Abstract
Introduction:
Hepatocellular carcinoma disproportionately affects minorities. Southern states have high proportions of black populations and prevalence of known risk factors. Further research is needed to understand the role of southern geography in hepatocellular carcinoma disparities. This paper examined racial disparities in hepatocellular carcinoma incidence, demographics, tumor characteristics, receipt of treatment, and all-cause mortality in southern and non-southern cancer registries.
Methods:
Surveillance Epidemiology and End Results data were probed in 2015 to identify 43,868 patients diagnosed with hepatocellular carcinoma from 2000 to 2012 (5,455 in southern registries [Atlanta, Louisiana, Rural and Greater Georgia]).
Results:
Southern registries showed steeper increases of age-adjusted hepatocellular carcinoma incidence (from 2.89 to 5.29 cases/100,000 people) versus non-southern areas (from 3.58 to 5.54 cases/100,000 people). Blacks were over-concentrated in southern registries (32% vs 10%). Compared with whites, blacks were significantly younger at diagnosis, more likely diagnosed with metastasis, and less likely to receive surgical therapies in both registry groups. After adjustment, blacks had significantly higher risk of all-cause mortality compared with whites in southern (hazard ratio=1.10, p=0.007) and non-southern areas (hazard ratio=1.08, p<0.001). For overall populations, southern registries had higher risk of all-cause mortality versus non-southern registries (hazard ratio=1.13, p<0.001).
Conclusions:
Age-adjusted incidence rates of hepatocellular carcinoma are plateauing overall, but are still rising in southern areas. Race and geography had independent associations with all-cause mortality excess risk among patients with hepatocellular carcinoma. Further studies are needed to understand the root causes of potential mortality risk excess among overall populations with hepatocellular carcinoma living in the South.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, diagnosed in approximately 20,000 people in the U.S. each year.1 The pathway leading to HCC generally begins with an acute hepatic insult that progresses over decades to fibrosis and cirrhosis, typical precursors of HCC.2 Most HCC cases are associated with alcohol-induced liver disease, chronic hepatitis C virus (HCV), hepatitis B virus infection, obesity, smoking, and type 2 diabetes.3 Among patients with localized stage cancer, treatment options may include resection, liver transplantation (LT) or chemoembolization, all reported to improve survival in well-selected patients.4 However, many patients have advanced disease at diagnosis and are only candidates for palliative care. This contributes to improving survival rates, but there was still a very low 5-year survival rate of approximately 22% for patients diagnosed with HCC from 2008 to 2013.5 Despite the overall decline in incidence and death rates of the most common cancers from 2003 to 2012 in the U.S., liver cancer incidence rates have followed opposite trends and increased 2.3% per year during the same period.6
HCC disproportionately affects disadvantaged populations, with the highest age-specific rates among racial/ethnic minorities, often clustered in areas of low SES.7,8 Contemporary state-level analysis has shown high prevalence of known risk factors for HCC, such as obesity, smoking, and drinking in southern states.9–11 Southern states also have the highest proportions of black populations.12 According to the National Health and Nutrition Examination Survey, blacks are more likely than other races/ethnicities combined to have chronic HCV infection, an important HCC risk factor.13 Furthermore, liver and intrahepatic bile duct cancer death rates are highly variable from state to state, compounded by state-level differences in racial composition, poverty level, and access to care.14
Geographic variations in demographics, healthcare quality, and access highlight the importance of detecting enhanced disparities within local contexts and across regions, and focus resource allocations to address racial disparities that are region specific.15 Further research is needed to understand the role of southern U.S. geography in creating or mitigating disparities in key HCC care outcomes. In this paper, the authors examine racial disparities in HCC demographics, tumor characteristics, receipt of treatment, incidence, and all-cause mortality by contrasting southern and non-southern areas of the Surveillance Epidemiology and End Results (SEER) cancer registry.
METHODSI
Study Sample
The SEER database is derived from cancer registries representing ≌28% of the U.S. population and is maintained by the National Cancer Institute (available at: www.seer.cancer.gov). The SEER population-based cancer registries contain information on cancer incidence and survival in selected geographic areas. Appropriate approval was obtained from the University of Minnesota IRB before data extraction.
