Abstract
Osteoarthritis is the most frequently diagnosed disease of the musculoskeletal system. Growing number of patients waiting for surgical treatment and the possible negative consequences resulting from long-term pharmacological therapy lead to the search for non-pharmacological methods aimed at alleviating pain and reducing doses of analgesics, among them physical therapy with use of magnetic fields.
The study involved 30 men aged 49 to 76 (mean age, 61.7 years) treated for idiopathic osteoarthritis of the hip joint. The subjects were divided into 2 groups (15 patients each) and underwent a cycle of magnetostimulation and magnetoledtherapy procedures, respectively. During the exposure cycle concentrations of β-endorphin were assessed 3 times and the mood was assessed 2 times. In addition, the assessment of pain intensity and the dose of analgesic drugs was performed before and after the end of therapy.
Statistically significant increase in plasma β-endorphins concentration was observed in both groups of patients (magnetostimulation—P < .01 vs magnetoledtherapy—P < .001). In the assessment of mood of respondents, no statistically significant differences were found. Significant reduction in intensity of perceived pain was observed in both groups of patients (P < .05). In the group of patients who underwent magnetoledtherapy cycle, the analgesic drug use was significantly lower by 13% (P < .05) as compared with initial values, which was not noted in group of patients who underwent magnetostimulation procedures.
The use of magnetic field therapy in the treatment of men with idiopathic osteoarthritis of hip joints causes a statistically significant increase in the concentration of plasma β-endorphins resulting in statistically significant analgesic effect in both magnetostimulation and magnetoledtherapy treated groups of patients, with accompanying decrease of need for analgetic drugs in magnetoledtherapy group, but without any significant changes regarding the patient's mood.
Keywords: β-endorphins, magnetic field therapy, magnetoledtherapy, magnetostimulation, mood, osteoarthritis of the hip joint, pain intensity
1. Introduction
Osteoarthritis is a non-inflammatory process with a chronic character and a multifactorial etiology. In its course, as a result of disturbed regenerative processes of the subchondral layer and joint cartilage, proliferative lesions (osteophytes) in joints occur, which lead to limitation of joint mobility with accompanying severe pain, mainly during movements but pain may also occur at rest.[1]
Osteoarthritis of hip joint (hip OA) affects approximately 3.2 million people around the world. In the population over 65 years of age, it is the most common cause of disability after cardiovascular disease. About 30% of patients with hip OA suffer from lesions located in both joints.[2] Arthrosis in joints of hand is more often found in younger women (50–55 years), while osteoarthritis of knee and hip joint is much more common in patients aged 70 to 75.[3] Obesity is an important risk factor contributing to the development and occurrence of early degenerative changes in knee joints.[4] Diagnosis of hip OA based on physical examination and confirmed by x-ray examination (pelvis + hip joints) is not synonymous with the final qualification of the patient to arthroplasty.[5] For advanced forms of this disease, the most efficient method of treatment is total hip arthroplasty.[6,7] In patients not qualified for arthroplasty due to numerous health problems of internal organs, a conservative treatment with use of analgesics and physiotherapy is applied. Effectiveness of physiotherapy in the treatment of pain in the course of degenerative disease depends to a large extent on the knowledge and selection of guidelines on the basis of which physicians recommend a visit to a physiotherapist.[8] Choice of a specific method of physical therapy or physiotherapy exercises should always take into account both the clinical condition of the patient and the physiotherapist's experience.[9] The use of analgesics improves the quality of life of patients but their long-term application can lead to many side effects (toxic effects on the liver and kidneys, hematopoietic system, and gastrointestinal mucosa), and also involves the cost of medication. Thorlund et al[10] showed that chronic (over 1 year) use of analgesics in patients with osteoarthritis of knee and hip joints affected the exacerbation of symptoms of comorbidities in the treated group of patients. Acute bleeding from the upper gastrointestinal tract is an acute complication of the chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs) in analgesic therapy, which is a life-threatening condition. Every year in the United States, 41,000 patients over 65 years are hospitalized, while in the Netherlands >5000 patients, with gastrointestinal bleeding caused by NSAIDs abuse, >10% of patients do not survive. The unit cost of treatment of this complication of analgesic pharmacotherapy was calculated at almost 43,000 Euro. In the light of the above data, the continuous search for non-invasive, safe, and cheap therapeutic methods that are an alternative to pharmacological methods of treatment of osteoarthritis appears to be fully justified.
Combining the calculation of costs of magnetic field treatment with the pain costs of paracetamol 1500 mg per day, it can be assumed that direct costs of both forms of therapy are comparable, while in the case of treatment with paracetamol (the basic painkiller) the cost of treatment should be additionally taken into account when calculating the total cost of therapy inflammatory and hemorrhagic disorders in the upper gastrointestinal tract and hematological complications.
