Abstract
Background
Millions of children are hospitalised due to respiratory syncytial virus (RSV) infection every year. Treatment is supportive, and current therapies (e.g. inhaled bronchodilators, epinephrine, nebulised hypertonic saline, and corticosteroids) are ineffective or have limited effect. Respiratory syncytial virus immunoglobulin is sometimes used prophylactically to prevent hospital admission from RSV‐related illness. It may be considered for the treatment of established severe RSV infection or for treatment in an immunocompromised host, although it is not licenced for this purpose. It is unclear whether immunoglobulins improve outcomes when used as a treatment for established RSV infection in infants and young children admitted to hospital.
Objectives
To assess the effects of immunoglobulins for the treatment of RSV‐proven lower respiratory tract infections in children aged up to three years, admitted to hospital.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, Ovid MEDLINE, Embase, CINAHL, and Web of Science (from inception to 6 November 2018) with no restrictions. We searched two trial registries for ongoing trials (to 30 March 2018) and checked the reference lists of reviews and included articles for additional studies.
Selection criteria
Randomised controlled trials comparing immunoglobulins with placebo in hospitalised infants and children aged up to three years with laboratory‐diagnosed RSV lower respiratory tract infection.
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias, and extracted data. We assessed evidence quality using GRADE.
Main results
We included seven trials involving 486 infants and children aged up to three years. The immunoglobulin preparations used in these trials included anti‐RSV immunoglobulin and the monoclonal antibody preparations palivizumab and motavizumab. We assessed the primary outcomes of mortality, length of hospital stay, and adverse events as providing low‐ or very low‐certainty evidence due to risk of bias and imprecision. All trials were conducted at sites in high‐income countries (USA, Chile, New Zealand, Australia), with two studies including a site in a middle‐income country (Panama). Five of the seven studies were "supported" or "sponsored" by the trial drug manufacturers.
We found no evidence of a difference between immunoglobulins and placebo for mortality (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.14 to 5.27; 3 trials; 196 children; 4 deaths; 2 deaths amongst 98 children receiving immunoglobulins, and 2 deaths amongst 98 children receiving placebo. One additional death occurred in a fourth trial, however, the study group of the child was not known and the data were not included in the analysis; very low‐certainty evidence), and length of hospitalisation (mean difference −0.70, 95% CI −1.83 to 0.42; 5 trials; 324 children; low‐certainty evidence). There was no evidence of a difference between immunoglobulins and placebo in adverse events of any severity or seriousness (reported in five trials) or serious adverse events (four trials) (RR for any severity 1.18, 95% CI 0.78 to 1.78; 340 children; low‐certainty evidence, and for serious adverse events 1.08, 95% CI 0.65 to 1.79; 238 children; low‐certainty evidence).
We found no evidence of a significant difference between immunoglobulins and placebo for any of our secondary outcomes. We identified one ongoing trial.
Authors' conclusions
We found insufficient evidence of a difference between immunoglobulins and placebo for any review outcomes. We assessed the evidence for the effects of immunoglobulins when used as a treatment for RSV lower respiratory tract infection in hospitalised infants and young children as of low or very low certainty due to risk of bias and imprecision. We are uncertain of the effects of immunoglobulins on these outcomes, and the true effect may be substantially different from the effects reported in this review. All trials were conducted in high‐income countries, and data from populations in which the rate of death from RSV infection is higher are lacking.
Plain language summary
Drug treatment for respiratory syncytial virus lung infections
Review question
Does the use of immunoglobulins in very young children hospitalised with a respiratory syncytial virus (RSV) lung infection reduce deaths and hospital stay without increased adverse events, compared with placebo (a similar‐appearing fake drug that has no effect)?
Background
Respiratory syncytial virus is a common virus that can infect lungs and airways. Millions of children are treated in hospital each year for RSV, which can result in severe illness and death. The majority of these deaths occur in low‐income countries. In high‐income countries, the majority of deaths associated with RSV lung infection occur in infants and young children with other illnesses.
Immunoglobulins, also known as antibodies, are a type of molecule normally produced by white blood cells when an infection is present. Immunoglobulins may recognise and attach to viruses (such as RSV) and help destroy them. Immunoglobulins can be produced artificially and given to children who are not making their own RSV antibodies. Some studies have shown that immunoglobulins are helpful in preventing RSV infection in children at high risk of becoming infected. They may also be used as a treatment when an RSV infection is already present, but the effectiveness and safety of immunoglobulins for this use is unknown.
Search date
We searched for evidence up to 6 November 2018.
Study characteristics
We included seven randomised controlled trials (studies in which participants are assigned to one of two or more treatment groups using a random method) that compared the effects of immunoglobulins with placebo in 486 young children hospitalised with RSV lung infections. All trials were conducted at sites in the USA; three trials included some children from South American countries (Chile and Panama); and one trial also included children from New Zealand and Australia. The trials were published between 1987 and 2014.
Study funding sources
Five trials were supported by the manufacturer of the immunoglobulin tested in the studies. One trial was supported by a government agency, and one trial did not describe how it was funded.
Key results
Immunoglobulins did not appear to be more effective than placebo in preventing deaths among young children with RSV infection, although few deaths occurred in the trials. Immunoglobulins given to children hospitalised with RSV lung infection did not decrease the time spent in hospital. Children treated with immunoglobulins experienced adverse effects of any severity or seriousness and adverse effects considered to be serious (such as respiratory failure) as often as children treated with placebo. There was no difference between immunoglobulins and placebo for any other outcomes measured in the trials, such as the need for oxygen or admission to the intensive care unit. Data from populations in which the rate of death from RSV infection is higher are lacking.
Quality of the evidence
The quality of the evidence was low or very low, which means that the true effect of immunoglobulin treatment for young children in hospital with RSV lung infection may be very different from the findings of this review.
Summary of findings
Summary of findings for the main comparison. Immunoglobulins compared to placebo for treatment of respiratory syncytial virus infection.
| Immunoglobulins compared to placebo for treatment of respiratory syncytial virus infection | ||||||
| Patient or population: children with respiratory syncytial virus infection Setting: hospital Intervention: immunoglobulins Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with immunoglobulins | |||||
| Mortality follow‐up: range 30 days to 60 days | 20 per 1000 | 18 per 1000 (3 to 108) | RR 0.87 (0.14 to 5.27) | 196 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 | Interventions: monoclonal immunoglobulin palivizumab in 2 trials, titres of neutralising antibodies to RSV in 1 trial Settings: study sites in high‐income country (USA) and middle‐income country (Panama) |
| Length of hospitalisation (days) follow‐up: range 30 days to 60 days | Mean length of hospitalisation range 5 to 12 days | MD 0.7 fewer (1.83 fewer to 0.42 more) | ‐ | 324 (5 RCTs) | ⊕⊕⊝⊝ LOW 2 | Interventions: monoclonal immunoglobulin palivizumab in 2 trials, monoclonal immunoglobulin motavizumab in 1 trial, titres of neutralising antibodies to RSV in 2 trials Settings: study sites in high‐income countries (USA, Chile) and middle‐income country (Panama) |
| Adverse events follow‐up: range 30 days to 90 days | 413 per 1000 | 488 per 1000 (322 to 736) | RR 1.18 (0.78 to 1.78) | 340 (5 RCTs) | ⊕⊕⊝⊝ LOW 3 | Interventions: monoclonal immunoglobulin palivizumab in 2 trials, monoclonal immunoglobulin motavizumab in 2 trials, titres of neutralising antibodies to RSV in 1 trial Settings: study sites in high‐income countries (USA, Chile, New Zealand, Australia) and middle‐income country (Panama) |
| Serious adverse events follow‐up: range 30 days to 90 days | 202 per 1000 | 218 per 1000 (131 to 362) | RR 1.08 (0.65 to 1.79) | 238 (4 RCTs) | ⊕⊕⊝⊝ LOW 3 | Interventions: monoclonal immunoglobulin palivizumab in 2 trials, monoclonal immunoglobulin motavizumab in 2 trials Settings: study sites in high‐income countries (USA, Chile, New Zealand, Australia) and middle‐income country (Panama) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; RSV: respiratory syncytial virus | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded to very low due to serious risk of bias (unclear random sequence generation, selective reporting, and other bias) and very serious imprecision (very small sample size compared to the optimal information size, few events, and wide confidence interval overlapping zones of no effect as well as potential harm or benefit). 2Downgraded to low due to serious risk of bias (unclear random sequence generation, selective reporting, and other bias) and serious imprecision. 3Downgraded to low due to serious risk of bias (unclear random sequence generation, selective reporting, and other bias) and serious imprecision (small sample size compared to the optimal information size and wide confidence intervals overlapping zones of no effect as well as potential harm or benefit).
Background
Description of the condition
The respiratory syncytial virus (RSV) is the most common cause of acute lower respiratory tract infections (LRTI), such as bronchiolitis and pneumonia, in infancy and childhood (Nair 2010). Nearly all children will have been infected with RSV by the age of two years (Greenough 2001). Respiratory syncytial virus infection carries a substantial disease burden, with 33 million (uncertainty range 22 to 50 million) episodes of RSV‐associated acute LRTI globally in 2015, resulting in about three million hospital admissions and approximately 59,000 deaths (Shi 2017). The societal burden associated with caring for the ill and healthcare costs due to RSV infection are substantial (Langley 1997; Paramore 2004).
The clinical manifestations of RSV infection vary according to age and health status. Amongst children aged over three years and adults, RSV causes only mild acute respiratory symptoms (such as common cold, sore throat, headache, cough, low‐grade fever, and malaise) (Mayo Clinic 2017). However, about 20% to 30% of younger children presenting with these symptoms can progress rapidly to diffuse small airways disease with low‐grade fever, cough, wheezing, shortness of breath, decreased oral intake, and diffuse crackles/rales on chest auscultation (American Academy of Pediatrics 2015). Infants aged up to six weeks may present with a non‐specific sepsis‐like picture (Oray‐Schrom 2003). Apnoea (transient period of breathing cessation) may also be present in these infants (Ralston 2009). Severe cases of RSV infection in young children and infants can cause oxygen starvation (hypoxia) and acute respiratory (ventilatory) failure, which may need mechanical ventilation in an intensive care unit. Most children with RSV infection make a full recovery, but some develop an increased risk of wheezing, asthma, and impaired lung function later in life (Zomer‐Kooijker 2014).
Treatment of acute RSV infection is primarily supportive, and includes suction to remove airways secretions, administration of supplemental oxygen, and fluid replacement (American Academy of Pediatrics 2018). Interventional agents are largely ineffective or of limited effectiveness. The evidence for inhaled bronchodilator therapy with beta‐agonists is unconvincing for bronchiolitis (Gadomski 2014), and there is insufficient evidence to support the use of epinephrine for the treatment of bronchiolitis amongst children admitted to hospital (Hartling 2011). Hypertonic saline and corticosteroids are also used. Nebulised hypertonic saline solution has a modest effect on length of hospital stay amongst infants hospitalised with acute bronchiolitis (Zhang 2017), and systemic or inhaled glucocorticoids have not been found to be effective for this condition (Fernandes 2013). The effects of the antiviral therapy ribavirin in children with respiratory infections caused by RSV are unclear, and ribavirin is currently reserved for immunosuppressed children with severe RSV infection (American Academy of Pediatrics 2018). The World Health Organization (WHO) has targeted RSV for vaccine development (Broadbent 2015).
Description of the intervention
Immunogloblin therapy involves the administration of preparations containing high levels of immunoglobulins, or antibodies. Administration of these antibodies confers passive resistance to infection by increasing the quantity or quality of antibodies the individual possesses. Immunoglobulin therapy may also be used to reduce the severity of symptoms of disease in autoimmune disorders (e.g. Guillain‐Barre syndrome), secondary immunodeficiencies (e.g. HIV), and acute infections (Jolles 2005). Immunoglobulin preparations for RSV contain high concentrations of antibodies against RSV. These can be pooled preparations, whereby the preparation is derived from the plasma of donors with naturally high circulating levels of RSV neutralising antibodies. These preparations also contain neutralising antibodies to other viruses and bacteria. Alternatively, the preparation can comprise humanised monoclonal antibodies directed only against the RSV‐F fusion protein expressed on the surface of the RSV viron (Griffiths 2017). Palivizumab (Synagis, MedImmune) is a monoclonal antibody preparation administered as an intramuscular injection (Synagis 2017). It was approved by the US Food and Drug Administration (FDA) for RSV prophylaxis of high‐risk children in 1998, and has since received approval in over 45 other countries (Resch 2017). In randomised controlled trials, palivizumab reduced hospitalisations for RSV in children with congenital heart disease (risk ratio reduction (RRR) 0.45) (Feltes 2003), prematurity (RRR 0.78), and bronchopulmonary dysplasia (RRR 0.39). The American Academy of Pediatrics recommends immunoglobulin prophylaxis for high‐risk infants and children (American Academy of Pediatrics 2014). Recommendations for palivizumab prophylaxis differ globally because of its high cost (USD 3000 to 5000 per child per year) (Wang 2011). Palivizumab is not licenced for the treatment of established RSV infection. However, it has nonetheless been used to treat children with severe infection or to prevent progression of the disease (Hu 2010; Turner 2014).
