Abstract
Purpose:
Fludarabine is an integral anticancer agent for patients with chronic lymphocytic leukemia (CLL) and those receiving conditioning regimens prior to allogeneic hematopoietic cell transplantation (HCT). An individual’s response to fludarabine may be influenced by the amount of CD4+ and CD8+ T-lymphocyte suppression. Fludarabine undergoes cellular uptake and activation to form the cytotoxic metabolite, fludarabine triphosphate (F-ara-ATP).
Methods:
We have previously developed a highly sensitive LC-MS method to quantitate intracellular F-ara-ATP concentrations in a leukemic cell line. However, quantitation of F-ara-ATP concentrations within CD4+ and CD8+ T-lymphocytes from pharmacokinetic blood samples obtained from patients receiving fludarabine therapy is not feasible because of the limited number of T-lymphocytes that can be isolated from each blood sample. Thus, we sought to determine F-ara-ATP accumulation after ex vivo exposure of freshly isolated human CD4+ or CD8+ T-lymphocytes to fludarabine. The method was optimized in T-lymphocytes obtained from healthy volunteers, and proved to be a feasible method to determine F-ara-ATP accumulation in patients undergoing HCT.
Results:
Considerable variability was observed in F-ara-ATP accumulation in HCT patients (10.5- and 12.5-fold in CD4+ and CD8+ cells, respectively), compared to healthy volunteers (1.6- and 1.9-fold in CD4+ and CD8+ cells, respectively). Larger variability was also observed in gene expression of transporters and enzymes involved in F-ara-ATP accumulation in HCT patients; however, F-ara-ATP accumulation was not correlated with gene expression, which is in agreement with previous studies.
Conclusions:
The quantitation of F-ara-ATP accumulation in T-lymphocytes provides a novel tool to evaluate patient sensitivity to fludarabine. This tool can be used in future studies to evaluate whether intracellular F-ara-ATP accumulation is associated with efficacy and/or toxicity in patients receiving fludarabine.
Keywords: fludarabine, T-lymphocytes, hematopoietic cell transplantation, nucleoside transporters, chronic lymphocytic leukemia
INTRODUCTION
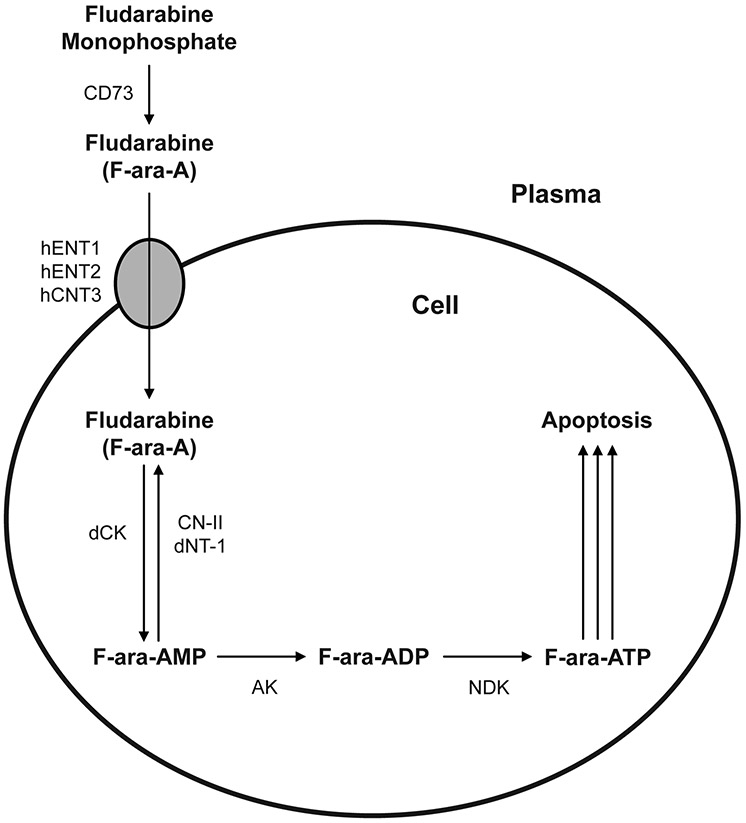
The purine nucleoside analog fludarabine has an established role as first-line therapy for chronic lymphocytic leukemia (CLL) and more recently has also been used in host conditioning regimens prior to allogeneic hematopoietic cell transplantation (HCT) or adoptive T-cell therapy [5, 11, 24]. Fludarabine is administered as its monophosphate form over a wide dosage range of 90-250 mg/m2 in HCT conditioning regimens [10]. Fludarabine must undergo active intracellular transport and metabolism to form fludarabine triphosphate (F-ara-ATP), which inhibits ribonucleotide reductase and DNA polymerase and ultimately leads to cellular apoptosis in both actively dividing and resting cells [15]. As represented in Figure 1, fludarabine (9-β-D-arabinofuranosyl-2-fluoroadenine, or F-ara-A) is administered as the monophosphate prodrug and is rapidly dephosphorylated in plasma to fludarabine by serum phosphatase and ecto-5’-nucleotidase (CD73) [15]. Intracellular uptake of fludarabine is mediated by several nucleoside transporters, including the nitrobenzylthioinosine (NBMPR)-sensitive human equilibrative nucleoside transporter 1 (hENT1), the NBMPR-insensitive human equilibrative nucleoside transporter 2 (hENT2), and the human concentrative nucleoside transporter 3 (hCNT3) [15, 29]. Once inside the cell, fludarabine is sequentially phosphorylated to the monophosphate (F-ara-AMP), diphosphate (F-ara-ADP), and triphosphate (F-ara-ATP) forms by deoxycytidine kinase (dCK), adenylate kinase (AK), and nucleoside diphosphate kinase (NDK), respectively [32]. F-ara-AMP can also be dephosphorylated to fludarabine by 5’-nucleotidase (CN-II) and deoxynucleotidase-1 (dNT-1).
Figure 1. Intracellular disposition of fludarabine.
The schematic displays the intracellular uptake and metabolism of fludarabine to the active metabolite fludarabine triphosphate (F-ara-ATP). Abbreviations: ecto-5’-nucleotidase (CD73), human equilibrative nucleoside transporter 1 (hENT1), human equilibrative nucleoside transporter 2 (hENT2), human concentrative nucleoside transporter 3 (hCNT3), deoxycytidine kinase (dCK), dephosphorylation by 5’-nucleotidase (CN-II), deoxynucleotidase-1 (dNT-1), adenylate kinase (AK), and nucleoside diphosphate kinase (NDK).
Substantial efforts have been made to optimize response and/or decrease toxicity to fludarabine as first-line therapy for CLL [15, 24, 30]. However, these results cannot be extrapolated to T-lymphocytes because CLL cells and T-lymphocytes (i.e. CD4+ and CD8+ cells) have different susceptibility to equivalent fludarabine concentrations [9]. The toxicity of fludarabine is not predicted by body-surface-area-based dosing [33]; and plasma fludarabine area under the plasma concentration-time curve (AUC) is not correlated with T-cell chimerism or engraftment in patients receiving fludarabine-based conditioning regimens [7]. Several lines of evidence suggest that the effects of fludarabine upon lymphocyte subsets may significantly influence outcomes in allogeneic HCT recipients [1, 4, 26, 31]. Therefore, developing novel methods of predicting an individual’s response to fludarabine is critical.
