Abstract
The monocarboxylate transporter 1 (MCT1) is highly expressed in the outer retina, suggesting that it plays a critical role in photoreceptors. We examined MCT1+/− heterozygotes, which express half of the normal complement of MCT1. The MCT1+/− retina developed normally and retained normal function, indicating that MCT1 is expressed at sufficient levels to support outer retinal metabolism.
Keywords: MCT1, Retina, Lactate, Transgenic mice, Electroretinogram
46.1. Introduction
The retina is among the most metabolically active tissues in the body and relies on glucose in both glycolytic and oxidative pathways to support the high-energy demands of visual transduction and outer segment renewal. Like cancer cells, the retina utilizes aerobic glycolysis to generate lactate (Weinhouse 1976). And the majority of glucose transported into the outer retina is metabolized to lactate (Wang et al. 1997). This raises the question: what becomes of the lactate? In the brain there is considerable evidence to suggest glucose is metabolized by astrocytes through aerobic glycolysis to generate lactate that is shuttled to adjacent neurons for oxidation (Pellerin and Magistretti 2012). A lactate shuttle could also support metabolism in the outer retina (Poitry-Yamate and Tsacopoulos 1991; Poitry-Yamate et al. 1995). In vitro studies showed isolated Müller glial cells (MGCs) metabolized glucose and released lactate into the media. When photoreceptor cells were added, lactate levels in the media decreased, suggesting photoreceptor cells utilized the lactate (Poitry-Yamate and Tsacopoulos 1991; Poitry-Yamate et al. 1995).
The cloning of MCT1 (SCL16A1) paved the way for identification of other members of the MCT family and the development of antibodies, siRNA, and inhibitors for studying their tissue-specific expression and activity (Adijanto and Philp 2012). Several MCT isoforms are expressed in the retina, and their specific distribution provides insight into the production and utilization of lactate in the outer retina. MCT1 is found in many cells but is expressed at the highest levels in cells that oxidize lactate such as type l muscle fibers and cardiac muscle. In the retina, MCT1 is expressed in the inner segment of photoreceptor cells and endothelial cells and in the apical membrane of the retinal pigment epithelium (RPE).
MCT1 is a heteromeric transporter that requires the accessory protein CD147 (Bsg) for trafficking to the plasma membrane (Kirk et al. 2000). Like other heteromeric transporters, the absence of one subunit results in the targeting of the other subunit for degradation. Mice lacking CD147 (Bsg−/−) have severely reduced electroretinograms (ERGs), progressive photoreceptor degeneration (Hori et al. 2000), and reduced levels of MCT1, MCT3, and MCT4 in the retina (Philp et al. 2003). These findings indicate that aerobic glycolysis and the lactate shuttle are essential for supporting photoreceptor cell activity and viability. To understand the specific contribution of MCT1 in supporting visual function, we examined the ocular phenotype of MCT1+/− mice, which have axonopathy due to disruption of the lactate shuttle between oligodendrocytes and motor neuron axons (Lee et al. 2012).
46.2. Materials and Methods
46.2.1. Mice
The generation of MCT1 mutant mice has been described (Lee et al. 2012). While MCT1 homozygous mutant mice do not survive, mice used in these studies were generated by crossing MCT1+/− and C57BL/6J (WT) mice.
46.2.2. Visual Electrophysiology
The methods used to record ERGs and visual evoked potentials (VEPs) have been described (Yu et al. 2012). In brief, after overnight dark adaptation, mice were anesthetized with ketamine (80 mg/kg) and xylazine (16 mg/kg) after which ERGs or VEPs were recorded to strobe flash stimuli presented in a ganzfeld bowl.
46.2.3. Western Blotting
RPE and retinas were microdissected from mouse eyes and homogenized in RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% Na deoxycholate, 0.1% SDS) containing protease inhibitors (Sigma) as described (Philp et al. 2003). Detergent soluble lysates (5 μg) were loaded onto a NuPAGE® 4–12% Tris-acetate gel (Invitrogen) for electrophoresis. Proteins were subsequently transferred onto PVDF membranes using XCell II™ Blot Module (Invitrogen). Nonspecific binding sites were blocked with TBS (+0.1% Tween 20) containing 5% w/v powdered milk. Antibodies used in this study were cyclophilin A antibody (Upstate Cell Signaling Solutions), Glut1 (Abcam), CD147 (G19; Santa Cruz), and MCT1 (Philp Lab) (Philp et al. 2003).
46.2.4. Immunofluorescence and Imaging
Mouse eyes were fixed using methanol/acetic at −80 °C as described (Sun et al. 2015). Cryosections (8 μm) were collected on glass slides and labeled with anti-MCT1 and CD147 antibodies as described (Philp et al. 2003).