β-endorphins are the opioid substances produced by the organism—derivatives of morphine (endogenous opiates), which on the one hand show an analgesic effect due to eliminating pain sensation by reducing the amount of nociceptive action potentials, and on the other hand—they just like opium can cause euphoria. Chemical composition of the so-called “happiness hormone” consists of at least 20 different endorphins called molecules of emotions. They are produced in the brain and spinal cord under the influence of stress stimuli. In case of disease, they effect on emotional and immune system of a human being, stimulating to fight the disease and being, in a sense, the body's response to the treatment.[11,12]
Chronic diseases of the musculoskeletal system with persistent pain, which lasts for years, and surgical procedures can cause depression, mood disorders, or anxiety. Each patient responds to the disease in an individual way, and negative reactions occur in varying degrees. Some of them may develop into chronic neurotic or depressive syndromes that occur even when the physical health prognosis is good.[13] In the perioperative period, a mood depression can be observed, which may be the result of the patients’ perception of the fact of surgery, analysis of the meaning of life, and their own situation. High severity of anxious-depressive reactions, exceeding adaptive abilities of patients may contribute to complications in the course of treatment and rehabilitation.[13,14]
Nowadays, the fight against pain has become one of the most common forms of doctor's activity. For complete elimination or significant reduction of pain perceived by patients, pharmacological (oral or intra-articular), surgical, and other methods of analgesic therapy are used. According to current trends in hip arthroplasty, total arthroplasty with a short metaphysis pin should be used in standard surgical procedures for patients with osteoarthritis of hip joint (hip OA). Patients awaiting arthroplasty or not eligible for it, for various reasons, may benefit from symptomatic, manual, and physical therapies.[6,15] Among methods of analgesic physical therapy, in medical practice due to confirmed positive influence of the magnetic field on the human body, also magnetic field therapy is applied. Actually the most commonly used magnetic fields have frequencies up to 100 Hz and magnetic field induction values up to 15 mT. Analgesic effects of low frequency magnetic fields are comparable with the analgesic effect of morphine in experimental studies carried out so far.[16,17]
The aim of the study was to compare the therapeutic efficacy of 2 forms of physical therapy with use of variable magnetic field (magnetostimulation and magnetoledtherapy) in the treatment of men suffering from idiopathic osteoarthrosis of hip joint, basing on assessment of plasma β-endorphin concentration, mood, and pain intensity.
2. Material and methods
The consent for the study protocol was obtained from the Bioethical Commission of the Medical University of Silesia in Katowice—permission no. NN-6501-195/I/06/07.
The study involved 30 men aged 49 to 76 years (mean age, 61.7 years) treated for idiopathic hip OA in Specialistic Orthopedic-Traumatic Clinic. The study group consisted of patients awaiting hip arthroplasty, whose pain related to degenerative lesions of 1 hip joint had been present for at least 1 year. Qualification of the above-mentioned patients to the research group was carried out by a physician—specialist in orthopedics, basing on the results of physical examination of the hip joint confirmed by x-ray examination.
Subjects were randomly divided into 2 groups (15 patients each) who were subjected to 15 procedures of magnetostimulation or magnetoledtherapy, respectively.[18,19]
Procedures of magnetostimulation and magnetoledtherapy were carried out 12 minutes daily at the same time in the morning hours, for 3 weeks with a break on Saturdays and Sundays.
Patients from the first group were subjected to magnetostimulation procedures applied to the patient with the use of large clinical ring-shaped applicator of Viofor JPS Standard device (Med&Life, Komorów, Poland) placed in the region of hip joints, which generated the variable magnetic field basing on the mechanism of ionic magnetic resonance.[20] Procedures were carried out using programme—P2 (application of variable magnetic field using JPS system basing on the phenomenon of ionic magnetic resonance, with saw-like shape of basic impulse and frequency of 180–195 Hz, grouped in packages with frequency of 12.5–29 Hz, groups of packages with frequency of 2.8–7.6 Hz, and series with frequency of 0.08–0.3 Hz) mode of application—M2 (application of variable magnetic field with magnetic field intensity increasing cyclically every 10 or 12 seconds from 0.5 to a selected value, during the whole procedure) and intensity—8, which corresponds to mean magnetic induction value of 42.7 μT and peak induction value of 480 μT.
Patients from the second group were subjected to magnetoled therapy procedures with use of local eliptic magnetic-light applicators of Viofor JPS Magnetic Light device (Med&Life, Komorów, Poland) placed in the region of hip joints which generated variable magnetic field with saw-like shape of basic impulse and frequency of 180 to 195 Hz, grouped in packages with frequency of 12.5 to 29 Hz, groups of packages with frequency of 2.8 to 7.6 Hz and series with frequency of 0.08 to 0.3 Hz and mean induction value of 45 μT in the distance of 10 cm from the surface of the applicator with simultaneous emission of light radiation generated by 48 LED diodes in frequency range of red light (634 ± 5 nm) with frequency of light impulses of 181.8 Hz, maximal power of radiation in impulse 880 mW and energy density of 0.48 J/cm2.
Plasma concentration of β-endorphins was assessed in blood samples collected from patients 1 hour before the initial exposure as well as in the morning on the day of last exposure and 2 weeks after the completion of exposure cycle. For the assessment of plasma β-endorphin concentration 4 mL of venous blood was collected from the ulnar vein using a closed Vacutainer system for tubes containing anticoagulant ethylenediaminetetraacetic acid (EDTA) and 0.6 TIU/mL of aprotinin. Plasma obtained from the collected blood samples by centrifugation (2500 rpm for 10 minutes) was transferred to Eppendorf tubes and stored in a freezer at –85 °C until the determinations were made. Determination of β-endorphin concentrations was preceded by the extraction of mentioned peptides from the plasma on SEP-COLUMN C18 columns. Quantitative determination of β-endorphins in the obtained extract was carried out by the radioimmunoenzyme method according to the instructions for the kit (Cat. No. RK-022-14, & #61538; Endorphin [Human] RIA Kit), from manufacturer Phoenix Pharmaceuticals, Inc., CA. Coefficient of variation between 2 lines and within the series was respectively: 8% and 2.6%.