In 1996, RSV immune globulin intravenous (RSV‐IGIV, RespiriGam, MedImmune) was approved by the FDA for use in the prevention of severe RSV infections in infants and children aged up to 24 months with bronchopulmonary dysplasia or history of premature birth following two randomised controlled trials in these high‐risk infants. RSV‐IGIV administered monthly during the RSV season resulted in a 40% to 65% reduction in hospitalisation rates (Groothuis 1993; PREVENT 1997). RSV‐IGIV was superseded by palivizumab in 2004.
Motavisumab is a monoclonal antibody against RSV that was derived from palivizumab in the early 2000s. It has been reported to offer greater potency against RSV in animal studies (Mejías 2005). However, its development was discontinued in 2010 after the FDA declined the manufacturer's request for licensure due to concerns about safety and non‐inferiority to palivizumab.
How the intervention might work
Immunoglobulins provide passive immunity when the antibodies bind and neutralise viral proteins responsible for viral attachment to cells (G protein) and cell fusion (F protein) (Roche 2003), which reduces viral replication (Rodriguez 1997). Palivizumab is a humanised monoclonal antibody specific for the envelope fusion protein (RS‐F) of RSV. As viruses need to fuse with living cells to replicate, this would reduce viral replication in the lungs of those infected with RSV.
Why it is important to do this review
Therapy for RSV infection of the lower respiratory tract in children is primarily supportive, with existing interventional agents not generally recommended or indicated. Although immunoglobulins are currently licenced for the prevention of RSV LRTI only, they may also be used as a management strategy. As such, an assessment of the efficacy and safety of immunoglobulins as a treatment for established RSV infection in children was necessary.
Objectives
To assess the effects of immunoglobulins for the treatment of RSV‐proven LRTIs in children aged up to three years, admitted to hospital.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that compared immunoglobulin treatment with a placebo control.
Types of participants
Infants and children (aged up to three years) hospitalised for bronchiolitis, pneumonia, or other LRTI with laboratory‐documented RSV infection.
Types of interventions
Treatments involving infusions with immunoglobulins. We did not apply any limits regarding immunoglobulin type, dose, or method of administration. The comparator was placebo.
Types of outcome measures
Primary outcomes
Mortality from any cause occurring during hospitalisation or follow‐up.
Length of hospitalisation.
-
Adverse events. We used definitions applied by study investigators for adverse events and serious adverse events. These were:
adverse events: the number of participants experiencing one or more adverse event of any severity or seriousness during the trial or follow‐up. Adverse events were any adverse changes from baseline occurring after study drug administration. These events may or may not have been related to the study drug; and
serious adverse events: the number of participants experiencing one or more adverse events considered by study investigators to be serious in nature. These were events that resulted in a substantial impairment of baseline function or death, required or prolonged hospitalisation, or were otherwise considered an important medical event, during the trial or follow‐up.
Secondary outcomes
Need for mechanical ventilation (for participants in studies where requirement for mechanical ventilation was not a study entry criterion).
Duration of mechanical ventilation (for those ventilated).
Need for supplemental oxygen.
Duration of supplemental oxygen (for those receiving supplemental oxygen).
Need for intensive care unit (ICU) admission.
Duration of stay in the ICU (for those admitted to the ICU).
Pulmonary function measured by spirometry.
Rehospitalisation for recurrent breathing difficulties in the long term.
The occurrence of reactive airway disease in the long term.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (Issue 10, October 2018 accessed 6 November 2018) in the Cochrane Library, which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, Ovid MEDLINE (1946 to 6 November 2018), Embase (Elsevier) (1974 to 6 November 2018), CINAHL (EBSCO) (1982 to 6 November 2018), and Web of Science (Clarivate Analytics) (1985 to 6 November 2018). There were no language restrictions.
We used the search strategy in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase (Appendix 2), CINAHL (Appendix 3), and Web of Science (Appendix 4).
Searching other resources
We searched two trials registers (US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)) for completed and ongoing trials on 30 March 2018. We conducted a forward citation search of included studies via Web of Science on 30 March 2018. We also searched the reference lists of included trials and relevant review articles.
Data collection and analysis
Selection of studies
Two review authors (SLS and one of the current review authors (CDM or MD) or one of two review authors who worked on an earlier draft of the review, (MK or MG)) independently screened titles and abstracts. We retrieved full‐text study reports of titles and abstracts considered by two review authors to be potentially relevant. Two review authors (SLS and either CDM or MD or MK or MG) independently screened the retrieved full‐text reports to identify studies for inclusion, and recorded the reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion or by consultation with a third review author (either CDM or MD) when necessary.
Data extraction and management
Two review authors (SLS and CDM or MK) independently extracted the following data from the included studies: study design and setting; location of study, inclusion and exclusion criteria, and characteristics of the participants; characteristics of the intervention and comparison (type of immunoglobulin, dosage, method of administration); and the primary and secondary outcomes specified and time points reported. Disagreements regarding data extraction were resolved by discussion. One review author (SLS) entered data into RevMan 5 (Review Manager 2014). Two other review authors (SA and MH) verified data extraction and entry.
Assessment of risk of bias in included studies
Two review authors (SLS and MD or CDM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreements were resolved by discussion with a third review author (MD or CDM). We considered the following seven domains in our 'Risk of bias' assessment.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias including bias related to study funding sources.
We assessed each study as being at low, high, or unclear risk of bias for each domain and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. For other bias relating to study funding sources, we rated a study as at high risk of bias if the report indicated that it was funded, supported, or sponsored by parties that may have had a vested interest in the results of the study (e.g. drug manufacturer). We rated studies as at unclear risk of bias if study authors or members of the study group had potential conflicts of interest (e.g. were employees of the drug manufacturer). We took into account the risk of bias for the studies contributing to each outcome when considering treatment effects.
Measures of treatment effect
We used Cochrane's Review Manager 5 (RevMan 5) software for all analyses (Review Manager 2014). We planned to calculate risk ratios (RRs) and 95% confidence intervals (CIs) for binary outcomes (i.e. mortality and adverse events). For continuous outcomes, such as length of hospitalisation, we calculated the mean difference (MD) and 95% CIs.
Unit of analysis issues
The participant was the unit of analysis in our meta‐analysis. We planned to apply any corrections for clustering as described in the Cochrane Handbook for Systematic Reviews of Interventions if the unit of randomisation was not the same as the unit of analysis in cluster‐randomised trials (Higgins 2011).
Dealing with missing data
For continuous outcomes where no standard deviations (SD) were reported, we obtained them from standard errors for group means using the method specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For continuous outcomes where median was reported instead of the group mean, we described outcomes for that study narratively for each outcome (see Results). For the primary outcome, length of hospital stay, where median was reported instead of group mean, we used the median and range to calculate a mean and SD employing the method of Hozo (Hozo 2005). The study was then included in the meta‐analysis in a post hoc sensitivity analysis.
Assessment of heterogeneity
We assessed the presence of clinical heterogeneity by comparing populations (age, immune status, severity of disease), interventions, and outcomes before deciding whether it was appropriate to pool data. We planned to describe studies that we judged to be too clinically heterogeneous and not combine them in a meta‐analysis. We assessed studies providing data on each outcome without substantial clinical heterogeneity for statistical heterogeneity by means of the I² statistic (I² greater than 50% was considered substantial heterogeneity) (Higgins 2011).
Assessment of reporting biases
We planned to assess publication bias using a funnel plot test if more than 10 studies contributed data. However, there were too few included studies to enable this assessment.
Data synthesis
We pooled outcome data from studies that we judged to be clinically homogeneous using RevMan 5 (Review Manager 2014). As we considered that a single true effect was not plausible due to variation in populations and interventions, we pooled study data using a random‐effects model.
For studies with more than one placebo or intervention group, we combined data according to the formula in Section 7.7.3.8 Combining groups of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We planned to report the number of participants who experienced one or more adverse events (whether the event was considered serious or not, or whether the event was attributed by the investigators to the study interventions or not) rather than the number of adverse events.
GRADE and 'Summary of findings' table
We created Table 1 for the following outcomes: mortality, length of hospitalisation, adverse events, and serious adverse events. We assessed the certainty of the evidence for each outcome included in the 'Summary of findings' table using the GRADE evidence grading system as described in the GRADE Handbook, Schünemann 2013, and Section 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used GRADEpro GDT software (GRADEpro GDT). We took the following factors into consideration when deciding whether or not to downgrade the certainty of evidence for each outcome: risk of bias, inconsistency of results, indirectness of evidence, imprecision of results, and publication bias.
We considered the number of events, the size of the confidence intervals and calculated a posteriori the optimal information size to assess imprecision. We considered a difference of 25% as the minimal clinically important difference for dichotomous outcomes to determine optimal information size (this is the threshold recommended by GRADE when there is no compelling rationale for an alternate threshold) (Schünemann 2013). For the continuous outcome length of stay, we considered one day as the minimum clinically important difference (the judgement that reductions in stay of one day are clinically important was based on the clinical experience of the review authors and agreed upon through discussion). Calculation of the optimal information size depends upon this difference and the resulting sample size required (Schünemann 2013). We assumed a 3% risk of mortality (median control event rate from trials providing these data); 30% risk of adverse event (median control event rate from trials providing these data); and an SD of 6.4 (median control SD from trials providing these data) with a power of 80% and a two‐sided alpha of 0.05. One review author (SLS) initially applied the GRADE criteria and then discussed the certainty of evidence ratings with other review authors (CDM, MD). Final decisions on the ratings were reached through discussion and consensus. We justified all decisions to downgrade the certainty of studies in table footnotes.
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following subgroup analyses.
Children aged six months or less versus children older than six months.
Children with a recurrent episode of RSV versus first episode.
Immunocompromised versus non‐immunocompromised children.
Children with congenital heart disease.
Children with bronchopulmonary dysplasia.
Palivizumab versus other immunoglobulin preparations.
However, the small number of included studies precluded these analyses.
Sensitivity analysis
We planned to perform sensitivity analyses to assess the impact of excluding studies at high risk of bias for allocation concealment based on the 'Risk of bias' assessment for the primary outcome estimates. However, all studies were judged to be at either low or unclear risk of bias for allocation concealment, so sensitivity analysis was not performed.
Results
Description of studies
Results of the search
Database searches (conducted 6 November 2018) yielded 4399 records, and 38 records were identified in trials register searches (conducted 30 March 2018). We screened 3336 records for eligibility after removal of duplicates, of which 15 were retrieved for full‐text screening. We also obtained two additional records identified from screening reference lists of previously published reviews and forward citation searching the included studies for full‐text screening. We therefore screened 17 full‐text records, and included seven studies in the review (Hemming 1987; Lagos 2009; Malley 1998; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b; Sáez‐Llorens 2004). We identified one ongoing study (NCT02442427). A flow diagram of the study selection process is presented in Figure 1.
1.
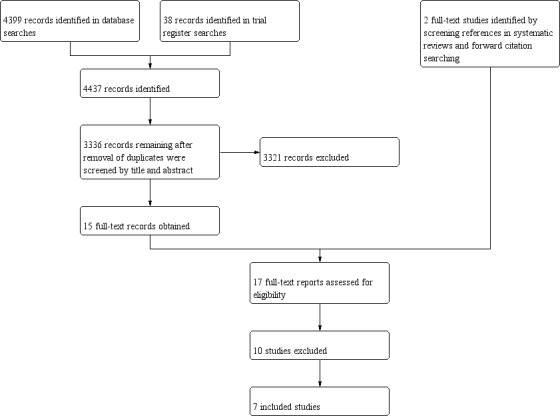
Study flow diagram.
