Abstract
Rationale:
Crohn disease includes 3 phenotypes, inflammatory, stricturing, and penetrating. In cases where corticosteroids and immunosuppressive agents are not suitable treatment options, enteral nutrition (EN) can be used to reduce disease severity and enhance barrier defense with fewer potential adverse effects.
Patient concerns:
A 23-year-old man with abdominal pain and diarrhea presented at our hospital in 2014. The frequency of defecation was 3 or 4 times a day without mucus or blood in the stool. His body mass index was 15.8, and in laboratory tests the erythrocyte sedimentation rate was 42.4 mm/h, serum C reactive protein was 65.2 mg/L, the leukocyte count was 11.64 × 109/L, and hemoglobin was 111 g/L.
Diagnosis:
In computed tomography (CT) enterography the ascending colon was thickened, and there was effusion and enlarged lymph nodes around the colon. Colonoscopy revealed ulcer, polypoid proliferation, and bowel stenosis in many segments. Chronic inflammation was evident in multiple biopsies. Crohn disease was diagnosed based on the above observations.
Interventions:
Mesalazine was administered at a dose of 4 g daily for 2 years. The patient was hospitalized again due to severe abdominal pain and ongoing fever. Intestinal perforation was detected via CT. Percutaneous drainage was performed followed by administration of intravenous metronidazole (0.5 g) and ciprofloxacin (0.2 g) twice a day. Peptison liquid was used as exclusive EN. After 2 weeks the antibiotics regimen was changed to metronidazole 0.4 g twice a day and ciprofloxacin 0.25 g 3 times a day, both administered orally.
Outcomes:
CT revealed that the infection was eliminated and the fistula was healed after 10 weeks, at which point antibiotics and exclusive EN was discontinued. Azathioprine was prescribed at a dose of 2 mg/kg daily to maintain clinical remission. The patient did not report any pain or diarrhea at a 1-year follow-up visit.
Lessons:
The present case suggests that exclusive EN combined with antibiotics is useful in inducing remission in Crohn disease patients with active disease and penetrating complications.
Keywords: abscess, Crohn disease, enteral nutrition, intestinal perforation
1. Introduction
Intra-abdominal fistula is found in more than a third of patients with Crohn disease (CD).[1] These patients often require hospitalization and surgery. Enteral nutrition (EN) has been used to induce remission in active CD patients, and can reportedly contribute to maintaining remission of quiescent CD by reducing the release of inflammatory cytokines and promoting mucosal healing.[2,3] Exclusive EN (EEN) can effectively reduce the need for surgery in patients with stricturing or penetrating complications.[3] It may also improve the effects of immunosuppressants and biologics as an adjunctive therapy.[4,5]
This case report describes the treatment of a young man presenting with intestinal perforation and abscess. Antibiotics combined with EEN were used after percutaneous drainage to induce remission in the patient, followed by oral azathioprine (AZA) to maintain remission.
2. Case presentation
A 23-year-old man with abdominal pain and diarrhea was admitted to the in-patient service at a Department of Gastroenterology in September 2014. The symptoms had reportedly been present for 2 months. The frequency of defecation was 3 or 4 times a day, and there was no mucus or blood in the stool. No family history of inflammatory bowel disease was reported by the patient. His body mass index (BMI) was 15.8 at the time of admission. Follow-up workup included computed tomography (CT) enterography (CTE), colonoscopy, and regular blood tests. The erythrocyte sedimentation rate (ESR) was 42.4 mm/h, serum C-reactive protein (CRP) was 65.2 mg/L, the leukocyte count was 11.64 × 109/L, and hemoglobin was 111 g/L. In CTE the ascending colon was thickened, and there was effusion and enlarged lymph nodes around the colon. Segmental lesions were detected via colonoscopy. Polypoid proliferation was found 45 to 48 cm from the anus and was more severe at 60 cm. Bowel stenosis was found approximately 70 cm from the anus. Chronic inflammation was evident in multiple biopsies. No erosions or ulcers were detected via peroral small bowel endoscopy. A diagnosis of CD was made, with a Clinical Disease Activity Index (CDAI) score of 240. The patient refused to take corticosteroids or biological agents reportedly based on consideration of potential side effects. Mesalazine was then administered at a dose of 1 g 4 times a day for 2 years. His abdominal pain was relieved, but his defecation pattern was similar to that on initial admission.