The state of mood was assessed twice: before the beginning and after the end of exposure cycle according to the modified 7-Item Hamilton Depression Rating Scale.
Pain was assessed according to the Husskinson 10-point visual analog scale (VAS) (0 points—the lack of pain, 10 points—the most severe pain that patient had in his life-time). The evaluation of the pain intensity was carried out according to following pattern: 1 hour before the start of the exposure and then 1 hour after the end of the exposure—on the 1st, 7th, 14th, 21st, 28th, and 35th day of the exposure cycle. The last 2 evaluations were carried out on the 7th and 14th day after the end of the exposure cycle.
Amount of analgesic drugs taken daily before and after treatment was also assessed. During the study, patients from both groups took their own analgesics. The dosage of analgesics for each patient was normalized to the amount of his/her uptake before the treatment.
3. Statistical analysis
Statistical analysis was carried out with use of the Statistica 7.1 PL package (StatSoft Polska Sp. z o.o., Kraków, Poland). Values calculated for measurable variables are presented as the arithmetic mean value with standard deviation (SD). Normality of the distribution was checked by the Shapiro-Wilk test and the homogeneity of the variance was checked by the Levene test. Comparative analysis between both groups was made using the Student t test for independent samples and Mann–Whitney U test. In each group of patients the Student t test for dependent samples and Wilcoxon pairs order test were used for comparison of related variables in particular time periods of the study. Analyzed differences at the significance level of P < .05 were considered statistically significant.
4. Results
The characteristics of the clinical state of the subjects from both groups before the beginning of physical therapy cycle are presented in the Table 1.
Table 1.
Characteristics of clinical state of patients with idiopathic hip osteoarthritis from both groups treated with magnetostimulation and magnetoledtherapy respectively, before the therapeutic cycle.
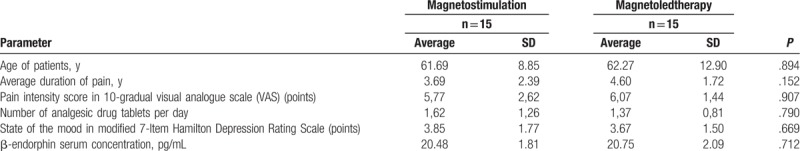
No statistically significant differences of initial values of analyzed parameters were observed between both groups of patients.
Results of determination of plasma concentration of β-endorphins in particular time periods of the study in both groups of magnetic field treated patients are presented in Fig. 1.
Figure 1.
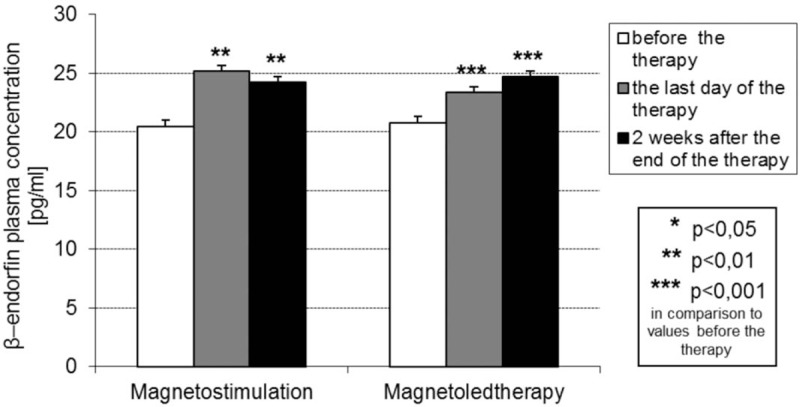
Plasma concentration of β-endorphins (mean value and SD) (pg/mL) in both groups of patients with idiopathic hip osteoarthritis treated with magnetostimulation and magnetoledtherapy respectively, at various time periods of the study.
In both groups of magnetic field treated patients with hip OA the mean value of plasma concentration of β-endorphins on the day of last procedure and also 14 days after the end of the cycle of procedures increased statistically significantly in comparison to the initial values before the beginning of the cycle of procedures. In group of patients subjected to magnetoled therapy procedures the mean value of plasma concentration of β-endorphins on the 14th day after the end of procedures cycle slightly increased as compared with the value on the last day of the exposure cycle, while in group of patients subjected to magnetostimulation procedures the mean value of plasma concentration of β-endorphins on the 14th day after the end of procedures cycle slightly decreased as compared with the value on the last day of the exposure cycle, but the observed differences were not statistically significant.
Results of assessment of the patient's mood in both groups of magnetic field treated patients are presented in Fig. 2.
Figure 2.
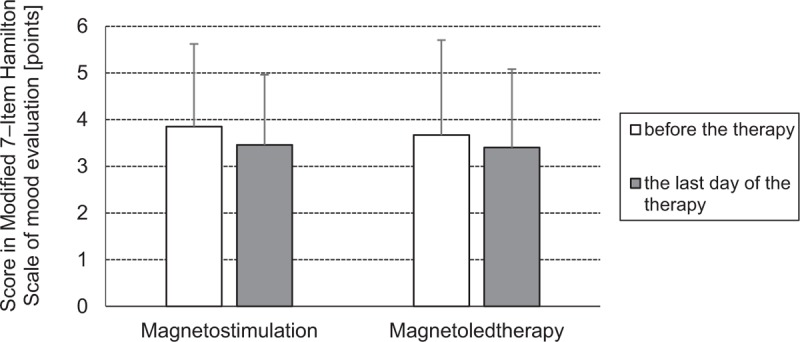
Comparison of the state of mood assessed basing on the score (mean value and SD) (points) in the Modified 7-Item Hamilton scale of mood evaluation in patients with idiopathic hip osteoarthritis from both groups treated with magnetostimulation and magnetoledtherapy respectively, before the beginning and on the last day of the therapeutic cycle.
