Abstract
Lung ultrasound is a useful tool for the assessment of patients with both acute and chronic heart failure. The use of different image acquisition methods, inconsistent reporting of the technique employed and variable quantification of “B-lines”, however, have all made it difficult to compare published reports. As a result, strategies to improve patient care by its use have been difficult to develop. There is a need to ensure future studies utilizing lung ultrasound in the assessment of heart failure adopt a standardized approach to reporting the quantification of pulmonary congestion. This consensus report includes a checklist to provide standardization in the preparation, review and analysis of manuscripts. This will serve as a guide for investigators and clinicians and enhance the quality and transparency of lung ultrasound research. Key aspects of standardization discussed include equipment used, number of chest zones assessed, the method of quantifying B-lines, the presence and timing of additional investigations (e.g., natriuretic peptides and echocardiography) and the impact of therapy.
Keywords: lung ultrasound, heart failure, methodology, reporting checklist
Introduction
Pulmonary congestion is one of the most important findings in heart failure (HF), yet traditional methods, e.g., clinical examination and chest x-ray, are relatively insensitive for its detection.1–3 Lately, there has been tremendous growth in the use of lung ultrasound (LUS) for the detection of pulmonary congestion in HF both in research and, more recently, in clinical practice.2,4–10 LUS has been proposed as a useful tool in the assessment of patients with both acute and chronic HF.2,5,8,10 This technique enables the detection of pulmonary congestion in patients presenting with acute dyspnea with higher accuracy than chest auscultation or chest x-ray.5 The LUS findings of pulmonary congestion, commonly called B-lines, change dynamically with treatment for acute HF and can provide prognostic information in both acute and chronic HF.11,12 However, different methods and inconsistent reporting of the LUS technique used and the quantification of B-lines make it difficult to compare existing studies. This lack of standardization impedes the development of strategies to reduce pulmonary congestion and improve patient care.11 One prior international consensus statement described a wide variety of LUS applications, but was not specifically focused on its use in HF or detailed in its description of the methodological aspects.4 With the anticipated growth in the use of LUS, and in subsequent potential publications, in patients with HF, there is a need to develop a standardized reporting guide for the quantification of pulmonary congestion by LUS in HF.
Methods and Aims
Our aim was to create a checklist to enhance the quality and transparency of LUS research and reporting. This consensus statement is intended to serve as a guide for investigators, reviewers, editors and readers in the preparation, evaluation and interpretation of manuscripts involving the use of LUS in HF.13 We convened a group of cardiologists and emergency physicians with expertise in LUS, HF, epidemiological studies, and clinical trials to review the current literature in this area. Following discussion and agreement, they composed a succinct evidence-based reporting checklist. In contrast to other existing guidelines, we focused on unique aspects of LUS research, including study design and image analysis.
Reporting Checklist
Title, abstract and study design
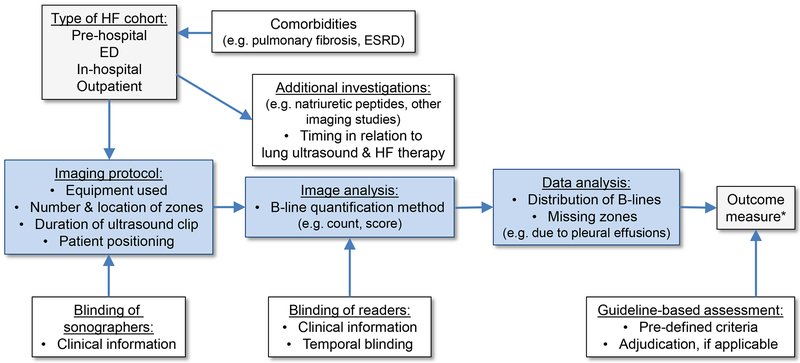
All reports should follow previously published guidelines regarding the use of a structured abstract and appropriate title.14 The relevant guidelines for the design of the study e.g. observational vs. randomized clinical trial should be used.14 For diagnostic studies, the reference standard should be clearly described and for prognostic studies, authors should report how the primary outcome was adjudicated, as applicable.15 A description of the key aspects of both the general study design and LUS-specific components is provided in the reporting checklist (Table 1 and Figure 1).
Table 1.