The patient was readmitted to the in-patient service in September 2016 due to severe abdominal pain and ongoing fever that he reported he had been experiencing for 1 week. His temperature was >39 °C. Masses could be felt via palpation at the right groin area and right posterior superior iliac crest. ESR and CRP were substantially elevated, and hemoglobin was reduced (Table 1). An abscess measuring approximately 9.1 × 4.0 cm was detected via abdominal ultrasonography. Two radiologists diagnosed the patient with penetrating CD independently, based on CT-depicted thickening and mucosa disruption of the ascending colon and distal ileum, and accumulation of gas and fluid mainly in the right abdomen. These are reliable radiological indicators of intestinal perforation. Because the boundaries between the right colon, ileum, and the iliopsoas or psoas muscle were not clear, it was difficult to identify the precise location of the perforation (Fig. 1). The CDAI score increased to 420. Percutaneous drainage was performed, followed by the administration of intravenous metronidazole (0.5 g) and ciprofloxacin (0.2 g) twice a day. Peptison liquid (Nutricia Company, Wuxi, China) was used as EEN via nasogastric feeding to avoid stimulation derived from food antigens. The total volume of the liquid administered daily was 1500 mL, which potentially provided 1500 kcal per day. After 2 weeks the antibiotics regimen was changed to metronidazole 0.4 g twice a day and ciprofloxacin 0.25 g 3 times a day, both administered orally.
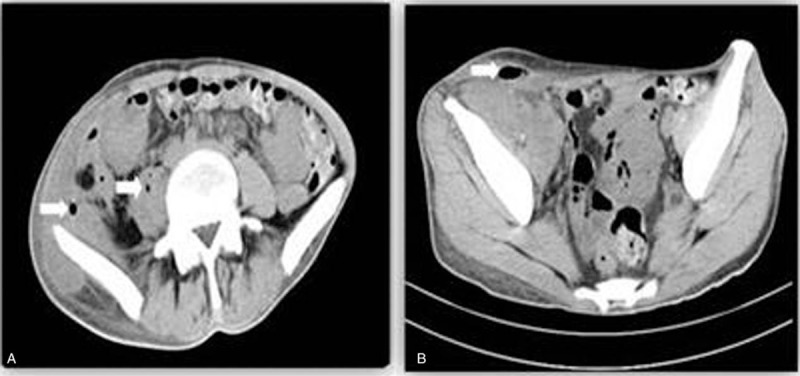
Table 1.
BMI and blood test results before and after the combined treatment.
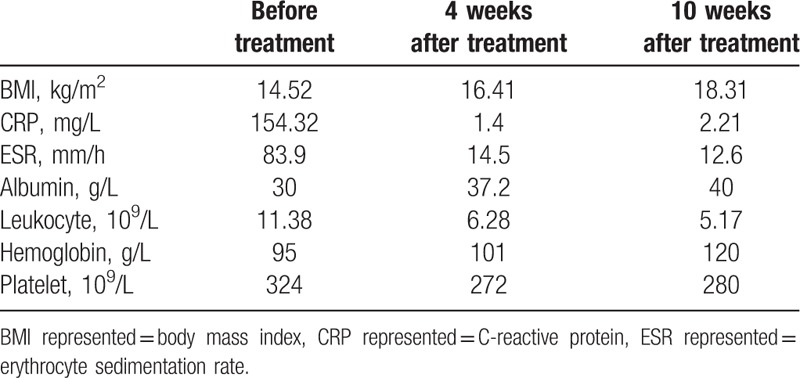
Figure 1.
The performance of intestinal perforation on CTE: thickening and mucosa disruption of the ascending colon and distal ileum, and accumulation of gas and fluid mainly in the right abdomen. CTE = CT enterography.
CT confirmed the disappearance of gas and shrinkage of the abscess 4 weeks after the treatment. CRP and ESR had decreased substantially and the anemia had improved (Table 1). The patient's weight increased by >10%, and his CDAI score decreased to 276. The above-described observations suggested that the treatment was effective in inducing remission, and that corticosteroids may not be needed in some cases. With regard to maintenance treatment, both anti-tumor necrosis factor agents and AZA were offered to the patient. Due to higher cost, the patient refused infliximab. Peptison liquid was increased to 2000 mL a day and AZA was administered at a daily dose of 1 mg/kg. The leukocyte count was not reduced 2 weeks later, so the daily dose of AZA was increased to 1.5 mg/kg.