Measures
This is a population-based, retrospective cohort study using data from the SEER database, based on the November 2014 submission, examining data from 2000 through 2012 of SEER 18 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, Rural Georgia, Greater Georgia, Alaska Native Tumor Registry, Greater California, Kentucky, Louisiana, and New Jersey). The SEER data set included information on patient demographics, tumor and disease characteristics, cancer-associated treatments (such as the use of cancer-directed surgery), and survival for individuals with cancer. No record of chemotherapy appeared in this database.
The SEER database was approached in a similar fashion as recently described by Njei and colleagues,16 by applying the same exclusion criteria. In November 2015, a total of 59,869 patients were identified with HCC (site code C 22.0, ICD-O histology codes 8170–8175).17,18 Patients with the fibrolamellar variant of HCC were excluded because they differ in clinical course and prognosis, compared with conventional HCC.19 Patients who were diagnosed within 1 month before death were included in incidence analyses, but excluded from survival analyses because SEER calculates survival time in months and not days (if included, these cases would be deemed to have a survival of zero). For the same reason, patients who were diagnosed on the basis of a death certificate only or for the first time at autopsy were excluded. Patients with another malignant primary tumor diagnosed within 5 years before their HCC diagnosis were excluded to minimize the chance that metastatic disease to the liver was misdiagnosed as HCC. To ensure a uniform cancer staging classification across all study years, SEER historic stage was used including: localized (confined to primary site), regional (spread to regional lymph nodes), and distant (cancer had metastasized). Unique to this study, HCC cases were allocated in two distinct groups of southern (Atlanta, Rural Georgia, Louisiana) and non-southern registries (Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, Alaska Native Tumor Registry, Greater California, Kentucky, and New Jersey) for comparative analysis.
Statistical Analysis
Summary statistics were used to describe and compare demographic information and disease characteristics of southern and non-southern SEER registries. The Kruskal–Wallis test was used to compare the medians of continuous variables and the chi-square test was applied to evaluate the difference of categorical variables between groups. The incidence was calculated as the number of new cancers divided by the U.S. Census population (in each year, registry, race and age group) and direct age adjustment was performed using the U.S. 2000 standard population. For survival analysis predicting mortality by race in non-southern and southern regions, Kaplan–Meier methods were used to calculate curves, which were analyzed using the log-rank test. A multivariate Cox proportional hazard regression model was used to identify the hazard ratio (HR) of death in blacks versus whites, with adjustments for location (southern registries versus non-southern registries), age, gender, stage, grade, and treatment regimen. Patients were censored at the end of the observation period (December 31, 2012). Stratified analysis was further conducted using multivariate Cox model for southern and non-southern registries separately. Study analyses were conducted in February 2016.
RESULTS
A total of 43,868 patients met the inclusion criteria for the descriptive and survival analysis (Appendix Figure 1), including 5,455 in southern registries (Atlanta, Louisiana, Rural and Greater Georgia). In terms of demographic and clinical characteristics of the study population, males and whites comprised the majority of HCC patients in both southern and non-southern SEER registries. In southern registries, blacks represented a much larger proportion of HCC patients compared with non-southern cohorts (32.4% vs 10.1%). Conversely, the proportion of other races (American Indian/Alaska Native, Asian/Pacific Islander) was higher in non-southern areas (22.5% vs 5.2%) as shown in Table 1. The median age at diagnosis was significantly lower in blacks compared with whites in both registry groups (southern registries: 58 years in blacks vs 61 years in whites, p<0.001 and non-southern registries: 59 years in blacks vs 61 years in whites, p<0.001).
Table 1.