46.3. Results
Figure 46.1a presents frozen sections of an MCT1+/− retina analyzed at 14 months of age. MCT1 antibody labels the apical membrane of the RPE, photoreceptor cell inner segments, and capillaries in the outer and inner plexiform layers. The overall appearance of the MCT1+/− retina showed no structural abnormalities, and there was no evidence of photoreceptor degeneration. Similar results were obtained by OCT imaging (not shown). Immunoblot analysis demonstrated that the MCT1+/− RPE and retina are both haploinsufficient for MCT1, as compared to WT littermates (Fig. 46.1b).
Fig. 46.1.
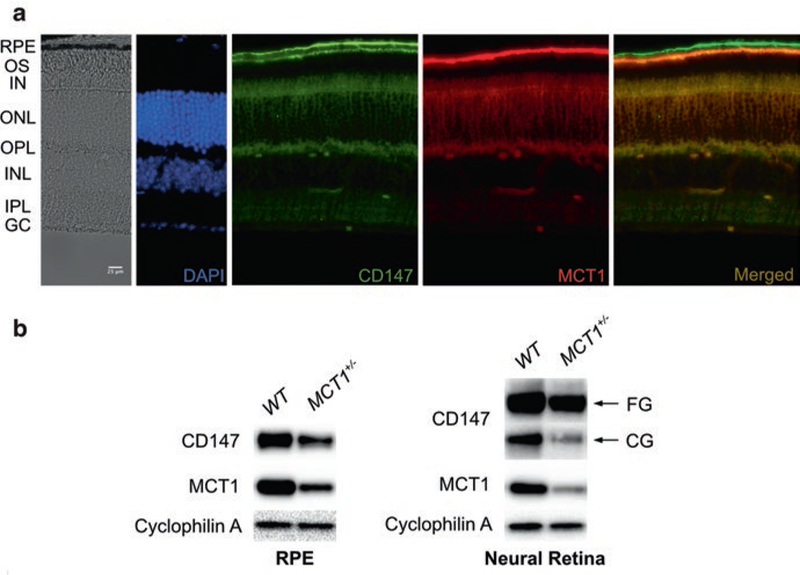
MCT1+/− retina has normal structure but reduced levels of MCT1. (a) Frozen section of 14-month-old MCT1+/− retina. Retinal structure is normal, and while the abundance of MCT1 is decreased relative to age-matched WT littermates, the distribution of MCT1 is not changed. (b) Immunoblot analysis of RPE and retinal lysates demonstrates greater than 50% reduction in MCT1 levels in MCT1+/− as compared to WT mice
To examine if there was a functional impact of MCT1 haploinsufficiency, we examined visual electrophysiology. ERGs recorded from MCT1+/− mice under dark-adapted conditions had a waveform that was not different from WT littermates (Fig. 46.2a). The amplitudes of the major ERG components did not differ in amplitude from those of WT littermates (Fig. 46.2b), indicating normal function of photoreceptors (a-wave) and rod bipolar cells (b-wave). Similar results were obtained for the light-adapted ERG (not shown). We also examined transmission through the visual pathway, by recording VEPs over the visual cortex. The VEP of MCT1+/− mice had a normal waveform (Fig. 46.2c), and implicit time measurements were not different from WT littermates (Fig. 46.2d).
Fig. 46.2.
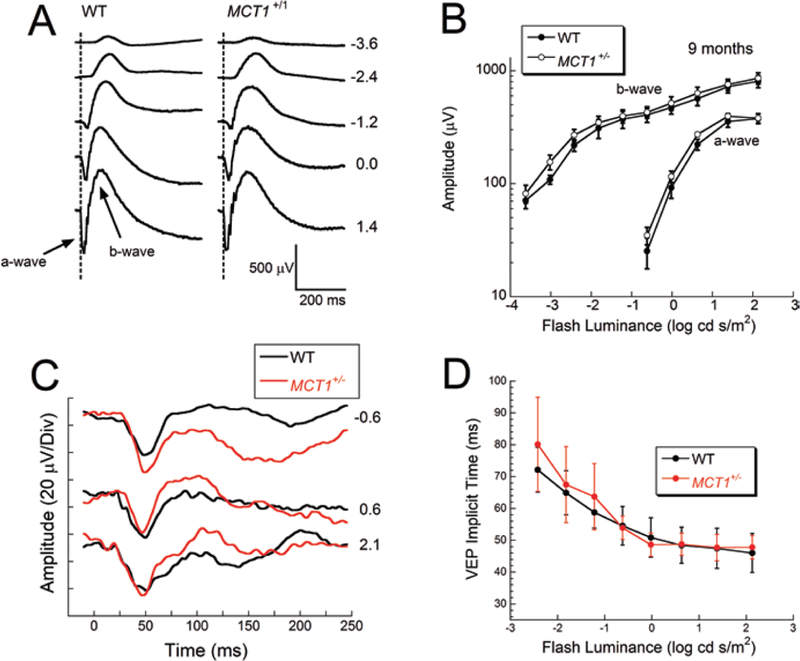
Normal visual electrophysiology in MCT1+/− mice. (a) Dark-adapted ERGs obtained from 9-month-old MCT1+/− and WT littermates. (b) Amplitude of the major components of the dark-adapted ERG. Data points indicate average ± sem from four mice per genotype. (c) VEPs obtained from 8-month-old MCT1+/− and WT littermates. (d) Implicit time of the major VEP component plotted as a function of flash luminance. Data points indicate average ± sem from 15 WT and 11 MCT1+/− mice
46.4. Discussion
In the central nervous system, MCT1 is expressed in oligodendrocytes and facilitates lactate transport to the axon to support their energy demands. The MCT1+/− haploinsufficient adult mice exhibit widespread axonopathy in the central nervous system that does not result from demyelination or oligodendrocyte death (Lee et al. 2012). In the retina and RPE, we and others have shown MCT1 is primarily expressed in the RPE and photoreceptor cells suggesting that lactate provides a key energy substrate for these cells (Philp et al. 2003). Mice expressing a single MCT1 allele had reduced levels of MCT1 in the retina, indicating the absence of a mechanism to upregulate expression of the single allele present in MCT1+/− mice or to stabilize the protein for a longer period of time. Despite this reduction, we detected no changes in retinal structure. Moreover, our visual electrophysiology data indicate that a single allele of MCT1 generates sufficient protein to support normal function of photoreceptors and bipolar cells, as the ERG a- and b-waves of MCT1+/− mice were comparable to WT. Transmission through the MCT1+/− visual pathway was also comparable to WT. These results, coupled with the absence of visual abnormalities in patients with GLUT1 haploinsufficiency (Levy et al. 2010), indicate that the retina enjoys high expression of transporters related to supplying this tissue with metabolic substrates.