The intracellular exposure to F-ara-ATP within CD4+ and CD8+ T-lymphocytes is of interest because F-ara-ATP is the primary intracellular, and only known cytotoxic, metabolite of fludarabine [15]. Determination of F-ara-ATP accumulation in CD4+ and CD8+ T-lymphocytes may provide a quantitative phenotypic tool to predict the efficacy and/or toxicity of fludarabine. Pharmacokinetic sampling of intracellular F-ara-ATP concentrations in T-lymphocytes obtained from patients receiving fludarabine therapy would be optimal. However, this approach is not feasible because low circulating CD4+ and CD8+ counts after fludarabine administration do not provide adequate cell number for quantitation. In addition, a highly acidic environment is required to stabilize F-ara-ATP in cells [19]. Thus, a novel method to estimate intracellular F-ara-ATP exposure is needed. Our laboratory recently developed a liquid chromatography-mass spectrometry (LC-MS) method to quantitate F-ara-ATP in Jurkat cells, a leukemic cell line, that is 250-1000 times more sensitive than previously published methods [19]. With Jurkat cells, there are an unlimited number of cells available to quantitate F-ara-ATP. However, in HCT recipients receiving fludarabine, the paucity of circulating T-lymphocyte concentrations may prevent isolation of an adequate number of T-lymphocytes to quantitate F-ara-ATP. However, isolation of the T-lymphocytes of interest – specifically CD4+ and CD8+ T-lymphocytes – followed by ex vivo exposure to pharmacologically relevant fludarabine concentrations may provide an estimation of F-ara-ATP exposure. Prediction of exposure via protein or mRNA expression of the transporters and enzymes involved in F-ara-ATP uptake and activation is not realistic because studies evaluating these as predictors for fludarabine response in CLL patients have yet to yield a consistent and biologically plausible association [24, 30].
Therefore, in this study, we have sought to develop a method to directly measure an individual’s functional ability to activate fludarabine to F-ara-ATP in T-lymphocytes. This quantitative method offers the ability to evaluate a patient’s cellular sensitivity to fludarabine and may act as a predictive marker to adjust the dosing of fludarabine-based conditioning regimens in HCT patients. The goals of these studies were to evaluate the feasibility of quantitating F-ara-ATP accumulation rate after ex vivo fludarabine exposure of CD4+ or CD8+ T-lymphocytes; to quantitate F-ara-ATP accumulation and variability in CD4+ and CD8+ T-lymphocytes isolated from healthy volunteers and patients awaiting HCT; and to assess possible mechanisms underlying the interindividual variability by analyzing gene expression levels of transporters (hENT1, hENT2, and hCNT3) and enzymes (dCK, CN-II, and dNT-1) involved in fludarabine uptake and activation. Achieving these goals are essential to subsequent pharmacodynamic studies to evaluate the relationship of F-ara-ATP exposure in CD4+ and CD8+ T-lymphocytes and clinical outcomes in patients receiving fludarabine-based conditioning regimens.
METHODS
Isolation of T-lymphocytes from healthy volunteers and patients awaiting HCT
Prior to study procedures, written consent was obtained using forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (Seattle, WA). Study participation did not influence the conditioning regimen administered to the HCT patients, and patients receiving any conditioning regimen were eligible for recruitment in this study. Cells were obtained from 12 healthy volunteers and 44 HCT patients. Cells from 34 of the 44 HCT patients underwent subsequent T-lymphocyte processing. Patient demographics for the 34 HCT patients used in these studies are presented in Table 1. Most of the 34 HCT patients had prior exposure to other chemotherapy agents; only 7 patients had no previous chemotherapy exposure. A variety of chemotherapeutics regimens were used in this patient group, including 3 patients who had previous exposure to fludarabine.
Table 1.
Patient demographics
| Characteristic | Number of patients (n = 34) |
|---|---|
| Gender | Male: 19 |
| Female: 15 | |
| Age | 21-67 years |
| Diseases | Acute myeloid leukemia: 13 |
| Myelodysplastic syndrome: 7 | |
| Myelofibrosis: 3 | |
| Acute lymphoid leukemia: 2 | |
| Chronic lymphocytic leukemia: 2 | |
| Non-hodgkin lymphoma: 2 | |
| Chronic myeloid leukemia: 1 | |
| Chronic myelomonocytic leukemia: 1 | |
| Hodgkin lymphoma: 1 | |
| Myeloma: 1 | |
| Paroxysmal nocturnal hemoglobinura: 1 | |
| Conditioning regimens | Fludarabine/TBI: 16 |
| Fludarabine/busulfan: 14 | |
| Cyclophosphamide/TBI: 3 | |
| Busulfan/cyclophosphamide: 1 |
A mononuclear-enriched apheresis product was obtained from the healthy volunteers to optimize the methods to enrich and evaluate CD4+ and CD8+ T-cells for intracellular F-ara-ATP accumulation. In patients awaiting HCT, a peripheral blood sample (60 mL) was obtained from 44 adults prior to the administration of the conditioning regimen, of which 34 had sufficient total cell numbers to proceed with CD4+ and CD8+ isolation (>90×106 cells in the 60 mL peripheral blood draw was arbitrarily determined as the minimum total cell count necessary to proceed with isolation of T-lymphocytes). Independently, total white blood cells and absolute CD4+ and CD8+ cells counts were obtained within 1 day of the peripheral blood draw for each HCT patient.
The same enrichment and purification procedure for CD4+ and CD8+ lymphocytes was used for both apheresis products from healthy volunteers and peripheral blood sample from HCT patients, with an additional step for peripheral blood samples, which were treated with NH4CL to lyse the red blood cells before proceeding onto the selection. CD4+ and CD8+ T-lymphocytes were purified using magnetic microbeads (Miltenyi Biotec Inc., Auburn, CA) labeled with anti-CD4+ or anti-CD8+ antibody and an autoMACS™ automatic magnetic cell sorter (Miltenyi Biotec Inc.) according to the manufacturer’s recommendations.
Fludarabine triphosphate accumulation in T-lymphocytes
The measurement of F-ara-ATP accumulation was initiated in each cell population independently within 2 hours of cell isolation. Cells were incubated in fludarabine (5 μM) in RPMI 1640 media (not containing phenol red) supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) antibiotic/antimycotic for 4 hours at 37°C in the presence of 5% CO2. This concentration is in the range of the peak fludarabine plasma concentration (3 μM) after fludarabine monophosphate administration of 30 mg/m2/day, the dose used in nonmyeloablative conditioning regimens. Starting cell numbers varied slightly based on yield from the isolation procedure. A minimum number of 1.25×105 cells were needed per incubation to form adequate amounts of F-ara-ATP for accurate quantitation. Incubations were conducted in at least two replicates. After fludarabine exposure, cells were washed twice in ice-cold phosphate buffered saline, centrifuged, and solubilized in 1 M perchloric acid. Samples were frozen at −70°C prior to F-ara-ATP quantitation.