Demographics and Hepatocellular Carcinoma Characteristics in Southern and Non-Southern Registries
| Southern registries n (%) or median (N=5,455)a | p-valueb | Non-Southern registries n (%) or median (N=38,413)c | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | White | Black | Otherd | White | Black | Otherd | ||
| Number of patients | 3,402 (62.4) | 1,770 (32.4) | 283 (5.2) | 25,877 (67.4) | 3,873 (10.1) | 8,663 (22.5) | ||
| Median age, years | 61 | 58 | 59 | <0.0001** | 61 | 59 | 63 | <0.0001** |
| Age, years | <0.0001** | <0.0001** | ||||||
| 0–49 | 384 (11.2) | 249 (14.0) | 57 (20.2) | 2,621 (10.2) | 451 (11.6) | 1,161 (13.4) | ||
| 50–79 | 2,701 (79.4) | 1,458 (82.4) | 216 (76.4) | 20,980 (81.0) | 3,275 (84.6) | 6,701 (77.4) | ||
| ≥80 | 317 (9.4) | 63 (3.6) | 10 (3.6) | 2,276 (8.8) | 147 (3.8) | 801 (9.2) | ||
| Sex | 0.0921 | <0.0001** | ||||||
| Male | 2,699 (79.4) | 1,380 (78.0) | 210 (74.2) | 20,166 (78.0) | 2,995 (77.4) | 6,298 (72.6) | ||
| Female | 703 (20.6) | 390 (22.0) | 73 (25.8) | 5,711 (22.0) | 878 (22.6) | 2,365 (27.4) | ||
| Stage | 0.0009** | <0.0001** | ||||||
| Local | 1,752 (51.4) | 815 (46.0) | 147 (52.0) | 12,612 (48.8) | 1,761 (45.4) | 4,205 (48.6) | ||
| Regional | 855 (25.2) | 500 (28.2) | 79 (28.0) | 6,789 (26.2) | 1,085 (28.0) | 2,493 (28.8) | ||
| Distant | 468 (13.8) | 300 (17.0) | 34 (12.0) | 3,393 (13.2) | 650 (16.8) | 1,070 (12.4) | ||
| Grade | 0.0052* | <0.0001** | ||||||
| Well differentiated | 479 (14.0) | 218 (12.4) | 36 (12.8) | 3,510 (13.6) | 493 (12.8) | 928 (10.8) | ||
| Moderately differentiated | 647 (19.0) | 295 (16.6) | 54 (19.0) | 3,898 (15.0) | 635 (16.4) | 1,422 (16.4) | ||
| Poorly differentiated | 313 (9.2) | 160 (9.0) | 42 (14.8) | 1,816 (7.0) | 313 (8.0) | 792 (9.2) | ||
| Undifferentiated | 38 (1.2) | 16 (1.0) | 3 (1.0) | 201 (0.8) | 30 (0.8) | 74 (0.8) | ||
| Unknown | 1,925 (56.6) | 1,081 (61.0) | 148 (52.2) | 24,301 (63.3) | 16,452 (63.6) | 2,402 (62.0) | ||
| Treatment group | <0.0001 | <0.0001 | ||||||
| None | 2,432 (71.4) | 1,386 (78.4) | 194 (68.6) | 18,429 (71.2) | 2,905 (75.0) | 5,844 (67.4) | ||
| Local destruction | 186 (5.4) | 100 (5.6) | 23 (8.2) | 2,893 (11.2) | 392 (10.2) | 1,017 (11.8) | ||
| Surgical resection | 333 (9.8) | 162 (9.2) | 38 (13.4) | 2,052 (8.0) | 353 (9.2) | 1,270 (14.6) | ||
| Liver transplant | 376 (11.0) | 89 (5.0) | 23 (8.2) | 1,860 (7.2) | 151 (3.8) | 378 (4.4) | ||
Note: Boldface indicates statistical significance (**p<0.01; ***p<0.001).
Missing data in Southern registries (Atlanta, Rural Georgia, and Louisiana): stage, unknown or unstaged, 9.3%; and treatment group, unknown, 2.1%.
p-value: Kruskal-Wallis test (medians) and the chi-square test (categorical).
Missing data in non-Southern registries (Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, Alaska Native Tumor Registry, Greater California, Kentucky, and New Jersey): stage, unknown or unstaged, 11.3%; and treatment group, unknown, 2.3%.
Other races in SEER (Surveillance, Epidemiology, and End Results Program) include American Indian/Alaska Native, Asian/Pacific Islander.