Included studies
A full description of all seven included studies is provided in Characteristics of included studies.
Design
All seven included studies were RCTs that used a parallel‐group design. Three trials compared two or more different doses of the intervention with placebo (Lagos 2009; Ramilo 2014; Sáez‐Llorens 2004), with escalation to a higher dose after a specified period of time in the absence of toxicity or serious adverse events in two studies (Lagos 2009; Sáez‐Llorens 2004).
Participants
A total of 486 children were included in the seven trials; the number of children per trial ranged from 31 to 118. All trials were conducted at sites in high‐income countries, with two trials including a study site in a middle‐income country (Panama). Six trials were conducted in the USA (Hemming 1987; Lagos 2009; Malley 1998; Rodriguez 1997a; Rodriguez 1997b; Sáez‐Llorens 2004); two of these studies had sites in South America (Sáez‐Llorens 2004 in Panama and Lagos 2009 in Chile). One trial was reported to have been conducted at "multiple sites", which included the USA, New Zealand, Chile, Panama, and Australia (Ramilo 2014).
The studies included children and infants hospitalised for pneumonia, bronchiolitis, or other LRTI with a documented positive RSV test. Participants were described as "previously healthy" in five studies (Hemming 1987; Lagos 2009; Ramilo 2014; Rodriguez 1997b; Sáez‐Llorens 2004); at "high risk for severe RSV infections" in one study (Rodriguez 1997a); and were a mix of previously healthy children and children with "chronic medical conditions" in one study (Malley 1998). High‐risk infants included those with severe bronchopulmonary dysplasia, chronic lung disease, congenital heart disease, or prematurity (< 32 weeks gestational age). One study included only children who required intubation and mechanical ventilation at study entry (Malley 1998), and another study included children who required more than 30% supplemental oxygen (Sáez‐Llorens 2004). Participants were aged up to 12 months in one study (Ramilo 2014); up to two years at randomisation in five studies (Lagos 2009; Malley 1998; Rodriguez 1997a; Rodriguez 1997b; Sáez‐Llorens 2004); and one study did not report an upper age limit, but included children with body weight up to 10 kg (Hemming 1987).
Interventions
The included studies evaluated different doses and types of immunoglobulin preparations. Three studies used titres of neutralising antibody to RSV administered intravenously (Hemming 1987; Rodriguez 1997a; Rodriguez 1997b), at doses of 1500 mg per kg of body weight in Rodriguez 1997b and Rodriguez 1997a and 2 g per kg of body weight in Hemming 1987. The monoclonal immunoglobulin motavizumab was administered intravenously in two studies at doses of 3 mg, 15 mg, or 30 mg per kg of body weight in Lagos 2009 and at doses of 30 mg or 100 mg per kg of body weight in Ramilo 2014. The monoclonal immunoglobulin palivizumab was administered intravenously at doses of 5 mg and 15 mg per kg in one study (Sáez‐Llorens 2004), and at doses of 15 mg per kg in another study (Malley 1998).
Placebo was normal saline (0.9% sodium chloride) or half‐normal saline (0.45%) in three studies, Lagos 2009; Malley 1998; Sáez‐Llorens 2004, and albumin (0.5% or 6%) in three studies (Hemming 1987; Rodriguez 1997a; Rodriguez 1997b). The composition of the placebo was not stated in one study (Ramilo 2014).
None of the studies provided detail on who was involved in delivering the interventions to participants. Only two studies with multiple study sites within the same country stated that "methods were standardized for all centres" (Rodriguez 1997a; Rodriguez 1997b).
Outcome measures
Four studies reported that deaths occurred during the trial or the follow‐up period (Hemming 1987; Malley 1998; Rodriguez 1997a; Sáez‐Llorens 2004). All seven included studies reported the duration of hospitalisation. Six studies reported the number of adverse events that occurred in the study groups (Lagos 2009; Malley 1998; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b; Sáez‐Llorens 2004). Five of six studies that involved children who did not require mechanical ventilation at study entry reported the need for mechanical ventilation (Hemming 1987; Lagos 2009; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b). Five studies reported the duration of mechanical ventilation (Lagos 2009; Malley 1998; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b). Four of five studies involving children who did not require intubation, mechanical ventilation, or supplemental oxygen at study entry reported the need for supplemental oxygen (Hemming 1987; Lagos 2009; Ramilo 2014; Rodriguez 1997a). Five studies reported duration of supplemental oxygen (Hemming 1987; Lagos 2009; Malley 1998; Ramilo 2014; Sáez‐Llorens 2004). Five of six studies involving children not in the ICU reported the need for admission to the ICU (Hemming 1987; Lagos 2009; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b), and four studies also reported the duration of stay in the ICU (Lagos 2009; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b). Two studies reported on rehospitalisations for recurrent breathing difficulties in the long term (Rodriguez 1997a; Rodriguez 1997b).
No studies reported on the outcomes of pulmonary function or the occurrence of reactive airway disease in the long term.
Excluded studies
We excluded 10 study reports from the review: six were reviews (AAP 1998; Faber 2008; Givner 1999; Harkensee 2006; Hu 2010; Wegzyn 2014); three were prophylaxis rather than treatment studies (Feltes 2011; Fernández 2010; Halsey 1997); and one study did not randomise participants to immunoglobulin and control groups (Helmink 2016). See Characteristics of excluded studies.
Ongoing studies
We identified one ongoing study (NCT02442427). This study included children aged up to three years presenting to an emergency department with acute bronchitis and positive RSV antigen test. The children were randomised to receive either a single intravenous dose of palivizumab or an identical saline placebo. The primary outcome is readmission within three weeks of discharge.
Risk of bias in included studies
A summary of the 'Risk of bias' assessment is presented in Figure 2. Risk of bias was unclear for random sequence generation in all studies. Risk of bias was mostly low for allocation concealment, blinding, and incomplete outcome data. Risk of bias from selective reporting was unclear in most studies. We assessed all studies as at unclear or high risk of other bias due to study funding sources and potential author conflicts of interest.
2.
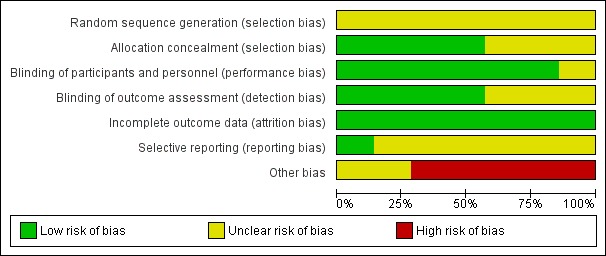
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
None of the included studies adequately described the method used to generate the randomisation sequence. Four included studies reported and used appropriate methods to conceal the allocation sequence and were rated as at low risk of bias for this domain (Malley 1998; Ramilo 2014; Rodriguez 1997a; Sáez‐Llorens 2004). The method of concealing the allocation sequence was unclear in three studies (Hemming 1987; Lagos 2009; Rodriguez 1997b).
Blinding
We rated six studies as at low risk of performance bias because appropriate steps were taken to ensure blinding of participants and personnel (e.g. identical intervention and placebo solutions) (Hemming 1987; Malley 1998; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b; Sáez‐Llorens 2004). We could not determine the adequacy of blinding of participants and personnel in one study, which we assessed as at unclear risk of bias (Lagos 2009). We rated the risk of detection bias as low for clinical outcomes in four studies (Malley 1998; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b). There was insufficient information in three studies to determine the risk of detection bias, although this was unlikely to impact objective outcomes such as mortality or length of stay (Hemming 1987; Lagos 2009; Sáez‐Llorens 2004). We assessed these studies as at unclear risk of bias.
Incomplete outcome data
All included studies either had no losses to follow‐up or exclusions, or had a small amount of attrition that was deemed unlikely to bias the results. We assessed all included studies to be at low risk of attrition bias (Hemming 1987; Lagos 2009; Malley 1998; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b; Sáez‐Llorens 2004).
Selective reporting
We assessed only one study as at low risk of reporting bias, which provided outcome data for all outcomes specified in the trial protocol (Ramilo 2014). In five studies (Lagos 2009; Malley 1998; Rodriguez 1997a; Rodriguez 1997b; Sáez‐Llorens 2004), the risk of reporting bias was unclear because data were reported for the outcomes specified in the methods section of the publication, but none of the studies had an available trial protocol. It was therefore unclear whether other outcomes were measured but not reported based on the results. Outcomes of interest were not specified in the methods section of one study (Hemming 1987).
Other potential sources of bias
We judged all seven included studies to be at unclear or high risk of other potential bias. Five studies either received financial "support" from or were "sponsored" by the intervention manufacturer to conduct the study. In the remaining two studies, study authors or members of the study group were employees of or had received funding from the manufacturer.
Effects of interventions
See: Table 1
See Table 1 for the main comparison intravenous immunoglobulin compared with placebo for RSV infection in infants and children.
Primary outcomes
1. Mortality
Five deaths were reported among 196 children in four included studies (Hemming 1987; Malley 1998; Rodriguez 1997a; Sáez‐Llorens 2004). We excluded data from Hemming 1987 from analysis because the study group of the one child who died was not reported. We found no evidence of a difference in mortality between children in the immunoglobulin and placebo groups (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.14 to 5.27; Analysis 1.1; Figure 3; very low‐certainty evidence, downgraded due to serious risk of bias and very serious imprecision). In Rodriguez 1997a, two children in the immunoglobulin group died; the deaths were considered by the authors to be unrelated to the administration of immunoglobulins (one death occurred after cardiac corrective surgery, and the other was caused by urosepsis in a child with bronchopulmonary dysplasia). Two deaths occurred among children in the placebo group in two studies due to progressive respiratory failure (Malley 1998), possibly complicated by bacterial superinfection (Sáez‐Llorens 2004). One child (study group unknown) died in an accident after discharge (Hemming 1987). A breakdown of the numbers of deaths that occurred in the included studies is presented in Table 2.
1.1. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 1 Mortality (any cause during hospitalisation or follow‐up).
3.
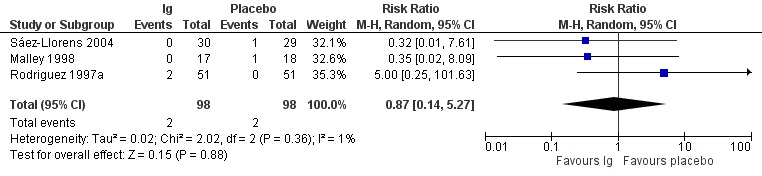
Forest plot of comparison: Immunoglobulins versus placebo, outcome: 1.1 Mortality.
1. Mortality from any cause during hospitalisation or follow‐up.
| Study | Number of deaths in the immunoglobulin group | Immunoglobulin group total | Number of deaths in the placebo group | Placebo group total |
| Hemming 1987 | 1 death (study group unknown) | |||
| Malley 1998 | 0 | 17 | 1 | 18 |
| Rodriguez 1997a | 2 | 51 | 0 | 50 |
| Sáez‐Llorens 2004 | 0 | 30 | 1 | 29 |
2. Length of hospitalisation
All seven included studies reported on length of hospital stay (in days). However, data from two studies could not be included in the meta‐analysis due to missing variability data or missing useable outcome data (median rather than mean was reported) (Hemming 1987; Ramilo 2014). The length of hospital stay ranged from 4.4 to 14.5 days with immunoglobulins and 4.9 to 7.4 days with placebo. There was no difference in length of hospitalisation (in days) between immunoglobulins and placebo (mean difference (MD) −0.70, 95% CI −1.83 to 0.42; Analysis 1.2, Figure 4; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision).
1.2. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 2 Length of hospitalisation (days).
4.
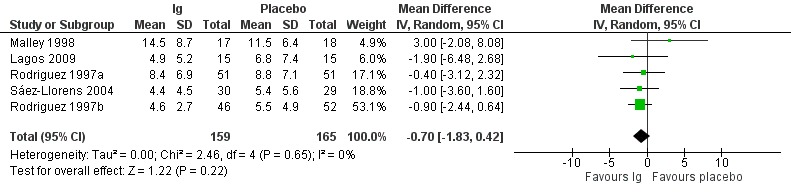
Forest plot of comparison: Immunoglobulins versus placebo, outcome: 1.2 Length of hospitalisation (days).