No statistically significant differences in the score (number of points) of 7-Item Hamilton Scale of mood evaluation assessed before the beginning and after the end of the cycle of magnetic field therapy procedures was observed in both groups of magnetic field treated patients with hip OA.
Results of assessment of pain intensity with use of VAS scale in both groups of magnetic field treated patients are presented in Fig. 3.
Figure 3.
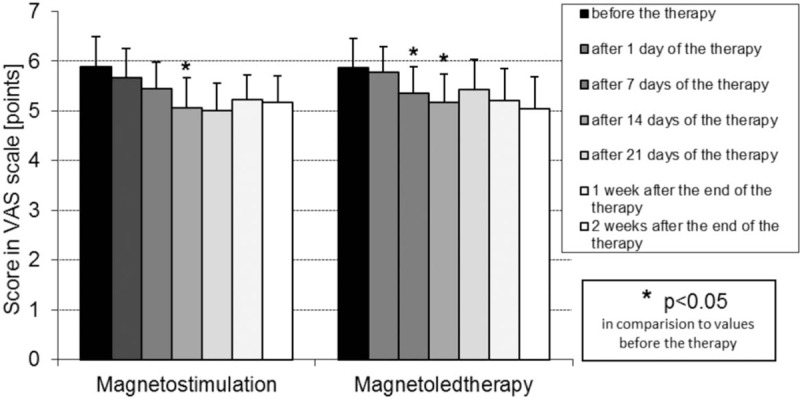
Pain intensity (mean value and SD) (points) assessed with use of the VAS scale in patients with idiopathic hip osteoarthritis from both groups treated with magnetostimulation and magnetoledtherapy respectively, at various time periods of the study. VAS = visual analog scale.
In both groups of magnetic field treated patients with hip OA in all time periods of assessment (on the 1st, 7th, 14th, and 21st day of procedures cycle, as well as on the 7th and 14th day after the end of procedures cycle) the score (mean number of points) in the VAS scale was lower in comparison to initial values on the day of beginning of procedures cycle, but the differences were statistically significant only on the 7th day of a cycle of procedures in magnetostimulation group and on the 7th and 14th days of a cycle of procedures in magnetoledtherapy group. No statistically significant persistent reduction in pain intensity was observed after the completion of a cycle of magnetic field therapy procedures in both groups of patients.
The results of percentage change of dose of analgesic drugs in relation to initial dose treated as 100% in both groups of magnetic field treated patients are presented in Fig. 4.
Figure 4.
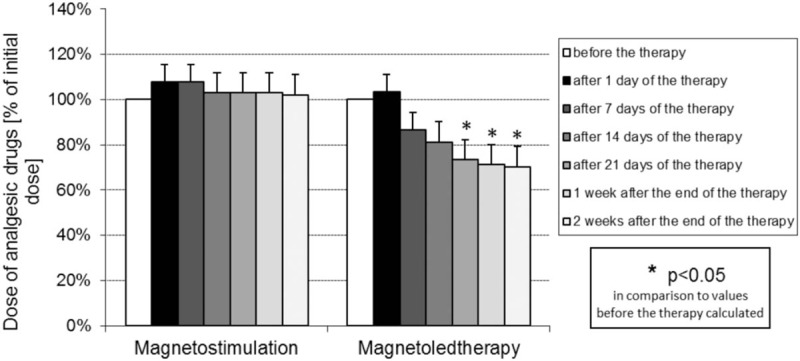
Percentage change of dose of analgesic drugs in relation to initial dose treated as 100% in patients with idiopathic hip osteoarthritis from both groups treated with magnetostimulation and magnetoledtherapy respectively, at various time periods of the study.
In group of patients subjected to magnetoledtherapy procedures a distinct tendency for reduction of the doses of analgesic drugs taken by patients in relation to initial dose, both during a cycle of the procedures and till the 14th day after the completion of a cycle was observed, with statistically significant differences on the 21st day of a cycle of the procedures as well as on the 7th and 14th days after completion of a cycle. On the contrary in group of patients subjected to magnetostimulation procedures no significant changes in the doses of analgesic drugs neither during a cycle of the procedures nor after the completion of the cycle were observed.