Acknowledgments
This work was supported by grants from NIH (R01EY12042) and Department of Veterans Affairs (I01BX2340) and by the Foundation Fighting Blindness and Research to Prevent Blindness.
Contributor Information
Neal S. Peachey, Louis Stokes Cleveland VA Medical Center, Cleveland, OH, USA Cole Eye Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH, USA.
Minzhong Yu, Cole Eye Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH, USA.
John Y.S. Han, Department of Pathology, Anatomy and Cell Biology, Thomas Jefferson University, Philadelphia, PA, USA
Sylvain Lengacher, Brain Mind Institute, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland.
Pierre J. Magistretti, Brain Mind Institute, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland Division of Biological and Environmental Sciences and Engineering, King Abdullah University of Science and Technology (KAUST), Thuwal, KSA, Saudi Arabia.
Luc Pellerin, Department of Physiology, University of Lausanne, Lausanne, Switzerland.
Nancy J. Philp, Department of Pathology, Anatomy and Cell Biology, Thomas Jefferson University, Philadelphia, PA, USA
References
- Adijanto J, Philp NJ (2012) The SLC16A family of monocarboxylate transporters (MCTs)--physiology and function in cellular metabolism, pH homeostasis, and fluid transport. Curr Top Membr 70:275–311 [DOI] [PubMed] [Google Scholar]
- Hori K, Katayama N, Kachi S et al. (2000) Retinal dysfunction in basigin deficiency. Invest Ophthal Vis Sci 41:3128–3133 [PubMed] [Google Scholar]
- Kirk P, Wilson MC, Heddle C et al. (2000) CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 19:3896–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y et al. (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B, Wang D, Ullner PM et al. (2010) Uncovering microdeletions in patients with severe Glut1 deficiency syndrome using SNP oligonucleotide microarray analysis. Mol Genet Metab 100:129–135 [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. Cereb Blood Flow Metab 32:1152–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp NJ, Ochrietor JD, Rudoy C et al. (2003) Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophth Vis Sci 44:1305–1311 [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate C, Tsacopoulos M (1991) Glial (Müller) cells take up and phosphorylate [3H]2-deoxy-D-glucose in mammalian retina. Neurosci Lett 122:241–244 [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M (1995) Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci 15:5179–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Shibata B, Hess JF, FitzGerald PG (2015) An alternative means of retaining ocular structure and improving immunoreactivity for light microscopic studies. Mol Vis 21:428–442 [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tornquist P, Bill A (1997) Glucose metabolism in pig outer retina in light and darkness. Acta Physiol Scand 160:75–81 [DOI] [PubMed] [Google Scholar]
- Weinhouse S (1976) The Warburg hypothesis fifty years later. Z Krebsforsch 87:115–126 [DOI] [PubMed] [Google Scholar]
- Yu M, Sturgill-Short G, Ganapathy P et al. (2012) Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp Eye Res 96:142–131 [DOI] [PMC free article] [PubMed] [Google Scholar]