In two studies that could not be meta‐analysed, the mean length of hospital stay in the treatment and placebo groups was 3.94 days and 3.06 days, respectively, in Hemming 1987, and the median duration of hospitalisation was 3.05 days for the motavizumab 30 mg/kg group, 2.99 days for the motavizumab 100 mg/kg group, and 2.88 days for the placebo group in Ramilo 2014. We conducted a post hoc sensitivity analysis including the study of Ramilo (Ramilo 2014). There was no difference in length of hospitalisation between immunoglobulins and placebo (MD −0.33, 95% CI −1.17 to 0.51).
3. Adverse events
All seven included studies reported on adverse events. Five studies provided data on the number of children who experienced one or more adverse events of any severity or seriousness (Lagos 2009; Malley 1998; Ramilo 2014; Rodriguez 1997a; Sáez‐Llorens 2004). Four studies provided data on the number of children who experienced one or more adverse events considered by study investigators to be serious in nature (Lagos 2009; Malley 1998; Ramilo 2014; Sáez‐Llorens 2004). Two studies did not provide data, stating only that "there were no serious adverse events associated with RSVIG therapy" in Rodriguez 1997b and "Follow up to date has revealed no harmful effects resulting from immunotherapy of RSV infections" in Hemming 1987.
The numbers of children who experienced one or more adverse events (of any severity) and the number who experienced one or more serious adverse events is presented in Table 3. Table 3 also shows the number of children who experienced one or more adverse events considered by the study investigators to be related to the study drug.
2. Adverse events.
| Study | Number of children/total number in group (%) experiencing ≥ 1 adverse event | Number of children/total number in group (%) experiencing ≥ 1 adverse event judged by study investigators to be serious in nature | Number of participants/total number in group (%) experiencing ≥ 1 adverse event judged by study investigators to be related to study drug | Narrative results provided by the study investigators | |||
| Immunoglobulin | Placebo | Immunoglobulin | Placebo | Immunoglobulin | Placebo | ||
| Lagos 2009 | 11/16 (69) | 6/15 (40) | 1/16 (6) | 1/15 (7) | 0 | 0 | "The frequency of AEs was similar between the combined motavizumab groups and the placebo group" (p. 836) |
| Ramilo 2014 | 60/76 (79) | 33/37 (89) | 13/76 (17) | 6/37 (16) | 6/76 (8) | 4/37 (11) | "The incidence rates of AEs and SAEs were similar for the 3 groups" (p. 706) |
| Rodriguez 1997a | 16/51 (31) | 10/51 (20) | NA | NA | 16 of 22 adverse events among the 16 immunoglobulin participants experiencing ≥ 1 adverse event and 8 of 11 adverse events among 10 placebo participants experiencing ≥ 1 adverse event were judged to be related to study drug. | "No significant differences in adverse events were reported in the RSVIG group... when compared with the control group" (p. 454) | |
| Malley 1998 | NA | NA | 7/17 (41) | 6/18 (33) | 0 | 0 | "The percentage of children reporting adverse events and the total number of adverse events were similar in the placebo and MEDI0493 groups" (p. 1559) |
| Sáez‐Llorens 2004 | NA | NA | 7/30 (23) | 7/29 (24) | 1/30 (3) | 3/30 (10) | "The incidence of individual adverse events was balanced between the placebo and palivizumab treatment groups for each dose" (p. 710) |
AE: adverse event, NA: not available, RSVIG: respiratory syncytial virus immunoglobulin, SAE: serious adverse event
There was no difference between the treatment and placebo groups in the number of children who experienced one or more adverse events of any severity or seriousness (RR 1.18, 95% CI 0.78 to 1.78; Analysis 1.3; Figure 5; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision). There was moderate heterogeneity (I² = 57%) amongst trials overall for this analysis. There was no difference between treatment and placebo groups in the number of children who experienced one or more adverse events judged by study investigators to be serious in nature (RR 1.08, 95% CI 0.65 to 1.79; Analysis 1.4; Figure 6; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision).
1.3. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 3 Adverse events of any severity or seriousness.
5.
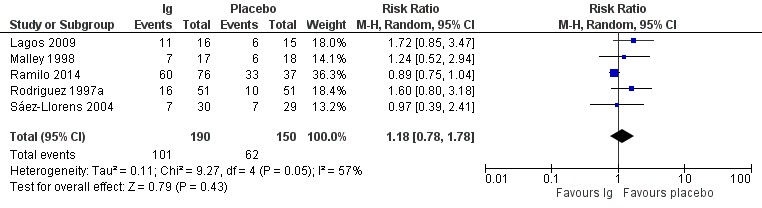
Forest plot of comparison: Immunoglobulins versus placebo, outcome: 1.3 Adverse events.
1.4. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 4 Serious adverse events.
6.
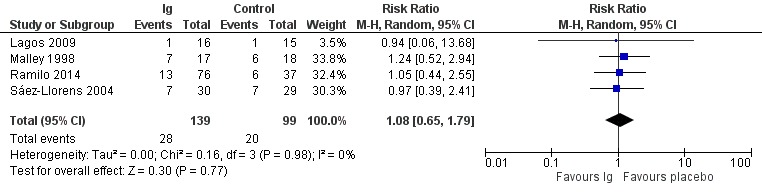
Forest plot of comparison: Immunoglobulins versus placebo, outcome: 1.4 Serious adverse events.
Secondary outcomes
1. Need for mechanical ventilation
Five of six studies that involved children who did not require mechanical ventilation at study entry reported the need for subsequent mechanical ventilation (Hemming 1987; Lagos 2009; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b). Hemming 1987 reported that "Neither group included infants who... needed ventilatory support". This study was therefore not included in the meta‐analysis (as per Section 16.9.3 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011). We do not know if there is a difference in the need for mechanical ventilation between children who received immunoglobulins and those who received placebo (RR 1.24, 95% CI 0.64 to 2.41; Analysis 1.5; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision).
1.5. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 5 Need for mechanical ventilation.
2. Duration of mechanical ventilation
Five included studies reported duration of ventilation (in days) (Lagos 2009; Malley 1998; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b). However, data from two studies could not be included in the meta‐analysis due to missing useable outcome data (median rather than mean was reported, or there were no variation data) (Lagos 2009; Ramilo 2014). It is unclear if there is a difference in duration of mechanical ventilation between immunoglobulins and placebo (MD −0.22, 95% CI −2.64 to 2.21; Analysis 1.6; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision).
1.6. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 6 Duration of mechanical ventilation.
In the two studies excluded from the meta‐analysis, the median duration of ventilation was 7.8 days for children in the motavizumab 30 mg/kg group and 4.6 days for children in the motavizumab 100 mg/kg group; no child in the placebo group required mechanical ventilation (Ramilo 2014). In Lagos 2009, one child in the motavizumab 30 mg/kg group required mechanical ventilation for a duration of 16 days. The mean duration of ventilation was five days for two children in the placebo group.
3. Need for supplemental oxygen
Four of five studies that included children who did not require intubation, mechanical ventilation, or supplemental oxygen at study entry reported the need for subsequent supplemental oxygen (Hemming 1987; Lagos 2009; Ramilo 2014; Rodriguez 1997a). Of these studies, two did not provide data but reported that "No significant differences were observed between the intravenous immunoglobulin (IVIG)‐treated and the placebo‐treated groups in the following: supplemental O₂ requirements..." in Hemming 1987 and "no differences between the respiratory syncytial virus immune globulin (RSVIG) and placebo groups were observed in... supplemental oxygen" in Rodriguez 1997a. We found no evidence of a difference in the need for supplemental oxygen between immunoglobulins and placebo (RR 1.18, 95% CI 0.94 to 1.49; Analysis 1.7; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision).
1.7. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 7 Need for supplemental oxygen.
4. Duration of supplemental oxygen
Five studies reported duration of supplemental oxygen (in days) (Hemming 1987; Lagos 2009; Malley 1998; Ramilo 2014; Sáez‐Llorens 2004). However, two of these studies were excluded from the meta‐analysis due to missing useable outcome data (Hemming 1987; Ramilo 2014). We found no evidence of a difference in duration of supplemental oxygen between children in the immunoglobulin and placebo groups (MD −0.54, 95% CI −2.26 to 1.17; Analysis 1.8; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision).
1.8. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 8 Duration of supplemental oxygen.
In the two studies that could not be included in the meta‐analysis, the median duration of supplemental oxygen was 3.0 days for the motavizumab 30 mg/kg and 100 mg/kg groups and the placebo group (Ramilo 2014), and the Hemming 1987 investigators stated that "no significant differences" were observed in supplemental oxygen requirements between children in the intravenous immunoglobulin and placebo groups, but did not provide numerical data.
5. Need for intensive care unit admission
Five studies reported the need for admission to the ICU (Hemming 1987; Lagos 2009; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b). Of these studies, one reported that "Neither group included infants who required admission to an intensive care unit" (Hemming 1987), therefore data from Hemming 1987 were not included in the meta‐analysis (as per Section 16.9.3 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011). There was no evidence of a difference in the need for ICU admission between children who received immunoglobulins and those who received placebo (RR 1.22, 95% CI 0.64 to 2.32; Analysis 1.9; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision).
1.9. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 9 Need for ICU admission.
6. Duration of stay in the intensive care unit
Four studies reported duration of stay (in days) in the ICU (Lagos 2009; Ramilo 2014; Rodriguez 1997a; Rodriguez 1997b). However, two studies could not be included in the meta‐analysis due to missing useable outcome data (Lagos 2009; Ramilo 2014). There was no evidence of a difference in duration of stay in the ICU between children in the immunoglobulin and placebo groups (MD −2.13, 95% CI −4.55 to 0.30; Analysis 1.10; low‐certainty evidence, downgraded due to serious risk of bias and serious imprecision).
1.10. Analysis.
Comparison 1 Immunoglobulins versus placebo, Outcome 10 Duration of stay in the ICU.
In the two studies excluded from the meta‐analysis, the median duration of stay in the ICU was 10 days for the motavizumab 30 mg/kg group, 5 days for the motavizumab 100 mg/kg group, with no ICU admissions amongst children in the placebo group (Ramilo 2014). In Hemming 1987, one child in the motavizumab group stayed in the ICU for 16 days; the mean duration of stay amongst children in the placebo group was five days.
7. Pulmonary function
None of the included studies reported pulmonary function or spirometry data.
8. Rehospitalisation for recurrent breathing difficulties in the long term
Two studies reported readmissions during the subsequent respiratory seasons (Rodriguez 1997a; Rodriguez 1997b). In one study, three (of 26) children in the RSV immunoglobulin group were hospitalised for LRTI during the subsequent respiratory season (all three LRTI hospitalisations were due to RSV), and three (of 26) children in the placebo group were hospitalised for LRTI during the subsequent respiratory infections season (two were due to RSV) (Rodriguez 1997b). In the second study, five (of 48) children in the RSV immunoglobulin group were hospitalised for LRTI during the subsequent respiratory infections season (three were due to RSV), and six (of 50) children from the placebo group were hospitalised for LRTI during the subsequent respiratory infections season (three were due to RSV) (Rodriguez 1997a).
9. The occurrence of reactive airway disease in the long term
None of the included studies reported the occurrence of reactive airway disease in the long term. One study reported that the incidence of wheezing (a symptom of reactive airway disease) was similar between children in the motavizumab and placebo groups in the 12 months after randomisation (Ramilo 2014).
Subgroup analyses
We planned to undertake subgroup analyses based on children's age, episode of RSV, type of immunoglobulin intervention, and existing comorbidities. These analyses were not possible due to lack of data.
Sensitivity analysis
We planned to conduct a sensitivity analysis to examine the effect of risk of bias (from allocation concealment) on outcome estimates. However, none of the included studies were judged to be at high risk of bias for allocation concealment.
Discussion
Summary of main results
We aimed to assess the effectiveness and safety of immunoglobulins as a treatment for RSV‐associated LRTIs in hospitalised infants and young children.
We searched the literature to 6 November 2018 and included seven studies (486 children) that met the review inclusion criteria: two studies compared palivizumab to placebo; two studies compared motavizumab to placebo; and three studies compared high RSV neutralising antibody titre immunoglobulin to placebo.
Very low‐certainty evidence from three studies (downgraded in the GRADE assessment for risk of bias and imprecision due to small sample size and low event rates) meant that it is unclear if there is a difference between immunoglobulins and placebo in mortality (from any cause during hospitalisation or follow‐up). Wide confidence intervals around the estimate did not rule out a null effect or potential harm from immunoglobulin treatment.