LC-MS quantitation of fludarabine triphosphate in T-lymphocytes
After thawing, the sample was centrifuged and the supernatant was neutralized to pH ~ 7 with 1 M potassium bicarbonate. F-ara-ATP was quantitated using the LC-MS method described previously, with the following modifications [19]. A YMC-Pack Hydrosphere C18 column (3 μm 2.0 mm × 150 mm) was used with a flow rate of 0.225 ml/min and the column temperature at 30°C (Waters, Milford, MA). The mobile phase consisted of 20 mM ammonium formate pH 8 (A) and methanol (B) with the following gradient: initial 96.5% A and 3.5% B to 12% B at 5 minutes, then 3.5% B at 8 minutes. Chloroadenosine triphosphate was used as an internal standard. The F-ara-ATP quantitation assay is highly sensitive and provides a limit of detection equivalent to 50 fmol F-ara-ATP. The units for all F-ara-ATP accumulation rates were pmol/1×106 cells/4 hours.
Gene expression analysis
Immediately after autoMACS™ isolation of the CD4+ and CD8+ cells, a separate aliquot of lymphocytes was taken for total RNA extraction. Thus, gene expression analysis occurred in lymphocytes not exposed to fludarabine. Total RNA was extracted from CD4+ and CD8+ cells from both healthy volunteers (n = 8) and HCT patients (n = 32) using RNAqueous®−4PCR (Ambion, Austin, TX), followed by generation of cDNA using SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Gene-specific TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA) were used to quantitate gene expression of key transporters and enzymes involved in F-ara-ATP accumulation (see Figure 1): hENT1 (Hs00191940_m1), hENT2 (Hs00155426_m1), hCNT3 (Hs00223220_m1), dCK (Hs00176127_m1), CN-II (Hs00366992_m1), and dNT-1 (Hs00274359_m1) relative to an internal endogenous control β-glucuronidase (GUS) (4333767F) on a 7900HT Fast Real-Time PCR System (Applied Biosystems). GUS was chosen as the endogenous control because its there is small variation in its expression levels across tissues [6]. Samples were performed in triplicate using 10 ng cDNA in a 25 μL reaction containing a TaqMan® Gene Expression Assay primer/probe set and TaqMan® Universal PCR Master Mix (Applied Biosystems). For samples with very low RNA concentrations, we used the maximum volume of template available and were able to get amplification of all target genes and the endogenous control. The threshold cycle (CT) is defined as the cycle number at which the fluorescence corresponding to the amplified PCR product is detected above a baseline signal. Relative quantitation was performed according to the ΔCT method to evaluate the differences in gene expression in CD4+ and CD8+ T-lymphocytes from healthy volunteers and HCT patients. The ΔCT value, calculated in order to normalize for different amounts of cDNA in each sample, was the difference between the mean CT of a target gene and the mean CT of the endogenous control (GUS). Relative gene expression was calculated as 2−ΔCT (arbitrary units) and multiplied by 103 to provide more manageable numbers. Statistical analyses were performed to evaluate associations between gene expression and F-ara-ATP accumulation in CD4+ and CD8+ T-lymphocytes from HCT patients. A power analysis was conducted with type I error rate set to 5%. We have 83% power to detect a ρ2 (coefficient of determination in a regression with 3 independent variables) of 0.30 with 32 HCT patients in this study. Analysis was performed using the 3 predictors of the two main determinants of F-ara-ATP accumulation: the genes of the uptake transporters pathway (hENT1, hENT2, and hCNT3) and the genes involved in the intracellular formation of F-ara-AMP (dCK, CN-II, and dNT-1).
Assessment of fludarabine-induced apoptosis
CD4+ and CD8+ T-lymphocytes were exposed to different concentrations of fludarabine (5, 10, and 25 μM) over several time points (4, 24, 36, and 48 hours) to evaluate induction of apoptosis/cell death. Untreated cells were used as a control to correct for cell death due to spontaneous apoptosis at each time point. At each fludarabine concentration and time point, 1.25×106 cells were incubated in RPMI media (not containing phenol red) at 37°C, and washed and resuspended in 1 mL PBS. Total cell counts were made prior to staining and flow cytometry. Cells were stained for 15 minutes with FITC-Annexin V (Becton Dickinson, Franklin Lanes, NJ) and 7-amino-actinomycin D (7AAD) (Becton Dickinson) followed by subsequent washing. Samples were analyzed by flow cytometry to estimate the percentage of viable cells, the percentage of cells undergoing apoptosis (staining with Annexin V only), the percentage of dead or necrotic cells (staining with 7AAD only), and the percentage of dead cells that had gone through apoptotic pathways (staining for both Annexin V and 7AAD).
RESULTS
F-ara-ATP accumulation in T-lymphocytes from healthy volunteers
Apheresis products from healthy volunteers were used initially to provide enriched CD4+ and CD8+ T-lymphocytes (>90% purity; data not shown) with which to optimize the fludarabine incubation and detection methods. Median cell yields after T-lymphocyte isolation in healthy volunteers were 2.98×107 and 2.67×107 for CD4+ and CD8+ cells, respectively. F-ara-ATP accumulation was linear over a starting cell number from 1.25×105 to 2×106 cells (data not shown). Further, in both CD4+ and CD8+ cells, incubation with fludarabine demonstrated a linear relationship between initial fludarabine concentration and F-ara-ATP accumulation in the range of 1.25 to 10 μM (data not shown). In addition, F-ara-ATP accumulation was also linear up to an incubation time of 4 hours (data not shown). We achieved reliable assay detection of intracellular F-ara-ATP concentrations with starting cell numbers of less than 1.25×105 cells incubated with 5 μM fludarabine for 4 hours. The replicates within each cell type displayed low variability, as calculated by dividing the lowest F-ara-ATP accumulation by the highest value in each cell type from each healthy volunteer. These replicates were within 71-100% of each other. The majority of the replicates (i.e. 88%) provided F-ara-ATP accumulation values that were at least within 90% of each other. Thus, we concluded that apheresis products with subsequent autoMACS™ isolation of CD4+ and CD8+ T-lymphocytes provided adequate cell numbers to quantitate F-ara-ATP accumulation.
