Abstract
Halogenated organic compounds are pervasive in natural and built environments. Despite restrictions on the production of many of these compounds in most parts of the world through the Stockholm Convention on Persistent Organic Pollutants (POPs), many “legacy” compounds, including polychlorinated biphenyls (PCBs), are routinely detected in human tissues where they continue to pose significant health risks to highly exposed and susceptible populations. A major concern is developmental neurotoxicity, although impacts on neurodegenerative outcomes have also been noted. Here, we review human studies of prenatal and adult exposures to PCBs and describe the state of knowledge regarding outcomes across domains related to cognition (e.g., IQ, language, memory, learning), attention, behavioral regulation and executive function, and social behavior, including traits related to attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASD). We also review current understanding of molecular mechanisms underpinning these associations, with a focus on dopaminergic neurotransmission, thyroid hormone disruption, calcium dyshomeostasis, and oxidative stress. Finally, we briefly consider contemporary sources of organohalogens that may pose human health risks via mechanisms of neurotoxicity common to those ascribed to PCBs.
I. Introduction
Epidemiologic [6, 12, 166] and preclinical [161, 195] studies have identified the brain as a vulnerable target of polychlorinated biphenyls (PCBs). This review focuses on the state of the science regarding the neurotoxicity of PCBs at epidemiologic, neuropathologic, and molecular levels. Much of the scientific literature describes the developmental neurotoxicity of PCBs, which are arguably among the most extensively studied developmental neurotoxicants of the nonmetallic persistent organic pollutants (POPs). While our knowledge of how early or mid-life exposures to PCBs influence the aging brain is not as comprehensive, there is evidence linking PCBs to increased risk of neurodegenerative outcomes, and this literature is also addressed in this review.
PCBs are a chemically-related class (chemotype) within a vast number of POPs of diverse anthropogenic origins. Common to all POPs is their chemical stability in the environment, and their resistance to metabolic degradation. These properties underlie the ubiquitous environmental distribution and bioaccumulation of POPs in human tissues, which have raised global concerns about adverse health consequences of widespread human exposures [36, 43, 181]. These concerns have led to restrictions on the production of POPs imposed by many governments through the Stockholm Convention on POPs signed in 2001 and appended in 2008 and 2014 [168, 169]. Despite these regulatory efforts, “legacy” PCBs persist in the environment and in human tissue, where they are still routinely detected, thus continuing to pose significant health risks, particularly among susceptible populations.
Chemically, PCBs are a complex mixture of isomers or congeners that differ significantly in their structure and modes of action. PCB congeners have been heuristically divided into two categories based on structure: PCBs with biphenyl rings substituted with zero chlorines in the ortho position that assume a coplanar biphenyl orientation in solution, and PCBs with one to four chlorines in the ortho position that assume increasing degrees of noncoplanar biphenyl orientation (Figure 1). Of the 209 possible PCB congeners, 19 congeners are stable atropisomer or enantiomers, with chiral asymmetry about their biphenyl bond axes. PCBs can be hydroxylated or sulfonated metabolically, and each enantiomer and metabolite has potentially distinct interactions with biological targets, contributing to overall PCB neurotoxicity.
Figure 1:
Exemplary non-coplanar and coplanar polychlorinated biphenyls (PCBs) of anthropogenic origin. Coplanar PCBs are also referred to as dioxin-like PCBs because of their ability to binding the AhR with high affinity, a mechanism considered to contribute to cancer risk, whereas non-coplanar PCBs with one or more ortho chlorine substitution have negligible AhR activity and have been shown to be neurotoxic through other mechanisms.
Coplanar PCBs have been shown to mimic dioxin in that they bind with relatively high affinity to the aryl hydrocarbon receptor (AhR), which regulates the transcription of a large group of dioxin-responsive genes [106, 159]. Although low-level exposures to dioxin and dioxin-like PCBs have been associated with a number of adverse outcomes in several organ systems, especially liver, skin, and immune function [11, 126, 206], and are probably carcinogenic [110], there is less evidence from epidemiologic and preclinical studies that they are associated with neurotoxic outcomes. Recent preclinical studies have demonstrated that dioxin alters neuronal migration [100] and ultrasonic vocalization [101] in mice, albeit at doses higher than those required for induction of other pathological endpoints. But whether dioxin-like PCBs have similar effects on these neurodevelopmental endpoints has yet to be determined. There is also emerging data linking dioxin-like compounds to increased risk of neurodegenerative disease, specifically amyotrophic lateral sclerosis (ALS) [4]. The most prevalent pathology of ALS is the accumulation of phosphorylated insoluble aggregates of transactive response DNA binding protein 43 (TDP-43) in neurons. Dioxin-like compounds were shown to significantly increase TDP-43 in induced pluripotent stem cells and mouse brains via AhR-dependent mechanism(s) [4]. Whether environmentally-relevant levels of dioxin-like PCBs similarly induce TDP-43 expression in neural tissues, and whether this effect translates into increased ALS risk remains to be determined.
In contrast, noncoplanar PCBs, also referred to as nondioxin-like PCBs (NDL PCBs) have little to no binding affinity for AhR. However, there is extensive scientific evidence linking NDL PCBs to neurotoxic outcomes [148, 182]. Further, NDL PCBs are predominant not only in contemporary environmental samples, but also in breast milk, serum, and adipose tissue from wildlife and humans [9, 148]. Given that NDL PCBs constitute the predominant congener subclass comprising contemporary human exposures, and that the weight of evidence implicates NDL PCBs in neurotoxic sequelae, this review largely focuses on the neurotoxicity of NDL PCBs.
While PCBs are among the most extensively studied POP chemotype, other major POPs have been shown to have neuropathogenic potential, including organochlorine insecticides (e.g., DDT, cyclodienes, hexachlorocyclohexanes) and, more recently, polybrominated diphenyl ether flame retardants (PBDEs) and newly discovered organohalogens produced as disinfectant byproducts [97, 148, 226]. Figure 1 compares PCBs with chemotypes that have emerged more recently as POPs of human health concern because of their potential as neuromodulators and neurotoxicants. Interestingly, many brominated organics from anthropogenic sources, especially those released as water disinfection byproducts, closely mimic organohalogens naturally produced in marine environments that function as primary ecological signaling molecules and secondary deterrence and defense toxins [1].
In this review, we present a critical evaluation of the state of the science regarding the neurotoxicity of PCBs from both a neurodevelopmental and neurodegenerative standpoint. We also review what is known about the underlying mechanisms of PCB neurotoxicity, including alterations in biogenic amines (dopamine in particular), endocrine disruption, altered calcium signaling in neurons, and oxidative stress. Our understanding about PCB neurotoxicity is likely to provide broad insights regarding the neurotoxic potential of a number of chemically related organohalogens that are eliciting scientific and public attention as emerging concerns, and these chemically related organohalogens are briefly discussed in the closing statements.
II. Mechanisms of PCB neurotoxicity
While significant research effort has focused on understanding how PCBs interfere with neurodevelopment or promote neurodegeneration, the molecular mechanisms by which PCBs cause neurotoxicity, and whether all or only a subset of PCB congeners are neurotoxic, remain key questions in the field. Prevailing mechanistic hypotheses of PCB neurotoxicity include: (1) altered dopamine (DA) signaling; (2) disruption of thyroid hormone signaling; (3) perturbation of calcium intracellular Ca2+ dynamics; and (4) oxidative stress. The mechanistic studies described below have been highlighted because the PCB exposure paradigms that elicited changes in these important biological pathways are relevant to the PCB levels detected in humans and wildlife as well as the congener profiles detected in contemporary environmental and biological samples [9, 68, 148]. Importantly, these mechanisms have also been implicated in the neurotoxicity of several contemporary POPs with NDL structures, including brominated diphenyl ethers (PBDEs) [34, 97] and bisphenols [225].
II.a. Evidence that PCBs alter DA neurotransmission
In vitro studies using pheochromocytoma cells (PC12) exposed to technical PCB mixtures, e.g., the Aroclors, provided the first evidence that PCBs significantly reduce cellular DA concentrations [176]. Subsequent structure-activity relationship studies revealed that ortho-substituted NDL PCBs, but not coplanar dioxin-like congeners, significantly depleted cellular DA [176, 192]. These in vitro observations were corroborated in vivo in Macca mulatta (rhesus monkeys) orally exposed to Aroclor 1254 [171]. Analysis of striatal and substantia nigral concentrations of DA using high performance liquid chromatography with electrochemical detection demonstrated significantly reduced nigroneostriatal levels of DA in PCB-exposed subjects, an effect that persisted following termination of PCB exposure [172]. Studies using Aroclor 1016 (a mixture of lightly chlorinated NDL PCB congeners) in non-human primates also demonstrated significant reductions in nigroneostriatal DA concentrations, providing additional verification of in vitro studies demonstrating that NDL PCB congeners impair DA neurotransmission [173]. Similar observations were reported in adult male mice orally exposed to Aroclor 1254 for 4 weeks [112]. Experimental evidence suggests that DA depletion may result from reduced levels of tyrosine hydroxylase, the rate limiting enzyme in the formation of DA [28], or from inhibition of vesicular monoamine transporter (VMAT) or the plasma membrane dopamine transporter (DAT) [22, 122, 175]. However, evidence for direct molecular interactions of NDL PCBs with one or more of these targets remains weak.
II.b. Evidence that PCBs alter thyroid hormone (TH) signaling
Maternal or infant hypothyroidism is linked to significantly impaired neurodevelopment, including intellectual disability [141, 208]. The importance of decreased circulating TH, primarily measured as changes in levels of thyroxine (T4) in serum, has been widely debated as the primary mechanism of PCB neurotoxicity since it was initially proposed [210]. Many studies, performed in a variety of species, including humans, have demonstrated a negative association between PCB exposures and serum T4 [19, 62, 63, 89, 227]. Developmental exposure of rats to Aroclor 1254 was shown to cause hypothyroxinemia (decreased serum T4) coincident with hearing loss, and supplementation with T4 throughout development attenuated PCB-induced motor and auditory deficits [62]. However, cognitive effects of developmental PCB exposure do not appear to be mediated by thyroid hormone-dependent mechanisms. For example, rats developmentally exposed to Aroclor 1254 at levels sufficient to reduce serum T4 levels had no deficits in learning and memory assessed in the T-maze or the Morris water maze [223]. Conversely, rats developmentally exposed to Aroclor 1254 at levels that did not significantly decrease serum T3 or T4 levels exhibited poorer performance in the Morris water maze [218]. Two additional studies of individual PCB congeners also failed to support a role for TH deficits in cognitive and behavioral deficits linked to PCBs [133, 165]. Consistent with these preclinical data are recently published results from a prospective birth cohort, the Hokkaido study, which included 222 mother-neonate pairs and was designed to determine whether there was a relationship between specific hydroxylated PCB metabolites (OH-PCBs) and maternal/neonate serum thyroxine levels [81]. Unexpectedly, and in direct opposition to the hypothyroidism theory of PCB developmental neurotoxicity, the Hokkaido study indicated that maternal and/or neonatal exposure to specific hydroxylated PCB metabolites (OH-PCBs) were positively correlated with free thyroxine levels [81]. Results from another recently published prospective study performed in Denmark indicated no significant association between the placental levels of 35 PCB congeners and placental T4 or T3, although one PCB, PCB 81, was positively associated with reverse T3 (rT3) [119].
Further complicating the thyroid hormone disruption theory of PCB neurotoxicity, PCBs have been shown to have both antagonistic and agonistic effects on thyroid hormone receptors, depending on the congener and the dose [63, 133, 227]. Moreover, direct molecular interactions of PCBs with TH receptors have not been convincingly demonstrated. These inconsistencies across both human and rodent studies raise significant questions regarding an association between PCB effects on serum thyroid hormone levels and adverse neurodevelopmental outcomes.
II.c. Evidence that PCBs alter Ca2+ channel function and Ca2+-dependent signaling
The precise spatial and temporal patterning of cytoplasmic Ca2+ signals are critical for normal neurodevelopment, the establishment and refinement of neural networks, and the dynamic synaptic plasticity associated with many behaviors [7, 17, 104]. Diverse biochemical, biophysical and cellular approaches across many laboratories have consistently shown that NDL PCBs potently alter intracellular Ca2+ dynamics [148], and a number of studies have linked PCB-induced Ca2+ dyshomeostasis to specific neurodevelopmental deficits [182]. An early hypothesis was that developmental exposures to NDL PCBs caused cognitive and behavioral deficits by disrupting Ca2+-dependent PKC signaling [102, 220]. This hypothesis derived in part from observations that PCB disruption of Ca2+ fluctuations caused rapid redistribution of protein kinase C (PKC) isoforms in astrocytoma cells [194]. Further structure-activity relationship studies demonstrated that NDL PCBs [102, 220], but not DL PCBs [35], increased the translocation of PKC to the plasma membrane of cultured cerebellar neurons. In vivo studies demonstrated that developmental exposure to Aroclor 1254 changed the subcellular distribution of PKC isoforms in the brain in a complex region-specific manner [192]. However, whether influences on PKC signaling represent a primary mechanism driving the behavioral and cognitive impairments observed following developmental PCB exposure remains unanswered.
Subsequent studies focused on the primary molecular mechanism(s) responsible for PCB effects on intracellular Ca2+ dynamics. In vitro studies using pharmacological blockade of specific Ca2+ channels have shown that NDL PCBs increase extracellular Ca2+ entry into cells through a number of mechanisms, including activation of L-type voltage-sensitive Ca2+ channels and NMDA receptors [79, 130]. However, such changes are elicited only at high PCB concentrations (≥10 μM), which have been demonstrated to produce nonspecific changes in membrane fluidity [103]. NDL-PCBs have also been shown to facilitate the release of Ca2+ from intracellular stores through sensitization of two genetically related Ca2+ channels localized to the endoplasmic/sarcoplasmic reticulum (ER/SR): ryanodine receptors (RyR) [211–214] and inositol 1,4,5-trisphosphate receptors (IP3R) [78]. Of these, RyR sensitization is more sensitive to NDL PCBs, and this interaction has been shown to exhibit a consistent stringent structure–activity relationship, including stereoselectivity, as determined using biochemical, electrophysiological, cellular and in vivo approaches [47, 53, 54, 72, 73, 136, 148, 217]. Nanomolar PCB concentrations directly interact with RyR channels to stabilize RyR channels in their full open conformation [164], which sensitizes ER/SR Ca2+ signals to physiological modulators including Ca2+, Mg2+, and ATP.
Sensitization of RyRs by NDL PCBs alters the fidelity of spontaneous Ca2+ oscillations [203] and the neuroplasticity of hippocampal CA1 neurons [212] in vitro. Consistent with this observation, picomolar to nanomolar concentrations of NDL PCB 95, which is among the most RyR active congeners, activate two Ca2+-dependent signaling pathways in cultured rat hippocampal neurons: (1) sequential activation of CaMKK, CaMKIα/γ, and MEK/ERK and CREB to increase transcription of Wnt2 [203]; and (2) CREB-mediated miR132 upregulation, which suppresses the translation of p250GAP [117]. In cultured rat hippocampal neurons, the former signaling pathway mediates PCB 95-induced dendritic growth [203], whereas the latter mediates PCB 95-induced synaptogenesis, which is evident as increased spine density and increased frequency of miniature excitatory post-synaptic currents [117]. Several lines of evidence indicate that PCB effects on RyR activity are causally linked to dendritic growth and spine formation: (1) RyR-active PCB congeners, such as PCB 95 and PCB 136, but not PCB congeners that lack activity at the RyR, such as PCB 66, enhance dendritic growth and promote spine formation in cultured hippocampal and cortical neurons; and (2) pharmacological blockade or siRNA knockdown of RyRs inhibit the dendrite and spine promoting activity of these NDL PCBs [117, 204]. An interesting observation from these studies is that PCB-induced dendritic growth exhibits a non-monotonic concentration-effect relationship, with effects observed in the pico- to nanomolar range but not at femto- or micromolar concentrations [204, 217]. Elucidating the mechanisms responsible for this non-monotonic concentration-effect relationship remains a critical data gap in the field.