Reporting checklist for lung ultrasound studies in heart failure cohorts
| No. | Aspects for consideration | Literature | |
|---|---|---|---|
| Title or abstract | 1 | Identification as a study employing lung ultrasound as a measure of pulmonary congestion | |
| Abstract | 2 | Structured summary of study design, methods, results, and conclusions | |
| Introduction | 3 | Scientific and clinical background, including the intended use and clinical role of lung ultrasound | |
| 4 | Study hypothesis and objectives | ||
| Methods | |||
| Study design | 5 | Whether data collection was planned before the lung ultrasound was performed (prospective study) or after (retrospective study) | |
| 6 | Description of how heart failure was defined | 16,17 | |
| 7 | Description of reference standard for the primary outcome and how it was adjudicated (if applicable) | 15 | |
| Participants | 8 | Inclusion and exclusion criteria with particular attention to factors that could confound lung ultrasound findings (e.g. interstitial lung disease, pneumonitis, ARDS, dialysis) | 18–22 |
| 9 | On what basis potentially eligible participants were identified (such as symptoms, results from previous tests, inclusion in registry) | ||
| 10 | Where and when potentially eligible participants were identified (setting, location and dates) | 5,11,23 | |
| Study size | 11 | Explain how the study size was arrived at | |
| Lung ultrasound method | 12 | Type of ultrasound equipment used (such as high-end ultrasound system or pocket size ultrasound device), including type and orientation (transverse vs. sagittal) of transducer | 24,25 |
| 13 | Patient positioning during lung ultrasound examination | 26 | |
| 14 | Number and location of lung ultrasound zones examined | 5,6,8,11,28 | |
| 15 | Duration of recorded lung ultrasound clips (if image analysis was performed offline) | 24,25 | |
| 16 | Whether clinical information was available to the performers of the lung ultrasound | ||
| Lung ultrasound image analysis | 17 | Whether clinical information was available to the readers of the lung ultrasound (image analysis blindly performed offline vs. real time) | |
| 18 | For serial lung ultrasound assessment: Whether the timing of the lung ultrasound was available to the readers (temporal blinding) | 11 | |
| 19 | Method of B-line quantification (e.g. sum of all B-lines across all lung zones, or score based on B-line number in a given zone), including inter- and intra-observer variability. If automated software was used, type and version of software. | 4,11 | |
| 20 | Describe how pleural effusions were assessed by ultrasound and report the number of patients with unilateral or bilateral pleural effusions on ultrasound. | ||
| Additional investigations | 21 | Describe any additional investigations supporting the diagnosis or degree of congestion (e.g. echocardiography, natriuretic peptides, invasive hemodynamics) performed and their temporal relationship to the LUS examination, as well as to therapy targeted at congestion. | |
| Data analysis | 22 | Number of patients with missing LUS zones & how these patients were handled in the analysis. How lung ultrasound zones with pleural effusions that interfered with B-line quantification were handled in the analysis. | |
| Results | 23 | Report the number of patients enrolled, excluded, patients with adequate images and those analyzed, as well as outcomes. Give reasons for non-participation at each stage. Consider using a flow diagram. | |
| 24 | Baseline demographic and clinical characteristics of participants | ||
| 25 | Number and variation of B-lines at baseline (and follow up, if applicable) | ||
| Discussion | 26 | Study limitations, including sources of potential bias, statistical uncertainty, and generalizability | |
| 27 | Implications for clinical practice, including the intended use and clinical role of lung ultrasound | ||
| Other information | 28 | Name of registry and registration number if applicable. | |
| 29 | Sources of funding and other support; role of funders |
Lung ultrasound-specific aspects are highlighted in light blue.
Figure 1. Overview of important methodological aspects in the quantification of pulmonary congestion by lung ultrasound in heart failure.
HF: heart failure, ED: emergency department, ESRD: end-stage renal disease
* Outcome measures could represent B-line count/score, a diagnosis or prognostically important event(s).
Participant characteristics, co-morbidities and study setting
In studies of patients with known or suspected HF, the definition of HF used should be described in detail and should be consistent with recognized definitions.16,17 Standard patient descriptors should be reported as should how and where the patients were recruited and whether any inclusion and exclusion criteria were applied. Reported patient characteristics should include general demographics, such as age, sex, and body mass index, vital signs including respiratory rate, blood pressure and heart rate, as well as important comorbidities, symptoms and signs of heart failure, measures of cardiac function and natriuretic peptides.