Low‐certainty evidence from five studies (downgraded in the GRADE assessment due to risk of bias and imprecision) indicated that immunoglobulins did not make a significant difference in reducing length of hospitalisation for children with RSV infection.
There was no difference in the number of children who experienced one or more adverse events (of any severity or seriousness) between the immunoglobulin and placebo groups based on evidence from five studies assessed as at low certainty (downgraded due to risk of bias and imprecision). There was no difference in the number of children who experienced one or more adverse events considered by study investigators to be serious in nature between the immunoglobulin and placebo groups based on evidence from three studies assessed as at low certainty (downgraded due to risk of bias and imprecision).
Low‐certainty evidence (downgraded in the GRADE assessment due to risk of bias and imprecision) demonstrated that immunoglobulins did not make a significant difference in the need for or duration of mechanical ventilation, the need for or duration of supplemental oxygen, and the need for or duration of stay in the ICU compared to placebo. We identified no studies providing data on the effect of immunoglobulins on pulmonary function or the occurrence of reactive airway disease in the long term.
Overall completeness and applicability of evidence
Seven small studies (involving a total of 486 children) met the review inclusion criteria. Uncertainty about the effect of immunoglobulins on mortality reflects the small sample sizes and low event rates. For most comparisons, confidence intervals were very wide, and we could not rule out the possibility of clinically relevant differences. In some studies, outcome data were not always reported in a way that could be analysed, for example reporting of medians and absence of variation data.
All studies included hospitalised children with laboratory‐confirmed RSV infection. All trials were conducted at sites in high‐income countries (USA, Chile, New Zealand, Australia), with two studies including a site in a middle‐income country (Panama). The applicability of findings to low‐ and middle‐income countries, where rates of mortality from RSV infection are higher, is therefore unclear.
There was variation in the populations in the included studies (children were "previously healthy", were considered "high risk" for RSV infections, or had chronic medical conditions) and in the severity of illness at study entry (in two studies children were mechanically ventilated or required more than 30% supplemental oxygen). Given this variation, it is not clear if a specific group of children (i.e. those with more severe illness) might benefit from immunoglobulin treatment.
There was variation in the immunoglobulin preparations evaluated in the studies. Four studies evaluated different doses of the monoclonal immunoglobulins motavizumab (which is no longer available) and palivizumab. The remaining studies evaluated the pooled immunoglobulin respiratory syncytial virus immune globulin (RSVIG) (which was superseded by palivizumab). We were unable to perform further investigation of the effect of the alternate preparations in planned subgroup analysis due to the small number of included studies.
We identified an ongoing trial comparing a single dose of palivizumab with placebo in infants aged up to three months with RSV bronchiolitis (NCT02442427). A primary outcome of this review is readmission to either observation or hospital or paediatric ICU during three weeks of follow‐up after discharge. Recruitment status is complete (last update posted 27 February 2018). This study will be assessed for inclusion and results presented in a future review update if appropriate.
The overall completeness and applicability of evidence was limited. There were few trials with small samples assessing the effects of immunoglobulins, predominantly in high‐income healthcare settings.
Quality of the evidence
Using the GRADE methodology, which provides outcome‐specific ratings of the certainty of evidence, we considered confidence in the estimate of effect to be low or very low for the primary outcomes assessed in this review, due primarily to serious risk of bias and imprecision.
For the primary outcomes of the review (mortality, length of hospitalisation, and adverse events), we considered risk of bias to be serious due to unclear random sequence generation (all studies), allocation concealment (in some studies), and selective reporting (all studies). Furthermore, we considered all studies to be at risk of other bias because they were funded by parties with vested interest in the results, and/or trial authors or members of the study group had notable conflicts of interest.
We downgraded the quality of the evidence for the primary outcomes of the review due to imprecision. The effect estimate for mortality was derived from few small studies, a low event rate with wide confidence intervals including the null effect for appreciable harm or benefit. The evidence for length of hospitalisation and adverse events was also imprecise owing to small sample size compared to the calculated optimal information size, and wide confidence intervals. Consequently, the estimates of effect that have been presented should be considered uncertain, with further research likely to change these estimates.
Potential biases in the review process
We attempted to limit bias in the review process by following the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, there were several possible limitations of this review related to the process of selecting studies, extracting data, and assessing risk of bias. Although two review authors worked independently on each step, the second review authors varied between and within steps (i.e. the review authors screening the titles and abstracts of the original and updated searches may have been different, and different review authors acted as second reviewers for the risk of bias and data extraction steps). The effect of this on the outcomes and conclusions of the review is unclear. In addition, we did not attempt to obtain data from studies reporting unuseable outcome data. Although the search was thorough, it is possible that published and unpublished studies were not identified. The impact of possible omission on the results of the review is uncertain.
Agreements and disagreements with other studies or reviews
We are aware of only one other systematic review that has examined immunoglobulins as a treatment for RSV infection (Hu 2010). This review included studies of any design published to mid‐2009 evaluating palivizumab in people of any age with RSV infection. The review included one case report, four case series, and two randomised trials (also included in this review). The primary outcomes were progression from upper respiratory tract infection (URTI) to lower respiratory tract infection (LRTI) and survival. The methods of the search were provided, although the methods of selecting, extracting, and appraising studies were not reported. From the 7 included studies, Hu 2010 reported deaths in 3 of 25 (12%) participants with URTI receiving palivizumab and 5 of 88 (6%) participants with LRTI receiving palivizumab. The authors concluded that larger RCTs are required before palivizumab can be recommended as therapy for RSV.
Authors' conclusions
Implications for practice.
Our review did not demonstrate that immunoglobulins improve important clinical outcomes for children younger than three years of age hospitalised with respiratory syncytial virus (RSV) infection. We found insufficient evidence of a difference between immunoglobulins and placebo for any review outcomes. We assessed the evidence for the effects of immunoglobulins when used as a treatment for RSV lower respiratory tract infection in hospitalised infants and young children as of low or very low certainty due to risk of bias and imprecision. We are uncertain of the effects of immunoglobulins on these outcomes, and the true effect may be substantially different from the effects reported in this review. All trials were conducted in high‐income countries, and data from populations in which the rate of death from RSV infection is higher are lacking. Due to the low certainty of the evidence, cautious interpretation of the findings of this review is suggested.
Implications for research.
Although there is no evidence of benefit in the studies included in this review, further research may consider studying the benefits and harms of immunoglobulins as a treatment for RSV in specific subgroups of children.
Given the substantial burden of RSV infection, and the lack of effective therapies at present, further research with newer monoclonal and pooled immunoglobulin preparations and in low‐income countries may be considered. Such studies should be rigorously designed to minimise bias and assist applicability (e.g. by documenting when in the course of the illness treatment commenced).
Acknowledgements
We would like to acknowledge the authors of the Cochrane Review protocol 'Immunoglobulin for treating respiratory syncytial virus infection' (Tan 1998).
This review was based on a published review by Fuller 2006, which was withdrawn because the first author was unable to complete the review.
We acknowledge former authors who worked on the draft of this review but did not complete the review: Myuri Kantharajah, Farah Diba Zaman, Danielle Samra, and Monica N Gunturu.
We wish to thank the following people for commenting on the draft protocol for this review: Anne Lyddiatt, Nancy Banasiak, Lenny Krilov, Mark Jones, and Inge Axelsson.
We would like to gratefully acknowledge the following peer reviewers for their considered comments on the 2019 draft of this review: Leonard R Krilov, David J Marchant, Simon Nadel, Teresa Neeman, Dee Shneiderman, and Menelaos Konstantinidis.
We would also like to sincerely thank the Contact Editor, Roderick P Venekamp, for his critical comments that helped improve the review.
Our thanks also to Liz Dooley and Ann Jones from the Cochrane Acute Respiratory Infections Group for editorial support and Mark Jones for statistical advice. Finally, we thank Rebecca Fortescue, joint Co‐ordinating Editor of the Cochrane Airways Group, for signing off on this review.
Appendices
Appendix 1. MEDLINE and CENTRAL search strategy
MEDLINE (Ovid)
1 exp Bronchiolitis/ 2 bronchiolit*.tw. 3 exp Pneumonia/ 4 (pneumon* or bronchopneumon* or pleuropneumon*).tw. 5 Respiratory Tract Infections/ 6 lower respiratory infection*.tw. 7 (lower respiratory tract infection* or lrti).tw. 8 respiratory syncytial viruses/ or respiratory syncytial virus, human/ 9 Respiratory Syncytial Virus Infections/ 10 (respiratory syncytial virus* or rsv).tw. 11 or/1‐10 12 exp Immunoglobulins/ 13 immunoglobulin*.tw,nm. 14 (immune adj2 globulin*).tw. 15 rsv‐igiv.tw,nm. 16 respigam.tw,nm. 17 palivizumab.tw,nm. 18 synagis.tw,nm. 19 or/12‐18 20 11 and 19
Appendix 2. Embase (Elsevier) search strategy
#19 #15 AND #18 #18 #16 OR #17 #17 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR (((singl* OR doubl*) NEXT/1 blind*):ab,ti) #16 'randomised controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #15 #9 AND #14 #14 #10 OR #11 OR #12 OR #13 #13 'rsv‐igiv':ab,ti OR respigam:ab,ti OR palivizumab:ab,ti OR synagis:ab,ti #12 (immune NEAR/2 globulin*):ab,ti #11 immunoglobulin*:ab,ti #10 'immunoglobulin'/exp #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #8 'respiratory syncytial virus':ab,ti OR 'respiratory syncytial viruses':ab,ti OR rsv:ab,ti #7 'respiratory syncytial pneumovirus'/de OR 'respiratory syncytial virus infection'/de #6 'lower respiratory tract infection':ab,ti OR 'lower respiratory tract infections':ab,ti OR 'lower respiratory infection':ab,ti OR 'lower respiratory infections':ab,ti OR lrti:ab,ti #5 'respiratory tract infection'/de OR 'lower respiratory tract infection'/exp #4 pneumon*:ab,ti OR bronchopneumon*:ab,ti OR pleuropneumon*:ab,ti #3 'pneumonia'/exp #2 bronchiolit*:ab,ti #1 'bronchiolitis'/exp
Appendix 3. CINAHL (EBSCO) search strategy
S26 S16 and S25 59 S25 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 S24 (MH "Quantitative Studies") S23 (MH "Placebos") S22 TI placebo* OR AB placebo* S21 TI random* OR AB random* S20 TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) S19 TI clinic* trial* OR AB clinic* trial* S18 PT clinical trial S17 (MH "Clinical Trials+") S16 S10 and S15 S15 S11 or S12 or S13 or S14 S14 TI (rsv‐igiv or respigam or palivizumab or synagis) OR AB (rsv‐igiv or respigam or palivizumab or synagis) S13 TI immune N2 globulin* OR AB immune N2 globulin* S12 TI immunoglobulin* OR AB immunoglobulin* S11 (MH "Immunoglobulins+") S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 16135 S9 TI (respiratory syncytial virus* or rsv) OR AB (respiratory syncytial virus* or rsv) S8 (MH "Respiratory Syncytial Virus Infections") S7 (MH "Respiratory Syncytial Viruses") S6 TI (lower respiratory tract infection* or lower respiratory infection* or lrti) OR AB (lower respiratory tract infection* or lower respiratory infection* or lrti) S5 (MH "Respiratory Tract Infections") S4 TI pneumon* OR AB pneumon* S3 (MH "Pneumonia+") S2 TI bronchiolit* OR AB bronchiolit* S1 (MH "Bronchiolitis+")
Appendix 4. Web of Science (Clarivate Analytics) search strategy
TOPIC: ((bronchiolit* or pneumon* or bronchopneumon* or pleuropneumon* or "lower respiratory tract infection*" or "lower respiratory infection*" or lrti or rsv or "respiratory syncytial virus" or "respiratory syncytial viruses")) AND TOPIC: ((immunoglobulin* or "immune globulin" or "rsv‐igiv" or respigam or palivizumab or synagis))
Refined by: TOPIC: ((random* or placebo* or "clinic* trial*" or "singl* blind*" or "doubl* blind*"))
Timespan: All years. Indexes: SCI‐EXPANDED, CPCI‐S.