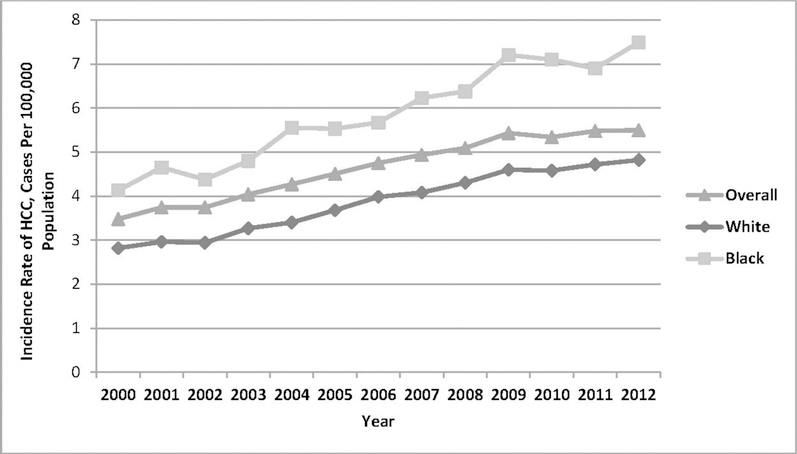
From 2000 to 2012, overall age-adjusted incidence was on the rise and remained higher in blacks compared with whites. During the same period, age-adjusted HCC incidence in southern SEER registries increased from 2.89 to 5.29 cases per 100,000 population, a steeper trend if compared with the incidence curve in non-southern SEER areas (from 3.58 to 5.54 cases per 100,000 population) as shown in Figure 1.
Figure 1.
Age-adjusted incidence rate, using U.S. 2000 standard population, by (A) race and (B) location (Southern versus non-Southern registries).
Notes: Figure 1A shows age-adjusted incidence rates, using the U.S. 2000 standard population in the overall population and stratified by race. From 2009, incidence rates remained stable for the overall cohort, whites, and blacks (the latter group with higher rates). Figure 1B shows age-adjusted incidence rates, using the U.S. 2000 standard population in the overall population and stratified by location. From 2009, incidence rates remained stable for the overall cohort and non-Southern cohorts, whereas Southern registries experienced rising rates in the same period.
In both registry groups, blacks were more likely to be diagnosed with advanced HCC stages compared with whites. In southern registries, 28.2% of blacks and 25.2% of whites had HCC in regional stage; 17.0% of blacks vs 13.8% of whites had HCC diagnosed in distant stage, (p<0.001). In non-southern areas, 28.0% of blacks and 26.2% of whites had HCC in regional stage; 16.8% of blacks compared with 13.2% of whites had HCC in distant stage (p<0.001). Similarly, blacks were less likely to receive treatment than whites. In southern registries, 78.4% of blacks and 71.4% of whites received no treatment (only cancer-directed surgery is captured in SEER); in non-southern registries, 75.0% of blacks and 71.2% of whites received no such treatments.
Blacks were also less likely to receive liver transplantation (LT). In southern registries, 5.0% of blacks and 11.0% of whites received LT (p<0.0001); in non-southern registries: 3.8% of blacks and 7.2% of whites received LT (p<0.0001). Compared to cases in non-southern registries, patients in southern registries were less likely to receive cancer-directed surgery overall (73.5% in southern vs. 70.7% in non-southern registries received no treatment, p<0.0001).
After adjusting for location, age, gender, stage, grade, and treatment regimen, there was no significant interaction (p=0.68) between race (blacks versus whites) X location (southern versus non-southern). Southern registries had higher overall risk of all-cause death compared with non-southern registries (HR=1.13, 95% CI=1.09, 1.16, p<0.001), and blacks had significantly higher risk of all-cause mortality compared with whites (HR=1.08, 95% CI=1.05, 1.12, p<0.001; results not shown in table). As shown in Table 2 and in overall survival curves of Figure 2, blacks had significantly higher risk of all-cause mortality compared with whites in both southern registries (HR=1.10, 95% CI=1.03, 1.17, p=0.007) and non-southern registries (HR=1.08, 95% CI=1.04, 1.12, p<0.001).
Table 2.