5. Discussion
Most of the research studies regarding the therapeutic effects of variable magnetic fields, that has been carried out so far, is of an experimental nature and evaluate mainly the analgesic effect of this physical factor.[21,22] Among the papers available in the literature there are no results of the studies assessing the changes in the concentration of endogenous opiates: β-endorphins in animals and humans under the influence of physical therapy methods, though β-endorphins show not only a strong analgesic effect, but also play an important role as a neurotransmitter in the transmission of stimuli within the nervous system.[11,22]
Assessment of the concentration of β-endorphin in blood plasma carried out in the present study in the examined men at particular time periods of the study confirmed that both analyzed methods of magnetic field therapy cause a statistically significant increase of β-endorphin level. Higher concentration of β-endorphins was observed in patients subjected to magnetoledtherapy—by 12% and 19%, on the last day of procedures cycle and on the 14th day after the end of procedures cycle, respectively (P < .001), in comparison to patients subjected to magnetostimulation—by 23% and 18% on the last day of procedures cycle and on the 14th day after the end of procedures cycle, respectively (P < .01). Different results were obtained by Ossenkopp and Cavaliers. They show that exposure of CF-1 and C57BL mice with activated by exposing mice to stressors endogenous opioid systems to variable magnetic field attenuates the daily rhythm in morphine-induced analgesia and inhibites the analgesic and locomotor effects of the endogenous opioids. According to the authors, this was due to changes in the level of calcium ion contents in brain tissue.[12] In turn Mohammed et al[23] evaluated the effectiveness of laser acupuncture in the treatment of 40 patients with bilateral knee osteoarthritis. Twenty patients from experimental group treated with laser radiation showed significant increase in serum β-endorphin concentration and a decrease in serum substance P concentration, with accompanying pain reduction estimated with use of the VAS scale, as compared with patients from control group exposed to sham exposure.
In present study a subjective assessment of the severity of pain in the hip joint confirmed that in the group of patients subjected to magnetostimulation procedures a statistically significant reduction of the pain intensity by 14% (P < .05) on the 14th day of a therapeutic cycle occurred. Similar results were obtained in the study performed by Sieroń et al[24] in which a treatment with use of a cycle of magnetostimulation procedures confirmed its beneficial analgesic effect in patients with peripheral OA, degenerative lesion of the spine joints as well as rheumatoid arthritis and posttraumatic lesions of the peripheral joints. In turn Thamsborg et al[25] proved high analgesic effectiveness of a treatment with use of pulsed magnetic field in patients with osteoarthritis of knee joint (knee OA).
Also in the group of patients subjected to magnetoledtherapy procedures the pain intensity decreased significantly on the 7th and 14th days of a therapeutic cycle, by 9% and 12%, respectively (P < .05). Similar results were obtained by Cieślar et al[26] applying magnetoledtherapy procedures in the treatment of degenerative and inflammatory lesions in joints of the limbs.
Confirmation of the above studies are the results of the studies of Pasek et al[17] and Beaulieu et al,[27] in which they showed a positive effect of a treatment with use of variable and static magnetic fields on the intensity of perceived pain of joints, also in patients suffering from OA of knee joints and joints of upper limbs.
The most important difference between both analyzed therapeutic methods is that in magnetoledtherapy apart from generation variable magnetic field basing on the phenomenon of ionic magnetic resonance, with saw-like shape of basic impulse and frequency of 180 to 195 Hz, grouped in packages with frequency of 12.5 to 29 Hz, groups of packages with frequency of 2.8 to 7.6 Hz and series with frequency of 0.08 to 0.3 Hz and low magnetic induction value of ca. 45 μT, also simultaneous emission of light radiation in frequency range of red light (634 ± 5 nm) with frequency of light impulses of 181.8 Hz, maximal power of radiation in impulse 880 mW and energy density of 0.48 J/cm2 is applied. Taking into account both own results presented in this paper and the results of above mentioned studies it seems that analgesic effect of low energy light radiation used in magnetoledtherapy with coexisting stimulation of β-endorphin secretion is more significant than that of variable magnetic fields applied in both analyzed methods.
It seems that in the mechanism of both analyzed methods using variable magnetic field an important role plays the influence of this physical factor on neuropathic-like symptoms in patients with OA. Blikman et al[28] determined the influence of neuropathic-like symptoms—adjusted for multiple influential covariates-on joint-specific function and health-related quality of life (HRQoL) in hip and knee OA patients. In this observational study 255 patients (117 with hip OA and 138 with knee OA) completed the modified pain DETECT questionnaire (mPDQ) to identify subjects with neuropathic-like symptoms (mPDQ score >12 was treated as possible neuropathic pain phenotype). The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the RAND 36-Item Health Survey questionnaires were also used to assess joint-specific function and HRQoL respectively. Final results showed that neuropathic-like symptoms deteriorate the subjective rating of pain-related quality of life in hip OA patients and significantly influence joint function in knee OA patients. In turn French et al[29] estimated the prevalence of neuropathic pain in people with hip or knee OA using systematical reviewing and meta-analyzing. Observational studies which measured neuropathic pain in people aged 18 years and older with hip or knee OA were considered for inclusion—among them 9 studies met the inclusion criteria. Authors concluded that neuropathic pain prevalence in people with knee or hip OA is considerable at 23% (95% confidence interval [CI]: 10–39%), with considerable heterogeneity (I2 = 97.9%, P < .001) and may be even higher when other potential causes of neuropathic pain are excluded.
In this study very important from the point of view of health safety and economy was to analyze the dynamics of use of analgesic drugs by patients. The assessment was carried out on the 1st, 7th, 14th, and 21st day of a cycle of procedures as well as on the 7th and 14th day after its completion. In the group of patients subjected to magnetoledtherapy, a statistically significant reduction of analgesic drugs doses was observed on the 21st day of a cycle of procedures by 29% (P < .05) and also during 2 weeks after the end of a cycle of magnetoledtherapy procedures, as at the end of the treatment, a final reduction of drug doses by 32% was obtained (P < .05). In turn in the group of patients subjected to magnetostimulation procedures, there was no statistically significant reduction of doses of analgesic drugs taken by patients during the study. Those results also indicate that analgesic effect of low energy light radiation is more significant than that of variable magnetic fields.