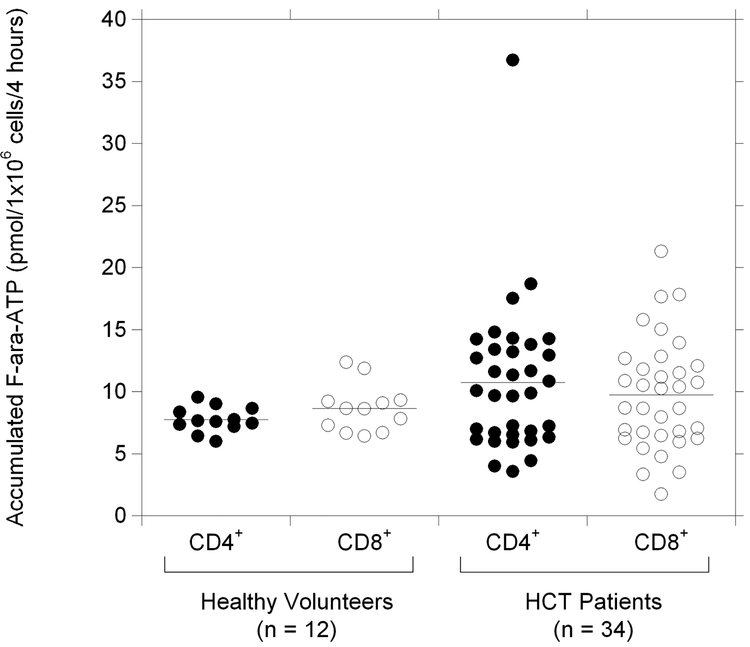
The mean (± s.d.) F-ara-ATP accumulation in healthy volunteers was 7.74 ± 1.02 and 8.66 ± 1.92 pmol/1×106 cells/4 hours in CD4+ and CD8+ cells, respectively. No correlation was observed between the F-ara-ATP accumulation in CD4+ cells and CD8+ cells isolated from the same individual (r2 = 0.135). Interindividual variability in F-ara-ATP accumulation varied 1.6-fold (range: 6.0 – 9.5) in CD4+ cells and 1.9-fold (range: 6.4 – 12.4) in CD8+ cells isolated from healthy volunteers (Figure 2).
Figure 2. Variability in F-ara-ATP accumulation in CD4+ and CD8+ T-lymphocytes.
Range of F-ara-ATP accumulation in CD4+ (●) and CD8+ (◯) T-lymphocytes isolated from healthy volunteers and patients awaiting HCT. Solid lines indicate the mean values (pmol/1×106 cells/4 hours) in each group.
F-ara-ATP accumulation in T-lymphocytes from patients awaiting HCT
After method optimization in the T-lymphocytes obtained from healthy volunteers, we determined the feasibility of obtaining enough CD4+ ad CD8+ cells from patients awaiting HCT to evaluate ex vivo F-ara-ATP accumulation (Table 1). A peripheral blood sample was obtained from 44 HCT patients prior to receiving HCT conditioning. Based on pre-set criteria (total cell numbers >90×106 cells in the 60 mL peripheral blood draw before starting CD4+ and CD8+ isolation), only 34 patients had adequate total cell numbers to proceed with T-lymphocyte purification. Retrospective analysis did not show a strong association between absolute CD4+ and CD8+ counts measured on the day of the blood draw and the number of T-lymphocyte subsets isolated from the peripheral blood; therefore, absolute starting cell counts may not be a good predictor of whether or not to enroll patients in these types of studies (data not shown). Median cell yields after T-lymphocyte isolation in HCT patients were 3.78×106 and 2.71×106 for CD4+ and CD8+ cells, respectively, with a purity >90% (data not shown). Accurate quantitation of F-ara-ATP accumulation was achieved in all of the 34 HCT patients that underwent CD4+ and CD8+ isolation. F-ara-ATP accumulation was assessed in two replicates per cell type. The replicates within each cell type displayed low variability, as calculated by dividing the lowest F-ara-ATP accumulation by the highest value in each cell type from each patient. These replicates were within 74-100% of each other. The majority of the replicates (i.e. 74%) provided F-ara-ATP accumulation values that were at least within 90% of each other.
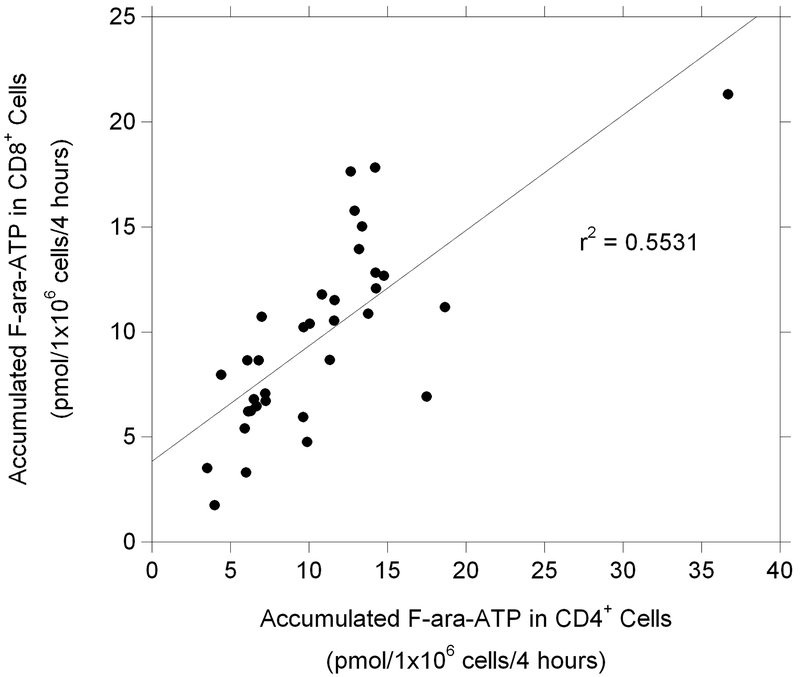
While the mean values (± s.d.) of F-ara-ATP accumulation in CD4+ and CD8+ cells were not statistically different in HCT patients compared to healthy volunteers (10.74 ± 6.05 and 9.74 ± 4.46 pmol/1×106 cells/4 hours in CD4+ and CD8+ cells from HCT patients, respectively), the interindividual variability was significantly larger in the HCT patients. F-ara-ATP accumulation varied 10.5-fold (range: 3.5 – 36.7) in CD4+ cells and 12.5-fold (range: 1.7 – 21.3) in CD8+ cells (Figure 2). In addition, a modest association was found between F-ara-ATP accumulation in individual patient’s matched CD4+ and CD8+ cells (Figure 3). No correlation was observed between F-ara-ATP accumulation and age, gender, or disease state from CD4+ or CD8+ cells in these HCT patients (data not shown).
Figure 3. F-ara-ATP accumulation in CD4+ and CD8+ T-lymphocytes from HCT patients.
Correlation between F-ara-ATP accumulation (pmol/1×106 cells/4 hours) in CD4+ and CD8+ T-lymphocytes in HCT patients (n = 34).