These observations suggest that PCB effects on dendritic growth contribute to PCB-induced behavioral deficits. In support of this possibility, developmental exposure of rats to Aroclor 1254 at 1, but not 6, mg kg−1 in the maternal diet was found to cause learning and memory deficits in the Morris water maze, and this dose of Aroclor 1254 also modulated RyR activity and dendritic arborization in brain regions that subserve Morris water maze behavior [218]. Several interesting findings emerged from this study. First, developmental PCB exposure promoted dendritic growth in cerebellar Purkinje cells and neocortical pyramidal neurons among untrained animals but attenuated or reversed experience-dependent dendritic growth in these brain regions among Morris water maze-trained littermates. Second, deficits in learning and memory were only seen in the 1 mg kg−1 group, and the most robust changes in dendritic arborization were also observed in this dose group, replicating the non-monotonic dose-response relationship observed in the in vitro studies. Third, this study demonstrated that the behavioral deficits correlated better with changes in RyR expression and activity than with changes in serum levels of thyroid hormone or sex hormones. In a separate study, a similar non-monotonic dose-response relationship was observed for dendritic arborization of CA1 pyramidal neurons in the hippocampus of rats exposed to PCB 95 in the maternal diet throughout gestation and lactation [204]. Developmental exposure to PCB 95 has also been shown to dramatically alter the tonotopy of the primary auditory cortex (A1) [92], presumably by tipping the balance of excitatory-inhibitory currents within A1 towards excitation during critical periods of its development [92, 98, 99, 212]. While these data strongly support the hypothesis that PCB effects on RyR activity contribute to behavioral deficits via modulation of calcium-dependent signaling pathways that regulate neuroplasticity, experimental evidence demonstrating that the molecular and cellular effects are causally linked to behavioral deficits are lacking. Testing this hypothesis using RyR knockout animals is not feasible because of embryo lethality, but alternative approaches using knock-in animals expressing human gain-of-function mutations that enhance RyR sensitization by organohalogens [44, 222] may increase confidence in causal inferences.
Although the current data support a primary mechanistic contribution of RyRs to the developmental neurotoxicity of NDL PCBs, their possible role in the induction and progression of neurodegenerative diseases, such as Parkinson’s disease (PD), is less clear. Although Ca2+ dyshomeostasis induced by ER stress is an important molecular mechanism of selective loss of dopaminergic (DA) neurons in PD, an etiological role for RyR channels has only recently received experimental attention. Using a 6-hydroxydopamine (6-OHDA)-induced in vitro SN4741 cell model of PD, Huang et al [76] showed that 6-OHDA significantly increased cytoplasmic Ca2+ levels, an effect that could be blocked by either an ER stress inhibitor or specific RyR blocker. The authors further demonstrated that 6-OHDA reduced the spike number and rheobase of DA neurons in acute brain slices, effects that were also reversed by RyR blockade. Furthermore, novel 6-aminoquinoxaline derivatives appear to confer neuroprotective effects on DA neurons in cellular animal models of PD that are at least partially mediated through interactions with RyRs [111]. Interestingly, blocking Ca2+ influx did not change PCB effects on intracellular dopamine levels in a catecholaminergic cell line [88], suggesting that PCBs may be more likely affecting dopamine homeostasis by mediating dysfunction of ER Ca2+ stores. Clearly this area needs more attention before inferences can be made about convergent mechanisms linking PCB neurotoxicity across life stages.
II.d. Evidence of PCB-induced oxidative stress
Oxidative stress occurs when the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) exceeds the antioxidant capacity of the system [8, 93, 139]. This imbalance can cause oxidative and nitrative damage to macromolecules, which in turn can cause cell damage or even cell death. Numerous in vitro studies have demonstrated that PCBs cause oxidative stress in neurons [35, 75, 123]. In cultured cerebellar granule neurons, exposure to the NDL congeners, PCB 4 or 153, or to Aroclor 1254, but not to dioxin-like PCBs, increased cellular ROS levels, which caused concentration-dependent cell death [35]. Similarly, Aroclor 1254 or the NDL PCB 47, but not the dioxin-like PCB 77, triggered neuronal cell apoptosis in primary hippocampal neurons that was blocked by co-exposure to the antioxidant alpha-tocopherol [75], suggesting that NDL PCBs induced apoptosis via increased ROS. Interestingly, this same study demonstrated that apoptosis induced by PCB 47 was also prevented by pharmacologic blockade of RyRs [75]. Whether and how RyRs and ROS interact to mediate PCB-induced apoptosis has yet to be determined. One possibility is that ROS is a consequence of RyR activation. As discussed above (Section II.c.), NDL PCBs stabilize RyRs in the open conductance state, which increases release of Ca2+ from the ER [148]. Elevated intracellular Ca2+ can trigger apoptosis either by directly activating caspases in neuronal cells and/or by increasing Ca2+ flux into mitochondria, thereby increasing ROS production, which triggers release of cytochrome c from mitochondria with subsequent activation of caspases [115]. Alternatively, ROS can act as signaling molecules that initiate apoptosis via targeted interactions with specific cellular components, one of which is the RyR [23, 146, 148]. ROS have been shown to interact directly with hyperreactive cysteine residues on the RyR to enhance its open probability [45, 138, 190, 216]. This suggests an alternative model of PCB-induced apoptotic signaling in which NDL PCBs activate RyRs indirectly as a consequence of PCB-induced increase in ROS [75]. There is precedence for this proposed mechanism in that ROS generated by quinone have been shown to increase the calcium conductance of RyR from rabbit sarcoplasmic reticulum membranes. In fact, RyR channels are tightly regulated by changes in the ER/SR membrane redox potential [45, 216] and are highly sensitive to modification by redox active environmental chemicals [46], providing a possible mechanistic link between Ca2+ dysregulation and oxidative stress. These may not be mutually exclusive models in that PCBs may independently influence both RyR activation and ROS generation, with each effect augmenting the other in a positive feedback mechanism.
There is conflicting evidence as to whether PCBs cause oxidative stress in the brain in vivo. Oral exposure of adult male mice to a relatively high dose (25 mg/kg/d) of Aroclor 1254 for 4 weeks was associated with increases in superoxide dismutase (SOD), heme oxygenase (HO-1), lipid peroxides, and protein carbonyl levels in the striatum and cerebellum [112]. In rats exposed throughout gestation and lactation to environmentally relevant low doses of Aroclor 1254 in the maternal diet (0.1 or 1.0 mg/kg/d), multiple brain regions showed increased expression of biomarkers of oxidative stress [219]. In contrast, a study of mice exposed to a mixture of six NDL PCBs via lactation failed to find any evidence of oxidative damage in the brain [39]. The discrepancy between these studies may be due to differences in species, timing and length of PCB exposure, dose and composition of PCB mixtures to which animals were exposed, as well as the use of different biomarkers to measure oxidative stress. Collectively, the available studies suggest that oxidative stress may contribute to the developmental neurotoxicity and neurodegenerative toxicity associated with NDL PCBs, potentially via RyR-dependent mechanisms.
III. The neuropathologic effects of PCBs
We performed a literature search using PubMed (searched December 2018) to identify human and animal studies of neuropathologic effects of PCBs. We used the following search terms: “polychlorinated biphenyls”, “PCBs but not printed circuit boards”, “neuropathology”, “neurophysiology”, and “neuroimaging”. Our search yielded seven unique articles, of which three were reviews [42, 120, 131, 152, 155, 174,184]. Inspection of the references cited in these three reviews yielded an additional two articles describing the neuropathology of PCBs. These articles, as well as other relevant publications describing PCB-induced effects on neuronal cytoarchitecture in vivo are discussed in this section.
III.a. The neuropathology of PCB developmental neurotoxicity
Currently, there is a very limited literature regarding pathologic effects of PCBs in the developing human brain. A pilot study that conducted magnetic resonance imaging (MRI) scans on 60 children age 4 years found stronger associations of PCBs with response inhibition among participants with smaller splenium size [184]. This indicates that children with suboptimal development of the splenium could be particularly vulnerable to exposure to PCBs, increasing their risk for poorer response inhibition, a core trait of attention-deficit hyperactivity disorder (ADHD). Another pilot study administered functional magnetic resonance imaging (fMRI) to twelve adolescent boys from a Faroese birth cohort assembled in 1986–1987 [207]. Boys with high exposure to both PCBs and methylmercury (MeHg) showed more activation across brain regions during visual and motor tasks than boys with lower mixed exposures to PCBs and MeHg. In a separate cohort of Inuit from Nunavik (Northern Québec) [40], cord blood concentrations of methylmercury or lead, but not PCBs, were associated with pattern-reversal visual evoked potentials (VEPs) recorded at the occipital cortex. Prenatal exposure to lead was associated with a delay of the N150 latency at most contrast levels. This study suggests that heavy metal exposure, in particular during the gestational period, but not PCB exposure, can impair the development of visual processing. Whether early-life exposures negatively impact healthy brain aging later in life [170] remains unanswered.
The earliest neuropathologic studies of developmental exposures to PCBs in preclinical models utilized very high PCB levels intended to mimic the exposures associated with the accidental mass poisonings due to ingestion of PCB-contaminated cooking oil in Japan in 1968 and Taiwan in 1979 [158]. In one of these studies [29], pregnant CD-1 mice were dosed orally with the dioxin-like PCB 77 at 32 mg/kg/d on days 10 through 16 of gestation. This exposure paradigm caused significant maternal toxicity as evident by significantly smaller litter sizes (60% of control) and higher neonatal mortality (40% at postnatal day 20). In approximately 55% of surviving offspring from PCB-exposed dams, a permanent motor disturbance, referred to as “spinning syndrome”, developed at weaning. Spinning syndrome was characterized by repetitive jerking of the head, hyperkinesia, and swift stereotyped circular or spinning movement, described as “chasing one’s tail”.
From over 100 spinners resulting from 60 PCB-exposed litters, 24 spinners ranging in age from 4 to 34 weeks along with age-matched controls and 12 littermates who did not develop spinning syndrome (nonspinners) were subjected to pathologic studies [29]. Interestingly, no detectable neuronal changes, gliotic foci or demyelinating lesions were detected in the CNS of either PCB-exposed spinners or nonspinners by light microscopy. However, cylindrical CNS peninsulas (CCPs) that projected into the cranial and spinal nerve roots were noted in all spinners and some nonspinners, but none of the control subjects. The CCPs were characterized by a cylindrical column of nerve fibers, myelinated or nonmyelinated, and/or glial bundles in varying proportions, which were well-circumscribed and delineated from the neighboring PNS-type nerve fibers. The diameter and length of CCPs varied considerably up to 100 μm in diameter and 5 mm in length. They were seen predominantly in the ventral roots, and to a lesser extent in the dorsal spinal roots, and most abundantly at lumbar levels. Large, apparently displaced, motor neurons were often detected within CCPs. Electron microscopy revealed that the CCPs in both the ventral and dorsal spinal roots were ensheathed in a glycocalyx layer that was indistinguishable from the basement membrane; and while collagen fibrils invested the outer surface of the CCPs, collagen fibrils were not observed within the CCPs. The myelinated fibers within the CCPs were distinguished by the lack of an outer basement membrane, the presence of oligodendroglia and shorter periodicity of myelin lamellae, all characteristics typical of CNS myelin. Signs of myelin sheath degeneration in the absence of macrophage infiltration were very common in CCPs, but not in the PNS-type myelinated fibers surrounding the CCP. All the cellular elements of the CNS (neurons, astroglial and oligodendroglia) were observed within CCPs. Some of the heterotopic neurons appeared normal with well-preserved terminal boutons detected along the cell surfaces. Except for frequent distal displacement in the ventral white matter, ventral horn motor neurons showed no ultrastructural changes suggestive of degenerative process in the perikaryon; however, along the cell surfaces, the nerve terminals appeared dense, amorphous and shrunken.
The authors noted that the glial outgrowth observed in the CCPs in the spinner mice was similar to that reported in pig spinal roots following transection and anastomosis but concluded that mechanical injury was unlikely to be the cause of CCP development in the spinner mice [29]. Rather, the neuropathology induced by developmental exposure to very high levels of PCB 77 appeared remarkably similar to that observed in Werdnig-Hoffman disease. The authors concluded that PCB 77 interfered with the organization rather than the differentiation of neurons and glia since no histopathologic abnormalities could be detected in the CNS of mice exposed developmentally to PCB 77. Specifically, the authors conjectured that PCB 77 selectively interfered with synaptogenesis of inhibitory dopaminergic terminals, and that PCB exposure may be relevant to the high incidence of aberrant motor development in hyperactive boys [29]. This hypothesis derived in part from pharmacologic observations in this study that, like hyperactivity in children, amphetamine was effective in attenuating spinning behavior in the spinner mice [29].
A later histopathologic study of Sprague-Dawley rats exposed to a more moderate dose of PCBs similarly concluded that PCBs did not cause overt damage to the developing brain, but rather PCBs interfered with the organization of neural networks [152]. In this study, pregnant Sprague-Dawley rats were fed chow containing Aroclor 1254 at 125 ppm throughout gestation and lactation. Upon weaning, offspring were maintained on chow containing 125 ppm Aroclor 1254 until euthanized for histological processing at 16, 30 and 60 days of age. Using Timm’s silver sulfide staining to visualize the hippocampal mossy fibers, the authors demonstrated that continuous exposure to Aroclor 1254 from conception significantly reduced the relative size of IIP mossy fibers in 16-, 30- and 60-day old rats. In contrast, developmental exposure to Aroclor 1254 had no effect on the size of the hilar or suprapyramidal mossy fibers or on cortical thickness. The authors speculated that PCBs decreased the growth of mossy fibers as a result of increased glutamate or delayed myelination secondary to PCB-induced hypothyroidism or as a result of altered Ca2+ homeostasis [152]. However, none of these postulated mechanisms explains the selective effect of Aroclor 1254 on a subpopulation of granule cells that give rise to the II-P mossy fibers.
Subsequent preclinical studies of neuropathologic changes induced by developmental exposures to more environmentally relevant doses of PCBs further support the hypothesis that PCB-induced functional deficits reflect subtle organizational and/or functional defects of key neural networks of the brain [58, 170, 182]. Classic histopathologic analyses of brains from weanling rats exposed to Aroclor 1254 at 1 or 6 mg/kg/d in the maternal diet throughout gestation and lactation did not detect overt structural damage in the brain [218]. However, Golgi analyses of rat CA1 hippocampal pyramidal neurons and cerebellar Purkinge cells indicated that exposure to Aroclor 1254 at 6 mg/kg/d in the maternal diet throughout gestation and lactation caused a pronounced age-related increase in dendritic growth. Thus, even though dendritic lengths were significantly attenuated in PCB-exposed animals at postnatal day 22, by postnatal day 60, dendritic growth was comparable to or exceeded that observed in vehicle controls [116]. A subsequent study of postnatal day 31 rats [218] demonstrated that developmental Aroclor 1254 exposure increased dendritic arborization of cerebellar Purkinje cells and neocortical pyramidal neurons in animals not trained in a Morris water maze but caused dose-dependent dendritic retraction in these same neuronal cell types in littermates trained in the Morris water maze. These dendritic effects were significantly more pronounced in the animals exposed to Aroclor 1254 at 1 mg/kg/d compared to 6 mg/kg/d in the maternal diet, and the lower dose, but not the higher dose of Aroclor 1254 elicited deficits in spatial learning and memory in the Morris water maze [218]. Interestingly, postmortem examinations of brains from Autism Spectrum Disorder (ASD) patients have revealed increased neuropil, which is comprised of dendrites, non-myelinated axons, synapses, vasculature and glial cell processes, in cortical regions [196]. Morphometric analyses of pyramidal neurons in various cortical regions altered in ASD, has revealed increased dendritic complexity, including increased dendritic spines [77, 80], on projection neurons. While studies from the 1990s indicated less complex dendritic arborization in the hippocampus of subjects with ASD [196], more recent studies of genetic mouse models of ASD have identified increased dendritic complexity of hippocampal neurons [27, 86, 91].