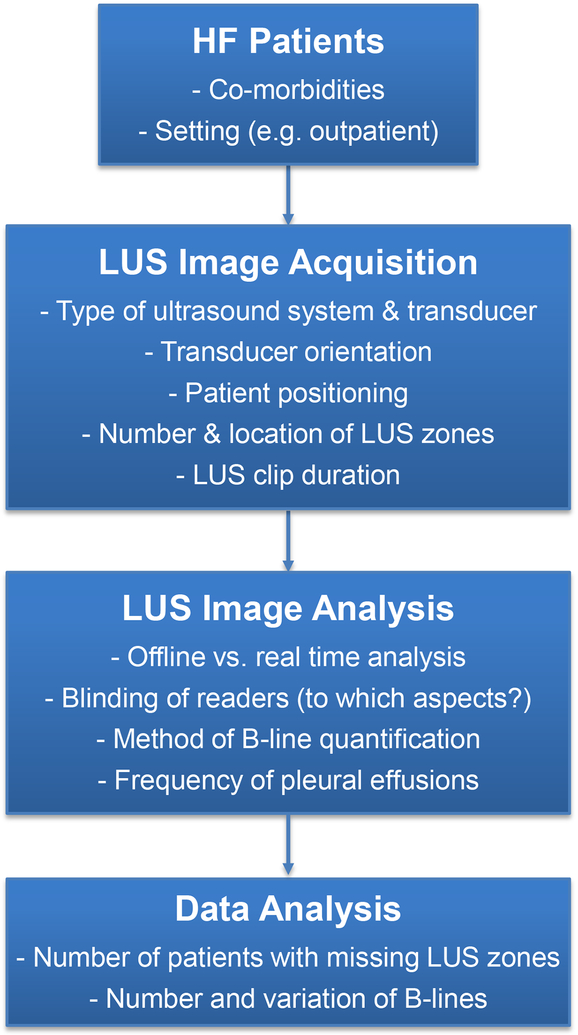
Diffuse B-lines, which usually reflect pulmonary congestion, can also be detected by LUS in other conditions such as pulmonary contusions, adult respiratory distress syndrome (ARDS), and interstitial lung disease.18–22 Pulmonary congestion can also result from conditions other than HF, e.g. end stage renal disease. Consequently, it is essential that studies designed to detect potential pulmonary congestion in patients with suspected or established HF also make a statement about the presence or absence of these other co-morbidities known to lead to B-lines on LUS (Table 1).11 Without a clear description of these variables, study results may be confounded or misleading. If these conditions are exclusion criteria, this should be clearly stated in the Methods section of the study. If patients having one of these conditions have been included, their potential significance must be evaluated, e.g. by undertaking stratified, sensitivity and other analyses to determine whether they have confounded the interpretation of potential pulmonary congestion and change in congestion over time and/or in response to treatment. Reporting of the setting of the study (e.g. pre-hospital, ambulatory care, emergency department, hospital ward, intensive care unit) is also important, as HF patients will demonstrate a different spectrum of B-lines reflecting the likely degree of pulmonary congestion in each setting and interpretation and comparison of studies must thus take study setting into account (Figures 1 and 2).5,11,23
Figure 2. Practical aspects of lung ultrasound in heart failure cohorts.
HF: heart failure, LUS: lung ultrasound
Ultrasound equipment, image acquisition and image analysis
The manufacturer and model of the ultrasound equipment used should be described. The type of transducer, transducer orientation (transverse vs. sagittal) and clip duration (which may be limited to shorter time periods on pocket ultrasound devices) can alter the number of detectable B-lines in patients with HF.24,25 Specifically, phased array transducers (as compared to curvilinear transducers) and longer clip duration (6–7 seconds/video clip) allow for observation of a higher number of B-lines in HF.24,25 Similarly, patient positioning during the LUS should be described and ideally performed in a standardized position due to its effect on B-line count, as patients with acute HF may have a higher number of B-lines in the supine vs. the sitting postion.26
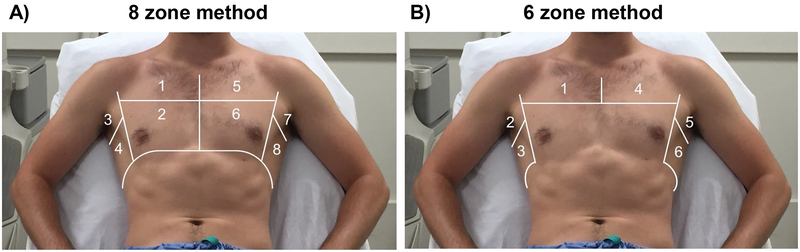
The number and location of chest zones examined should be clearly described. Prior studies in HF cohorts have reported 4 to 28 chest zones (Table 2), and in 2012 an international guideline recommended either the use of 8 or 28 zones (Figure 3A).4 Different approaches have since been described e.g. using 6 zones in the assessment of dyspneic patients in the emergency department and without apparent loss of diagnostic accuracy.4,5 Based on the currently available data, we suggest that at least 3 zones on each hemithorax (6 zones total; Figure 3B) should be examined and the B-line number reported in patients with HF.5
Table 2.