Gene expression in CD4+ and CD8+ T-lymphocytes
We then evaluated the mRNA expression levels of the transporters (hENT1, hENT2, hCNT3) and enzymes (dCK, CN-II, and dNT-1) important in the uptake of fludarabine and subsequent formation of F-ara-ATP (see Figure 1). Relative quantitation of the expression of these genes in CD4+ and CD8+ T-lymphocytes is presented in Table 2 for both healthy volunteers and HCT patients. We observed wide variability in the expression levels of several of the genes, with hCNT3 displaying the highest variability, followed by hENT1 and dNT-1. Less variability was observed in the expression of hENT2, dCK, and CN-II. Similar to what we observed with F-ara-ATP accumulation in CD4+ and CD8+ T-lymphocytes, we saw much higher interindividual variability in gene expression in the HCT patients compared to healthy volunteers. The difference in variability between healthy volunteers and HCT was particularly evident in the hENT1, hCNT3, and dNT-1 genes. The average expression of hENT1 was significantly decreased in CD8+ cells compared to CD4+ cells (p < 0.0001) isolated from HCT patients; however, none of the other genes displayed such a differential gene expression between the two cells types. Statistical analysis showed no association between gene expression levels for the transporters and enzymes involved in fludarabine uptake and activation and intracellular accumulation of F-ara-ATP in CD4+ and CD8+ lymphocytes from HCT patients. The mRNA expression of dNT-1 was associated with F-ara-ATP accumulation in CD8+ cells (p < 0.0005), but the slope of the association was very small; therefore, it is unlikely that dNT-1 mRNA expression is a good marker for T-lymphocyte F-ara-ATP accumulation.
Table 2.
Variability in mRNA expression of the transporters and enzymes involved in F-ara-ATP accumulation in CD4+ and CD8+ T-lymphocytes
| mRNA expression relative to GUS, arbitrary units | ||||
|---|---|---|---|---|
| Healthy volunteers, n = 8 |
HCT patients, n = 32 |
|||
| CD4+ cells | CD8+ cells | CD4+ cells | CD8+ cells | |
| hENT1 | ||||
| Mean ± s.d. | 16.6 ± 30.6 | 2.42 ± 1.76 | 37.8 ± 38.0 | 9.00 ± 9.30* |
| Range | 4.32 – 92.0 | 0.920 – 6.11 | 3.00 – 163 | 0.291 – 30.1 |
| Fold-variability | 21 | 6.6 | 54 | 103 |
| hENT2 | ||||
| Mean ± s.d. | 119 ± 85 | 99.8 ± 40.9 | 63.7 ± 39.7 | 48.2 ± 25.7 |
| Range | 42.2 – 279 | 45.4 – 164 | 19.7 – 195 | 25.4 – 144 |
| Fold-variability | 6.6 | 3.6 | 9.9 | 5.7 |
| hCNT3 | ||||
| Mean ± s.d. | 0.685 ± 1.22 | 0.359 ± 0.260 | 2.81 ± 3.90 | 1.31 ± 2.80 |
| Range | 0.118 – 3.68 | 0.032 – 0.802 | 0.083 – 17.3 | 0.052 – 12.3 |
| Fold-variability | 31 | 25 | 208 | 237 |
| dCK | ||||
| Mean ± s.d. | 1423 ± 475 | 1908 ± 1269 | 866 ± 324 | 890 ± 421 |
| Range | 778 – 2124 | 983 – 4985 | 319 – 1647 | 409 – 2038 |
| Fold-variability | 2.7 | 5.1 | 5.2 | 5.0 |
| CN-II | ||||
| Mean ± s.d. | 593 ± 220 | 633 ± 240 | 372 ± 130 | 341 ± 141 |
| Range | 383 – 1059 | 483 – 1175 | 188 – 688 | 188 – 731 |
| Fold-variability | 2.8 | 2.4 | 3.7 | 3.9 |
| dNT-1 | ||||
| Mean ± s.d. | 72.7 ± 66.0 | 102 ± 93 | 79.3 ± 76.4 | 93.4 ± 93.6 |
| Range | 12.2 – 182 | 22.0 – 264 | 2.60 – 271 | 3.27 – 266 |
| Fold-variability | 15 | 12 | 104 | 81 |
Significant difference observed between mRNA expression in CD4+ and CD8+ T-lymphocytes, p< 0.0001.
Fludarabine-induced apoptosis in lymphocytes
By virtue of inhibition of ribonucleotide reductase and DNA polymerase, fludarabine induces apoptosis and cell death [15]. One hypothesis would be that an increased rate of accumulation of the active metabolite F-ara-ATP in T-lymphocytes would presumably lead to more rapid and/or a greater degree of cell death. This was investigated using isolated CD4+ and CD8+ cells from 3 healthy volunteers. Annexin V staining identifies cells undergoing early stages of apoptosis, while the dye 7AAD marks cells in later stages of apoptosis or necrosis. Cells that stain for both Annexin V and 7AAD indicate cells that have died via apoptotic pathways. Cells that did not stain for either dye were considered viable. No apoptosis was observed after a 4 hour incubation with 5 μM fludarabine which were the conditions used to evaluate intracellular F-ara-ATP accumulation (described in the methods), which indicates that little cell death is occurring during our incubation time for F-ara-ATP quantitation. We observed a consistent concentration-dependent decrease in viable CD4+ and CD8+ T-cells after 24 hour incubations with 5, 10, and 25 μM fludarabine (Figure 4A). Furthermore, a time-dependent decrease in viable cells was also observed with incubations at the lowest fludarabine concentration (5 μM), with very few viable cells remaining after 48 hours (Figure 4B).
Figure 4. Fludarabine-induced apoptosis in CD4+ and CD8+ T-lymphocytes from healthy volunteers.
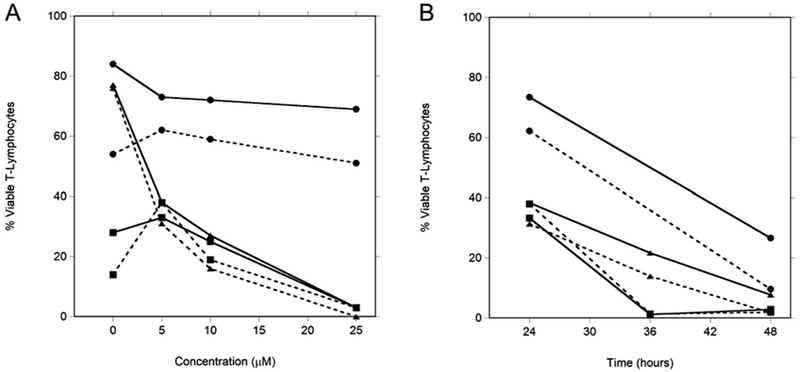
Concentration-dependent (panel A) and time-dependent (panel B) fludarabine-induced apoptosis was evaluated by determining percentage of viable T-lymphocytes (CD4+: solid line; CD8+: dotted line) from 3 healthy volunteers (designated ●, ∎, and ▴) at variable fludarabine concentrations over a range of incubation times. Concentration dependence was evaluated with a 24 hour exposure to fludarabine. Time dependence was evaluated in T-lymphocytes exposed to 5μM fludarabine. Viable cells were those that did not bind either Annexin V or 7AAD dyes.
DISCUSSION
F-ara-ATP is the major cytotoxic metabolite of fludarabine; therefore, estimating F-ara-ATP exposure in CD4+ and CD8+ T-lymphocytes could be a more predictive measure of an individual’s response to fludarabine. Unfortunately, quantitation of F-ara-ATP in T-lymphocytes from pharmacokinetic samples obtained from patients receiving fludarabine has not been feasible to date. We have demonstrated the feasibility of measuring F-ara-ATP accumulation rate in CD4+ and CD8+ T-lymphocytes isolated from healthy volunteers or HCT recipients. Determining intracellular F-ara-ATP accumulation may provide a direct quantitative method to evaluate the degree of fludarabine-induced immunosuppression and subsequent clinical efficacy and toxicity. This method will also allow for estimation of an individual’s intracellular F-ara-ATP exposure by establishing a ratio between plasma fludarabine and the measured ex vivo F-ara-ATP accumulation rate.