In patients from both magnetic field treated groups no significant changes of state of mood, assessed according to the modified 7—Item Hamilton Depression Rating Scale, were observed, whereas Talarowska-Bogusz et al[30] showed a relationship between perceived pain complaints and depression intensity, stating that a reduction in the severity of pain affects the mood of patients with mild and moderate depressive symptoms. Similar results were obtained by Pasek et al,[31] who after applying a combination of a magnetostimulation with pharmacological treatment in patients with drug-resistant depression showed statistically significant reduction in the severity of drug-resistant depression symptoms of the treated patient, and the visible clinical improvement, that progressed with the duration of exposure. Also Sieroń et al,[32] applying magnetostimulation, obtained a reduction in the severity of depressive symptoms in patients with depressive syndromes resistant to pharmacological treatment. Moreover in double blinded and randomized clinical trial of Khedr et al[33] a significant pain relief and mood improvement in patients with fibromyalgia treated with 10 sessions of transcranial direct current stimulation was observed, with coexisting increase in serum β-endorphin level and negative correlations between changes in serum β-endorphin level and the changes in various scales rating the mood (Hamilton depression and anxiety scales—HAM-D and HAM-A) and intensity of pain (widespread pain index—WPI, symptom severity of fibromyalgia—SS, and visual analogue scale—VAS).
Basing on both animal experiments and clinical trials it is suggested that β-endorphin plays an important role in the pathophysiology of major depressive disorder and most of the etiological theories of depression and behavioral disorders include the interaction of brain regions and neural systems with endogenous opioid systems and β-endorphin. β-endorphin inputs to regulatory pathways involving feeding behaviors, motivation, and specific types of motor activity have important implications in defining the biological foundations for specific depressive symptoms, probably due to its impact on the hypothalamic-pituitary-adrenal axis activity, as it was suggested that dysregulation of this axis may account for depressive symptoms in some individuals.[34]
It was proved that less effective coping behavior (described as the behavioral and physiological mechanisms that occur to return an organism to a basal state following stress exposure) and vulnerability to stress may have a biological basis in hypothalamic-pituitary-adrenal axis function and the modulating effects of the endogenous β-endorphin on the stress response.[35,36] Activation of the hypothalamic-pituitary-adrenal axis as a result of exposure to stressful stimuli mediates an adaptive response through a hormonal cascade of behavioral and physiological changes aimed at the maintenance of homeostasis in the body, among others by the secretion of corticotropin-releasing hormone (CRH) with subsequent stimulation expression of the proopiomelanocortin gene in the anterior pituitary and activation of adrenocorticotropic hormone and β-endorphin release.[37,38] While ACTH activates the adrenal gland to initiate the peripheral response to stress, β-endorphin attenuates the stress response among others by inhibiting secretion of CRH and blocking stress-induced nociception.[39,40]
Merenlender-Wagner et al,[41] basing on actual neurobiological and behavioral evidences suggest that β-endorphin neurotransmission, apart from its confirmed anti-nociceptive effect, is also involved in mediating of stress-related psychiatric disorders, depression, and posttraumatic stress disorder, probably as a modulating element of distress. This effect may occur via its interaction with the mesolimbic monoaminergic system and also by its effects on learning and memory.
In turn Bali et al[42] define the important role of endogenous opioids in modulating stress-associated behavior as related to release of β-endorphins in the amygdala in response to stress that helps to cope with a stressor by inhibiting the over-activation of hypothalamic-pituitary-adrenal axis. They emphasize that application of mu opioid agonists reduces the risk of developing posttraumatic stress disorder by inhibiting fear-related memory consolidation and similarly, the release of endogenous enkephalin and nociceptin in the basolateral amygdala and the nucleus tends to produce the anti-stress effects.
Moreover the results of clinical study of Kubryak et al[43] indicated that increased blood plasma level of β-endorphin in patients with nonpsychotic unipolar depression after 2 weeks of treatment correlates with the positive response to therapy.
Taking into account the results of our study (in which long-term pain syndrome could be treated as a stressor) and the data presented above, it seems that the impact of variable magnetic field on the stimulation of endogenous β-endorphin release could be one of the most important mechanisms of both analgesic effect and improvement of mood in patients with hip OA treated with both analyzed methods of physical therapy.
6. Conclusions
-
1.
The application of magnetostimulation and magnetoledtherapy in patients with idiopathic osteoarthritis of hip joints causes a significant increase in the concentration of plasma β-endorphins during the cycle of therapeutic procedures.
-
2.
The application of magnetostimulation and magnetoledtherapy does not affect the mood of men with idiopathic osteoarthritis of the hip joint.
-
3.
Magnetostimulation and magnetoledtherapy show significant analgesic activity in men with idiopathic osteoarthritis of hip joints awaiting for hip arthroplasty.
-
4.
Magnetoledtherapy in spite of magnetostimulation reduces the need for analgesic drugs taking.
Author contributions
Conceptualization: Bogdan Koczy, Tomasz Stołtny, Jarosław Pasek, Sławomir Kasperczyk, Grzegorz Cieślar.
Data curation: Bogdan Koczy, Tomasz Stołtny, Jarosław Pasek, Maria Leksowska-Pawliczek, Szymon Czech, Monika Białkowska, Grzegorz Cieślar.