Another pathologic feature associated with ASD is an imbalance of excitatory to inhibitory neural circuits [61]. Kenet et al., [92] reported that rats developmentally exposed to NDL PCB 95 at 6 mg/kg/d in the maternal diet during gestation and lactation exhibited profoundly abnormal development of the primary auditory cortex (A1) without impairment of hearing sensitivity and brainstem auditory responses of pups. A1 was irregularly shaped and marked by internal nonresponsive zones, with grossly abnormal or reversed topographic organization in about half of the exposed pups. Changes in A1 network organization occurred concomitantly with changes in the balance of neuronal inhibition to excitation for A1 neurons, with significant alterations in the critical period plasticity that underlies normal postnatal auditory system development [92].
Histologic studies in preclinical models of PCB developmental neurotoxicity have also detected increased apoptosis in the brains of rat pups exposed to Aroclor 1254 at 0.1 or 1.0 mg/kg/d in the maternal diet throughout gestation and lactation [219]. TUNEL staining of sections from the cortex, hippocampus and cerebellum revealed significantly increased apoptosis in all three brain regions, with the most pronounced effect in the cerebellum, at postnatal day 1 but not postnatal day 21 [219]. These findings were confirmed by assays of caspase 3 activity. Histologic evidence of increased apoptosis coincided spatiotemporally with biochemical evidence of oxidative stress, specifically increased levels of 4-HNE and 3-NT as detected by western blotting [219].
Collectively, the available neuropathologic data suggest that neither overtly toxic levels of PCBs nor more environmentally relevant levels of PCBs cause major structural changes in the developing brain. Rather, developmental exposures to PCBs interfere with the organization of neural networks, evident by CCPs in spinal roots in response to very high PCB levels, or by changes in axonal and dendritic morphology or altered patterns of apoptosis elicited by more environmentally relevant PCB exposures.
III.b. The neuropathology of PCB-induced neurodegeneration
A [123Iodine] 2β-carbomethoxy-3β-[4-iodophenyl) tropane] single-photon emission computed tomography ([123I] β-CIT SPECT) imaging study of former capacitor workers with documented PCB exposures found that relative to non-exposed controls, individuals with occupational exposure to PCBs had significantly decreased striatal dopamine terminal densities [174], suggesting loss of dopaminergic neurons. Interestingly, this association was sex-specific. After statistical correction for a number of confounders, including age, only women show a significant inverse relationship between lipid-adjusted total serum PCB concentrations and β-CIT densities in the caudate, putamen or combined caudate and putamen, despite the fact that neither serum PCB levels nor age was statistically different between men and women. In a separate cohort of women participating in the Nun Study who had no clinical signs or symptoms of PD at death, elevated levels of NDL PCBs 138, 153, and 180 were observed in brain tissue that exhibited moderate nigral depigmentation compared to subjects with mild or no depigmentation [69].
Preclinical studies of neuropathologic changes in adult subjects exposed to PCBs at levels approximating the high end of occupational exposures are consistent with the limited neuropathologic findings in humans. As discussed above (section II.a.), studies in non-human primates demonstrated that long-term exposure to PCBs significantly reduced basal ganglia DA concentrations [171, 172]. To determine whether subchronic PCB exposure would cause cell loss in midbrain DAergic systems, ventral midbrain neurons were quantified by stereologic analyses at 2 weeks after a month-long exposure of adult male mice to Aroclor 1254 at 6, 12 and 25 mg/kg/d [112]. Moderate to high doses of Aroclor 1254 elicited death of both tyrosine hydroxylase (TH) immunopositive and TH immunonegative neurons in the ventral tegmental area. In the substantia nigra, the low and moderate doses of Aroclor 1254 significantly reduced only TH immunopositive cells, while the highest dose significantly decreased both TH immunopositive and TH immunonegative cells. The loss of DAergic and non-DAergic neurons correlated with increased levels of oxidative stress biomarkers in these same brain regions [112]. However, PCB exposure caused persistent increases in locomotor activity that correlated with dose-dependent increases in DA levels in the ventral midbrain. The authors interpreted these paradoxical data as suggesting the existence of compensatory mechanisms to maintain DA homeostasis in the face of decreased numbers of DAergic neurons [112].
Collectively, the limited literature available regarding the neuropathologic effects of PCBs on the aging brain suggest that PCBs promote the loss of DAergic and, at high exposure levels, non-DAergic neurons, within brain regions critically involved in motor function. Whether PCBs promote neuronal cell loss in other regions of the adult brain remains an open question.
IV. Epidemiological evidence of PCB neurotoxicity
IV.a. PCB developmental neurotoxicity
Accidental mass poisonings due to ingestion of PCB-contaminated cooking oil in Japan in 1968 and Taiwan in 1979 [158], resulted in a number of clinical morbidities, including adverse neurodevelopment in response to prenatal exposure [25, 26]. These incidents were the basis for classifying PCBs and other organochlorine compounds as suspected developmental neurotoxicants. The epidemiologic literature on lower-level PCB exposure, mostly via diet (e.g., fish, meat and dairy) [108, 121], and neurodevelopment is among the richest in environmental neuroepidemiology. Results of these studies, while generally supportive of an adverse impact of PCBs on the developing brain, have been mixed, with differences based on methodologic considerations, such as study design (prospective vs. cross-sectional), window of exposure (e.g., prenatal vs. postnatal), neurobehavioral domain (e.g., language, memory, executive function, inhibitory control) and age of neuropsychological assessment. PCBs that bioaccumulate in maternal tissues efficiently cross the placenta during pregnancy and are transferred postnatally through breast milk. This makes them a particular concern for the developing central nervous system, which undergoes rapid development during gestation and early life. Here, we review a subset of the available literature on PCBs and neurodevelopment with the aim of focusing on studies with more statistical power and methodologic rigor. To this end, we reviewed the state of the literature to date on prospective epidemiologic studies of prenatal PCB exposure measured in biospecimens collected during pregnancy and neurodevelopment across cognitive and behavioral endpoints in children age 3 years or older.
Selection of studies for review
We performed a literature search using PubMed (searched June 2018) to identify human studies of prenatal exposure to PCBs and neurodevelopmental outcomes across domains related to cognition (e.g., IQ, language, memory, learning), attention, behavioral regulation and executive function, and social behavior, including traits related to attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASD). We used the following search terms: “polychlorinated biphenyls”, “PCBs”, “organochlorines”, “prenatal”, “pregnancy”, “neurodevelopment”, “attention, “cognition”, “behavior”, “impulsivity”, “hyperactivity”, “attention deficit hyperactivity disorder”, and “autism”. We also inspected references of previously published review papers on PCBs and neurodevelopment [5, 41, 166, 210] to confirm that we identified all papers that met our criteria (detailed below). We limited our search to papers published in English-language journals after 1990.
For this review, we included a select body of literature to identify studies more robust in methodologic rigor and study power. Our inclusion criteria were: 1) studies that included analysis sample sizes of 100 or more participants, to focus on studies with more statistical precision; 2) prospective cohort studies that measured PCB exposure in the prenatal period, as neurodevelopment is generally recognized to be most sensitive to environmental insults during this period [64]; 3) studies that measured PCB exposure in biospecimens, primarily serum or plasma from blood collected from the mother during pregnancy or at delivery, including from the umbilical cord and placenta; and 4) studies of children 3 years of age or older, as neurodevelopmental outcomes can be measured more reliably in older children.
Review Findings
We identified 29 papers that met our inclusion criteria. These papers represented 14 different prospective cohort studies. Geographically, these studies were diverse and included countries in North America (Canada, Greenland and the U.S.), Europe (Belgium, Denmark and the Faroe Islands, Germany, the Netherlands, Spain, and Ukraine), and Asia (Japan). Studies were also diverse with respect to cohort birth year and, therefore, pregnancy PCB concentrations, which have been slowly decreasing since PCBs were banned. Studies most often presented findings for the sum of PCB congeners, though the number of congeners included and the limit of detection for these individual congeners varied widely.
Prenatal PCB exposure and child cognition
We identified 12 papers from 9 different cohort studies that examined associations of PCBs with cognitive function among children ranging from ages 3 to 11 years. As shown in Table 1, most studies conducted tests of general intelligence (most commonly the Wechsler Intelligence Scale for Children, Kaufman Assessment Battery for Children and McCarthy Scales of Children’s Abilities), though a number also looked at specific cognitive domains, such as language and memory. Among these 12 papers, most (8) found associations of PCBs with at least one aspect of cognition, including poorer scores on overall intelligence [51, 82, 144, 186, 187], language [65], processing speed [85], short-term memory [189], and reading comprehension [82]. Four papers report null effects of PCBs on all reported measures of cognition [67, 142, 201, 223]. In addition, two studies which found associations of PCBs with IQ at age 3 years [144, 187], found that these associations attenuated to the null at slightly older ages (ages 4 and 6 years) [187, 201]; though, for one of the studies, associations re-emerged when children were older (age 9) [186].
Table 1.
Prenatal PCBs and cognitive function.
| Location | Reference | Sample size | PCB exposure matrix (congeners) | Median/mean concentrations | Outcome measure | Age at assessment | Study results |
|---|---|---|---|---|---|---|---|
| Denmark (Faroe Islands) | Grandjean et a. 2001 [65] | 435 | cord blood ΣPCBs (118, 138, 153, 170, 180) | Mean=1.88 ug/L wet | NES-2 Finger Tapping, Hand-Eye Coordination; BVMGa; WISC-Ra Digit Span, Similarities, Block Design; CVLTa; BNTa | 7y | ↓ BNT |
| Japan (Tohoku) | Tatsuta et al. 2014 [191] | 387 | cord blood ΣPCBs | Mean=46.5 ng/g lipid | K-ABCa | 3y | ↓ sequential and mental processing scores with highly chlorinated PCBs |
| Netherlands (Rotterdam and Groningen) | Patandin et al. 1999 [144] | 395 | maternal plasma in last month of pregnancy (n=415) and cord (n=373) ΣPCBs (118,138,153,180) | Median=2.04 ug/L in maternal plasma and 0.38 ug/L in cord wet | K-ABCa (n=395), RDLSa (n=193) | 3y | ↓ overall cognitive and processing on K-ABC, ↓ verbal comprehension on RDLS |
| Vreugdenhil et al. 2002 [201] | 372 | maternal plasma in last month of pregnancy and cord ΣPCBs (118,138,153,180) | Median=2.04 ug/L in maternal plasma and 0.38 ug/L in cord wet | MCSAa | 6y | ∅ (though possible effect modification by parental/home characteristics) | |
| Spain (Menorca) | Forns et al. 2012 [51] | 405 | cord serum ΣPCBs (118,138,153,180) | Median=0.71 ng/ml wet | MCSAa | 11y | ↓ MCSA scores, especially for PCB 153 |
| United States (Collaborative Perinatal Project: 12 states) | Gray et al. 2005 [67] | 894 | maternal serum ΣPCBs (28,52,74,105,118,138,153,170,180,194,203) | Median-2.85 ug/L wet | WISCa, WRAT-Ra | 7y | ∅ |
| United States (Cincinnati, Ohio) | Zhang et al. 2017 [224] | 239 | maternal serum ΣPCBs (118,138,153,158,180) | Median=31.3 ng/g lipid | WJ-IIIa (5y), WRAT- 4a (8y), WISV-IV (8y) | 5–8y | ∅ |
| United States (Michigan) | Jacobson et al. 1992 [85] | 226 | cord serum ΣPCBs (70% non detects) | Mean=0.4 ng/mL ng/mL serum | MCSA*, Sternberg (memory), Kagan’s (visual discrimination), vigilance task | 4y | ↓ visual discrimination, ↑errors in short term memory, ∅ sustained attention |
| Jacobson et al. 1996 [82] | 212 | cord serum ΣPCBs (118,138,153,180) | Mean=3 ng/ml serum | WISC-Ra, WRAT-Ra, WRMT-Ra | 11y | ↓ Full Scale, ↓ Verbal IQ, ↓ Freedom from Distractibility, ↓ word/reading comprehension | |
| United States (New Bedford, Massachusetts) | Orenstein et al. 2014 [142] | 393 | cord serum ΣPCBs | Median=0.3 ng/g serum | WRAMLa | 8y | ∅ |
| United States (Oswego, New York) | Stewart et al. 2003 [187] | 293 | cord blood ΣPCBs | Median=0.52 ng/g wet | MSCAa | 3y, 4y | ↓ MCSA scores at 3y but not 4y |
| Stewart et al. 2008 [186] | 189 | placental ΣPCBs | Median=1.5 ng/g wet | WISC-IIIa | 9y | ↓ Full Scale and Verbal IQ |
BNT=Boston Naming Test, BVMG=Bender Visual Motor Gestalt; CVLT=California Verbal Learning Test; K-ABC=Kaufman Assessment Battery for Children; MSCA=McCarthy Scales of Children’s Abilities; RLDS=Reynell Language Development Scales; WISC-R=Wechsler Intelligence Scale for Children-Revised; WISC-III=Wechsler Intelligence Scale for Children-Third Edition; WISC-IV=Wechsler Intelligence Scale for Children-Fourth Edition; WJ-III=Woodcock-Johnson III Tests of Achievement; WRAT-R=Wide Range Achievement Test-Revised; WRAT-4=Wide Range Achievement Test-Fourth Edition; WRAML=Wide Range Assessment of Memory and Learning; WRMT-R=Woodcock Reading Mastery Tests-Revised
Prenatal PCB exposure and child attention, behavioral regulation and social behavior
Table 2 summarizes results of 17 studies from 11 different cohorts that examined associations of prenatal PCBs with child attention, behavioral regulation and social behavior among children ranging mostly from ages 3 to 12 years (one study included participants up to age 22 years [189]). The majority of studies (10 of the 17) reported PCB-related associations with traits related to ADHD, including problems with impulse control [14, 83, 163, 184, 185, 188], hyperactivity [160, 163], and attention [51, 65, 83, 134, 198, 162, 163]. However, one of these studies, which reported more omission errors (indicating poorer attention) on a continuous performance test, also reported the opposite result when looking at parent-reported behaviors, with PCBs related to fewer parent-reported ADHD-related behaviors [134]. Four studies reported null associations of PCBs with behavioral problems, including ADHD diagnosis [13, 179, 189, 224]. Only 2 studies examined prenatal PCBs in relation to social behavior and autistic traits, with one study reporting that PCBs were associated with fewer autistic traits [137] and another study reporting mixed associations with autistic traits, depending on the specific PCB congener [16].
Table 2.