CTE was performed 10 weeks after the initial treatment, and it revealed segmental thickening and stricture in the terminal ileum with non-uniform enhancement on the wall (Fig. 2). There was no comb sign. Effusion was limited and the right iliopsoas and psoas muscles were swollen. The CDAI score was <150. Because infection was eliminated and the fistula had healed, antibiotics and EEN were terminated. AZA was prescribed at a dose of 2 mg/kg daily, to maintain clinical remission. The patient did not report any pain or diarrhea at a 1-year follow-up visit.
Figure 2.
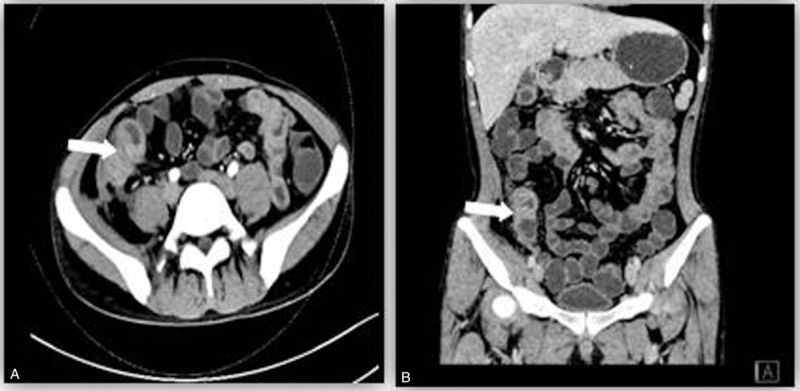
The improvement of intestinal lesion on CTE: the disappearance of gas and abscess after treatment. Segmental thickening and stricture could be found in the terminal ileum with non-uniform enhancement on the wall. CTE = CT enterography.
3. Discussion and conclusions
The active luminal inflammation associated with CD may lead to severe complications, which may reduce intestinal absorption and cause malnutrition. EEN has positive benefits with regard to controlling inflammation and improving overall nutritional status. It is useful for reducing bowel wall edema, relieving inflammatory stricture, and promoting fistula closure.[6,7]
Percutaneous drainage followed by antibiotics has been recommended as first-line treatment if an abdominal abscess is formed.[8] If a patient does not respond to this treatment, additional surgery or ostomy is needed. Notably however, patients often decline surgery due to the risk of postoperative complications and a comparatively high recurrence rate. Corticosteroids and immunosuppressive agents are not suitable in such circumstances because they can lead to heavy infection, then the subsequent combined use of antibiotics and EEN may have synergistic effects on inflammation.[3]
More than 50% of patients with active CDA exhibit a negative nitrogen balance.[9] Lower BMI indicates malnutrition, and it appears to be a severe risk factor for a poor prognosis.[2] Previous reports suggest that EEN can be useful for inducing clinical, biochemical, and radiological remission in patients with intestinal fistula/abdominal abscess.[7,10] The therapy is safe, and it is associated with improved quality of life. It can provide complete nutritional support in patients with distal fistula.[2,11,12] In the present case Peptison liquid was applied. It is a polymeric diet including short-chain peptide, vegetable oil, medium-chain triglycerides, and maltose. Because there was no substantial difference in efficacy between an elemental and a non-elemental diet,[13] this enteral formula was suitable for the present patient.
The mechanisms of action of EEN in the management of adults or children with abdominal phlegmon involve anti-inflammatory effects on the mucosa, and modification of the intestinal microenvironment.[2,14] Good nutritional status is associated with improvement of systemic immune responses and the facilitation of fistula closure.[11,15] In the present case abscess drainage and antibiotics were the main components of the treatment, and EEN played an assistive role. Disease severity was reduced and fistula closure was achieved after the combined treatment. The improved nutritional status was confirmed via assessments of hemoglobin and serum albumin levels.
This report is limited in that it only describes the outcome of treatment in a single young man. The benefits of EEN in CD patients with a dysfunctional intestinal barrier are yet to be clarified. Combined use of EN therapy may be a valid option to consider in patients with active CD and complex complications. Further prospective studies are needed to evaluate the effects of EEN.
Acknowledgments
The authors acknowledge Dr Owen Proudfoot of Editage for editing the final English draft of this manuscript prior to submission to the journal.