Data and analyses
Comparison 1. Immunoglobulins versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (any cause during hospitalisation or follow‐up) | 3 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.14, 5.27] |
| 2 Length of hospitalisation (days) | 5 | 324 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.83, 0.42] |
| 3 Adverse events of any severity or seriousness | 5 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.78, 1.78] |
| 4 Serious adverse events | 4 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.65, 1.79] |
| 5 Need for mechanical ventilation | 4 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.64, 2.41] |
| 6 Duration of mechanical ventilation | 3 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐2.64, 2.21] |
| 7 Need for supplemental oxygen | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.49] |
| 8 Duration of supplemental oxygen | 3 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐2.26, 1.17] |
| 9 Need for ICU admission | 4 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.64, 2.32] |
| 10 Duration of stay in the ICU | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐4.55, 0.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hemming 1987.
| Methods |
Study design: parallel‐group RCT Setting: children's hospital Duration: from recruitment to discharge variable. Follow‐up 6 weeks and 1 year after discharge. |
|
| Participants |
Location: USA Inclusion criteria
Exclusion criteria
Baseline characteristics (N = 35) Mean age (SD), months: treatment: 4.4 (4.3); comparator: 4.4 (4.1) Proportion male: not reported Health status/disease severity: not reported |
|
| Interventions | Treatment (N = 17): IV immunoglobulins containing high titres of RSV‐neutralising antibody (geometric mean neutralising antibody titres of approximately 1:5000) 2 g/kg body weight administered over 12 to 24 hours Comparator (N = 18): placebo 2 g/kg body weight administered over 12 to 24 hours |
|
| Outcomes |
|
|
| Notes | This study was supported by the manufacturer of the immunoglobulin used in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were assigned to 1 of 2 equal‐size treatment groups based on a table of random numbers." (p. 1883) Comment: there was insufficient information on the method used to generate the randomisation sequence to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information on how the allocation sequence was concealed to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Only the study monitors (Sandoz Inc, East Hanover, NJ) knew the contents of the bottles of drug infused into each participant. The codes were not broken until the completion of each portion of the study." (p. 1883) "Lyophised human albumin, prepared in identical bottles and with protein concentrations identical to that of the IVIG, was used as the placebo drug." (p. 1882) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of outcome assessment was not described. There is insufficient information to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Complete 2 g/kg infusions were not completed in three patients, two IVIG treated and one placebo, because of problems maintaining venous access. IgG levels rose in both of these IVIG‐treated children... suggesting receipt of most of the planned dose" (p. 1883) Comment: 35 participants were randomised. The number of participants not completing the study treatments was small (3 of 35), and analysis was based on all randomised participants for the outcomes RSV‐neutralising antibody titres, IgG levels, and nasopharyngeal RSV infectivity titres. For the outcome of oximetry, only participants completing the infusion were included in the analysis (32/35). Follow‐up was completed for 30 of the 35 children at 6 weeks and 1 year. The review authors judge that attrition is unlikely to have an important impact on the observed results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcomes were not specified in the methods section. Only a description of the tests carried out during hospitalisation was provided. The reporting of outcomes does not appear to be related to whether the results were significant or not, as both were presented. Also, without a trial protocol it is unclear if other outcomes were measured but not reported based on the nature of the results. |
| Other bias | High risk | Quote: "This research was supported by Sandoz Pharmaceutical Corp." (p. 1885) (manufacturer of the IVIG used in this study) and the Children's Hospital National Medical Center. This may lead to bias in favour of the intervention group. |
Lagos 2009.
| Methods |
Study design: parallel‐group RCT Setting: hospital, not further described Duration: recruitment to discharge variable. Adverse events "monitored through study day 30". (p. 835) |
|
| Participants |
Location: USA and Chile Inclusion criteria
Exclusion criteria
Baseline characteristics Mean age (range), months: treatment: 7.6 (1.0 to 21.8); comparator: 7.4 (0.6 to 22.6) Proportion male: treatment 80%; comparator: 53% Health status/disease severity: children described as "previously healthy" (p. 835). Lower Respiratory Infection Score (6‐point scale ranging from 0 = no respiratory infection to 5 = requiring mechanical ventilation): treatment: 2.5; comparator: 2.5 |
|
| Interventions | Treatment (N = 5): single IV infusion of motavizumab at a dose of 3 mg/kg Treatment (N = 5): single IV infusion of motavizumab at a dose of 15 mg/kg Treatment (N = 5): single IV infusion of motavizumab at a dose of 30 mg/kg Dose escalation occurred after ≥ 7 days of safety follow‐up of the previous dose group. Comparator (N = 15): single IV infusion of 0.45% NaCl, which was identical in appearance to the motavizumab |
|
| Outcomes |
|
|
| Notes | This study was funded by the manufacturer of the immunoglobulin used in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomised in 1:1 to groups..." (p. 835) Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were randomised in 1:1 to groups..." (p. 835) Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "...identically appearing placebo" (p. 835) Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All virologic assays were performed blind with respect to treatment assignment" (p. 835) Comment: blinding occurred for the RSV quantification outcomes (RSV quantification by viral culture and reverse transcriptase polymerase chain reaction), but blinding was not described for clinical outcomes. The risk of bias for the clinical outcomes is unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "thirty one children were randomised... Twenty‐nine patients completed the study." (p. 836) Comment: 1 participant randomised to motavizumab was discontinued at day 0 because the study drug could not be administered within the protocol‐specified time. 1 child was lost to follow‐up at day 8 after dosing but was included in the analysis, so outcomes are reported for 30 of the 31 randomised participants. The small number (N = 1) and reason for missing outcome data is unlikely to have an important impact on the observed results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data are reported for all outcomes specified in the methods section of the publication. However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results. |
| Other bias | High risk | Quote: "This research was funded by MedImmune" (p. 835) (manufacturer of motavizumab), and several authors were employees of MedImmune. This may lead to bias in favour of the treatment group. |
Malley 1998.
| Methods |
Study design: parallel‐group RCT Setting: children's hospitals Duration: follow‐up 30 days after administration of study treatments |
|
| Participants |
Location: USA Inclusion criteria
Exclusion criteria
Baseline characteristics (N = 35) Median age (range), months: treatment: 3.2 (1.2 to 23.8); comparator: 1.7 (0.8 to 15.4) Proportion male: treatment: 59%; comparator: 72% Health status/disease severity: children required intubation and mechanical ventilation at study entry. 3 children (18%) in the treatment group and 3 children (17) in the comparator group had "significant chronic medical conditions at the time of randomization" (p. 1557). For the children in the treatment group, this included congenital anomalies in 1 child; microcephaly, developmental delay, and a seizure disorder in a second child; and a third child was quadriplegic. For the children in the comparator group, this included trisomy 21 in 2 children and Pierre Robin syndrome in 1 child. |
|
| Interventions | Treatment (N = 17): intravenous palivizumab 15 mg/kg Comparator (N = 18): intravenous 0.9% saline |
|
| Outcomes |
|
|
| Notes | This study was funded by the manufacturer of the immunoglobulin used in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the method used to generate the allocation sequence is unclear. There was insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Low risk | Quote: "The study was centrally randomized in blocks of six per site." (p. 1556) Comment: an adequate method to conceal the allocation sequence was likely used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All clinical and laboratory personnel, the participants, and families were blinded to the treatment assignment; the pharmacist at each site was unblinded." (p. 1556) Comment: it is likely that participants and caregivers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All clinical and laboratory personnel, the participants, and families were blinded to the treatment assignment; the pharmacist at each site was unblinded." (p. 1556) Comment: it is likely that the personnel responsible for outcome data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 35 randomized children were included in all analyses for which appropriate data were available." (p. 1557) Comment: all randomised children had data for clinical outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data are reported for all outcomes specified in the methods section of the publication. However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results. |
| Other bias | High risk | Quote: "Financial support: MedImmune, Inc." (p. 1555) Comment: the trial was supported by MedImmune (manufacturer of MEDI‐493). This may lead to bias in favour of the intervention group. |
Ramilo 2014.
| Methods |
Design: multicentre, parallel‐group, 3‐arm RCT Setting: hospitals, not further described Duration: 1 year from randomisation to final follow‐up |
|
| Participants |
Location: "Northern and Southern Hemispheres", "5 countries". Trial registry indicates study sites in USA, Panama, Chile, New Zealand, and Australia. Inclusion criteria
Exclusion criteria
Baseline characteristics (N = 118) Median age (range), months: treatment: 2.0 (0.4 to 11.2) for the 30 mg/kg arm and 2.2 (0.3 to 11.3) for the 100 mg/kg arm; comparator: 2.7 (0.5 to 10.3) Proportion male: treatment: 51% for the 30 mg/kg arm and 51% for the 100 mg/kg arm; comparator: 73% Health status/disease severity: children were described as "previously healthy" (p. 703). Median (range) Respiratory Distress Assessment Instrument (RDAI) score (17‐point scale, with higher score indicating more severe wheezing and retractions): treatment: 6 (0 to 17) for the 30 mg/kg arm and 6 (0 to 13) for the 100 mg/kg arm; comparator: 4 (0 to 15) |
|
| Interventions | Treatment (N = 39): single IV dose of motavizumab 30 mg/kg Treatment (N = 39): single IV dose of motavizumab 100 mg/kg Comparator (N = 40): placebo (not further described) |
|
| Outcomes |
|
|
| Notes | This study was sponsored by the manufacturer of the immunoglobulin used in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible subjects were randomized to 1:1:1..." (p. 704) Comment: the method used to generate the allocation sequence is unclear. There is insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible subjects were randomized to 1:1:1 using an interactive voice response system to receive... The interactive voice response system was also used for assignment of patient identification number and assignment of blinded study drug kits." (p. 704) Comment: an adequate method was used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...assignment of blinded study drug kits ...Subjects parents/guardians, clinical site staff and protocol‐associated personnel were blinded to group assignment." (p. 704) Comment: it is likely that participants and care providers were blinded, although the appearance of the interventions is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects parents/guardians, clinical site staff and protocol‐associated personnel were blinded to group assignment... At a central laboratory, personnel who were blinded to treatment assignment tested nasal specimens..." (p. 704) Comment: personnel responsible for the virologic and clinical outcome data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 118 subjects were randomized... and 113 subjects received study drug. One hundred and seven subjects completed through study day 90 and 98 subjects completed through study day 360. Similar rates of non‐completion were observed among subjects treated with motavizumab or placebo." (p. 704) Comment: 91% (107/118) of randomised participants remained in the study at day 90, with a similar number of non‐completers in the study groups. Clinical outcome data are provided for 112 of 113 participants who received the study drug (1 participant was found to be negative for RSV at study day 0). Of the 112 participants with clinical outcome data, duration of hospitalisation data are available for 111 (1 participant in the motavizumab 30 mg/kg group withdrew consent). The review authors judge that the reasonably small number of participants randomised but not included in the analysis and the similar numbers lost to follow‐up in the study groups is unlikely to have an important impact on the observed results. |
| Selective reporting (reporting bias) | Low risk | Comment: outcome data were fully reported for all outcomes specified in ClinicalTrials.gov registry entry NCT00421304. |
| Other bias | High risk | Quote: "This study was sponsored by MedImmune." (p. 703) Comment: the study was sponsored by MedImmune (the manufacturer of motavizumab), and a number of the study investigators received funding from or were employees of MedImmune. This may lead to bias in favour of the intervention group. |
Rodriguez 1997a.