Prenatal PCBs and child attention, behavioral regulation and social behavior.
| Location | Reference | Sample size | PCB exposure matrix (congeners) | Median/mean concentrations | Outcome measure | Age at assessment | Study results |
|---|---|---|---|---|---|---|---|
| Belgium (Flanders) | Sioen et al. 2013 [179] | 270 | cord blood ΣPCBs (138,153,180) | Median=74.5 ng/g lipid | SDQa | 7–8y | ∅ |
| Canada (Nunavik) | Boucher et al. 2012 [12] | 279 | cord blood PCB 153 | Median=93.6 ug/kg lipid | CBCLa (teacher report form) | 8–14y | ∅ |
| Boucher et al. 2012 [13] | 191 | cord blood PCB 153 | Median=93.3 ug/kg lipid | Go/No-go | 9–12y | ↓ error monitoring (aspect of behavioral regulation) | |
| Denmark (Aarus) | Strøm et al. 2014 [189] | 876 | maternal serum ΣPCBs (118,138,153,154,170,180) | Median=3.4 pmol/ml | Clinical diagnosis/prescribed medication for ADHD or depression | up to 22y | ∅ |
| Denmark (Faroe Islands) | Grandjean et al. 2001 [65] | 435 | cord blood ΣPCBs (118, 138, 153, 170, 180) | Mean=1.88 ug/L wet | CPTa (NES2) | 7y | ↑ (slower) reaction time only in the presence of high mercury exposure |
| Germany (Duisburg) | Neugebauer et al. 2015 [134] | 116 | maternal serum (23 weeks) ΣPCBs (138,153,180) | Median=0.16 lipid | KITAPa (8y) and FBB-ADHSa (9y) | 8–9y | ↑ omission errors for attention, but protective of parent reported ADHD behavior |
| Nowack et al. 2015 [137] | 100 | maternal serum (28–42 weeks) ΣPCBs | Median=6.85 wet | SRSa | 10y | ↓ autistic traits | |
| Greenland and Ukraine (Khariv) | Rosenquist et al. 2017 [160] | maternal serum (25 weeks gestation in Greenland, 23 weeks in Ukraine) PCB 153 | Median=107 (Greenland) and 27 (Ukraine) ng/g lipid | SDQa | 5, 9y | ↑ conduct problems and hyperactivity | |
| Spain (Menorca) | Forns et al. 2012 [51] | 393 | cord serum ΣPCBs (118,138,153,180) | Median=0.54 ng/ml serum | Conners’ CPTa | 11y | slower response time |
| United States (Cincinatti, Ohio) | Zhang et al. 2017 [224] | 239 | maternal serum ΣPCBs (118,138,153,158,180) | Median=31.3 ng/g lipid | BASC-2a | 5–8y | ∅ |
| Braun et al. 2014 [16] | 175 | maternal serum PCBs (25 different congeners, including 138 and 153) | Median 138/158=7.7 and 153=11 ng/g lipid | SRSa | 4–5y | Inconsistent associations with SRS scores across PCB congeners. 138/158 associated with ↑ autistic traits and 153 associated with ↓ autistic traits, though very imprecise. | |
| United States (Michigan) | Jacobson et al. 2003 [82] | 154 (age 4y), 148 (age 11y) | cord serum ΣPCBs (70% non detects) | Mean=2.7 ng/mL serum | CPTa, WCSTa, SWCTa | 4, 11y | ↑ impulsivity, ↓ concentration, verbal, visual and auditory working memory |
| United States (New Bedford, Massacusetts) | Sagiv et al. 2010 [163] | 573 | cord serum ΣPCBs (118,138,153,180) | Median=0.19 ng/g serum | CRS-Ta | 8y | ↑ ADHD-related behaviors (attention, impulsivity/hyperactivity) |
| Sagiv et al. 2012 [162] | 578 (CPT), 584 (WISC) | cord serum ΣPCBs (118,138,153,180) | Median=0.19 ng/g serum | CPTa, WISC-IIIa Processing Speed, Freedom from Distractibility | 8y | ↑ errors of omission (inattention) and slower processing speed among boys only | |
| United States (Oswego, New York) | Stewart et al. 2003 [187] | 189 | cord blood ΣPCBs | Median=0.52 ng/g wet | CPTa (Catch the Cat) | 4y | ↓ response inhibition (more commission errors) |
| Stewart et al. 2005 [185] | 182 (age 8y), 183 (age 9y) | cord blood ΣPCBs | categorized as ND, low, med and high | CPTa (NES2a 8y), Extended CPT (9y) | 8 and 9y | ↓ response inhibition | |
| Stewart et al. 2006 [188] | 167 | cord blood ΣPCBs | Mean=0.96 ng/g wet | DRLa | 9y | ↓ response inhibition (shorter interresponse times and reinforcements earned) |
BASC-2=Behavioral Assessment System for Children, Second Edition; CBCL=Child Behavior Checklist; CPT=Continuous Performance Test; CRS-T=Conners Rating Scale for Teachers; DRL=Differential Reinforcement of Low Rates Task; FBB-ADHS=parent rating scale for attention-deficit hyperactivity disorder; KITAP=Computerized test battery for attentional performance for children; NES-2=Neuropsychological Examination System-2; SRS=social responsiveness scale; SDQ=Strengths and Difficulties Questionnaire; SRS=Social Responsiveness Scale; SWCT=Stroop Color-Word Test; WCST=Wisconsin Card Sort Test; WISC-III=Wechsler Intelligence Scale for Children-Third Edition
The epidemiologic literature on PCBs and neurodevelopment is extensive and continues to grow. We present a focused summary based on studies we determined to be the most rigorous in study design and statistical precision. Despite these narrow inclusion criteria, we found a large number of studies for this review (29 papers representing 14 prospective cohort studies). While the results of these studies were mixed, overall our review showed that most studies reported that prenatal PCBs were related to poorer cognitive function and more behavior problems. We chose to focus our review on prospective birth cohort studies with PCB exposure measured during the prenatal period. Studies have shown that gestation may be the most sensitive period of neurodevelopment [64], when the central nervous system is rapidly developing. However, neurodevelopment continues through childhood into early adulthood and is also vulnerable to chemical exposures. Early infancy, in particular, may be a sensitive window of exposure due to high levels of PCBs in breastmilk. In addition, PCBs in dust from caulking materials and light ballasts used in schools and other buildings constructed before 1977 may be a significant source of exposure among children [20, 67]. Studies of associations of PCBs from breastmilk with neurodevelopment show mixed findings, with many studies reporting null associations [5, 83, 84, 151, 197]. In addition, disentangling the impact of prenatal and postnatal exposure on neurodevelopment, particularly when it comes from breastmilk, which may be highly correlated with transplacental exposure, presents a challenge.
We also chose to limit our review to studies of slightly older children (age 3 years or older). A sizable literature reports association of PCBs with neurodevelopment under the age of 3 using tests such as the Bayley Scales of Infant Development [15, 33, 59, 60, 105, 143, 209]; however, neuropsychological measures of infants and toddlers tend to be noisy, in part due to difficulty in achieving a desired state for testing in younger children [180]. Measured endpoints are therefore more likely to be reliable among older children. In addition, as demonstrated by three studies included in this review [144, 187, 201], PCB-related decrements in neurodevelopment may be transient, where decrements in cognitive or behavioral function found in early childhood recede in later childhood due to compensation by other brain regions or more stable measures of neurodevelopment at older ages.
The main limitation of any review article is publication bias, and this review is no exception. Pressures in the scientific and editorial processes promotes publication of positive results over null findings, which can skew the balance when trying to represent the weight of the evidence. Our stringent inclusion criteria may have offset this slightly, by filtering out smaller, methodologically less rigorous studies that are at greater risk for producing spurious findings. Yet, no review is immune to publication bias, and it is likely that a number of studies that would have met our inclusion criteria, but found null associations of PCBs and neurodevelopment, remain unpublished. Findings of this review paper should therefore be interpreted with this significant caveat in mind.
Another limitation of this review is that we did not evaluate associations with neurodevelopment for specific PCB congeners, or mechanism-based classes of congeners. This limitation is underscored by emerging studies that suggest it is specific RyR-active NDL PCBs and related organohalogens that may be associated with increased risk of heritable neurodevelopmental disorders, including ASDs [21, 64, 66, 71, 178, 182], and may be at least in part mediated by cellular Ca2+ dyshomeostasis, mitochondrial dysfunction and alterations in gene-specific patterns of DNA methylation [38, 118, 128, 132, 148, 215]. The majority of studies included in this review used a sum of PCB congeners, which may be dominated by specific individual congeners in the mix, such as PCB 153. Correlations across individual congeners also tends to be high, which makes disentangling individual congeners difficult due to collinearity. In addition, the mechanism for PCB neurotoxicity remains unclear and may be different across congeners and cognitive/behavioral domains, making a review of congener-specific associations challenging.
Summary and priorities for future epidemiologic study of PCBs and neurodevelopment
In summary, we found that among larger studies with more methodologic rigor, most showed that PCBs were associated with poorer cognitive and behavioral development. Though PCB levels have been steadily decreasing since their ban in the 1970s, their persistence in the environment and in human tissue and continued exposure in older building materials make PCBs a continued public health concern. In addition, as analytic methods improve, measurement of congeners at lower limits of detection is possible. This may make it possible to examine outcomes in relation to lower-level congeners that could not be previously quantified, such as lower chlorinated congeners, which may also be related to neurodevelopment [135]. In addition, assessment of neurodevelopment continues to improve, in particular neuroimaging modalities, which have not yet been explored in relation to PCBs. This presents the opportunity to examine PCB-related associations with different, and potentially more sensitive, neurodevelopmental endpoints, including neuroimaging [74]. Finally, this review may inform the potential neurodevelopmental impact of more contemporary and emerging chemicals with structural and toxicological properties similar to PCBs.
IV.b. Epidemiology of occupational exposures to PCBs
In contrast to a wealth of epidemiological data showing associations of perinatal PCB exposures with poorer neurodevelopmental outcomes, there are considerably fewer published studies that have examined PCB exposures in relation to impaired brain aging. Negative consequences have been reported in adults exposed via consumption of PCB-contaminated foods. Subtle cognitive and behavioral changes have been reported in recreational fishermen from the Great Lakes [167] and in adults who consumed PCB-contaminated fish from the upper Hudson River [49, 50]. Similarly, humans who consumed high amounts of whale meat, which typically has very high PCB levels, were found to be at increased risk for PD [150]. With respect to the latter, it should be noted that a prospective study reported no association between PCB exposure history and serum levels of PCBs before PD diagnosis [205].
A number of studies have examined the effects of occupational PCB exposures on adult neurologic function. Several studies provide suggestive evidence that occupational PCB exposures exert subtle influences on adult behavior and well-being. For example, PCB exposures during adulthood were associated with moderately lower well-being scores for teachers who worked in PCB-contaminated school buildings [145]. In another study, PCB-exposed firefighters exhibited higher rates of depressive disorders compared with respective control groups [95]. Five additional studies have provided evidence of a positive correlation between PCB body burden and depressive symptoms in older PCB exposed individuals [48, 49, 56, 57, 154]. Results from one of these studies, a cross-sectional study, indicated a significant negative influence of NDL PCB exposure on urinary levels of homovanillic acid, which is a metabolite of dopamine [154].
There have also been indications of an association between PCBs and increased risk of Parkinson’s disease (PD). An early study of the caudate nucleus obtained post mortem from PD patients and from controls not diagnosed with PD showed significantly higher concentrations of NDL PCB 153 in the PD tissue [30]. More recently, a study of postmortem brain tissues from control patients and those diagnosed with PD disease obtained from the Emory University Brain Bank showed NDL PCB congeners 153 and 180 were significantly elevated in the brains of PD patients. When stratified by sex, the female PD group demonstrated significantly elevated concentrations of total PCBs and specifically congeners NDL PCB 138, 153, and 180 compared to controls, whereas PCB concentrations in males were not significantly different between control and PD groups [69]. Epidemiologic studies have similarly noted sex differences in neurodegenerative outcomes related to PCB exposures. For example, Steenland and colleagues [183] demonstrated that female electrical capacitor workers were more likely to die from either Parkinson’s disease (PD) or amyotrophic lateral sclerosis than their male counterparts. Seegal and colleagues [174] reported that occupational exposure to PCBs significantly decreased striatal dopamine terminal densities only in women. In a separate cohort of women participating in the Nun Study who had no clinical signs or symptoms of PD at death, elevated levels of the same three PCB congeners were observed in brain tissue that exhibited moderate nigral depigmentation compared to subjects with mild or no depigmentation [69]. The reasons for these sexually-dimorphic findings remain unexplained. One potential explanation, particularly for older women who were either peri-or post-menopausal during occupational exposure, is decreased neuroprotection because of reduced levels of circulating estrogen [200]. Another possible explanation is that PCBs are lipophilic [94], and women typically have higher PCB body burdens due to a higher body fat content compared to men [10].
The limited human epidemiological data available regarding associations between PCB exposure and frank neuropathological changes associated with Parkinson’s disease is mixed with studies supporting [30, 69, 150] and not supporting [183, 205] an association between occupational PCB exposure with Parkinson’s disease. It should be emphasized that the available occupational literature is limited by a paucity of specific PCB biomarkers and exposures likely included other organohalogens. Nevertheless, the environmental persistence of PCBs and evidence of continued human exposure to PCBs, combined with robust animal and human evidence of PCB neurotoxicity, provide strong justification for further longitudinal studies of early life exposures and later neurodegenerative pathology in aging. Importantly, future epidemiological studies need to incorporate in their sampling and exposure assessments the more recent literature that clearly indicates that not all PCB congeners are equivalent in their neurotoxic potential. As reviewed above, there is strong experimental evidence that NDL PCB congeners are significantly more neuroactive than DL congeners. Among the NDL PCBs there appears to be a stringent structure-activity relationship that includes a significant degree of stereoselectivity among the 19 chiral PCBs. Thus, using the sum of the PCBs or a small number of sentinel PCBs, such as PCB 153, to reflect exposure may limit or underestimate associations with neurotoxicity throughout the lifespan.
V. Are PCBs the tip of the NDL-POP iceberg?
Anthropogenic and naturally synthesized polybrominated diphenyl ether (PBDEs)
PBDE flame retardants share several common chemical properties with NDL PCBs, including a noncoplanar orientation of the phenyl rings (Figure 1). Halogen and hydroxy substitutions at ortho-, meta- and para-positions exhibit similar structure-activity profiles toward triggering Ca2+ release from microsomal vesicles [97] as have been reported for NDL-PCBs [47, 72, 53, 54, 73, 149]. PBDEs were shown to cause intracellular Ca2+ dyshomeostasis in vitro by altering both ionotropic glutamate receptors [32] and RyR channels [95], and perturbations in the functional coupling of these channels has been linked to degenerative neuropathology [155]. Like PCBs, PBDEs alter TH signaling [34], and increase oxidative stress [31, 132]. Several studies have indicated that the neurotoxic activities of NDL PCBs, PBDEs and their hydroxylated metabolites are additive, and that a dose addition model for estimating risk may be appropriate [34, 72]. However, unlike NDL PCBs, which selectively alter dendritic complexity, neuronal-glial co-cultures from rat hippocampus exposed to BDE-47 or BDE-49 exhibited delayed neuronal polarization resulting in significant inhibition of axonal outgrowth during the first few days in vitro, but no later influences on dendritic complexity [24]. Interestingly, the axon inhibitory effects of these PBDEs were blocked by pharmacological antagonism of RyR or siRNA knockdown of RyR2, indicating common molecular mechanisms affecting two distinct developmental processes of neuronal connectivity. The explanation for the differential cellular effects of NDL PCBs and PBDEs despite a common molecular mechanism of action remains unknown but may possibly reflect differential effects on distinct RyR isoforms that are spatially segregated [7, 114]. A recent systematic review regarding the effects of developmental exposure to POPs on intelligence or ADHD and attention-related behavioral conditions in humans concluded that there is sufficient evidence supporting an association between developmental PBDE exposure and reduced IQ, but a similar association was not observed for PCBs [224].