Overview of common B-line quantification methods in patients with heart failure
| Zones, n | Location of zones | Method | B-line quantification | Sample studies |
|---|---|---|---|---|
| 28 | Anterior & lateral chest | Count | Sum of B-lines in all zones | 8,9,34,35* |
| Score | Mild: 6–15 B-lines in all zones Moderate: 16–30 B-lines in all zones Severe: >30 B-lines in all zones |
7,8 | ||
| 11 | Anterior & lateral chest | Score | 0 points: <3 B-lines per zone 1 point: ≥3 B-lines per zone Score: Number of points |
31 |
| 8 | Anterior & lateral chest | Count | Sum of B-lines in all zones | 8,10,23 |
| Score | 0 points: <3 B-lines per zone 1 point: ≥3 B-lines per zone Score: Number of points |
8,9,27,28 | ||
| 6 | Anterior & lateral chest | Score | 0 points: <3 B-lines per zone 1 point: ≥3 B-lines per zone Score: Number of points |
5 |
| 5 | Anterior & posterior chest | Count | Sum of B-lines in all zones | 6 |
| Score | 0 points: ≤3 B-lines per zone 1 point: >3 B-lines per zone Score: Number of points |
6 | ||
| 4 | Anterior & lateral chest | Score | 0 points: <3 B-lines per zone 1 point: ≥3 B-lines per zone Score: Number of points |
36 |
Some studies used semi-quantitative count based approaches.
Figure 3. Example of 8 and 6 chest zones for lung ultrasound imaging.
Adapted from: Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail. 2017;19(9):1154–1163.
For B-line quantification, two general approaches have been reported in HF cohorts (Table 2):
A count-based method, in which the sum of B-lines in one intercostal space per zone across all zones is reported.10,23
A scoring system, in which a minimum number of B-lines in one intercostal space per zone is used to define a zone as “positive”. Positive zones are then summed to delineate a cut-off value. For example, ≥3 B-lines in 2 zones on each hemithorax are consistent with a diagnosis of pulmonary edema in dyspneic patients presenting to the emergency department.5,27,28
If software is used to quantify the number of B-lines, the manufacturer and version of the software should be reported, as the type of software could potentially contribute to variability in B-line number between vendors. In addition, the cut-off definition process or decision limits for the detection of HF should be accurately described, if applicable. As large pleural effusions may interfere with B-line quantification, the presence of pleural effusions (overall frequency of unilateral or bilateral pleural effusions) and how pleural effusions were assessed should be reported, when possible.
Blinding & central image interpretation
Blinding is an important methodologic feature in diagnostic and prognostic studies to minimize bias and maximize the validity of results. Sonographer knowledge of the findings on clinical examination or results of other diagnostic modalities, therapies and medical history, should be described when reporting image acquisition. Blinding to these same aspects should be reported with respect to the individuals undertaking B-line quantification. The temporal aspects of blinding should be described for studies involving serial LUS examinations. Although HF studies investigating the impact of reader experience on both real-time and offline quantification of B-lines have demonstrated similar results between novice and expert readers, with high inter-reader agreement, the experience of the personnel involved in analyses and the setting in which the analyses are performed should be reported.25,29 Specifically, whether the LUS images were interpreted in real-time (at the bedside), off-line by investigators not involved in the image acquisition, or at a central core laboratory should be reported. In order to obtain unbiased results, blinded reading in a central core laboratory clearly is preferable.
