Scholar]
- 14.Tukmechi A, Bandboni M. Effects of Saccharomyces cerevisiae supplementation on immune response, hematologi- cal parameters, body composition and disease resistance in rainbow trout, Oncorhynchus mykiss (Walbaum, 1792) Journal of Applied Ichthyology. 2014;30(1):55–61. [Google Scholar]
- 15.Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept) Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataria J, Li N, Wynn JL, Neu J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev. 2009;67(9):546–50. doi: 10.1111/j.1753-4887.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim JU, Kim YH, Han KS, OH SJ, Wang KU, Kim JN, Kim SN. Function of cell-bound and released exopolysac- charide produced by Lactobacillus rhamnosus ATCC9595. Microbiol Biotechnol . 2006;16:939–945. [Google Scholar]
- 18.Choi SS, Kim Y, Han KS, You S, Oh S, et al. Effects of Lactobacillus strains on cancer cell proliferation and oxida- tive stress in vitro. Lett Appl Microbiol . 2006;42(5):452–458. doi: 10.1111/j.1472-765X.2006.01913.x. [DOI] [PubMed] [Google Scholar]
- 19.Seockmo K, Hyun JY, Geun EJ. Enhancement of Anti-tumorigenic Polysaccharide Production, Adhesion, and Branch Formation of Bifidobacterium bifidum BGN4 by Phytic Acid. Food Sci Biotechnol. 2009;18(3):749–754. [Google Scholar]
- 20.Walton E, Gibson R. PREBIOTICS AND BOWEL CANCER. Current Topics in Nutraceutical Research . 2007;5(1):19–28. [Google Scholar]
- 21.Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, et al. Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr . 2004;91(6):915–923. doi: 10.1079/BJN20041150. [DOI] [PubMed] [Google Scholar]
- 22.BlenguerA , DuncanSH , CalderAG , HoltrabG , LouisP , etal Tworoutesofmetaboliccross-feedingbetweenBifidobacteri- um adolescentisandbutyrate-producinganaerobesfromthehumangut. ApplEnvironMicrobiol. 2006;72(5):3593–3599. [Google Scholar]
- 23.Siavoshian S, Blottiere HM, Cherbut C, Galmiche JP. Butyrate stimulates cyclin D and p21 and inhibits cyclin- dependent kinase 2 expression in HT-29 colonic epithelial cells. Biochem Biophys Res Commun. 1997;232(1):169–172. doi: 10.1006/bbrc.1997.6255. [DOI] [PubMed] [Google Scholar]
- 24.Lan A, Lagadic-Gossman D, Leaire C, Brenner C, Jan G. Acidic extracellular pH shifts colorectal cancer cell death from apoptosis to necrosis upon exposure to propionate and acetate, major end-products of the human probiotic pro- pionibacteria. Apoptsis. 2007(3):573–591. doi: 10.1007/s10495-006-0010-3. [DOI] [PubMed] [Google Scholar]