Finally, contemporary sources of organohalogens produced as disinfection by-products [107, 221] and biosynthesized by marine organisms [1] are receiving considerable attention as emerging health risks because of their abundance, persistence, and potential to mimic natural organohalogens produced by bacteria that serve signaling and/or toxicological functions in marine environments. Recently 34 organohalogens from anthropogenic and marine sources, including hydroxylated PBDEs, bromopyrroles and bromobipyrroles (Figure 1), and bromoindoles were tested to identify compounds active towards RyRs. Several of these compounds were found to be highly active in not only sensitizing RyR channel activation, but also inhibiting microsomal sarcoplasmic/endoplasmic reticulum (SR / ER) Ca2+ ATPase (SERCA) [226]. The temporal and spatial properties of SR/ER Ca2+ signals not only depend on the density and activities of RyRs and IP3R channels, but also the activity of ATP-dependent driven SERCA pumps, which are necessary for accumulating luminal Ca2+ and setting the ionic force that drives Ca2+ signal properties. Several halopyrroles formed as disinfectant byproducts that are detected in waste and drinking water treatment streams have recently been shown to exert cytotoxic, genotoxic and carcinogenic activity in mammalian cell culture models [156, 157, 221]. Whether they have neurotoxic effects as predicted by their mechanistic similarity to NDL PCBs remains to be determined. However, the risk of developmental neurotoxicity is plausible given recent evidence that organohalogens of biosynthetic and anthropogenic origins accumulate in marine mammals and human breast milk [3, 177, 199].
VI. Concluding Remarks
There is compelling evidence from animal research and human epidemiological studies that PCBs are developmental neurotoxicants that are associated with a number of early cognitive and motor deficits later in life. Although less well studied, the influence of PCB exposure on the risk, onset and severity of late-onset neurodegenerative disorders are suggestive. It is important to note that the diversity of PCB chemistry (degree and position of chlorination, and their major hydroxylated and sulfonated metabolites) make the design of animal and epidemiological studies more challenging, as few studies include analysis of the major DL- and NDL-congeners and metabolites present in human tissues for their biochemical and/or behavioral outcomes, which can vary significantly by population demographics, age, sex and specific life-stages of exposure.
Experiments designed to assess the consequences of long-term exposures to PCBs and related environmental organohalogens on aging-related neuropathology present unique set of challenges. Aging-related declines in cognitive capacity, clinical dementia, and the major neurodegenerative disorders such as Parkinson’s disease (PD), Alzheimer’s disease (AD) and Amyotrophic Lateral Sclerosis (ALS) have both genetic and environmental components that contribute to temporal onset, progression and severity [70, 124, 202]. Gene by environment interactions have also been implicated in several neurodevelopmental disorders [71, 115, 178] Future research should encourage approaches that test convergent biologically plausible mechanisms. For example, the transactive response DNA binding protein 43 (TDP-43) has been implicated in the etiology of several aging-related neurodegenerative disorders, including PD [140], AD [129], ALS [87], frontotemporal dementia [18], and idiopathic late-onset dementia [125].
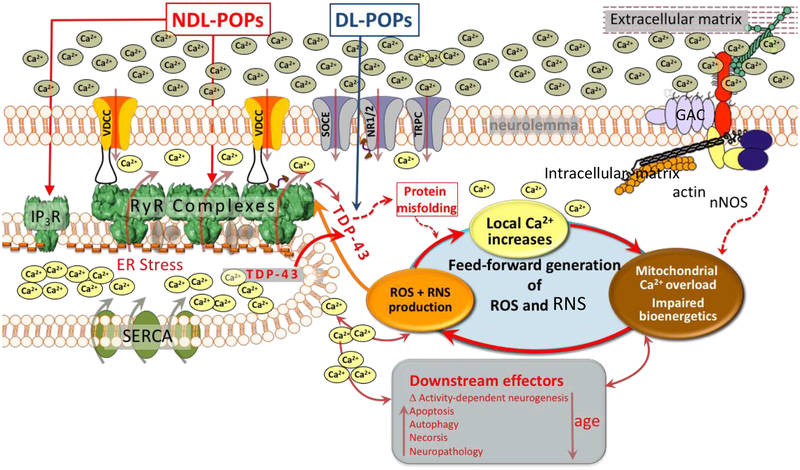
RyR dysfunction has been implicated in several aging disorders and may provide a focal point for more detailed studies of chronic exposures to NDL organohalogens and neuropathological markers. ALS may serve as a proof of principle for studying convergent mechanisms. Expression of TDP-43 is significantly upregulated by a variety of dioxin-like environmental chemicals [4] and TDP-43 mediated neurotoxicity proceeds by Ca2+ dyshomeostasis and ER stress [37, 90] via mechanisms requiring RyR channel activity [2]. Considering the widespread distribution of dioxin-like and NDL environmental chemicals, TD-43 and RyR (Figure 2) could serve as a basis for improving our understanding of adverse outcome pathways that contribute to risk for neurodevelopmental and neurodegenerative disorders. A better understanding of the relationships between the neurodevelopmental impairments highlighted in this review and their potential impact on neuropathological sequelae later in life are needed.
Figure 2.
Heuristic hypothesis modeling known mechanisms by which nondioxin-like (NDL) and dioxin-like (DL) persistent organic pollutants (POPs) influence mechanisms converging through their respective activities on ryanodine receptor (RyR) calcium channel complexes and expression of TDP-43. Expression of TDP-43 is significantly upregulated by a variety of dioxin-like environmental chemicals [4] and TDP-43 mediated neurotoxicity proceeds by Ca2+ dyshomeostasis and ER stress [37, 90] via mechanisms requiring RyR channel activity [2]. Such a model could serve as a basis for improving our understanding of adverse outcome pathways that contribute to risk for neurodevelopmental and neurodegenerative disorders, and the relationships between them. Abreviations: GAP, glycoprotein anchoring complex; IP3R, inositol 1,4,5-trisphosphate receptor; nNOS, neuronal nitric oxide synthase; ROS, reactive oxygen species; RNS, reactive nitrogen species; SOCE, store-operated calcium channel; SERCA, sarcoplasmic/endoplasmic reticulum calcium ATPase; TDP-43, transactive response DNA binding protein 43; TRPC, canonical transient receptor protein; VDCC, voltage-dependent calcium channel.
Acknowledgements
Supported by the National institute of Environmental Health Sciences (R01 ES014901, P01 ES011269, R01 ES030318 and P42 ES04699).
References
- 1.Agarwal V, Miles ZD, Winter JM, Eustáquio AS, El Gamal AA, Moore BS (2017) Enzymatic halogenation and dehalogenation reactions: Pervasive and mechanistically diverse. Chem Rev 117:5619–5674. 10.1021/acs.chemrev.6b00571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggad D, Vérièpe J, Tauffenberger A, Parker JA (2014) TDP-43 toxicity proceeds via calcium dysregulation and necrosis in aging Caenorhabditis elegans motor neurons. J Neurosci 34: 12093–103. https://doi.10.1523/JNEUROSCI.2495-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso MB, Maruya KA, Dodder NG, Lailson-Brito J Jr, Azevedo A, Santos-Neto E, Torres JP, Malm O, Hoh E (2017) Nontargeted Screening of Halogenated Organic Compounds in Bottlenose Dolphins (Tursiops truncatus) from Rio de Janeiro, Brazil. Environ Sci Technol 51:1176–1185. 10.1021/acs.est.6b04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ash PEA, Stanford EA, Al Abdulatif A, Ramirez-Cardenas A, Ballance HI, Boudeau S, Jeh A, Murithi JM, Tripodis Y, Murphy GJ, Sherr DH, Wolozin B (2017) Dioxins and related environmental contaminants increase TDP-43 levels. Mol Neurodegener 12:35 10.1186/s13024-017-0177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell MR (2014) Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr Opin Pharmacol 19:134–144. 10.1016/j.coph.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berghuis SA, Bos AF, Sauer PJ & Roze E (2015) Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol, 89, 687–709. 10.1007/s00204-015-1463-3 [DOI] [PubMed] [Google Scholar]
- 7.Berridge MJ (2006) Calcium microdomains: organization and function. Cell Calcium 40:405–12. 10.1016/j.ceca.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Betteridge DJ (2000) What is oxidative stress? Metabolism 49:3–8. 10.1016/S0026-0495(00)80077-3 [DOI] [PubMed] [Google Scholar]
- 9.Beyer A, Biziuk M (2009) Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol 201:137–58. 10.1007/978-1-4419-0032-6_5 [DOI] [PubMed] [Google Scholar]
- 10.Blaak E (2001) Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4:499–502. [DOI] [PubMed] [Google Scholar]
- 11.Bock KW (2016) Toward elucidation of dioxin-mediated chloracne and Ah receptor functions. Biochem Pharmacol 112:1–5. 10.1016/j.bcp.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 12.Boucher O, Muckle G & Bastien CH (2009) Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect, 117, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL (2012) Response inhibition and error monitoring during a visual go/no-go task in inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environ Health Perspect 120:608–615. 10.1289/ehp.1103828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boucher O, Jacobson SW, Plusquellec P, Dewailly E, Ayotte P, Forget-Dubois N, Jacobson JL, Muckle G (2012) Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Quebec. Environ Health Perspect 120:1456–1461. 10.1289/ehp.1204976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boucher O, Muckle G, Jacobson JL, Carter RC, Kaplan-Estrin M, Ayotte P, Dewailly E, Jacobson SW (2014) Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect 122:310–316. 10.1289/ehp.1206323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, Hauser R, Webster GM, Chen A, Lanphear BP (2014) Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect 122:513–520. 10.1289/ehp.1307261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brini M, Cali T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71:2787–814. 10.1007/s00018-013-1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briston T, Hicks AR.(2018) Mitochondrial dysfunction and neurodegenerative proteinopathies: mechanisms and prospects for therapeutic intervention. Biochem Soc Trans 46:829–842. https://dx.doi.org/10.1042%2FBST20180025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouwer A, Longnecker MP, Bimbaum LS, Cogliano J, Kostyniak P, Moore J, Schantz S, Winneke G (1999) Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ. Health Perspect 107(Suppl 4): 639–649. 10.1289/ehp.99107s4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown KW, Minegishi T, Cummiskey CC, Fragala MA, Hartman R, MacIntosh DL (2016) PCB remediation in schools: a review. Environ Sci Pollut Res Int 23:1986–1997. 10.1007/s11356-015-4689-y [DOI] [PubMed] [Google Scholar]
- 21.Brown AS, Cheslack-Postava K, Rantakokko P, Kiviranta H, Hinkka-Yli-Salomäki S, McKeague IW, Surcel HM, Sourander A (2018) Association of maternal insecticide levels with autism in offspring from a national birth cohort. Am J Psychiatry. 2018. August 16:appiajp201817101129. doi: 10.1176/appi.ajp.2018.17101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, Miller GW (2006) Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson’s disease-associated dopamine toxicity. Toxicol Sci 92:490–9. 10.1093/toxsci/kfl018 [DOI] [PubMed] [Google Scholar]
- 23.Carmody RJ, Cotter TG (2001) Signaling apoptosis: a radical approach. Redox. Rep 6:77–90. 10.1179/135100001101536085 [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Streifel KM, Singh V, Yang D, Mangini L, Wulff H, Lein PJ (2017). From the Cover: BDE-47 and BDE-49 inhibit axonal growth in primary rat hippocampal neuron-glia co-cultures via ryanodine receptor-dependent mechanisms. Toxicol Sci 156:375–386. 10.1093/toxsci/kfw259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YC, Guo YL, Hsu CC (1992) Cognitive development of children prenatally exposed to polychlorinated biphenyls (Yu-Cheng children) and their siblings. J Formos Med Assoc 91:704–707. [PubMed] [Google Scholar]
- 26.Chen YC, Yu ML, Rogan WJ, Gladen BC, Hsu CC (1994) A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am J Public Health 84:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng N, Alshammari F, Hughes E, Khanbabaei M, Rho JM. (2017) Dendritic overgrowth and elevated ERK signaling during neonatal development in a mouse model of autism. PLoS One. 2017. June 13;12(6):e0179409 https://doi10.1371/journal.pone.0179409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choksi NY, Kodavanti PR, Tilson HA, Booth RG (1997) Effects of polychlorinated biphenyls (PCBs) on brain tyrosine hydroxylase activity and dopamine synthesis in rats. Fundam Appl Toxicol 39:76–80. 10.1006/faat.1997.2351 [DOI] [PubMed] [Google Scholar]
- 29.Chou SM, Miike T, Payne WM, Davis GJ (1979) Neuropathology of “spinning syndrome” induced by prenatal intoxication with a PCB in mice. Ann N Y Acad Sci 320: 373–95. 10.1111/j.1749-6632.1979.tb56619.x [DOI] [PubMed] [Google Scholar]
- 30.Corrigan FM, Murray L, Wyatt CL, Shore RF (1998) Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Exp Neurol 150:339–342. 10.1006/exnr.1998.6776 [DOI] [PubMed] [Google Scholar]
- 31.Costa LG, Pellacani C, Dao K, Kavanagh TJ, Roque PJ (2015) The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. Neurotoxicology 48:68–76. 10.1016/j.neuro.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa LG, Tagliaferri S, Roqué PJ, Pellacani C (2016) Role of glutamate receptors in tetrabrominated diphenyl ether (BDE-47) neurotoxicity in mouse cerebellar granule neurons. Toxicol Lett 241:159–66. 10.1016/j.toxlet.2015.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels JL, Longnecker MP, Klebanoff MA, Gray KA, Brock JW, Zhou H, Chen Z, Needham LL (2003) Prenatal exposure to low-level polychlorinated biphenyls in relation to mental and motor development at 8 months. Am J Epidemiol 157:485–492. 10.1093/aje/kwg010 [DOI] [PubMed] [Google Scholar]
- 34.Dingemans MM, Kock M, van den Berg M (2016) Mechanisms of action point towards combined PBDE/NDL-PCB risk assessment. Toxicol Sci 153:215–24. 10.1093/toxsci/kfw129 [DOI] [PubMed] [Google Scholar]
- 35.Do Y, Lee DK (2012) Effects of polychlorinated biphenyls on the development of neuronal cells in growth period; structure-activity relationship. Exp Neurobiol 21:30–6. 10.5607/en.2012.21.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domingo JL, Bocio A (2007) Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: A literature review. Environ Int 33:397–405. 10.1016/j.envint.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 37.Dreser A, Vollrath JT, Sechi A, Johann S, Roos A, Yamoah A, Katona I, Bohlega S, Wiemuth D, Tian Y, Schmidt A, Vervoorts J, Dohmen M, Beyer C, Anink J, Aronica E, Troost D, Weis J, Goswami A (2017) The ALS-linked E102Q mutation in Sigma receptor-1 leads to ER stress-mediated defects in protein homeostasis and dysregulation of RNA-binding proteins. Cell Death Differ. 24:1655–1671. 10.1038/cdd.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunaway K,* Islam MS,* Lopez SJ, Coulson RL, Ciernia AV, Chu RG, Yasui DH, Pessah IN, Lott P, Mordaunt C, Meguro-Horike M, Horike S, Korf I, and LaSalle JM (2016) Cumulative impact of large chromosomal duplications and polychlorinated biphenyl exposure on DNA methylation, chromatin, and expression of autism candidate genes. Cell Reports 17, 3035–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elnar AA, Desor F, Legay S, Nemos C, Yen FT, Oster T, Bohn T, Soulimani R (2016) No evidence for oxidative stress in the cerebellar tissues or cells of juvenile male mice exposed via lactation to the 6 non-dioxin-like PCBs at levels below the regulatory safe limits for humans. Toxicol Lett 245:7–14. 10.1016/j.toxlet.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 40.Ethier AA, Muckle G, Bastien C, Dewailly É, Ayotte P, Arfken C, Jacobson SW, Jacobson JL, Saint-Amour D (2012) Effects of environmental contaminant exposure on visual brain development: a prospective electrophysiological study in school-aged children. Neurotoxicology 2012 33:1075–85. 10.1016/j.neuro.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 41.Eubig PA, Aguiar A, Schantz SL (2010) Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ Health Perspect 118:1654–1667. 10.1289/ehp.0901852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evangelista de Duffard AM, Duffard R. (1996) Behavioral toxicology, risk assessment, and chlorinated hydrocarbons. Environ Health Perspect 104 Suppl 2: 353–60. 10.1289/ehp.96104s2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faroon O, Ruiz P (2016) Polychlorinated biphenyls: New evidence from the last decade. Tox Ind Health 32:1825–1847. 10.1177/0748233715587849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng W, Barrientos GC, Cherednichenko G, Yang T, Padilla IT, Truong K, Allen PD, Lopez JR, Pessah IN (2011). Functional and biochemical properties of ryanodine receptor type 1 channels from heterozygous R163C malignant hyperthermia-susceptible mice. Mol Pharmacol 79:420–31. 10.1124/mol.110.067959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng W, Liu G, Allen PD, Pessah IN (2000) Transmembrane redox sensor of ryanodine receptor complex. J Biol Chem 275:35902–7. 10.1074/jbc.C000523200 [DOI] [PubMed] [Google Scholar]