| Methods |
Design: multicentre, parallel‐group RCT Setting: children's and university hospitals Duration: recruitment to discharge variable. Children were followed up in the next RSV season. |
|
| Participants |
Location: USA Inclusion criteria
Exclusion criteria
Baseline characteristics (N = 102 (of 107 children randomised)) Mean age (SE), months: treatment: 0.55 (0.07); comparator: 0.58 (0.06) Proportion male: treatment: 45%; comparator: 57% Health status/disease severity: children were described as high risk for severe RSV infection. High‐risk children included those with severe bronchopulmonary dysplasia, chronic lung disease, congenital heart disease, or prematurity. Mean (SE) respiratory score (score ranges from 0 to 5, with higher scores indicating more severe disease): treatment: 3.4 (0.2); comparator: 3.1 (0.1). Proportion with Lower Respiratory Tract Infection Score 5 (score ranges from 0 to 5, with 5 indicating respiratory failure): treatment: 31%; comparator: 18% |
|
| Interventions | Treatment (N = 54): 30 mL/kg RSVIG (1.5 mg/kg IVIG) given intravenously over 12 hours Comparator (N = 54): 0.15 mg/kg of albumin given intravenously over 12 hours | |
| Outcomes |
Primary
Secondary
|
|
| Notes | Some members of the study group were employees of the manufacturer of the immunoglobulin used in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the method used to generate the allocation sequence is unclear. There was insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Low risk | Quote: "Each year of the study, MPHBL coded vials by one of six letters... Only MPHBL and the Data and Safety Monitoring Board member knew the contents of the vials until the study code was broken... Each centre received from MedImmune Inc a randomization schedule that ensured that each center enrolled nearly equal numbers of RSVIG and placebo patients by balancing randomisation in blocks of six. Patients who fit the inclusion criteria were assigned to the next lettered vial specified in the randomizations scheme for each centre." (p. 456) Comment: an adequate method was likely used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Bottles containing respiratory syncytial virus immunoglobulin or placebo were coded by the MPHBL so that contents were unknown to the investigators, sponsor, and study participants... A 0.5% solution of albumin bottled identically to the RSVIG was used as the placebo solution." (p. 456) Comment: it is likely that participants and care providers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Bottles containing respiratory syncytial virus immunoglobulin or placebo were coded by the MPHBL so that contents were unknown to the investigators, sponsor, and study participants... A 0.5% solution of albumin bottled identically to the RSVIG was used as the placebo solution... Attending physicians not associated with the study were responsible for routine treatment... Furthermore, they determined when to administer supplemental oxygen, the level of oxygen therapy, or the need for mechanical ventilation. Likewise, the decision for hospital discharge was made by the attending physicians." (p. 456) Comment: it is likely that the personnel responsible for outcome data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Fifty four patients were randomized to receive RSVIG, and 53 were randomized to receive placebo. Three children in the RSVIG group and 2 in the placebo group received less than 75% of the ordered dose and those were not evaluable for efficacy." (p. 457) Comment: the review authors judge that owing to the small number of participants not completing study treatments and excluded from the analysis, the similar numbers in each study group, and for reasons unlikely to be related to the outcomes, this is unlikely to have an important impact on the observed results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data are reported for all outcomes specified in the methods section of the publication. However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results. |
| Other bias | Unclear risk | Quote: "This work was supported by grant H5 MO1RR0069, General Clinical Research Centers program, from the National Institutes of Health (University of Colorado)." (p. 460) Comment: a number of members of the RSVIG Study Group were employees of MedImmune (manufacturer of RSVIG). This may lead to bias in favour of the intervention group. |
Rodriguez 1997b.
| Methods |
Design: multicentre, parallel‐group RCT Setting: children's and university hospitals Duration: recruitment to discharge variable. Follow‐up 8 weeks after discharge and during the following respiratory season. |
|
| Participants |
Location: USA Inclusion criteria
Exclusion criteria
Baseline characteristics (N = 101) Mean age (SE), months: treatment: 0.20 (0.03); comparator: 0.19 (0.03) Proportion male: treatment 48%; comparator 50% Health status/disease severity: children were described as "previously healthy" (p. 938). Mean (SE) Respiratory score (score ranges from 0 to 5, with higher scores indicating more severe disease): treatment: 3.69 (0.13); comparator: 3.77 (0.13). Proportion with Lower Respiratory Tract Infection Score 5 (score ranges from 0 to 5, with 5 indicating respiratory failure): treatment: 28%; comparator: 33% |
|
| Interventions | Treatment (N = 47): 30 mL/kg (1500 mg/kg) infusion of RSVIG Comparator (N = 54): 30 mL/kg (1500 mg/kg) infusion of albumin placebo |
|
| Outcomes |
Primary
Secondary
Other
|
|
| Notes | This study was supported by the manufacturer of the immunoglobulin used in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the method used to generate the allocation sequence is unclear. There was insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Bottles containing RSVIG or placebo were coded by the Massachusetts Public Health Biological Laboratories so that controls were unknown to investigators, sponsor and study participants." (p. 938) Comment: there was insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A one half percent (0.5%) solution of albumin bottled identically to the RSBIG was utilized as the placebo control solution." (p. 938) Comment: it is likely that participants and care providers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Bottles containing respiratory syncytial virus immunoglobulin or placebo were coded by the Massachusetts Public Health Biological Laboratories so that contents were unknown to the investigators, sponsor, and study participants. ...A 0.5% solution of albumin bottled identically to the RSVIG was used as the placebo solution... Attending physicians determined whether and when supplemental oxygen or mechanical ventilation was required. The decision for hospital discharge was also made by the attending physicians." (p. 938) Comment: it is likely that the personnel responsible for the primary and secondary outcome data were blinded. It is unclear who evaluated participants by the analogue scale, LRI, and respiratory score and if they were blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One hundred one patients were enrolled in the trial, 47 in the RSVIG group and 54 in the placebo group. Forty‐six RSVIG (98%) and 52 placebo recipients (96%) could be evaluated. Excluded from the evaluation were 1 infant in the RSVIG group who received less than 75% of the infusion, 1 placebo recipient who had an admission respiratory score < 2.5, and 1 placebo patient on whom we were unable to start an intravenous infusion." (p. 939) Comment: the review authors judge that owing to the small number of participants not completing study treatments and excluded from the analysis, the similar numbers in each study group, and for reasons unlikely to be related to the outcomes, this is unlikely to have an important impact on the observed results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data are reported for all outcomes specified in the methods section of the publication. However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results. |
| Other bias | High risk | Quote: "This study was supported by MedImmune, Inc. and by Grant H5 MO1RR0069, General Clinical Research Centers Program National Institutes of Health (University of Colorado)." (p. 941) Comment: the study is supported in part by MedImmune, and a number of members of the RSVIG Study Group are employees of MedImmune (manufacturer of RSVIG). This may lead to bias in favour of the intervention group. |
Sáez‐Llorens 2004.
| Methods |
Design: multicentre, parallel‐group RCT Setting: hospitals, not further described Study duration: follow‐up for 30 days after study drug administration and during the following RSV season |
|
| Participants |
Location: USA and Panama Inclusion criteria
Exclusion criteria
Baseline characteristics (N = 59) Mean age (SE), months: treatment: 1.5 (0.4) for palivizumab 5 mg/kg arm and 5.2 (0.9) for palivizumab 15 mg/kg arm; comparator: 2.9 (0.7) for the group compared to palivizumab 5 mg/kg arm and 4.2 (0.8) for the group compared to palivizumab 15 mg/kg arm Proportion male: treatment: 75% for palivizumab 5 mg/kg arm and 59% for palivizumab 15 mg/kg arm; comparator: 13% for the group compared to palivizumab 5 mg/kg arm and 48% for the group compared to the palivizumab 15 mg/kg arm Health status/disease severity: children described as "previously healthy" (p. 707). Children required > 30% supplemental oxygen at study entry. Proportion with Lower Respiratory Infection score ≥ 3 (score ranges from 0 to 5, with 0 indicating no respiratory illness, 3 indicating moderate lower respiratory infection, and 5 indicating the need for mechanical ventilation): treatment: 100% for palivizumab 5 mg/kg arm and 36% for palivizumab 15 mg/kg arm; comparator: 88% for the group compared to palivizumab 5 mg/kg arm and 33% for the group compared to palivizumab 15 mg/kg arm |
|
| Interventions | Treatment (N = 8): 5 mg/kg intravenous palivizumab Treatment (N = 22): 15 mg/kg intravenous palivizumab. The dose of palivizumab was increased to 15 mg/kg after the first 12 children receiving the 5 mg/kg dose had been followed for at least 5 days after treatment without the occurrence of dose‐limiting toxicity or serious adverse event. Comparator (N = 29): placebo (0.9% normal saline) |
|
| Outcomes |
|
|
| Notes | Members of the study group were employees of the manufacturer of the immunoglobulin used in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomized centrally 1:1..." (p. 707) Comment: although randomisation appeared to be independent of study investigators, the way the sequence was generated was not described. There was insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible patients were randomized 1:1 centrally by the investigator calling Pharmaceutical Products Development Inc. and obtaining the next available patient identification number with specified study drug." (p. 707) Comment: an adequate method was likely used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study drug was dispensed from the pharmacy in a blinded manner; i.e., the study drug assignment was not on the label" (p. 708) Comment: it is likely that participants and care providers were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Adverse events considered by the blinded investigator to be possibly related..." (p. 710) Comment: the study states that adverse events were classified by a blind investigator, however it is unclear whether clinical outcomes were reported by blind clinicians, and the risk of detection bias is unclear for these outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 60 children were randomized and 59 received the study drug. One child was randomized but did not receive any study drug because of a protocol violation: this patient was not included in any of the analyses... Overall 56 patients (95%) were followed through 30 days after study drug administration. One placebo patient died during RSV hospitalisation and 2 patients, one placebo and the other 15 mg/kg palivizumab were lost to follow‐up during the 30 day post treatment period" (p. 709) Comment: only 1 randomised participant was not included in the analysis for adverse events and clinical outcomes, and this was due to a protocol violation. Attrition in this study is unlikely to have an important impact on the observed results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome data are reported for all outcomes specified in the methods section of the publication. However, without a trial protocol it is unclear whether other outcomes were measured but not reported based on the nature of the results. |
| Other bias | Unclear risk | Quote: "We thank Barbara Shepherd, PhD of MedImmune, Inc. for assistance with preparation of the manuscript" (p. 712) Comment: the paper acknowledges an employee of MedImmune (palivizumab manufacturer) for assistance with preparation of the manuscript. A member of the MEDI‐493 Study Group is an employee of MedImmune. This may lead to bias in favour of the intervention group. |
ELISA: enzyme‐linked immunosorbent assay ICU: intensive care unit IFA: immunofluorescence assay IgG: immunoglobulin G IV: intravenous IVIG: intravenous immunoglobulins LRI: lower respiratory infection LRTI: lower respiratory tract infection N: number (of people) NaCl: normal saline PO₂: partial pressure of oxygen RCT: randomised controlled trial RNA: ribonucleic acid RSV: respiratory syncytial virus RSVIG: respiratory syncytial virus immunoglobulins SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AAP 1998 | Not an RCT (review) |
| Faber 2008 | Not an RCT (review) |
| Feltes 2011 | This study looked at prophylaxis, not treatment. |
| Fernández 2010 | This study looked at prophylaxis, not treatment. |
| Givner 1999 | Not an RCT (review) |
| Halsey 1997 | This study looked at prophylaxis, not treatment. |
| Harkensee 2006 | Not an RCT (review) |
| Helmink 2016 | This study did not randomise participants to palivizumab or control. |
| Hu 2010 | Not an RCT (review) |
| Wegzyn 2014 | Not an RCT (review) |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02442427.
| Trial name or title | Palivizumab therapy for RSV‐bronchiolitis |
| Methods | Randomised placebo‐controlled trial |
| Participants | Infants 3 years of age or younger presenting to an emergency department with acute bronchitis and positive RSV rapid antigen test |
| Interventions | Single‐dose intravenous palivizumab versus saline placebo comparator |
| Outcomes | Readmission during 3‐week follow‐up after discharge |
| Starting date | September 2014 |
| Contact information | Information provided by: Hamad Medical Corporation |
| Notes | Sponsor: Hamad Medical Corporation |
RSV: respiratory syncytial virus
Differences between protocol and review
New authors joined the review (SLS, SA, and MH).
We made several changes to the secondary outcomes specified in the protocol. We changed adverse effects of the treatments from being a secondary outcome in the protocol to a primary outcome in the review. The protocol also specified secondary outcomes related to the duration of ventilation and attendance in the intensive care unit. We included the secondary outcome "need for ventilation" in addition to the existing "duration of ventilation" outcome in the review. We included the secondary outcome "need for intensive care unit admission" and renamed the outcome "days admitted to the intensive care unit" to "duration of stay in the intensive care unit". We renamed the secondary outcome "oxygen dependence" to "need for supplemental oxygen" and "duration of supplemental oxygen". We added the secondary outcomes related to need for mechanical ventilation, supplemental oxygen, and intensive care unit admission, as we felt these were patient‐important outcomes. We also identified the limitation in duration‐related outcomes. Duration data were only available for those children requiring mechanical ventilation, supplemental oxygen, or intensive care admission. This means the comparisons between the interventions (immunoglobulins and placebo) were not randomised comparisons, and the outcomes are at risk of selection bias. The need for ventilation, supplemental oxygen, and intensive care unit admission outcomes are randomised comparisons.