Identifying determinants of T-lymphocyte suppression after fludarabine administration is critical. Complete remission rates and infections are associated with the amount of T-cell suppression after fludarabine administration to patients with CLL [22]. Fludarabine has also proven to be a key component of reduced-intensity HCT conditioning regimens, in which fludarabine appreciably improves engraftment rates from 80% with total body irradiation (TBI) alone to >95% with fludarabine/TBI [4, 10, 18, 27]. Although fludarabine decreases rejection rates, its use is associated with slower immune recovery and an increased susceptibility to infections [4, 10, 27]. The success of reduced-intensity conditioning regimens for allogeneic HCT relies on achieving a fine balance between recipient and donor cells such that there is sufficient immunosuppression of the recipient, maximal anti-tumor effect, and minimal toxicity. The ultimate goal is to achieve acceptably low rates of graft rejection and graft-versus-host disease, and maximize the graft-versus-tumor effect, which involves the immunoreactivity of donor cells against recipient tumor cells [8, 10, 17, 21, 28, 34, 35, 37]. Following nonmyeloablative conditioning, short-term mixed chimerism develops in the patient after infusion of the donor cells. Mixed chimerism is a state in which donor and host hematological cells co-exist in the blood of the recipient. The level and rate of change in donor T-cell chimerism has been correlated with several clinical outcomes such as graft rejection, graft-versus-host disease, disease relapse/progression (i.e. graft-versus-tumor effect), and progression-free survival [4]. Current research is focusing upon identifying the optimal blood cell population(s) to monitor for chimerism, including CD3+, CD4+, CD8+, granulocytes, and natural killer cells [8, 34, 37]. Some of the observed associations between donor T-cell chimerism levels and subsequent clinical responses could, in part, reflect differences in each recipient’s sensitivity to the conditioning regimen. One example of such interindividual variability could be the rate of cellular uptake, activation, and response to fludarabine. For instance, if less F-ara-ATP accumulates in the recipient’s cells during the conditioning, fewer of their immunologically competent cells will die, leading to lower donor T-cell chimerism, which may increase the risk of graft rejection, relapse, or progression of the recipient’s underlying malignancy due to low graft-versus-tumor effect [2, 3]. Conversely, increased F-ara-ATP accumulation might result in slower recovery of immune function and increased infections, leading to higher morbidity and mortality. Thus, the development of quantitative methods to predict dosing and drug response is critical to optimizing fludarabine treatment.
With our highly sensitive method to quantitate F-ara-ATP, we were able to evaluate the degree of variability in intracellular F-ara-ATP accumulation. We observed that while both healthy volunteers and HCT patients exhibit considerable variability in the accumulation of F-ara-ATP in T-lymphocytes, the variability was much greater in HCT patients. Several reasons may account for this increased variability in HCT patients, including disease state, prior chemotherapy treatment, and concomitant medications.
Fludarabine has been traditionally used in the treatment of CLL. Peak concentrations of fludarabine are reached in myeloblasts and leukemic lymphoblasts 4-6 hours after fludarabine infusion, and the intracellular half-life of fludarabine is about 15 hours [14, 15]. Lymphopenia is a well-recognized effect of fludarabine administration in CLL patients, with a similar degree of suppression between CD4+ and CD8+ lymphocytes occurring for >24 months after fludarabine administration [9, 20]. However, the immediate effects of fludarabine administration upon circulating T-cells have not been well characterized. In vitro, CD8+ T-lymphocytes appear to be more sensitive to fludarabine; short-term exposure of CD4+ and CD8+ T-lymphocytes isolated from healthy volunteers led to a higher proportion of CD8+ cells undergoing apoptosis than CD4+ cells [13]. Variability in the cellular uptake and activation of fludarabine in CD4+ and CD8+ cells may account for this differential cellular sensitivity, but our data suggest a similar degree of F-ara-ATP accumulation between the two lymphocyte subsets (Figure 2). We did, however, observe significant interindividual variability in F-ara-ATP accumulation in HCT patients in both lymphocyte subsets. This large variability we observed (>10-fold), as well as the 2-fold variability in plasma AUC of fludarabine [7], is clearly enough to expect fludarabine responses to vary as a result of variable F-ara-ATP exposure.
There have been mixed results amongst the in vitro and in vivo studies that evaluated if drug-induced apoptosis or clinical outcomes are associated with the expression of genes involved in intracellular uptake and activation of nucleoside analogs [12, 16, 24, 25, 30, 36]. Therefore, a biologically plausible and consistent association has not been found between clinical responses and protein or mRNA expression of transporters and enzymes involved in F-ara-ATP formation. Our results in T-lymphocytes qualitatively support these findings because we also did not observe a relationship between mRNA expression and F-ara-ATP accumulation. We observed significant variability in the gene expression of the transporters and enzymes involved in fludarabine uptake and activation, and as with the F-ara-ATP accumulation measurements, this variability was more pronounced in HCT patients than it was in healthy volunteers. This greater variability in HCT patients could be due to the prior administration of chemotherapy, however the specific effects of an individual chemotherapeutic agent could not be studied due to the limited sample size. However, we did not find significant associations between the levels of intracellular F-ara-ATP and the mRNA expression level of specific genes. Therefore, gene expression does not appear to be a good indicator of F-ara-ATP accumulation in CD4+ and CD8+ T-lymphocytes. The wide variability that we observed is consistent with another report that estimated gene expression of nucleoside transporters in CLL patients and observed substantial variability in the expression of hENT1, hENT2, and hCNT3 [29]. These researchers also found no association with gene expression levels of nucleoside transporters and fludarabine-induced cytotoxicity [29]. Another report evaluated dCK gene expression in peripheral leukocytes from CLL patients and found no association between dCK gene expression and enzymatic activity, suggesting that dCK mRNA levels are not a good indicator for dCK protein expression [23]. We also observed a significant increase in hENT1 expression in CD4+ cells isolated from HCT patients compared to CD8+ cells, which is consistent with a previous report [13]. However, none of the other genes of interest displayed a significantly different expression pattern between CD4+ and CD8+ cells. These data together suggest that while substantial variability exists, gene expression may not be a good predictor of protein expression and functional activity that influences fludarabine uptake and activation. Unfortunately, a high number of T-lymphocytes would be needed to evaluate protein expression of the relevant transporters and enzymes. Thus, the association of gene expression with protein expression and functional activity was not feasible.