- 46.Feng W, Liu G, Xia R, Abramson JJ, Pessah IN (1999) Site-selective modification of hyperreactive cysteines of ryanodine receptor complex by quinones. Mol Pharmacol 55:821–31. [PubMed] [Google Scholar]
- 47.Feng W, Zheng J, Robin G, Dong Y, Ichikawa M, Inoue Y, Mori T, Nakano T, Pessah IN. (2017) Enantioselectivity of 2,2’,3,5’,6-pentachlorobiphenyl (PCB 95) atropisomers toward ryanodine receptors (RyRs) and their influences on hippocampal neuronal networks. Environ Sci Technol 51:14406–14416. 10.1021/acs.est.7b04446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fimm B, Sturm W, Esser A, Schettgen T, Willmes K, Lang J, Gaum PM, Kraus T (2017) Neuropsychological effects of occupational exposure to polychlorinated biphenyls. Neurotoxicology 63:106–119. 10.1016/j.neuro.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 49.Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, Jansing RL, Hwang SA, Hicks HE (2008). Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ Health Perspect 116:209–15. 10.1289/ehp.10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzgerald EF Belanger EE, Gomez MI, Hwang SA, Jansing RL, Hicks HE (2007) Environmental exposures to polychlorinated biphenyls (PCBs) among older residents of upper Hudson River communities. Environ. Res 104, 352–360. 10.1016/j.envres.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 51.Forns J, Torrent M, Garcia-Esteban R, Grellier J, Gascon M, Julvez J, Guxens M, Grimalt JO, Sunyer J (2012) Prenatal exposure to polychlorinated biphenyls and child neuropsychological development in 4-year-olds: an analysis per congener and specific cognitive domain. Sci Total Environ 432:338–343. 10.1016/j.scitotenv.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 52.Freitas J, Cano P, Craig-Veit C, Goodson ML, Furlow JD, Murk AJ (2011) Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol In Vitro 25:257–66. 10.1016/j.tiv.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 53.Fritsch EB, Pessah IN (2013) Structure-activity relationship of non-coplanar polychlorinated biphenyls toward skeletal muscle ryanodine receptors in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 140–141:204–12. 10.1016/j.aquatox.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fritsch EB, Stegeman JJ, Goldstone JV, Nacci DE, Champlin D, Jayaraman S, Connon RE, Pessah IN (2015) Expression and function of ryanodine receptor related pathways in PCB tolerant Atlantic killifish (Fundulus heteroclitus) from New Bedford Harbor, MA, USA. Aquat Toxicol 159:156–66. 10.1016/j.aquatox.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fritsche E, Cline JE, Nguyen NH, Scanlan TS, Abel J (2005) Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: clue for involvement of thyroid hormone receptors. Environ Health Perspect 113:871–6. 10.1289/ehp.7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaum PM, Esser A, Schettgen T, Gube M, Kraus T, Lang J (2014) Prevalence and incidence rates of mental syndromes after occupational exposure to polychlorinated biphenyls. Int J Hyg Environ Health 217:765–74. 10.1016/j.ijheh.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 57.Gaum PM, Gube M, Schettgen T, Putschögl FM, Kraus T, Fimm B, Lang J (2017) Polychlorinated biphenyls and depression: cross-sectional and longitudinal investigation of a dopamine-related Neurochemical path in the German HELPcB surveillance program. Environ Health 16:106 10.1186/s12940-017-0316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilbert ME, Mundy WR, Crofton KM (2000) Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol Sci 57:102–111. 10.1093/toxsci/57.1.102 [DOI] [PubMed] [Google Scholar]
- 59.Gladen BC, Rogan WJ (1991) Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethene on later development. J Pediatr 119:58–63. 10.1016/S0022-3476(05)81039-X [DOI] [PubMed] [Google Scholar]
- 60.Gladen BC, Rogan WJ, Hardy P, Thullen J, Tingelstad J, Tully M (1988) Development after exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene transplacentally and through human milk. J Pediatr 113:991–995. 10.1016/S0022-3476(88)80569-9 [DOI] [PubMed] [Google Scholar]
- 61.Golden CE, Buxbaum JD, De Rubeis S (2018) Disrupted circuits in mouse models of autism spectrum disorder and intellectual disability. Curr Opin Neurobiol 48:106–112. 10.1016/j.conb.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldey ES, Crofton KM (1998) Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci 45:94–105. 10.1006/toxs.1998.2495 [DOI] [PubMed] [Google Scholar]
- 63.Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM (1995) Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol 135:77–88. 10.1006/taap.1995.1210 [DOI] [PubMed] [Google Scholar]
- 64.Grandjean P, Landrigan PJ (2014) Neurobehavioural effects of developmental toxicity. Lancet Neurol 13:330–338. 10.1016/S1474-4422(14)70121-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, Debes F, Murata K, Simonsen H, Ellefsen P, Budtz-Jørgensen E, Keiding N, White RF (2001) Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol 23:305–317. 10.1016/S0892-0362(01)00155-6 [DOI] [PubMed] [Google Scholar]
- 66.Granillo L, Sethi S, Keil KP, Lin Y, Ozonoff S, Iosif A-M, Puschner B, Schmidt RJ (2019) Polychlorinated biphenyls influence on autism spectrum disorder risk in the MARBLES cohort. Env Health Persp (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gray KA, Klebanoff MA, Brock JW, Zhou H, Darden R, Needham L, Longnecker MP (2005) In utero exposure to background levels of polychlorinated biphenyls and cognitive functioning among school-age children. Am J Epidemiol 162: 17–26. 10.1093/aje/kwi158 [DOI] [PubMed] [Google Scholar]
- 68.Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman Å, Robertson LW (2015) Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol 45:245–72. 10.3109/10408444.2014.999365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatcher-Martin JM, Gearing M, Steenland K, Levey AI, Miller GW, Pennell KD (2012) Association between polychlorinated biphenyls and Parkinson’s disease neuropathology. Neurotoxicology 33:1298–304. 10.1016/j.neuro.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. (2017) Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology. 61:143–187. https://doi:10.1016/j.neuro.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 71.Hertz-Picciotto I, Schmidt RJ, Krakowiak P (2018) Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Res 11:554–586. https://doi:10.1002/aur.1938 [DOI] [PubMed] [Google Scholar]
- 72.Holland EB, Feng W, Zheng J, Dong Y, Li X, Lehmler HJ, Pessah IN (2017) An Extended Structure-activity relationship of nondioxin-like PCBs evaluates and supports modeling predictions and identifies picomolar potency of PCB 202 towards ryanodine receptors. Toxicol Sci 155:170–181. 10.1093/toxsci/kfw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holland EB, Goldstone JV, Pessah IN, Whitehead A, Reid NM, Karchner SI, Hahn ME, Nacci DE, Clark BW, Stegeman JJ (2017) Ryanodine receptor and FK506 binding protein 1 in the Atlantic killifish (Fundulus heteroclitus): A phylogenetic and population-based comparison. Aquat Toxicol 192:105–115. 10.1016/j.aquatox.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horton MK, Margolis AE, Tang C, Wright R (2014) Neuroimaging is a novel tool to understand the impact of environmental chemicals on neurodevelopment. Curr Opin Pediatr 26:230–236. 10.1097/MOP.0000000000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, Lein PJ (2003) Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol 190:72–86. 10.1016/S0041-008X(03)00156-X [DOI] [PubMed] [Google Scholar]
- 76.Huang L, Xue Y, Feng D, Yang R, Nie T, Zhu G, Tao K, Gao G, Yang Q (2017) Blockade of RyRs in the ER Attenuates 6-OHDA-Induced Calcium Overload, Cellular Hypo-Excitability and Apoptosis in Dopaminergic Neurons. Front Cell Neurosci 3:11:52. 10.3389/fncel.2017.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hutsler JJ, Zhang H (2010) Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res 1309:83–94. 10.1016/j.brainres.2009.09.120 [DOI] [PubMed] [Google Scholar]
- 78.Inglefield JR, Mundy WR, Shafer TJ (2001) Inositol 1,4,5-triphosphate receptor-sensitive Ca(2+) release, store-operated Ca(2+) entry, and cAMP responsive element binding protein phosphorylation in developing cortical cells following exposure to polychlorinated biphenyls. J Pharmacol Exp Ther 297:762–73. http://jpet.aspetjournals.org/content/297/2/762.long [PubMed] [Google Scholar]
- 79.Inglefield JR, Shafer TJ (2000) Polychlorinated biphenyl-stimulation of Ca(2+) oscillations in developing neocortical cells: a role for excitatory transmitters and L-type voltage-sensitive Ca(2+) channels. J Pharmacol Exp Ther 295:105–13. http://jpet.aspetjournals.org/content/295/1/105 [PubMed] [Google Scholar]
- 80.Irwin SA, Patel B, Idupulapati M, Harris Jb, Crisostomo Ra, Larsen Bp, Kooy F, Willems Pj, Cras P, Kozlowski Pb, Swain Ra, Weiler Ij, Greenough Wt (2001) Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitiative examination. Am J Med Genet 98, 161–167. [DOI] [PubMed] [Google Scholar]
- 81.Itoh S, Baba T, Yuasa M, Miyashita C, Kobayashi S, Araki A, Sasaki S, Kajiwara J, Hori T, Todaka T, Fujikura K, Nakajima S, Kato S, Kishi R (2018) Association of maternal serum concentration of hydroxylated polychlorinated biphenyls with maternal and neonatal thyroid hormones: The Hokkaido birth cohort study. Environ Res 167:583–590. 10.1016/j.envres.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 82.Jacobson JL, Jacobson SW (1996) Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med 335:783–789. 10.1056/NEJM199609123351104 [DOI] [PubMed] [Google Scholar]
- 83.Jacobson JL, Jacobson SW (2003) Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr 143:780–788. 10.1067/S0022-3476(03)00577-8 [DOI] [PubMed] [Google Scholar]
- 84.Jacobson JL, Jacobson SW, Humphrey HE (1990) Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicol Teratol 12:319–326. 10.1016/0892-0362(90)90050-M [DOI] [PubMed] [Google Scholar]
- 85.Jacobson JL, Jacobson SW, Padgett RJ, Brumitt GA, Billings RL (1992) Effects of prenatal PCB exposure on cognitive processing efficiency and sustained attention. Developmental Psychology 28:297–306. 10.1111/j.1467-8624.1992.tb01656.x [DOI] [Google Scholar]
- 86.Jawaid S, Kidd GJ, Wang J, Swetlik C, Dutta R, Trapp BD. (2018) Alterations in CA1 hippocampal synapses in a mouse model of fragile X syndrome. Glia 66:789–800. 10.1002/glia.23284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jawaid A, Khan R, Polymenidou M, Schulz PE (2018) Disease-modifying effects of metabolic perturbations in ALS/FTLD. Mol Neurodegener. 13:63 https://doi:10.1186/s13024-018-0294-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang JH, Park IS, Oh WY, Lim HK, Wang SY, Lee SY, Choi KH, Kim JI, Jung SY, Suh CK, Kim DS (2004) Inhibition of Aroclor 1254-induced depletion of stored calcium prevents the cell death in catecholaminergic cells. Toxicology 200: 93–101. 10.1016/j.tox.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 89.Kato Y, Haraguchi K, Yamazaki T, Ito Y, Miyajima S, Nemoto K, Koga N, Kimura R, Degawa M (2003) Effects of polychlorinated biphenyls, kanechlor-500, on serum thyroid hormone levels in rats and mice. Toxicol Sci 72:235–41. 10.1093/toxsci/kfg025 [DOI] [PubMed] [Google Scholar]
- 90.Kaus A, Sareen D. (2015) ALS Patient Stem Cells for Unveiling Disease Signatures of Motoneuron Susceptibility: Perspectives on the Deadly Mitochondria, ER Stress and Calcium Triad. Front Cell Neurosci 9:448 https://doi:10.3389/fncel.2015.00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keil KP, Sethi S, Wilson MD, Silverman JL, Pessah IN, Lein PJ (2019) Genetic mutations in Ca2+ signaling alter dendrite morphology and social approach in juvenile mice. Genes Brain Behav Oct 12:e12526 10.1111/gbb.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM (2007) Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc Natl Acad Sci U S A 104:7646–51. 10.1073/pnas.0701944104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kennedy KA, Sandiford SD, Skerjanc IS, Li SS (2012) Reactive oxygen species and the neuronal fate. Cell Mol Life Sci 69:215–21. 10.1007/s00018-011-0807-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khadikar PV, Singh S, Shrivastava A (2002) Novel estimation of lipophilic behaviour of polychlorinated biphenyls. Bioorg Med Chem Lett 12:1125–8. 10.1016/S0960-894X(02)00086-0 [DOI] [PubMed] [Google Scholar]
- 95.Kilburn KH, Warsaw RH, Shields MG (1989) Neurobehavioral dysfunction in firemen exposed to polychlorinated biphenyls (PCBs): Possible improvement after detoxification. Arch Environ Health 44:345–50. 10.1080/00039896.1989.9935904 [DOI] [PubMed] [Google Scholar]
- 96.Kim D, Amy GL, Karanfil T (2015) Disinfection by-product formation during seawater desalination: A review. Water Res 81:343–55. 10.1016/j.watres.2015.05.0407 [DOI] [PubMed] [Google Scholar]
- 97.Kim KH, Bose DD, Ghogha A, Riehl J, Zhang R, Barnhart CD, Lein PJ, Pessah IN (2011). Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ Health Perspect 119:519–26. 10.1289/ehp.1002728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim KH, Inan SY, Berman RF, Pessah IN (2009) Excitatory and inhibitory synaptic transmission is differentially influenced by two ortho-substituted polychlorinated biphenyls in the hippocampal slice preparation. Toxicol Appl Pharmacol 237:168–77. 10.1016/j.taap.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim KH, Pessah IN (2011) Perinatal exposure to environmental polychlorinated biphenyls sensitizes hippocampus to excitotoxicity ex vivo. Neurotoxicology 32:981–5. 10.1016/j.neuro.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kimura E, Kubo KI, Endo T, Nakajima K, Kakeyama M, Tohyama C (2017) Excessive activation of AhR signaling disrupts neuronal migration in the hippocampal CA1 region in the developing mouse. J Toxicol Sci 42:25–30. 10.2131/jts.42.25 [DOI] [PubMed] [Google Scholar]
- 101.Kimura E, Tohyama C (2018) Vocalization as a novel endpoint of atypical attachment behavior in 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed infant mice. Arch Toxicol 92:1741–1749. 10.1007/s00204-018-2176-1 [DOI] [PubMed] [Google Scholar]
- 102.Kodavanti PR, Tilson HA (2000) Neurochemical effects of environmental chemicals: in vitro and in vivo correlations on second messenger pathways. Ann N Y Acad Sci, 919:97–105. 10.1111/j.1749-6632.2000.tb06872.x [DOI] [PubMed] [Google Scholar]
- 103.Kodavanti PR, Ward TR, Mckinney JD, Tilson HA (1996) Inhibition of microsomal and mitochondrial Ca2+-sequestration in rat cerebellum by polychlorinated biphenyl mixtures and congeners. Structure-activity relationships. Arch Toxicol 70:150–7. https://link.springer.com/article/10.1007/s002040050254 [DOI] [PubMed] [Google Scholar]