Formal analysis: Tomasz Stołtny, Jarosław Pasek, Szymon Czech, Alina Ostałowska, Sławomir Kasperczyk, Monika Białkowska.
Funding acquisition: Bogdan Koczy.
Investigation: Bogdan Koczy, Jarosław Pasek, Maria Leksowska-Pawliczek, Szymon Czech, Alina Ostałowska.
Methodology: Jarosław Pasek, Maria Leksowska-Pawliczek, Alina Ostałowska.
Project administration: Tomasz Stołtny, Jarosław Pasek.
Resources: Alina Ostałowska.
Visualization: Jarosław Pasek.
Writing – original draft: Tomasz Stołtny, Jarosław Pasek, Maria Leksowska-Pawliczek, Szymon Czech, Monika Białkowska.
Writing – review & editing: Bogdan Koczy, Tomasz Stołtny, Jarosław Pasek, Sławomir Kasperczyk, Grzegorz Cieślar.
Footnotes
Abbreviations: ACTH = adrenocorticotropic hormone, CRH = corticotropin-releasing hormone, EDTA = ethylenediaminetetraacetic acid, HAM-A = Hamilton anxiety scale, HAM-D = Hamilton depression and anxiety scale, hip OA = osteoarthritis of hip joint, HRQoL = health-related quality of life, knee OA = osteoarthritis of knee joint, NSAIDs = nonsteroidal anti-inflammatory drugs, SS = symptom severity of fibromyalgia, VAS = visual analog scale, WPI = widespread pain index.
All data are included in the tables and figures in the article.
The authors have no conflicts of interest to disclose.
References
- [1].Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 2006;20:3–25. [DOI] [PubMed] [Google Scholar]
- [2].Thomas AC, Hubbard-Turner T, Wilkstrom EA, et al. Epidemiology of posttraumatic osteoarthritis. J Athl Train 2017;52:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Prieto-Alhambra D, Judge A, Javaid MK, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014;73:1659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reyes C, Garcia-Gil M, Elorza JM, et al. Socio-economic status and the risk of developing hand, hip or knee osteoarthritis: a region-wide ecological study. Osteoarthritis Cartilage 2015;23:1323–9. [DOI] [PubMed] [Google Scholar]
- [5].Chitnavis J, Sinsheimer JS, Suchard MA, et al. End-stage coxarthrosis and gonarthrosis. Aethiology, clinical patterns and radiological features of idiopathic osteoarthrosis. Rheumatology (Oxford) 2000;39:612–9. [DOI] [PubMed] [Google Scholar]
- [6].Goldberg SH, Von Feldt JM, Lonner JH. Pharmacologic therapy for osteoarthritis. Am J Orthop 2002;31:673–80. [PubMed] [Google Scholar]
- [7].Berge DJ, Dolin SJ, Williams AC, et al. Pre-operative and post-operative effect of a pain management program prior to total hip replacement: a randomized controlled trial. Pain 2004;110:33–9. [DOI] [PubMed] [Google Scholar]
- [8].Holden MA, Whittle R, Waterfield J, et al. A mixed methods exploration of physiotherapist's approaches to analgesic use among patients with hip osteoarthritis. Physiotherapy 2018;12:134–9. [DOI] [PubMed] [Google Scholar]
- [9].Kloek CJJ, Bossen D, de Vries HJ, et al. Physiotherapists’ experiences with a blended osteoarthritis intervention: a mixed methods study. Physiother Theory Pract 2018;28:1–8. [DOI] [PubMed] [Google Scholar]
- [10].Thorlund JB, Turkiewicz A, Prieto-Alhambra D, et al. Opioid use in knee or hip osteoarthritis: a region-wide population-based cohort study. Osteoarthritis Cartilage 2019;22:871–7. [DOI] [PubMed] [Google Scholar]
- [11].Karpel E. The place of systemic inflammatory response mediators in intensive care. Anestezjol Intens Ter 2001;33:181–90. [Google Scholar]
- [12].Ossenkopp KP, Kavaliers M. Clinical and applied aspects of magnetic field exposure: Possible role for the endogenous opioid systems. J Bioelectr 1988;7:189–208. [Google Scholar]
- [13].Badura-Brzoza K, Zając P, Kasperska-Zając A, et al. Anxiety and depression and their influence on the quality of life after total hip replacement: preliminary report. Int J Psychiatry Clin Pract 2008;12:280–4. [DOI] [PubMed] [Google Scholar]
- [14].Hinrichs-Rocker A, Schulz K, Jarvinen I, et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - a systematic review. Eur J Pain 2009;13:719–30. [DOI] [PubMed] [Google Scholar]
- [15].Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007;370:1508–19. [DOI] [PubMed] [Google Scholar]
- [16].Pasek J, Mucha R, Sieroń A. Magnetoledtherapy in the treatment of knee arthritis. Acta Bio-Opt Inform Med 2006;12:93–6. [Google Scholar]
- [17].Pasek J, Szajkowski S, Zięba A, et al. Variable and static magnetic fields in the treatment of osteoarthritis changes. J Ecol Health 2011;2:79–82. [Google Scholar]
- [18].Pasek J, Mucha R, Sieroń A. Magnetostimulation – the modern form in physiotherapy and medicine. Physiotherapy 2006;14:3–8. [Google Scholar]
- [19].Sieroń A, Pasek J, Mucha R. Magnetic fields and light energy in medicine and rehabilitation – magnetoledtherapy. Pol Balneol 2007;1:1–7. [Google Scholar]