Additional investigations
The results of additional investigations assessing hemodynamic or clinical congestion, such as chest radiography, echocardiography, invasive hemodynamic measurements or natriuretic peptide levels, should be documented. Importantly, the temporal relationship between these investigations and the assessment of pulmonary congestion by LUS should be reported. This information will also facilitate a better understanding of the sequence of the dynamic changes of these congestion markers.30 For example, whether the chest radiograph was performed at the same time as the LUS study or whether it was performed 24 hours later affects the interpretation of the relationship between these investigations. Similarly, the initiation of any therapy directed at congestion, and any response that occurred between the LUS study and supporting investigations should be clearly documented (e.g. if pulmonary artery pressures were measured, after which the patient received diuretics, followed by the LUS study, should be documented).
Data reporting and analysis
Sonographic B-lines in patients with HF are known to be differentially distributed.12,31 As a higher prevalence of B-lines occurs in more dependent chest zones, the reporting of missing data in zones that could not be analyzed (e.g., due to cardiomegaly or large pleural effusions) is essential. More dependent zones are also those most likely influenced by the presence of pleural effusions or, in the left hemithorax, by cardiomegaly. The method or methods used to deal with missing B-line data or missing zones should be clearly described.
Statistical methods appropriate for the quantification method (e.g. score or count data) should be used and detailed in the statistical analysis section. As B-lines are frequently not normally distributed, the analysis should consider their distribution among the patients studied.
Presentation of results
The presentation of results should include the number of patients enrolled and excluded from analysis or follow-up, the proportion with adequate images and the number analyzed. Authors should provide reasons for non-participation at each stage, preferably using a CONSORT flow diagram for illustration.32 The LUS data description should include the number and variation of B-lines at baseline and at follow up, if applicable. In addition to the main study results, sources of potential bias and the generalizability of study findings should be discussed, as well as any implications for clinical practice with respect to the role of LUS.
Gaps in current knowledge
While there is general agreement on how to diagnose pulmonary edema with LUS in patients with undifferentiated dyspnea presenting to the emergency department, the wide range of LUS methods used, has made the establishment of a standardized approach and cut-off values in other settings challenging. This hampers the performance of meta-analyses of available evidence and consequently the genesis of a widely accepted consensus. Studies with larger sample sizes comparing different imaging protocols with respect to the number of zones and B-line quantification method, in both ambulatory and hospitalized HF patients, including on admission and pre-discharge, would be useful to inform clinical guidelines and future clinical trials. Specifically, whether LUS provides incremental diagnostic or prognostic information beyond current methods in patients with suspected or known HF should be further addressed through well-designed, prospective investigations, with appropriate statistical analyses, e.g. including comprehensive multivariable models incorporating other important diagnostic and prognostic variables. In addition, studies investigating treatment response and the adequacy of decongestive therapy, for instance at the time of hospital discharge in large, well-defined HF cohorts will be important. Specifically, outcome randomized controlled trials assigning patients to a treatment intervention designed to maximize B-line resolution vs. standard of care could inform clinical practice in the future. Similarly, the value and frequency of LUS use during outpatient clinic follow up warrants further investigation. While B-lines can be detected irrespective of ejection fraction in both ambulatory and hospitalized patients with heart failure, recent reports in patients with reduced vs. preserved ejection fraction demonstrated differing results with respect to the number of B-lines in these HF cohorts23,33 These findings could be due to different degrees of pulmonary congestion or other confounders. Further research is needed to better understand the impact of these factors on LUS findings in patients with HF and how to best integrate LUS in the management of these patients.
Conclusions
Lung ultrasound can provide useful information regarding the presence and degree of pulmonary congestion in patients with heart failure. Consistent reporting of certain methodological aspects should be considered in studies employing lung ultrasound in heart failure populations to assure high-quality research result dissemination and allow for future standardization.
Funding
The writing of this manuscript was supported by a grant from the National Heart, Lung and Blood Institute (Grant number K23HL123533) (Platz). The sponsors had no input or contribution in the development of the research and manuscript.