Adjusted Hazard Ratio of All-Cause Mortality Among HCC Patients: Southern Versus Non-Southern Registries
| Southern registriesa | Non-Southern registriesb | |||
|---|---|---|---|---|
| Covariates | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Race | ||||
| White (ref) | 1.000 | 1.000 | ||
| Black | 1.097 (1.026, 1.172) | 0.0066** | 1.078 (1.037, 1.122) | 0.0002*** |
| Otherc | 0.847 (0.729, 0.983) | 0.0286* | 0.832 (0.808, 0.857) | <0.0001*** |
| Age | ||||
| 0–49 (ref) | 1.000 | 1.000 | ||
| 50–79 | 1.129 (1.027, 1.240) | 0.012* | 1.066 (1.025,1.108) | 0.0013** |
| ≥80 | 1.43 (1.241, 1.648) | <0.0001*** | 1.509 (1.431, 1.591) | <0.0001*** |
| Sex | ||||
| Male (ref) | 1.000 | 1.000 | ||
| Female | 0.893 (0.827, 0.965) | 0.0044** | 0.937 (0.911, 0.964) | <0.0001*** |
| Stage | ||||
| Local (ref) | 1.000 | 1.000 | ||
| Regional | 1.605 (1.487, 1.731) | <0.0001*** | 1.68 (1.632, 1.729) | <0.0001*** |
| Distant | 2.195 (2.004, 2.404) | <0.0001*** | 2.635 (2.542, 2.731) | <0.0001*** |
| Unknown/unstaged | 1.426 (1.274, 1.596) | <0.0001*** | 1.589 (1.527, 1.655) | <0.0001*** |
| Grade | ||||
| Well differentiated (ref) | 1.000 | 1.000 | ||
| Moderately differentiated | 1.186 (1.054, 1.336) | 0.0048** | 1.206 (1.150, 1.264) | <0.0001*** |
| Poorly differentiated | 1.702 (1.491, 1.943) | <0.0001*** | 1.569 (1.486, 1.657) | <0.0001*** |
| Undifferentiated | 2.231 (1.670, 2.981) | <0.0001*** | 1.643 (1.441, 1.872) | <0.0001*** |
| Unknown | 1.244 (1.126, 1.374) | <0.0001*** | 1.202 (1.157, 1.248) | <0.0001*** |
| Treatment | ||||
| None (ref) | 1.000 | 1.000 | ||
| Local tumor destruction | 0.574 (0.499, 0.660) | <0.0001*** | 0.503 (0.482, 0.524) | <0.0001*** |
| Surgical resection | 0.401 (0.354, 0.454) | <0.0001*** | 0.364 (0.346, 0.383) | <0.0001*** |
| Liver transplant | 0.154 (0.129, 0.185) | <0.0001*** | 0.153 (0.142, 0.166) | <0.0001*** |
| Unknown | 0.983 (0.740, 1.306) | 0.9072 | 1.185 (1.058, 1.327) | 0.0034** |
Notes: Boldface indicates statistical significance (*p<0.05; **p<0.01; ***p<0.001).
Southern registries: Atlanta, Rural Georgia, and Louisiana.
Non-Southern registries Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, Alaska Native Tumor Registry, Greater California, Kentucky, and New Jersey.
Other races in SEER (Surveillance, Epidemiology, and End Results Program) include American Indian/Alaska Native, Asian/Pacific Islander.
HCC, Hepatocellular Carcinoma; HR, hazard ratio.
Figure 2.
Kaplan-Meier overall survival curves by race in non-Southern and Southern registries.
Notes: In multivariable analysis, blacks had similar and significantly higher risk of all-cause death compared to whites in both non-Southern (A) and Southern (B) registries. Overall populations of hepatocellular carcinoma patients in Southern registries had higher risk of all-cause death compared to those in non-Southern registries.
OS, overall survival
DISCUSSION
This study investigated racial disparities in HCC demographics, tumor characteristics, receipt of treatment, incidence, and all-cause mortality by contrasting southern and non-southern registry areas in the SEER database from 2000 to 2012. In both regions, blacks were similarly more likely to be diagnosed with advanced HCC stages, and not to receive cancer-directed surgery treatments (including LT) compared with whites. Comparative analysis did not suggest that racial disparities in the HCC outcomes under study in southern registries were of higher magnitude than in non-southern areas. Nevertheless, southern registries showed overrepresentation of blacks (comprising nearly a third of HCC cases), steeper and rising HCC age-adjusted incidence rates, and higher overall risk of all-cause death that was independent from age, gender, tumor stage and grade, and treatment regimen.