Fludarabine-induced apoptosis was also evaluated in CD4+ and CD8+ T-lymphocytes obtained from healthy volunteers. Both fludarabine concentration- and duration of exposure time-dependence were observed. As these preliminary studies suggest, cells exposed to higher levels of F-ara-ATP would undergo more rapid and greater cell death. Therefore, we would expect that the greater than 10-fold differences seen in intracellular accumulation of F-ara-ATP in individual HCT patients would result in differences in the degree and rate of apoptosis seen in their CD4+ and CD8+ T-cells. This in turn could have a significant impact on the extent of immunosuppression resulting after the conditioning regimen, and subsequent clinical outcomes such as graft rejection, delayed engraftment, and anti-tumor responses. However, since there was variability in the percentage of viable cells in the T-lymphocytes not treated with fludarabine (Figure 4A), this variability may contribute to the ability of these lymphocytes to form F-ara-ATP since fludarabine uptake and its enzymatic conversion to F-ara-ATP are active processes. The ideal experiment of correlating F-ara-ATP accumulation with the percentage of viable T-lymphocytes at each concentration and time point is not feasible because of the high number of T-lymphocytes that would be required from each donor. Future studies are needed to directly evaluate the association with F-ara-ATP accumulation and cellular apoptosis in HCT patients. An improved understanding of the pharmacodynamics of fludarabine in patients receiving fludarabine/TBI will also be gained by future studies directed to determine the association between F-ara-ATP accumulation and circulating CD4+ and CD8+ cells and T-cell donor chimerism.
In conclusion, we have shown that adequate number of T-lymphocytes can be isolated from healthy volunteers and from patients awaiting HCT to quantitate F-ara-ATP accumulation using our novel, highly sensitive method originally developed in cell lines. We are the first to demonstrate considerable interindividual variability in intracellular F-ara-ATP accumulation rates within CD4+ and CD8+ T-lymphocytes. Healthy volunteers exhibited a small range of accumulation values; however, the variability in F-ara-ATP accumulation was significantly greater in patients awaiting HCT. Subsequent translational studies will be necessary to evaluate whether F-ara-ATP accumulation or estimated exposure provides a novel diagnostic tool to predict an individual’s sensitivity and response to fludarabine, with the long-range goal of individualizing fludarabine doses to improve the efficacy and/or toxicity of this important nucleoside analog.
ACKNOWLEDGMENTS
The authors would like to thank Dr. John T. Slattery for his thoughtful discussions about the manuscript, Dr. Aaron G. Ren for his methodical experiments to show linearity over fludarabine concentration incubation, and Thomas Kalhorn for his contribution to the initial development of the LC-MS method.
Supported by grants from the National Institutes of Health (CA18029, CA15704, CA78902, DK56465, HL36444, and HL91744). ELW is supported by the Elmer M. and Joy B. Plein Fellowship for Excellence in Pharmacy Education, School of Pharmacy, Seattle, WA.
ABBREVIATIONS
- AK
adenylate kinase
- AUC
area under the plasma concentration-time curve
- CD73
ecto-5’-nucleotidase
- CLL
chronic lymphocytic leukemia
- CN-II
5’-nucleotidase
- dCK
deoxycytidine kinase
- dNT-1
deoxynucleotidase-1
- F-ara-ADP
fludarabine diphosphate
- F-ara-AMP
fludarabine monophosphate
- F-ara-ATP
fludarabine triphosphate
- GUS
β-glucuronidase
- hCNT3
human concentrative nucleoside transporter 3
- HCT
hematopoietic cell transplantation
- hENT1
human equilibrative nucleoside transporter 1
- hENT2
human equilibrative nucleoside transporter 2
- LC-MS
liquid chromatography-mass spectrometry
- NBMPR
nitrobenzylthioinosine
- NDK
nucleoside diphosphate kinase
REFERENCES
- [1].Auletta JJ, Lazarus HM (2005) Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant 35: 835–57 [DOI] [PubMed] [Google Scholar]
- [2].Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, Woolfrey AE, Chauncey TR, Flowers ME, Mielcarek M, Maloney DG, Storb R (2005) Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 23: 1993–2003 [DOI] [PubMed] [Google Scholar]
- [3].Baron F, Maris MB, Storer BE, Sandmaier BM, Stuart MJ, McSweeney PA, Radich JP, Pulsipher MA, Agura ED, Chauncey TR, Maloney DG, Shizuru JA, Storb R (2005) HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with chronic myeloid leukemia. Biol Blood Marrow Transplant 11: 272–9 [DOI] [PubMed] [Google Scholar]
- [4].Baron F, Sandmaier BM (2006) Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia 20: 1690–700 [DOI] [PubMed] [Google Scholar]
- [5].Baron F, Storb R (2006) Allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning as treatment for hematologic malignancies and inherited blood disorders. Mol Ther 13: 26–41 [DOI] [PubMed] [Google Scholar]
- [6].Barrachina M, Castano E, Ferrer I (2006) TaqMan PCR assay in the control of RNA normalization in human post-mortem brain tissue. Neurochem Int 49: 276–84 [DOI] [PubMed] [Google Scholar]
- [7].Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J, Martin PJ, McDonald GB, Nichols WG, Radich J, Woolfrey A, Jenke A, Schleyer E, Thiede C, Ehninger G, Anasetti C (2003) Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood 102: 820–6 [DOI] [PubMed] [Google Scholar]
- [8].Champlin R, Khouri I, Anderlini P, De Lima M, Hosing C, McMannis J, Molldrem J, Ueno N, Giralt S (2003) Nonmyeloablative preparative regimens for allogeneic hematopoietic transplantation. Biology and current indications. Oncology (Williston Park) 17: 94–100; discussion 103–7 [PubMed] [Google Scholar]
- [9].Consoli U, El-Tounsi I, Sandoval A, Snell V, Kleine HD, Brown W, Robinson JR, DiRaimondo F, Plunkett W, Andreeff M (1998) Differential induction of apoptosis by fludarabine monophosphate in leukemic B and normal T cells in chronic lymphocytic leukemia. Blood 91: 1742–8 [PubMed] [Google Scholar]
- [10].Deeg HJ, Maris MB, Scott BL, Warren EH (2006) Optimization of allogeneic transplant conditioning: not the time for dogma. Leukemia 20: 1701–5 [DOI] [PubMed] [Google Scholar]
- [11].Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA (2005) Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 23: 2346–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Galmarini CM, Thomas X, Calvo F, Rousselot P, Jafaari AE, Cros E, Dumontet C (2002) Potential mechanisms of resistance to cytarabine in AML patients. Leuk Res 26: 621–9 [DOI] [PubMed] [Google Scholar]