Conflict of interest
Dr. Platz reports grants from NHLBI, during the conduct of the study; Dr. Jhund reports personal fees from Novartis, grants and personal fees from Boehringer Ingelheim, personal fees from Vifor pharma, personal fees from Amgen, outside the submitted work; and is Director of GCTP Ltd.; Dr. Girerd reports personal fees from Novartis, outside the submitted work; Dr. Maisel reports personal fees from critical diagnostics, personal fees from Abbott, outside the submitted work; Dr. Bueno reports grants from Instituto de Salud Carlos III, personal fees from Bayer, personal fees from Novartis, grants, personal fees and non-financial support from AstraZeneca, grants and personal fees from BMS-Pfizer, personal fees from Ferrer, personal fees from MEDSCAPE-the Heart-org, personal fees from Janssen, outside the submitted work; Dr. Mebazaa reports personal fees from Novartis, personal fees from Orion, personal fees from Roche, personal fees from Servier, grants and personal fees from Adrenomed, grants and personal fees from Abbott, personal fees from Sanofi, outside the submitted work; Dr. Gualandro reports personal fees and non-financial support from Servier, grants from FAPESP (Research Foundation of the State of Sao Paulo), outside the submitted work; Dr. Metra reports personal fees from Novartis, Bayer, outside the submitted work; Dr. Coats reports personal fees from Astra Zeneca, personal fees from Vifor, personal fees from Respicardia, personal fees from Impulse Dynamics, personal fees from Faraday, personal fees from WL Gore, personal fees from Actimed, personal fees from Menarini, personal fees from Novartis, personal fees from Servier, personal fees from Nutricia, personal fees from Stealth Peptides, personal fees from Verona, outside the submitted work; Dr. Ruschitzka reports grants and personal fees from SJM / Abbott, grants and personal fees from Servier, personal fees from Zoll, personal fees from Astra Zeneca, personal fees from Sanofi, grants and personal fees from Novartis, personal fees from Amgen, personal fees from BMS, personal fees from Pfizer, personal fees from Fresenius, personal fees from Vifor, personal fees from Roche, grants and personal fees from Bayer, personal fees from Cardiorentis, personal fees from Boehringer Ingelheim, other from Heartware, grants from Mars, outside the submitted work; Dr. Mueller reports grants, personal fees and non-financial support from several diagnostic companies, outside the submitted work; Dr. Pivetta, Dr. McMurray, Dr. Peacock, Dr. Masip, Dr. Martin-Sanchez, Dr. Miro, Dr. Price, Dr. Cullen, Dr. Vrints, Dr. Cowie, Dr. Di Somma, Dr. Tavares, and Dr. Severovic have no competing interests.
References
- 1.Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care Ultrasonography for the Diagnosis of Acute Cardiogenic Pulmonary Edema in Patients Presenting With Acute Dyspnea: A Systematic Review and Meta-analysis. Acad Emerg Med 2014;21:843–52. [DOI] [PubMed] [Google Scholar]
- 2.Martindale JL, Wakai A, Collins SP, et al. Diagnosing Acute Heart Failure in the Emergency Department: A Systematic Review and Meta-analysis. Acad Emerg Med 2016;23:223–42. [DOI] [PubMed] [Google Scholar]
- 3.Platz E, Jhund PS, Campbell RT, McMurray JJ. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: a systematic review. Eur J Heart Fail 2015;17:906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577–91. [DOI] [PubMed] [Google Scholar]
- 5.Pivetta E, Goffi A, Lupia E, et al. Lung Ultrasound-Implemented Diagnosis of Acute Decompensated Heart Failure in the ED: A SIMEU Multicenter Study. Chest 2015;148:202–10. [DOI] [PubMed] [Google Scholar]
- 6.Gustafsson M, Alehagen U, Johansson P. Imaging Congestion With a Pocket Ultrasound Device: Prognostic Implications in Patients With Chronic Heart Failure. J Card Fail 2015;21:548–54. [DOI] [PubMed] [Google Scholar]
- 7.Gargani L, Pang PS, Frassi F, et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound 2015;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coiro S, Rossignol P, Ambrosio G, et al. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 2015;17:1172–81. [DOI] [PubMed] [Google Scholar]
- 9.Cogliati C, Casazza G, Ceriani E, et al. Lung ultrasound and short-term prognosis in heart failure patients. Int J Cardiol 2016;218:104–8. [DOI] [PubMed] [Google Scholar]
- 10.Platz E, Lewis EF, Uno H, et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 2016;37:1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail 2017;19:1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortellaro F, Ceriani E, Spinelli M, et al. Lung ultrasound for monitoring cardiogenic pulmonary edema. Intern Emerg Med 2017;12:1011–7. [DOI] [PubMed] [Google Scholar]
- 13.Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest 2010;40:35–53. [DOI] [PubMed] [Google Scholar]
- 14.Equator network. (Accessed June 20, 2018, at https://www.equator-network.org/reporting-guidelines/.)