This contemporary SEER analysis shows that racial disparities persist in HCC care utilization, as reported in prior registry studies of similar methodologies. By utilizing the California Cancer Registry data, Zak et al.20 verified that blacks were 58% and 36% less likely to undergo LT or liver resection respectively, when compared with whites. Likewise, SEER cancer registry studies from previous years, representing a large, ethnically and geographically diverse cohort in the U.S., have strongly suggested that blacks with HCC tend to present to medical care with a more advanced tumor at diagnosis, have lower rates of surgical intervention, and worse prognosis.21–24
It has been increasingly recognized that geographic variations in health care are responsible for a substantial component of the observed racial disparities in care, because blacks may disproportionately live in parts of the country that have lower-quality care.25 The authors set out to contrast southern and non-southern SEER areas, assessing whether there was increased racial disparities in tumor characteristics, receipt of surgical treatment, and all-cause mortality in southern areas (with higher prevalence of blacks) versus non-southern areas. This comparative analysis demonstrated similar degree of racial disparities among blacks and whites in both regions. Multivariable analysis showed no significant interactions between race and location influencing all-cause mortality among HCC patients. These findings build on prior observations by Sonnenday and colleagues,22 who reported on patterns of surgical therapy utilization for HCC from SEER (between 1998 and 2004). Despite blacks being 24% less likely to receive surgical therapy than white individuals, these racial disparities did not change when analysis were refined by geographic adjustment based on 171 Health Service Areas.22 Taken together, these observations support the notion that some of the roles played by race and location in HCC outcomes are independent.
In the present study, it was observed that HCC patients from southern SEER cohorts had a 13% greater HR of all-cause mortality compared with the patients from non-southern areas, further suggesting that location matters in HCC health disparities. The root causes for these geographic differences in mortality are not clear and likely multifactorial. HCC is a condition that carries high mortality rates and the need for multidisciplinary and specialized care only available in tertiary centers. In reference centers, differences in processes of care may lead to “surgical signatures” or differences in the rates at which certain surgical procedures are performed, even in adjacent geographic regions with very similar patient populations.25 In the study by Sonnenday and colleagues, for example, the adjusted probability of surgical therapy by each specific Health Service Area averaged 22%, but varied widely from less than 10% to nearly 80%.22
The rise in incidence of HCC in the U.S. has been well documented over decades. But a recent study by Njei et al.20 examined recent temporal trends (SEER database from 1973 to 2011) and observed a deceleration in the incidence rates of HCC since 2006; and there was no increase in incidence-based mortality rates for HCC since 2009 (for the first time in four decades). These findings suggest that the peak of the HCC epidemic could be near in the U.S., driven by earlier detection at a curative stage and greater utilization of ablation, resection, and LT. Nevertheless, this study verified persisting, steeper increasing trends of HCC incidence in southern registries. The reasons for such trends are not clear. Precursors of HCC, such as HCV, disproportionally affects blacks and is a potential reason for the accelerated trends of HCC incidence in these regions.25 Further studies are needed to confirm the presence of hot beds of HCC morbidity in the South.
Limitations
The present study is limited by not capturing the etiology of HCC cases and inability to estimate the current proportion of HCC cases that are attributed to HCV, hepatitis B virus, or other etiologies across demographic groups in different locations. Further studies utilizing the SEER-Medicare database could overcome these limitations by matching HCC care utilization to underlying comorbidity and risk factors in population studies.26 As mentioned above, analysis did not account for local provider and hospital variations in HCC care delivery. In the case of HCC treatment, these variations could potentially affect multiple steps in the continuum of care, from provider referral practices to LT uptake, not mentioning the inability to measure other specific treatment modalities not captured in SEER (chemoembolization and radiofrequency ablation), variations in organ availability, and case selection in each tertiary center. Likewise, the study is limited by the absence of data on comorbidities and underlying liver disease that affect clinical decision making about treatment options. Data were collected in November 2015, based on SEER 2014 Submission that includes HCC cases from 2000 to 2012. Therefore, these results did not include cases occurring in 2013 and 2014, which are included in the latest SEER Submission available by the time of publication of this study.