- [13].Gamberale R, Galmarini CM, Fernandez-Calotti P, Jordheim L, Sanchez-Avalos J, Dumontet C, Geffner J, Giordano M (2003) In vitro susceptibility of CD4+ and CD8+ T cell subsets to fludarabine. Biochem Pharmacol 66: 2185–91 [DOI] [PubMed] [Google Scholar]
- [14].Gandhi V, Kemena A, Keating MJ, Plunkett W (1993) Cellular pharmacology of fludarabine triphosphate in chronic lymphocytic leukemia cells during fludarabine therapy. Leuk Lymphoma 10: 49–56 [DOI] [PubMed] [Google Scholar]
- [15].Gandhi V, Plunkett W (2002) Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet 41: 93–103 [DOI] [PubMed] [Google Scholar]
- [16].Giovannetti E, Mey V, Nannizzi S, Pasqualetti G, Marini L, Del Tacca M, Danesi R (2005) Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol 68: 110–8 [DOI] [PubMed] [Google Scholar]
- [17].Giralt S, Estey E, Albitar M, van Besien K, Rondon G, Anderlini P, O’Brien S, Khouri I, Gajewski J, Mehra R, Claxton D, Andersson B, Beran M, Przepiorka D, Koller C, Kornblau S, Korbling M, Keating M, Kantarjian H, Champlin R (1997) Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 89: 4531–6 [PubMed] [Google Scholar]
- [18].Giralt S, Logan B, Rizzo D, Zhang MJ, Ballen K, Emmanouilides C, Nath R, Parker P, Porter D, Sandmaier B, Waller EK, Barker J, Pavletic S, Weisdorf D (2007) Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biol Blood Marrow Transplant 13: 844–52 [DOI] [PubMed] [Google Scholar]
- [19].Kalhorn TF, Ren AG, Slattery JT, McCune JS, Wang J (2005) A highly sensitive high-performance liquid chromatography-mass spectrometry method for quantification of fludarabine triphosphate in leukemic cells. J Chromatogr B Analyt Technol Biomed Life Sci 820: 243–50 [DOI] [PubMed] [Google Scholar]
- [20].Keating MJ, O’Brien S, Lerner S, Koller C, Beran M, Robertson LE, Freireich EJ, Estey E, Kantarjian H (1998) Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood 92: 1165–71 [PubMed] [Google Scholar]
- [21].Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O’Brien S, Giralt S, Ippoliti C, von Wolff B, Gajewski J, Donato M, Claxton D, Ueno N, Andersson B, Gee A, Champlin R (1998) Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 16: 2817–24 [DOI] [PubMed] [Google Scholar]
- [22].Landau H, Lamanna N, Hobart S, Weiss MA (2006) High level T cell suppression following purine analog therapy for patients with CLL correlates with important clinical benefit American Society of Hematology, Orlando, FL. p. abstract #2784. [Google Scholar]
- [23].Lotfi K, Karlsson K, Fyrberg A, Juliusson G, Jonsson V, Peterson C, Eriksson S, Albertioni F (2006) The pattern of deoxycytidine- and deoxyguanosine kinase activity in relation to messenger RNA expression in blood cells from untreated patients with B-cell chronic lymphocytic leukemia. Biochem Pharmacol 71: 882–90 [DOI] [PubMed] [Google Scholar]
- [24].Mackey JR, Galmarini CM, Graham KA, Joy AA, Delmer A, Dabbagh L, Glubrecht D, Jewell LD, Lai R, Lang T, Hanson J, Young JD, Merle-Beral H, Binet JL, Cass CE, Dumontet C (2005) Quantitative analysis of nucleoside transporter and metabolism gene expression in chronic lymphocytic leukemia (CLL): identification of fludarabine-sensitive and -insensitive populations. Blood 105: 767–74 [DOI] [PubMed] [Google Scholar]
- [25].Mansson E, Spasokoukotskaja T, Sallstrom J, Eriksson S, Albertioni F (1999) Molecular and biochemical mechanisms of fludarabine and cladribine resistance in a human promyelocytic cell line. Cancer Res 59: 5956–63 [PubMed] [Google Scholar]
- [26].Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M, Sandmaier B, Maloney D, Storb R, Storek J (2003) Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol 31: 941–52 [DOI] [PubMed] [Google Scholar]
- [27].Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, Petersdorf E, McSweeney P, Pulsipher M, Woolfrey A, Chauncey T, Agura E, Heimfeld S, Slattery J, Hegenbart U, Anasetti C, Blume K, Storb R (2003) HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood 102: 2021–30 [DOI] [PubMed] [Google Scholar]
- [28].McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, Gooley TA, Hegenbart U, Nash RA, Radich J, Wagner JL, Minor S, Appelbaum FR, Bensinger WI, Bryant E, Flowers ME, Georges GE, Grumet FC, Kiem HP, Torok-Storb B, Yu C, Blume KG, Storb RF (2001) Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 97: 3390–400 [DOI] [PubMed] [Google Scholar]
- [29].Molina-Arcas M, Bellosillo B, Casado FJ, Montserrat E, Gil J, Colomer D, Pastor-Anglada M (2003) Fludarabine uptake mechanisms in B-cell chronic lymphocytic leukemia. Blood 101: 2328–34 [DOI] [PubMed] [Google Scholar]
- [30].Molina-Arcas M, Marce S, Villamor N, Huber-Ruano I, Casado FJ, Bellosillo B, Montserrat E, Gil J, Colomer D, Pastor-Anglada M (2005) Equilibrative nucleoside transporter-2 (hENT2) protein expression correlates with ex vivo sensitivity to fludarabine in chronic lymphocytic leukemia (CLL) cells. Leukemia 19: 64–8 [DOI] [PubMed] [Google Scholar]
- [31].Montagna D, Locatelli F, Moretta A, Lisini D, Previdere C, Grignani P, DeStefano P, Giorgiani G, Montini E, Pagani S, Comoli P, Maccario R (2004) T lymphocytes of recipient origin may contribute to the recovery of specific immune response toward viruses and fungi in children undergoing cord blood transplantation. Blood 103: 4322–9 [DOI] [PubMed] [Google Scholar]
- [32].Plunkett W, Saunders PP (1991) Metabolism and action of purine nucleoside analogs. Pharmacol Ther 49: 239–68 [DOI] [PubMed] [Google Scholar]
- [33].Seymour L, Eisenhauer E (2001) A review of dose-limiting events in phase I trials: antimetabolites show unpredictable relationships between dose and toxicity. Cancer Chemother Pharmacol 47: 2–10 [DOI] [PubMed] [Google Scholar]
- [34].Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R (1998) Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91: 756–63 [PubMed] [Google Scholar]
- [35].Spitzer TR, McAfee S, Sackstein R, Colby C, Toh HC, Multani P, Saidman S, Weyouth DW, Preffer F, Poliquin C, Foley A, Cox B, Andrews D, Sachs DH, Sykes M (2000) Intentional induction of mixed chimerism and achievement of antitumor responses after nonmyeloablative conditioning therapy and HLA-matched donor bone marrow transplantation for refractory hematologic malignancies. Biol Blood Marrow Transplant 6: 309–20 [DOI] [PubMed] [Google Scholar]
- [36].Stam RW, den Boer ML, Meijerink JP, Ebus ME, Peters GJ, Noordhuis P, Janka-Schaub GE, Armstrong SA, Korsmeyer SJ, Pieters R (2003) Differential mRNA expression of Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL gene-rearranged infant acute lymphoblastic leukemia. Blood 101: 1270–6 [DOI] [PubMed] [Google Scholar]
- [37].Storb R (2002) Mixed allogeneic chimerism and graft-versus-leukemia effects in acute myeloid leukemia. Leukemia 16: 753–4 [DOI] [PubMed] [Google Scholar]