- 104.Konur S, Ghosh A (2005) Calcium signaling and the control of dendritic development. Neuron 46:401–5. 10.1016/j.neuron.2005.04.022 [DOI] [PubMed] [Google Scholar]
- 105.Koopman-Esseboom C, Weisglas-Kuperus N, de Ridder MA, Van der Paauw CG, Tuinstra LG, Sauer PJ (1996) Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics 97:700–706. http://pediatrics.aappublications.org/content/97/5/700..info [PubMed] [Google Scholar]
- 106.Kreitinger JM, Beamer CA, Shepherd DM (2016) Environmental immunology: Lessons learned from exposure to a select panel of immunotoxicants. J Immunol. 196:3217–25. 10.4049/jimmunol.1502149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kumar A, Borgen M, Aluwihare LI, Fenical W (2017) Ozone-activated halogenation of mono- and dimethylbipyrrole in seawater. Environ Sci Technol 51:589–595. 10.1021/acs.est.6b03601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laden F, Neas LM, Spiegelman D, Hankinson SE, Willett WC, Ireland K, Wolff MS, Hunter DJ (1999) Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women. Environ Health Perspect 107:75–81. 10.1289/ehp.9910775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, Davidson L, Daniels N, Sen S, Woodruff TJ (2017) Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ Health Perspect 125(8):086001 10.1289/EHP1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim- Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K (2013) Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol 14:287–288. 10.1016/S1470-2045(13)70104-9 [DOI] [PubMed] [Google Scholar]
- 111.Le Douaron G, Ferrié L, Sepulveda-Diaz JE, Amar M, Harfouche A, Séon-Méniel B, Raisman-Vozari R, Michel PP, Figadère B (2016) New 6-Aminoquinoxaline Derivatives with Neuroprotective Effect on Dopaminergic Neurons in Cellular and Animal Parkinson Disease Models. J Med Chem 59:6169–86. 10.1021/acs.jmedchem.6b00297 [DOI] [PubMed] [Google Scholar]
- 112.Lee DW, Notter SA, Thiruchelvam M, Dever DP, Fitzpatrick R, Kostyniak PJ, Cory-Slechta DA, Opanashuk LA (2012) Subchronic polychlorinated biphenyl (Aroclor 1254) exposure produces oxidative damage and neuronal death of ventral midbrain dopaminergic systems. Toxicol Sci 125:496–508. 10.1093/toxsci/kfr313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee HK (2006) Synaptic plasticity and phosphorylation. Pharmacol Ther 112:810–32. 10.1016/j.pharmthera.2006.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee KF, Soares C, Thivierge JP, Béïque JC (2016) Correlated synaptic inputs drive dendritic calcium amplification and cooperative plasticity during clustered synapse development. Neuron 89:784–99. 10.1016/j.neuron.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 115.Lein PJ, Supasai S, Guignet M (2018) Chapter 9: Apoptosis as a mechanism of developmental neurotoxicity In: Handbook of Developmental Neurotoxicology, 2nd Edition (Edited by Slikker W Jr, Paule MG and Wang C), Elsevier, Inc., Amsterdam, pp. 91–112. [Google Scholar]
- 116.Lein PJ, Mervis RF, Bachstetter AD, Yang D, Tilson HA, Harry GJ, Kodavanti PRS (2007) Ontogenetic alterations in molecular and structural correlates of dendritic growth after developmental exposure to polychlorinated biphenyls. Environ Health Perspect 115:556–563. 10.1289/ehp.9773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, Wayman GA (2014). The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci 34:717–25. 10.1523/JNEUROSCI.2884-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leung YK, Ouyang B, Niu L, Xie C, Ying J, Medvedovic M, Chen A, Weihe P, Valvi D, Grandjean P, Ho SM (2018) Identification of sex-specific DNA methylation changes driven by specific chemicals in cord blood in a Faroese birth cohort. Epigenetics 13:290–300. doi: 10.1080/15592294.2018.1445901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li ZM, Hernandez-Moreno D, Main KM, Skakkebæk NE, Kiviranta H, Toppari J, Feldt-Rasmussen U, Shen H, Schramm KW, De Angelis M (2018) Association of in utero persistent organic pollutant exposure with placental thyroid hormones. Endocrinology 159:3473–3481. 10.1210/en.2018-00542 [DOI] [PubMed] [Google Scholar]
- 120.Liu CH, Huang CY, Huang CC (2012) Occupational neurotoxic diseases in Taiwan. Saf Health Work. 3:257–67. 10.5491/SHAW.2012.3.4.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Malisch R, Kotz A (2014) Dioxins and PCBs in feed and food--review from European perspective. Sci Total Environ 491–492:2–10. 10.1016/j.scitotenv.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 122.Mariussen E, Fonnum F (2001) The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology 159:11–21. 10.1016/S0300-483X(00)00374-7 [DOI] [PubMed] [Google Scholar]
- 123.Mariussen E, Myhre O, Reistad T, Fonnum F (2002) The polychlorinated biphenyl mixture aroclor 1254 induces death of rat cerebellar granule cells: the involvement of the N-methyl-D-aspartate receptor and reactive oxygen species. Toxicol Appl Pharmacol 179:137–44. 10.1006/taap.2002.9353 [DOI] [PubMed] [Google Scholar]
- 124.Martino R, Candundo H, Lieshout PV, Shin S, Crispo JAG, Barakat-Haddad C (2017) Onset and progression factors in Parkinson’s disease: A systematic review. Neurotoxicology. 61:132–141. https://doi:10.1016/j.neuro.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Mattson MP (2015) Late-onset dementia: a mosaic of prototypical pathologies modifiable by diet and lifestyle. NPJ Aging Mech Dis 2015:1 pii: 15003. 10.1038/npjamd.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mellor CL, Steinmetz FP, Cronin MT. The identification of nuclear receptors associated with hepatic steatosis to develop and extend adverse outcome pathways. Crit Rev Toxicol 2016. February;46(2):138–52. 10.3109/10408444.2015.1089471 [DOI] [PubMed] [Google Scholar]
- 127.Mengeling BJ, Furlow JD (2015) Pituitary specific retinoid-X receptor ligand interactions with thyroid hormone receptor signaling revealed by high throughput reporter and endogenous gene responses. Toxicol In Vitro 29:1609–18. 10.1016/j.tiv.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 128.Mitchell MM, Woods R, Chi L-H, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM (2012) Levels of select PCB and PBDE congeners in human post-mortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Env Molec Mutagenesis 53:589–98. https://doi.10.1002/em.21722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, Cummings J, Fagan AM, Hampel H, Mielke MM, Mikulskis A, O’Bryant S, Scheltens P, Sevigny J, Shaw LM, Soares HD, Tong G, Trojanowski JQ, Zetterberg H, Blennow K (2018) Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol 136:821–853. doi: 10.1007/s00401-018-1932-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mundy WR, Shafer TJ, Tilson HA, Kodavanti PR (1999) Extracellular calcium is required for the polychlorinated biphenyl-induced increase of intracellular free calcium levels in cerebellar granule cell culture. Toxicology 136:27–39. 10.1016/S0300-483X(99)00052-9 [DOI] [PubMed] [Google Scholar]
- 131.Nandipati and Litvan 2016, Int J Environ Res Public Health 13(9): pii: E881 10.3390/ijerph13090881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Napoli E, Hung C, Wong S, Giulivi C (2013) Toxicity of the flame-retardant BDE-49 on brain mitochondria and neuronal progenitor striatal cells enhanced by a PTEN-deficient background. Toxicol Sci 132:196–210. 10.1093/toxsci/kfs339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ness DK, Schantz SL, Moshtaghian J, Hansen LG (1993) Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol Lett 68:311–23. 10.1016/0378-4274(93)90023-Q [DOI] [PubMed] [Google Scholar]
- 134.Neugebauer J, Wittsiepe J, Kasper-Sonnenberg M, Schoneck N, Scholmerich A, Wilhelm M (2015) The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: results from the Duisburg Birth Cohort Study. Int J Hyg Environ Health 218:153–162. 10.1016/j.ijheh.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 135.Newman J, Gallo MV, Schell LM, DeCaprio AP, Denham M, Deane GD, Akwesasne Task Force on E (2009) Analysis of PCB congeners related to cognitive functioning in adolescents. Neurotoxicology 30:686–696. 10.1016/j.neuro.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Niknam Y, Feng W, Cherednichenko G, Dong Y, Joshi SN, Vyas SM, Lehmler HJ, Pessah IN (2013) Structure-activity relationship of selected meta- and para-hydroxylated nondioxin like polychlorinated biphenyls: from single RyR1 channels to muscle dysfunction. Toxicol Sci. 136:500–13. 10.1093/toxsci/kft202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nowack N, Wittsiepe J, Kasper-Sonnenberg M, Wilhelm M, Scholmerich A (2015) Influence of Low-Level Prenatal Exposure to PCDD/Fs and PCBs on Empathizing, Systemizing and Autistic Traits: Results from the Duisburg Birth Cohort Study. PLoS One 10: e0129906 10.1371/journal.pone.0129906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okabe E, Tsujimoto Y, Kobayashi Y (2000) Calmodulin and cyclic ADP-ribose interaction in Ca2 signaling related to cardiac sarcoplasmic reticulum: superoxide anion radical-triggered Ca2 release. Antioxid Redox Signal 2:47–54. 10.1089/ars.2000.2.1-47 [DOI] [PubMed] [Google Scholar]
- 139.Olguin-Albuerne M, Moran J (2015) ROS produced by NOX2 control in vitro development of cerebellar granule neurons development. ASN Neuro 7:1–28. 10.1177/1759091415578712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Olsson B, Portelius E, Cullen NC, Sandelius Å, Zetterberg H, Andreasson U, Höglund K, Irwin DJ, Grossman M, Weintraub D, Chen-Plotkin A, Wolk D, McCluskey L, Elman L, Shaw LM, Toledo JB, McBride J, Hernandez-Con P, Lee VM, Trojanowski JQ, Blennow K (2018) Association of Cerebrospinal Fluid Neurofilament Light Protein Levels With Cognition in Patients With Dementia, Motor Neuron Disease, and Movement Disorders. JAMA Neurol. 2018. December 3 https://doi:10.1001/jamaneurol.2018.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oppenheimer JH, Schwartz HL (1997) Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 18:462–75. 10.1210/edrv.18.4.0309 [DOI] [PubMed] [Google Scholar]
- 142.Orenstein ST, Thurston SW, Bellinger DC, Schwartz JD, Amarasiriwardena CJ, Altshul LM, Korrick SA (2014) Prenatal organochlorine and methylmercury exposure and memory and learning in school-age children in communities near the New Bedford Harbor Superfund site, Massachusetts. Environ Health Perspect 122: 1253–1259. 10.1289/ehp.1307804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T (2010) Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ Health 9:51 10.1186/1476-069X-9-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patandin S, Lanting CI, Mulder PG, Boersma ER, Sauer PJ, Weisglas-Kuperus N (1999) Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J Pediatr 134: 33–41. 10.1016/S0022-3476(99)70369-0 [DOI] [PubMed] [Google Scholar]
- 145.Peper M, Klett M, Morgenstern R (2005) Neuropsychological effects of chronic low- dose exposure to polychlorinated biphenyls (PCBs): a cross-sectional study. Environ. Health. 2005. Environ Health 4:22 10.1186/1476-069X-4-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pessah IN (2001) Ryanodine receptor acts as a sensor for redox stress. Pest Manag Sci. 57:941–5. 10.1002/ps.391 [DOI] [PubMed] [Google Scholar]
- 147.Pessah IN, Beltzner C, Burchiel SW, Sridhar G, Penning T, Feng W (2001) A bioactive metabolite of benzo[a]pyrene, benzo[a]pyrene-7,8-dione, selectively alters microsomal Ca2+ transport and ryanodine receptor function. Mol Pharmacol. 59:506–13. [DOI] [PubMed] [Google Scholar]
- 148.Pessah IN, Cherednichenko G, Lein PJ (2010) Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther 125:260–85. 10.1016/j.pharmthera.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW (2006) Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1). Chem Res Toxicol 19:92–101. 10.1021/tx050196m [DOI] [PubMed] [Google Scholar]
- 150.Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Neilsen F, Jorgensen PJ, Budtz-Jorgensen E, Grandjean P (2008) Impact of dietary exposure to food contaminants on the risk of Parkinson’s disease. Neurotoxicology 29:584–590. 10.1016/j.neuro.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 151.Plusquellec P, Muckle G, Dewailly E, Ayotte P, Begin G, Desrosiers C, Despres C, Saint-Amour D, Poitras K (2010) The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology 31:17–25. 10.1016/j.neuro.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 152.Pruitt DL, Meserve LA, Bingman VP (1999) Reduced growth of intra- and infra-pyramidal mossy fibers is produced by continuous exposure to polychlorinated biphenyl. Toxicology 138:11–7. 10.1016/S0300-483X(99)00073-6 [DOI] [PubMed] [Google Scholar]
- 153.Purkayastha S, Fernando SS, Diallo S, Cohen L, Ranasinghe B, Levano K, Banerjee P (2009) Regulation of protein kinase C isozymes during early postnatal hippocampal development. Brain Res 1288:29–41. 10.1016/j.brainres.2009.06.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Putschögl FM, Gaum PM, Schettgen T, Kraus T, Gube M, Lang J (2015) Effects of occupational exposure to polychlorinated biphenyls on urinary metabolites of neurotransmitters: A cross-sectional and longitudinal perspective. Int J Hyg Environ Health 218:452–60. 10.1016/j.ijheh.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 155.Raymond LA (2017) Striatal synaptic dysfunction and altered calcium regulation in Huntington disease. Biochem Biophys Res Commun 483:1051–1062. 10.1016/j.bbrc.2016.07.058 [DOI] [PubMed] [Google Scholar]
- 156.Richardson SD, Plewa MJ, Wagner ED, Schoeny R, Demarini DM (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636:178–242. 10.1016/j.mrrev.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 157.Richardson SD, Thruston AD Jr, Rav-Acha C, Groisman L, Popilevsky I, Juraev O, Glezer V, McKague AB, Plewa MJ, Wagner ED (2003) Tribromopyrrole, brominated acids, and other disinfection byproducts produced by disinfection of drinking water rich in bromide. Environ Sci Technol 37:3782–93. 10.1021/es030339w [DOI] [PubMed] [Google Scholar]
- 158.Rogan WJ (1982) PCBs and cola-colored babies: Japan, 1968, and Taiwan, 1979. Teratology 26:259–261. 10.1002/tera.1420260307 [DOI] [PubMed] [Google Scholar]
- 159.Roman ÁC, Carvajal-Gonzalez JM, Merino JM, Mulero-Navarro S, Fernández-Salguero PM (2018) The aryl hydrocarbon receptor in the crossroad of signalling networks with therapeutic value. Pharmacol Ther 185:50–63. 10.1016/j.pharmthera.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 160.Rosenquist AH, Hoyer BB, Julvez J, Sunyer J, Pedersen HS, Lenters V, Jonsson BAG, Bonde JP, Toft G (2017) Prenatal and Postnatal PCB-153 and p,p’-DDE Exposures and Behavior Scores at 5–9 Years of Age among Children in Greenland and Ukraine. Environ Health Perspect 125(10):107002 10.1289/EHP553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sable HJK, Schantz SL (2006) Executive Function following Developmental Exposure to Polychlorinated Biphenyls (PCBs): What Animal Models Have Told Us In: Levin ED & Buccafusco JJ (eds.) Animal Models of Cognitive Impairment. Boca Raton (FL) https://www.ncbi.nlm.nih.gov/books/NBK2531/ [PubMed] [Google Scholar]
- 162.Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA (2012) Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ Health Perspect 120:904–909. 10.1289/ehp.1104372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA (2010) Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol 171:593–601. 10.1093/aje/kwp427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Samsó M, Feng W, Pessah IN, Allen PD (2009) Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol 7(4):e85 10.1371/journal.pbio.1000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schantz SL, Moshtaghian J, Ness DK (1995) Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation. Fundam Appl Toxicol 26:117–26. 10.1006/faat.1995.1081 [DOI] [PubMed] [Google Scholar]