- [20].Liboff AR, Rozek RJ, Sherman ML, et al. Ca2+ cyclotron resonance in human lymphocytes. J Bioelectr 1987;6:13–22. [DOI] [PubMed] [Google Scholar]
- [21].Sieroń A, Cieślar G, Krawczyk-Krupka A. The Use of Magnetic Fields in Medicine. Ed. IIBielsko–Biała: α-medica press; 2002. [Google Scholar]
- [22].Prato FS, Kavaliers M, Thomas AW. Extremely low frequency magnetic fields can either increase and decrease analgaesia in the land snail depending on field and light conditions. Bioelectromagnetics 2000;21:287–301. [DOI] [PubMed] [Google Scholar]
- [23].Mohammed N, Allam H, Elghoroury E, et al. Evaluation of serum beta-endorphin and substance P in knee osteoarthritis patients treated by laser acupuncture. J Complement Integr Med 2018;15:2–7. [DOI] [PubMed] [Google Scholar]
- [24].Sieroń A, Sieroń-Stołtny K, Biniszkiewicz T, et al. Analysis of therapeutic efficacy of magnetostimulation provided by Viofor JPS system in selected diseases. Acta Bio–Opt Inform Med 2001;7:1–8. [Google Scholar]
- [25].Thamsborg G, Florescu A, Oturai P, et al. Treatment of knee osteoarthritis with pulsed electromagnetic fields: a randomised, double – blind, placebo – controlled study. Osteoarthritis Cartilage 2005;13:575–81. [DOI] [PubMed] [Google Scholar]
- [26].Cieślar G, Rozmus-Kuczia I, Łatka U, et al. Estimation of clinical efficacy of Viofor JPS System Magnetic & Light Therapy – device for magnetostimulation connected with light energy in the treatment of degenerative and inflammatory diseases of joints. Pol Balneol 2004;46:42–58. [Google Scholar]
- [27].Beaulieu K, Beland P, Pinard M, et al. Effect of pulsed electromagnetic field therapy on experimental pain: a double-blind, randomized study in healthy young adults. Electromagn Biol Med 2016;35:237–44. [DOI] [PubMed] [Google Scholar]
- [28].Blikman T, Rienstra W, van Raay JA, et al. Neuropathic-like symptoms and the association with joint-specific function and quality of life in patients with hip and knee osteoarthritis. PLoS One 2018;13:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].French HP, Smart KM, Doyle F. Prevalence of neuropathic pain in knee or hip osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2017;47:1–8. [DOI] [PubMed] [Google Scholar]
- [30].Talarowska-Bogusz M, Florkowski A, Radomska A, et al. Depression and pain in arthrosis of spine and hip in elderly patients. Pol Merkur Lekarski 2006;21:566–9. [PubMed] [Google Scholar]
- [31].Pasek J, Mucha R, Sieroń A. Variable magnetic fields in the treatment of drug-resistance depression – case report. Pol Balneol 2006;8:235–8. [Google Scholar]
- [32].Sieroń A, Hese RT, Sobiś J, et al. Estimation of therapeutic efficacy of weak variable magnetic fields with low value of induction in patients with depression. Psychiatr Pol 2004;38:217–25. [PubMed] [Google Scholar]
- [33].Khedr EM, Omran EAH, Ismail NM, et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimul 2017;10:893–901. [DOI] [PubMed] [Google Scholar]
- [34].Hegadoren KM, O’Donnell T, Lanius R, et al. The role of beta-endorphin in the pathophysiology of major depression. Neuropeptides 2009;43:341–53. [DOI] [PubMed] [Google Scholar]
- [35].Van Santen A, Vreeburg SA, Van der Does AJ, et al. Psychological traits and the cortisol awakening response: results from the Netherlands study of depression and anxiety. Psychoneuroendocrinology 2011;36:240–8. [DOI] [PubMed] [Google Scholar]
- [36].Grisel JE, Bartels JL, Allen SA, et al. Influence of β-endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology 2008;200:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Low MJ. Role of proopiomelanocortin neurons and pep- tides in the regulation of energy homeostasis. J Endocrinol Invest 2004;276 suppl:95–100. [PubMed] [Google Scholar]
- [38].Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005;67:259–84. [DOI] [PubMed] [Google Scholar]
- [39].Plotsky PM. Pathways to the secretion of adrenocorticotropin: a review from the portal. J Endocrinol 1991;3:1–9. [DOI] [PubMed] [Google Scholar]
- [40].Parikh D, Hamid A, Friedman TC, et al. Stress-induced analgesia and endogenous opioid peptides: the importance of stress duration. Eur J Pharmacol 2011;650:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Merenlender-Wagner A, Dikshtein Y, Yadid G. The beta-endorphin role in stress-related psychiatric disorders. Curr Drug Targets 2009;10:1096–108. [DOI] [PubMed] [Google Scholar]
- [42].Bali A, Randhawa PK, Jaggi AS. Stress and opioids: role of opioids in modulating stress-related behavior and effect of stress on morphine conditioned place preference. Neurosci Biobehav Rev 2015;51:138–50. [DOI] [PubMed] [Google Scholar]
- [43].Kubryak OV, Umriukhin AE, Emeljanova IN, et al. Increased β-endorphin level in blood plasma as an indicator of positive response to depression treatment. Bull Exp Biol Med 2012;153:758–60. [DOI] [PubMed] [Google Scholar]