Author contributions
Data curation: Chenxi Xie, Jinzhou Lin, Jingling Su.
Formal analysis: Chenxi Xie, Jinzhou Lin.
Funding acquisition: Jianlin Ren.
Investigation: Chenxi Xie, Jingling Su.
Methodology: Jinzhou Lin.
Writing – original draft: Chenxi Xie.
Writing – review & editing: Jianlin Ren.
Footnotes
Abbreviations: AZA = azathioprine, BMI = body mass index, CD = Crohn disease, CDAI = clinical disease activity index, CRP = C reactive protein, CTE = CT enterography, EEN = exclusive enteral nutrition, EN = enteral nutrition, ESR = erythrocyte sedimentation rate, IBD = inflammatory bowel disease, PN = parenteral nutrition.
Ethics approval and consent to participate: This case report does not include personally identifying information throughout. Accordingly, formal approval from the Ethics Committee of Zhongshan Hospital, Xiamen University was not required. Written informed consent was obtained from the patient.
Consent for publication: We have obtained data release authorization from the patient to report the data in this article.
The datasets used and/or analyzed are available from the corresponding author on reasonable request.
There were no funding sources.
The authors have no conflicts of interest to disclose.
References
- [1].Schwartz DA, Loftus EJ, Tremaine WJ, et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology 2002;122:875–80. [DOI] [PubMed] [Google Scholar]
- [2].Forbes A, Escher J, Hébuterne X, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr 2017;36:321–47. [DOI] [PubMed] [Google Scholar]
- [3].Heerasing N, Thompson B, Hendy P, et al. Exclusive enteral nutrition provides an effective bridge to safer interval elective surgery for adults with Crohn's disease. Aliment Pharmacol Ther 2017;45:660–9. [DOI] [PubMed] [Google Scholar]
- [4].Cerqueira RM, Lago PM. Clinical factors predictive of Crohn's disease complications and surgery. Eur J Gastroenterol Hepatol 2013;25:129–34. [DOI] [PubMed] [Google Scholar]
- [5].Gomollon F, Dignass A, Annese V, et al. 3Rd European evidence-based consensus on the diagnosis and management of Crohn's Disease 2016: Part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- [6].Hu D, Ren J, Wang G, et al. Exclusive enteral nutritional therapy can relieve inflammatory bowel stricture in Crohn's disease. J Clin Gastroenterol 2014;48:790–5. [DOI] [PubMed] [Google Scholar]
- [7].Yang Q, Gao X, Chen H, et al. Efficacy of exclusive enteral nutrition in complicated Crohn's disease. Scand J Gastroenterol 2017;52:995–1001. [DOI] [PubMed] [Google Scholar]
- [8].Gionchetti P, Dignass A, Danese S, et al. 3Rd European evidence-based consensus on the diagnosis and Management of Crohn's disease 2016: Part 2: surgical management and special situations. J Crohns Colitis 2017;11:135–49. [DOI] [PubMed] [Google Scholar]
- [9].Patel KV, Darakhshan AA, Griffin N, et al. Patient optimization for surgery relating to Crohn's disease. Nat Rev Gastroenterol Hepatol 2016;13:707–19. [DOI] [PubMed] [Google Scholar]
- [10].Zhu Y, Xu L, Liu W, et al. Safety and efficacy of exclusive enteral nutrition for percutaneously undrainable abdominal abscesses in Crohn's disease. Gastroenterol Res Pract 2017;2017:6360319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Forbes A, Goldesgeyme E, Paulon E. Nutrition in inflammatory bowel disease. JPEN J Parenter Enteral Nutr 2011;35:571–80. [DOI] [PubMed] [Google Scholar]
- [12].Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- [13].Smith MA, Smith T, Trebble TM. Nutritional management of adults with inflammatory bowel disease: practical lessons from the available evidence. Frontline Gastroenterol 2012;3:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Day AS, Brown SC. The adjunctive role of nutritional therapy in the management of phlegmon in two children with Crohn's disease. Front Pediatr 2017;5:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yan D, Ren J, Wang G, et al. Predictors of response to enteral nutrition in abdominal enterocutaneous fistula patients with Crohn's disease. Eur J Clin Nutr 2014;68:959–63. [DOI] [PubMed] [Google Scholar]