- 15.Hicks KA, Mahaffey KW, Mehran R, et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation 2018;137:961–72. [DOI] [PubMed] [Google Scholar]
- 16.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 18.Zoccali C, Torino C, Tripepi R, et al. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol 2013;24:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogliati C, Antivalle M, Torzillo D, et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology (Oxford) 2014;53:1497–503. [DOI] [PubMed] [Google Scholar]
- 20.Reissig A, Copetti R, Mathis G, et al. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: a prospective, multicenter, diagnostic accuracy study. Chest 2012;142:965–72. [DOI] [PubMed] [Google Scholar]
- 21.Haddam M, Zieleskiewicz L, Perbet S, et al. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med 2016;42:1546–56. [DOI] [PubMed] [Google Scholar]
- 22.Platz E, Cydulka R, Werner S, Resnick J, Jones R. The effect of pulmonary contusions on lung sliding during bedside ultrasound. Am J Emerg Med 2009;27:363–5. [DOI] [PubMed] [Google Scholar]
- 23.Dwyer KH, Merz AA, Lewis EF, et al. Pulmonary Congestion by Lung Ultrasound in Ambulatory Patients With Heart Failure With Reduced or Preserved Ejection Fraction and Hypertension. J Card Fail 2018;24:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platz E, Pivetta E, Merz AA, Peck J, Rivero J, Cheng S. Impact of device selection and clip duration on lung ultrasound assessment in patients with heart failure. Am J Emerg Med 2015;33:1552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pivetta E, Baldassa F, Masellis S, Bovaro F, Lupia E, Maule MM. Sources of Variability in the Detection of B-Lines, Using Lung Ultrasound. Ultrasound Med Biol 2018;44:1212–6. [DOI] [PubMed] [Google Scholar]
- 26.Frasure SE, Matilsky DK, Siadecki SD, Platz E, Saul T, Lewiss RE. Impact of patient positioning on lung ultrasound findings in acute heart failure. Eur Heart J Acute Cardiovasc Care 2015;4:326–32. [DOI] [PubMed] [Google Scholar]
- 27.Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med 2009;16:201–10. [DOI] [PubMed] [Google Scholar]
- 28.Pivetta E, Goffi A, Nazerian P, et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail 2019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Chiem AT, Chan CH, Ander DS, Kobylivker AN, Manson WC. Comparison of expert and novice sonographers’ performance in focused lung ultrasonography in dyspnea (FLUID) to diagnose patients with acute heart failure syndrome. Acad Emerg Med 2015;22:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picano E, Scali MC. The lung water cascade in heart failure. Echocardiography 2017;34:1503–7. [DOI] [PubMed] [Google Scholar]
- 31.Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med 2008;26:585–91. [DOI] [PubMed] [Google Scholar]
- 32.The CONSORT flow diagram. (Accessed July 30, 2018, at http://www.consort-statement.org/consort-statement/flow-diagram.)
- 33.Palazzuoli A, Ruocco G, Beltrami M, Nuti R, Cleland JG. Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol 2018;107:586–96. [DOI] [PubMed] [Google Scholar]
- 34.Facchini C, Malfatto G, Giglio A, Facchini M, Parati G, Branzi G. Lung ultrasound and transthoracic impedance for noninvasive evaluation of pulmonary congestion in heart failure. J Cardiovasc Med (Hagerstown) 2016;17:510–7. [DOI] [PubMed] [Google Scholar]
- 35.Miglioranza MH, Gargani L, Sant’anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013;6:1141–51. [DOI] [PubMed] [Google Scholar]
- 36.Ohman J, Harjola VP, Karjalainen P, Lassus J. Assessment of early treatment response by rapid cardiothoracic ultrasound in acute heart failure: Cardiac filling pressures, pulmonary congestion and mortality. Eur Heart J Acute Cardiovasc Care 2018;7:311–20. [DOI] [PubMed] [Google Scholar]