We used GRADE to assess the certainty of the body of evidence and included a 'Summary of findings' table. We did not contact trial authors for missing trial information or unpublished studies as was intended when the protocol was written due to resource constraints.
Missing data due to losses to follow‐up or protocol deviation were minimal; we took missing data into account in the 'Risk of bias' assessment and did not apply any imputation measures as intended in the protocol. We expressed dichotomous outcomes as risk ratios rather than odds ratios as stated in the protocol for their ease of interpretation.
We were unable to conduct prespecified subgroup analysis due to lack of data.
Contributions of authors
Sharon L Sanders: screened searches, extracted data, assessed risk of bias, analysed results, and drafted the final review Sushil Agwan: extracted data, verified data entry, drafted sections of the final review, and reviewed draft Mohamed Hassan: extracted data, verified data entry, drafted sections of the final review, and reviewed draft Mieke L van Driel: screened searches, extracted data, assessed risk of bias, and reviewed draft Chris B Del Mar: screened searches, extracted data, assessed risk of bias, and reviewed draft
Sources of support
Internal sources
Cochrane Acute Respiratory Infections Review Group, Australia.
Centre for Research in Evidence Based Practice, Australia.
External sources
No external funding received, Other.
Declarations of interest
Sharon L Sanders: none known Sushil Agwan: none known Mohamed Hassan: none known Mieke L van Driel: none known Chris B Del Mar: none known
New
References
References to studies included in this review
Hemming 1987 {published data only}
- Hemming VG, Rodriguez W, Kim HW, Brandt CD, Parrott RH, Burch B, et al. Intravenous immunoglobulin treatment of respiratory syncytial virus infections in infants and young children. Antimicrobial Agents and Chemotherapy 1987;31(12):1882‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lagos 2009 {published data only}
- Lagos R, DeVincenzo JP, Muñoz A, Hultquist M, Suzich JA, Connor EM, et al. Safety and antiviral activity of motavizumab, a respiratory syncytial virus (RSV)–specific humanized monoclonal antibody, when administered to RSV‐infected children. Pediatric Infectious Disease Journal 2009;28(9):835‐7. [DOI: 10.1097/INF.0b013e3181a165e4] [DOI] [PubMed] [Google Scholar]
Malley 1998 {published data only}
- Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner C, Gruber WC, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. Journal of Infectious Diseases 1998;178(6):1555‐61. [DOI] [PubMed] [Google Scholar]
Ramilo 2014 {published data only}
- Ramilo O, Lagos R, Sáez‐Llorens X, Suzich J, Wang CK, Jensen KM, et al. Motavizumab Study Group. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatric Infectious Disease Journal 2014;33(7):703‐9. [DOI: 10.1097/INF.0000000000000240] [DOI] [PubMed] [Google Scholar]
Rodriguez 1997a {published data only}
- Rodriguez WJ, Gruber WC, Welliver RC, Groothuis JR, Simoes EAF, Meissner HC, et al. Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections. Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics 1997;99(3):454‐61. [DOI] [PubMed] [Google Scholar]
Rodriguez 1997b {published data only}
- Rodriguez WJ, Gruber WC, Groothuis JR, Simoes EAF, Rosas AJ, Lepow M, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics 1997;100(6):937‐42. [DOI] [PubMed] [Google Scholar]
Sáez‐Llorens 2004 {published data only}
- Sáez‐Llorens X, Moreno MT, Ramilo O, Sánchez PJ, Top FH Jr, Connor EM, MEDI‐493 Study Group. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatric Infectious Disease Journal 2004;23(8):707‐12. [DOI: 10.1097/01.inf.0000133165.85909.08] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AAP 1998 {published data only}
- Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV‐IGIV. American Academy of Pediatrics Committee on Infectious Diseases and Committee of Fetus and Newborn. Pediatrics 1998;102(5):1211‐6. [DOI] [PubMed] [Google Scholar]
Faber 2008 {published data only}
- Faber TE, Kimpen JL, Bont LJ. Respiratory syncytial virus bronchiolitis: prevention and treatment. Expert Opinion on Pharmacotherapy 2008;9(14):2451‐8. [DOI] [PubMed] [Google Scholar]
Feltes 2011 {published data only}
- Feltes TF, Sondheimer HM, Tulloh RM, Harris BS, Jensen KM, Losonsky GA, et al. Motavizumab Cardiac Study Group. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatric Research 2011;70(2):186‐91. [DOI] [PubMed] [Google Scholar]
Fernández 2010 {published data only}
- Fernández P, Trenholme A, Abarca K, Griffin MP, Hultquist M, Harris B, et al. Motavizumab Study Group. A phase 2, randomized, double‐blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatrics 2010;10:38. [DOI: 10.1186/1471-2431-10-38] [DOI] [PMC free article] [PubMed] [Google Scholar]
Givner 1999 {published data only}
- Givner LB. Monoclonal antibodies against respiratory syncytial virus. Pediatric Infectious Disease Journal 1999;18(6):541‐2. [DOI] [PubMed] [Google Scholar]
Halsey 1997 {published data only}
- Halsey NA, Abramson JS, Chesney PJ, Fisher MC, Gerber MA, Gromisch DS, et al. Respiratory syncytial virus immune globulin intravenous: indications for use. Pediatrics 1997;99(4):645‐50. [PubMed] [Google Scholar]
Harkensee 2006 {published data only}
- Harkensee C, Brodlie M, Embleton ND, Mckean M. Passive immunisation of preterm infants with palivizumab against RSV infection. Journal of Infection 2006;52(1):2‐8. [DOI] [PubMed] [Google Scholar]
Helmink 2016 {published data only}
- Helmink BJ, Ragsdale CE, Peterson EJ, Merkel KG. Comparison of intravenous palivizumab and standard of care for treatment of respiratory syncytial virus infection in mechanically ventilated pediatric patients. Journal of Pediatric Pharmacology and Therapeutics 2016;21(2):146‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2010 {published data only}
- Hu J, Robinson JL. Treatment of respiratory syncytial virus with palivizumab: a systematic review. World Journal of Pediatrics 2010;6(4):296‐300. [DOI] [PubMed] [Google Scholar]
Wegzyn 2014 {published data only}
- Wegzyn C, Toh LK, Notario G, Biquenet S, Unnebrink K, Park C, et al. Safety and effectiveness of palivizumab in children at high risk of serious disease due to respiratory syncytial virus infection: a systematic review. Infectious Diseases and Therapy 2014;3(2):133‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02442427 {published data only}
- NCT02442427. Palivizumab therapy for RSV‐bronchiolitis. clinicaltrials.gov/ct2/show/NCT02442427 (first received 13 May 2015).
Additional references
American Academy of Pediatrics 2014
- American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134(2):415‐520. [DOI] [PubMed] [Google Scholar]
American Academy of Pediatrics 2015
- American Academy of Pediatrics. Respiratory syncytial virus. In: Kimberlin DW, Brady MT, Jackson MA, Long SS editor(s). Red Book: 2015 Report of the Committee on Infectious Diseases. 30th Edition. Elk Grove Village, IL: American Academy of Pediatrics, 2015:667‐76. [Google Scholar]
American Academy of Pediatrics 2018
- American Academy of Pediatrics. Respiratory syncytial virus. In: Kimberlin DW, Brady MT, Jackson MA, Long SS editor(s). Red Book: 2018 Report of the Committee on Infectious Diseases. 1st Edition. Itasca, IL: American Academy of Pediatrics, 2018:682‐92. [Google Scholar]
Broadbent 2015
- Broadbent L, Groves H, Shields MD, Power UF. Respiratory syncytial virus, an ongoing medical dilemma: an expert commentary on respiratory syncytial virus prophylactic and therapeutic pharmaceuticals currently in clinical trials. Influenza and Other Respiratory Viruses 2015;9(4):169‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feltes 2003
- Feltes TF, Cablka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. Journal of Pediatrics 2003;143(4):532‐40. [DOI] [PubMed] [Google Scholar]
Fernandes 2013
- Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD004878.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadomski 2014
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001266.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime).
Greenough 2001
- Greenough A, Cox S, Alexander J, Lenney W, Turnbull F, Burgess S, et al. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Archives of Disease in Childhood 2001;85(6):463‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2017
- Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection and new options for prevention and treatment. Clinical Microbiology Reviews 2017;30:277‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groothuis 1993
- Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high‐risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. New England Journal of Medicine 1993;329(21):1524‐30. [DOI] [PubMed] [Google Scholar]
Hartling 2011
- Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003123.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jolles 2005
- Jolles S, Sewell WAC, Misbah SA. Clinical uses of intravenous immunoglobulin. Clinical and Experimental Immunology 2005;142:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langley 1997
- Langley JM, Wang EE, Law BJ, Stephens D, Boucher FD, Dobson S, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: a Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. Journal of Pediatrics 1997;131:113‐7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mayo Clinic 2017
- Mayo Clinic. Respiratory syncytial virus. www.mayoclinic.org/diseases‐conditions/respiratory‐syncytial‐virus/symptoms‐causes/syc‐20353098 (accessed prior to 9 January 2019).
Mejías 2005
- Mejías A, Chávez‐Bueno S, Ríos AM, Aten MF, Raynor B, Peromingo E, et al. Comparative effects of two neutralizing anti‐respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency. Antimicrobial Agents and Chemotherapy 2005;49(11):4700‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2010
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 2010;375(9725):1545‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oray‐Schrom 2003
- Oray‐Schrom P, Phoenix C, Martin D, Amoateng‐Adjepong Y. Sepsis workup in febrile infants 0‐90 days of age with respiratory syncytial virus infection. Pediatric Emergency Care 2003;19(5):314‐9. [DOI] [PubMed] [Google Scholar]
Paramore 2004
- Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus‐related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22:275‐84. [DOI] [PubMed] [Google Scholar]
PREVENT 1997
- PREVENT Study Group. Reduction of RSV hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 1997;99(1):93‐9. [DOI] [PubMed] [Google Scholar]
Ralston 2009
- Ralston S, Hill V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: a systematic review. Journal of Pediatrics 2009;155(5):728‐33. [DOI] [PubMed] [Google Scholar]
Resch 2017
- Resch B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Human Vaccines and Immunotherapeutics 2017;13(9):2138‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roche 2003
- Roche P, Lambert S, Spencer J. Surveillance of viral pathogens in Australia: respiratory syncytial virus. Communicable Diseases Intelligence 2003;27(1):117‐22. [DOI] [PubMed] [Google Scholar]
Rodriguez 1997
- Rodriguez WJ, Gruber WC, Groothuis JR, Simoes EA, Rosas AJ, Lepow M, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics 2007;100(6):937‐42. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shi 2017
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390(10098):946‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Synagis 2017
- Synagis. Highlights of prescribing information. www.azpicentral.com/synagis/synagis.pdf (accessed prior to 5 December 2018).
Turner 2014
- Turner TL, Kopp BT, Paul G, Landgrave LC, Hayes D, Thompson R. Respiratory syncytial virus: current and emerging treatment options. ClinicoEconomics and Outcome Research 2014;6:217‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2011
- Wang D, Bayliss S, Meads C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high‐risk infants and young children: a systematic review and additional economic modelling of subgroup analyses. Health Technology Assessment 2011;15(5):1‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2017
- Zhang L, Mendoza‐Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD006458.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zomer‐Kooijker 2014
- Zomer‐Kooijker K, Ent CK, Ermers MJ, Uiterwaal CS, Rovers MM, Bont LJ, RSV Corticosteroid Study Group. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLOS ONE 2014;9(1):e87162. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Fuller 2004
- Fuller H, Mar CB. Immunoglobulin treatment for respiratory syncytial virus infection. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004883] [DOI] [PubMed] [Google Scholar]
Fuller 2006
- Fuller H, Mar CB. Immunoglobulin treatment for respiratory syncytial virus infection. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004883.pub2] [DOI] [PubMed] [Google Scholar]
Kantharajah 2011
- Kantharajah M, Zaman FD, Samra D, Gunturu MN, Mar CB, Driel ML. Immunoglobulin treatment for respiratory syncytial virus infection. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009417] [DOI] [Google Scholar]
Tan 1998
- Tan D, Wang E, Ohlsson A. Immunoglobulin for treatment of respiratory syncytial virus. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000981] [DOI] [Google Scholar]