CONCLUSIONS
Racial disparities in HCC incidence, demographics, tumor characteristics, receipt of treatment, and all-cause mortality were examined and compared in southern and non-southern registries. Age-adjusted incidence rates of HCC have plateaued overall, but are still rising in southern areas. Racial and geographic disparities had independent association with excess all-cause mortality risk among patients with HCC. Further studies are needed to understand the root causes of the observed excess mortality risk among overall populations with HCC living in the southern areas.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the Center for Healthy African American Men through Partnerships, funded by the National Institute of Minority Health and Health Disparities through a grant from NIH under award number U54MD008620. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
This article is part of a supplement entitled African American Men’s Health: Research, Practice, and Policy, which is sponsored by the National Institutes of Health.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94(2):153–156. 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Seeff LB. Introduction: the burden of hepatocellular carcinoma. Gastroenterol 2004;127(5 suppl 1):S1–S4. 10.1053/j.gastro.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Davila JA, Morgan RO, Shaib Y, et al. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterol 2004;127(5):1372–1380. 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362(9399):1907–1917. 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 5.Shah C, Mramba LK, Bishnoi R, et al. Survival differences among patients with hepatocellular carcinoma based on the stage of disease and therapy received: pre and post sorafenib era. J Gastrointest Oncol 2017;8(5):789–798. 10.21037/jgo.2017.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016;122(9):1312–1337. 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim AK, Singal AG. Health disparities in diagnosis and treatment of hepatocellular carcinoma. Clin Liver Dis 2014;4(6)143–145. 10.1002/cld.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shebl FM, Capo-Ramos DE, Graubard BI, McGlynn KA, Altekruse SF. Socioeconomic status and hepatocellular carcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2012;21(8):1330–1335. 10.1158/1055-9965.EPI-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. Prevalence of self-reported obesity (body mass index ≥30 kg/m2) in the United States by state/territory and race/ethnicity, both sexes combined, 2013 to 2015. Behavioral Risk Factor Surveillance System, CDC www.cdc.gov/obesity/data/prevalence-maps.html. Updated 2017. Accessed May 22, 2018.
- 10.Islami F, Ward EM, Jacobs EJ, et al. Potentially preventable premature lung cancer deaths in the USA if overall population rates were reduced to those of educated whites in lower-risk states. Cancer Causes Control 2015;26(3):409–418. 10.1007/s10552-014-0517-9. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Prevalence and intensity of binge alcohol drinking among adults in the United States by state, both sexes combined, 2015, age-adjusted to the 2000 U.S. standard population. Behavioral Risk Factor Surveillance System, CDC www.cdc.gov/alcohol/data-stats.htm. Updated 2017. Accessed May 22, 2018.
- 12.Rastogi S, Johnson TD, Hoeffel EM, Drewery MP Jr.. The Black Population: 2010. U.S. Census Bureau. 2010 Census interactive population search www.census.gov/prod/cen2010/briefs/c2010br-06.pdf. Published September 2011. Accessed June 16, 2018.
- 13.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160(5):293–300. 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin 2017;67(4):273–289. 10.3322/caac.21402. [DOI] [PubMed] [Google Scholar]
- 15.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med 2005;48(1 suppl):S42–S53. 10.1353/pbm.2005.0020. [DOI] [PubMed] [Google Scholar]
- 16.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61(1):191–199. 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz A, Percy C, Jack A, eds. International Classification of Diseases for Oncology 3rd ed. Geneva, Switzerland: WHO; 2000. [Google Scholar]
- 18.Percy C, Van Holten V, Muir CS, eds. International Classification of Diseases for Oncology, 2nd ed. Geneva, Switzerland: WHO; 1990. [Google Scholar]
- 19.Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg 2012;215(6):820–830. 10.1016/j.jamcollsurg.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Zak Y, Rhoads KF, Visser BC. Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type. Arch Surg 2011;146(7):778–784. 10.1001/archsurg.2011.37. [DOI] [PubMed] [Google Scholar]
- 21.Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl 2014;20(5):528–535. 10.1002/lt.23820. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenday CJ, Dimick JB, Schulick RD, Choti MA. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. J Gastrointest Surg 2007;11(12):1636–1646. 10.1007/s11605-007-0315-8. [DOI] [PubMed] [Google Scholar]
- 23.Mathur AK, Osborne NH, Lynch RJ, et al. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg 2010;145(12):1158–1163. 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 24.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009:27(9):1485–1491. 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med 2005;48(1 suppl):S42–S53. 10.1353/pbm.2005.0020. [DOI] [PubMed] [Google Scholar]
- 26.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40(8):IV-3–IV-18. 10.1097/00005650-200208001-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.