- 166.Schantz SL, Widholm JJ, Rice DC (2003) Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111:357–576. 10.1289/ehp.5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schantz SL, Gasior DM, Polverejan E, McCaffrey RJ, Sweeney AM, Humphrey HE, Gardiner JC (2001) Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environ. Health Perspect. 109:605–611. 10.1289/ehp.01109605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Secretariat of the Stockholm (2008) Secretariat of the Stockholm Convention Stockholm Convention – Protecting Human Health and the Environment from Persistent Organic Pollutants. http://chm.pops.int/Home/tabid/2121/mctl/ViewDetails/EventModID/1126/EventID/468/xmid/6922/Default.aspx
- 169.Secretariat of the Stockholm (2014) Secretariat of the Stockholm Convention The New POPs under the Stockholm Convention. http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx
- 170.Seegal RF (1996) Epidemiological and laboratory evidence of PCB-induced neurotoxicity. Crit Rev Toxicol 26:709–737. 10.3109/10408449609037481 [DOI] [PubMed] [Google Scholar]
- 171.Seegal RF, Bush B, Brosch KO (1991) Comparison of effects of Aroclors 1016 and 1260 on non-human primate catecholamine function. Toxicology 66:145–63. 10.1016/0300-483X(91)90215-M [DOI] [PubMed] [Google Scholar]
- 172.Seegal RF, Bush B, Brosch KO (1994) Decreases in dopamine concentrations in adult non-human primate brain persist following removal from polychlorinated biphenyls. Toxicology 86:71–87. 10.1016/0300-483X(94)90054-X [DOI] [PubMed] [Google Scholar]
- 173.Seegal RF, Bush B, Shain W (1990) Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol Appl Pharmacol 106:136–44. 10.1016/0041-008X(90)90113-9 [DOI] [PubMed] [Google Scholar]
- 174.Seegal RF, Marek KL, Seibyl JP, Jennings DL, Molho ES, Higgins DS, Factor SA, Fitzgerald EF, Hills EA, Korrick SA, Wolff MS, Haase RF, Todd AC, Parsons P, McCaffrey RJ (2010) Occupational exposure to PCBs reduces striatal dopamine transporter densities only in women: a beta-CIT imaging study. Neurobiol Dis 38:219–25. 10.1016/j.nbd.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Seegal RF, Okoniewski RJ, Brosch KO, Bemis JC (2002) Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: an in vivo microdialysis study. Environ Health Perspect 110:1113–7. 10.1289/ehp.021101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shain W, Bush B, Seegal R (1991) Neurotoxicity of polychlorinated biphenyls: structure-activity relationship of individual congeners. Toxicol Appl Pharmacol 111:33–42. 10.1016/0041-008X(91)90131-W [DOI] [PubMed] [Google Scholar]
- 177.Shaul NJ, Dodder NG, Aluwihare LI, Mackintosh SA, Maruya KA, Chivers SJ, Danil K, Weller DW, Hoh E (2015) Nontargeted biomonitoring of halogenated organic compounds in two ecotypes of bottlenose dolphins (Tursiops truncatus) from the Southern California Bight. Environ Sci Technol 49:1328–38. 10.1021/es505156q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shelton JF, Hertz-Picciotto I, Pessah IN (2012) Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environ Health Perspect 120, 944–51. 10.1289/ehp.1104553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N, Nawrot TS, Schoeters G (2013) Prenatal exposure to environmental contaminants and behavioural problems at age 7–8years. Environ Int 59:225–231. 10.1016/j.envint.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 180.Skovgaard AM, Houmann T, Landorph SL, Christiansen E (2004) Assessment and classification of psychopathology in epidemiological research of children 0–3 years of age: a review of the literature. Eur Child Adolesc Psychiatry 13:337–346. 10.1007/s00787-004-0393-z [DOI] [PubMed] [Google Scholar]
- 181.Stahl L, Snyder B, Olsen A, and Pitt J (2009). Contaminants in fish tissue from US lakes and reservoirs: A National Probabilistic Study. Environ Monit Assess 150:3–19. 10.1007/s10661-008-0685-8 [DOI] [PubMed] [Google Scholar]
- 182.Stamou M, Streifel KM, Goines PE, Lein PJ (2013) Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol Teratol 36:3–16. 10.1016/j.ntt.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Steenland K, Hein MJ, Cassinelli RT 2nd, Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM, Schnorr TM (2006) Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology 17:8–13. 10.1097/01.ede.0000190707.51536.2b [DOI] [PubMed] [Google Scholar]
- 184.Stewart P, Fitzgerald S, Reihman J, Gump B, Lonky E, Darvill T, Pagano J, Hauser P (2003) Prenatal PCB exposure, the corpus callosum, and response inhibition. Environ Health Perspect 111: 1670–1677. 10.1289/ehp.6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J (2005) Response inhibition at 8 and 9 ½ years of age in children prenatally exposed to PCBs. Neurotoxicol Teratol 27:771–780. 10.1016/j.ntt.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 186.Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T (2008) The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect 116:1416–1422. https://dx.doi.org/10.1289%2Fehp.11058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J (2003) Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol Teratol 25:11–22. 10.1016/S0892-0362(02)00320-3 [DOI] [PubMed] [Google Scholar]
- 188.Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J (2006) Response inhibition during Differential Reinforcement of Low Rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ Health Perspect 114:1923–1929. 10.1289/ehp.9216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Strom M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, Halldorsson TI (2014) Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes--a prospective study with long-term follow-up. Environ Int 68:41–48. 10.1016/j.envint.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 190.Suzuki YJ, 6 Ford GD (1999). Redox regulation of signal transduction in cardiac and smooth muscle. J. Mol Cell Cardiol 31:345–353. 10.1006/jmcc.1998.0872 [DOI] [PubMed] [Google Scholar]
- 191.Tatsuta N, Nakai K, Murata K, Suzuki K, Iwai-Shimada M, Kurokawa N, Hosokawa T, Satoh H (2014) Impacts of prenatal exposures to polychlorinated biphenyls, methylmercury, and lead on intellectual ability of 42-month-old children in Japan. Environ Res 133: 321–326. 10.1016/j.envres.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 192.Tilson HA, Kodavanti PR (1997) Neurochemical effects of polychlorinated biphenyls: an overview and identification of research needs. Neurotoxicology 18:727–43. [PubMed] [Google Scholar]
- 193.Traglia M, Croen LA, Lyall K, Windham GC, Kharrazi M, DeLorenze GN, Torres AR, Weiss LA (2017) Independent Maternal and Fetal Genetic Effects on Midgestational Circulating Levels of Environmental Pollutants. G3 (Bethesda) 7:1287–1299. 10.1534/g3.117.039784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Trilivas I, Brown JH (1989) Increases in intracellular Ca2+ regulate the binding of [3H]phorbol 12,13-dibutyrate to intact 1321N1 astrocytoma cells. J Biol Chem 264:3102–7. [PubMed] [Google Scholar]
- 195.Ulbrich B, Stahlmann R (2004) Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol 78:252–68. 10.1007/s00204-003-0519-y [DOI] [PubMed] [Google Scholar]
- 196.Varghese M, Keshav N, Jacot-Descombes S, Warda T, Wicinski B, Dickstein DL, Harony-Nicolas H, De Rubeis S, Drapeau E, Buxbaum JD, Hof PR. Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol. 2017. October;134(4):537–566. doi: 10.1007/s00401-017-1736-4. 10.1007/s00401-017-1736-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Verner MA, Hart JE, Sagiv SK, Bellinger DC, Altshul LM, Korrick SA (2015) Measured Prenatal and Estimated Postnatal Levels of Polychlorinated Biphenyls (PCBs) and ADHD-Related Behaviors in 8-Year-Old Children. Environ Health Perspect 123:888–894. 10.1289/ehp.1408084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Verner MA, Plusquellec P, Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL, Charbonneau M, Haddad S (2010) Alteration of infant attention and activity by polychlorinated biphenyls: unravelling critical windows of susceptibility using physiologically based pharmacokinetic modeling. Neurotoxicology 31:424–431. 10.1016/j.neuro.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 199.Vetter W, Alder L, Kallenborn R, Schlabach M (2000) Determination of Q1, an unknown organochlorine contaminant, in human milk, Antarctic air, and further environmental samples. Environ Pollut 110:401–9. 10.1016/S0269-7491(99)00320-6 [DOI] [PubMed] [Google Scholar]
- 200.Villa A, Vegeto E, Poletti A, Maggi A (2016) Estrogens, Neuroinflammation, and Neurodegeneration. Endocr Rev 37:372–402. 10.1210/er.2016-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vreugdenhil HJ, Lanting CI, Mulder PG, Boersma ER, Weisglas-Kuperus N (2002) Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J Pediatr 140:48–56. 10.1067/mpd.2002.119625 [DOI] [PubMed] [Google Scholar]
- 202.Wang MD, Little J, Gomes J, Cashman NR, Krewski D (2017) Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology. 61:101–130. 10.1016/j.neuro.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 203.Wayman GA, Bose DD, Yang D, Lesiak A, Bruun D, Impey S, Ledoux V, Pessah IN, Lein PJ (2012) PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ Health Perspect 120:1003–9. 10.1289/ehp.1104833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun D, Pessah IN, Lein PJ (2012). PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms. Environ Health Perspect 120:997–1002. 10.1289/ehp.1104833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Weisskopf MG, Knekt P, O’Reilly EJ, Lyytinen J, Reunanen A, Laden F, Altshul L Ascherio A (2012) Polychlorinated biphenyls in prospectively collected serum and Parkinson’s disease risk. Mov Disord 27:1659–1665. 10.1002/mds.25217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wheeler MA, Rothhammer V, Quintana FJ (2017) Control of immune-mediated pathology via the aryl hydrocarbon receptor. J Biol Chem 292:12383–12389. 10.1074/jbc.R116.767723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.White RF, Palumbo CL, Yurgelun-Todd DA, Heaton KJ, Weihe P, Debes F, Grandjean P (2011) Functional MRI approach to developmental methylmercury and polychlorinated biphenyl neurotoxicity. Neurotoxicology 32:975–80. 10.1016/j.neuro.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Williams GR (2008) Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20:784–94. 10.1111/j.1365-2826.2008.01733.x [DOI] [PubMed] [Google Scholar]
- 209.Winneke G, Bucholski A, Heinzow B, Kramer U, Schmidt E, Walkowiak J, Wiener JA, Steingruber HJ (1998) Developmental neurotoxicity of polychlorinated biphenyls (PCBS): cognitive and psychomotor functions in 7-month old children. Toxicol Lett 102-103:423–428. 10.1016/S0378-4274(98)00334-8 [DOI] [PubMed] [Google Scholar]
- 210.Winneke G, Walkowiak J, Lilienthal H (2002) PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology 181-182:161–165. 10.1016/S0300-483X(02)00274-3 [DOI] [PubMed] [Google Scholar]
- 211.Wong PW, Brackney WR, Pessah IN (1997) Ortho-substituted polychlorinated biphenyls alter microsomal calcium transport by direct interaction with ryanodine receptors of mammalian brain. J Biol Chem. 272:15145–53. 10.1074/jbc.272.24.15145 [DOI] [PubMed] [Google Scholar]
- 212.Wong PW, Joy RM, Albertson TE, Schantz SL, Pessah IN (1997) Ortho-substituted 2,2’,3,5’,6-pentachlorobiphenyl (PCB 95) alters rat hippocampal ryanodine receptors and neuroplasticity in vitro: evidence for altered hippocampal function. Neurotoxicology 18:443–56. [PubMed] [Google Scholar]
- 213.Wong PW, Pessah IN (1997) Noncoplanar PCB 95 alters microsomal calcium transport by an immunophilin FKBP12-dependent mechanism. Mol Pharmacol 51:693–702. 10.1124/mol.51.5.693 [DOI] [PubMed] [Google Scholar]
- 214.Wong PW, Pessah IN (1996) Ortho-substituted polychlorinated biphenyls alter calcium regulation by a ryanodine receptor-mediated mechanism: structural specificity toward skeletal- and cardiac-type microsomal calcium release channels. Mol Pharmacol 49:740–51. http://molpharm.aspetjournals.org/content/49/4/740/tab-article-info [PubMed] [Google Scholar]
- 215.Woods R, Vallero RO, Golub M, Suarez JK, Ta TA, Yasui DH, Chi L-H, Kostyniak PJ, Pessah IN, Berman RF, LaSalle JM (2012) Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum Mol Genet 21, 2399–411. 10.1093/hmg/dds046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xia R, Stangler T, Abramson JJ (2000) Skeletal muscle ryanodine receptor is a redox sensor with a well defined redox potential that is sensitive to channel modulators. J Biol Chem 275:36556–61. 10.1074/jbc.M007613200 [DOI] [PubMed] [Google Scholar]
- 217.Yang D, Kania-Korwel I, Ghogha A, Chen H, Stamou M, Bose DD, Pessah IN, Lehmler HJ, Lein PJ (2014). PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci 138:379–92. 10.1093/toxsci/kft334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ (2009) Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect 117:426–35. 10.1289/ehp.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yang D, Lein PJ (2010) Polychlorinated biphenyls increase apoptosis in the developing rat brain. Curr Neurobiol 1:70–76. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3775291/#__ffn_sectitle [PMC free article] [PubMed] [Google Scholar]
- 220.Yang JH, Kodavanti PR (2001) Possible molecular targets of halogenated aromatic hydrocarbons in neuronal cells. Biochem Biophys Res Commun 280:1372–7. 10.1006/bbrc.2001.4283 [DOI] [PubMed] [Google Scholar]
- 221.Yang M, Zhang X (2014) Halopyrroles: a new group of highly toxic disinfection byproducts formed in chlorinated saline wastewater. Environ Sci Technol 48:11846–52. 10.1021/es503312k [DOI] [PubMed] [Google Scholar]
- 222.Yuen B, Boncompagni S, Feng W, Yang T, Lopez JR, Matthaei KI, Goth SR, Protasi F, Franzini-Armstrong C, Allen PD, Pessah IN (2012) Mice expressing T4826I-RYR1 are viable but exhibit sex- and genotype-dependent susceptibility to malignant hyperthermia and muscle damage. FASEB J 26:1311–22. 10.1096/fj.11-197582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zahalka EA, Ellis DH, Goldey ES, Stanton ME, Lau C (2001) Perinatal exposure to polychlorinated biphenyls Aroclor 1016 or 1254 did not alter brain catecholamines nor delayed alternation performance in Long-Evans rats. Brain Res Bull 55:487–500. 10.1016/S0361-9230(01)00548-2 [DOI] [PubMed] [Google Scholar]
- 224.Zhang H, Yolton K, Webster GM, Sjodin A, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A (2017) Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Environ Health Perspect 125: 746–752. 10.1289/EHP478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang R, Pessah IN (2017) Divergent mechanisms leading to signaling dysfunction in embryonic muscle by bisphenol A and tetrabromobisphenol A. Mol Pharmacol 91:428–436. 10.1124/mol.116.107342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zheng J, McKinnie SMK, El Gamal A, Feng W, Dong Y, Agarwal V, Fenical W, Kumar A, Cao Z, Moore BS, Pessah IN (2018) Organohalogens naturally biosynthesized in marine environments and produced as disinfection byproducts alter sarco/endoplasmic reticulum Ca2+ dynamics. Environ Sci Technol 52:5469–5478. 10.1021/acs.est.8b00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zoeller RT, Dowling AL, Vas AA (2000) Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology 141:181–9. 10.1210/endo.141.1.7273 [DOI] [PubMed] [Google Scholar]