Supplemental Digital Content is available in the text
Keywords: acute exacerbation of chronic obstructive pulmonary disease, ambient pollutants, generalized additive model, myocardial infarction, stroke
Abstract
To analyze the short-term effects of air pollution on the hospitalization rates of individuals with acute exacerbation of chronic obstructive pulmonary disease (AECOPD), stroke, and myocardial infarction (MI) after adjusting for confounding factors including weather, day of the week, holidays, and long-term trends in Jinan, China.
Hospitalization information was extracted based on data from the primary class 3-A hospitals in Jinan from 2013 to 2015. The concentrations of PM2.5, PM10, SO2, NO2, and O3 were obtained from Jinan Environment Monitoring Center. The relative risk and 95% confidence intervals of AECOPD, stroke, and MI were estimated using generalized additive models with quasi-Poisson distribution in the mgcv package, using R software, version 1.0.136.
The incremental increased concentrations of particulate pollutants including PM2.5 and PM10 were significantly associated with increased risk of hospitalization of AECOPD, stroke, and MI, and the adverse influences of PM2.5 on these diseases were generally stronger than that of PM10. The incremental increased concentrations of gaseous pollutants including SO2, NO2, and O3 were significantly associated with increased risk of hospitalization of stroke and MI in this population.
Air pollution has significant adverse effects on hospitalization rates of individuals with AECOPD, stroke, and MI in Jinan, China.
1. Introduction
Accumulating evidence suggests that air pollution is a primary risk factor for hospitalization of individuals with respiratory and cardiovascular diseases in the general population.[1–4] Acute respiratory and cardiovascular diseases, such as acute exacerbation of chronic obstructive pulmonary disease (AECOPD), stroke, and myocardial infarction (MI), have become the leading causes of mortality worldwide. Substantial epidemiological evidence has demonstrated that ground-level ambient pollutants are associated with AECOPD, stroke, and MI in developed countries such as in Europe and North America.[5–7] However, for developing countries like China, research has been inconsistent, probably due to the limited sample sizes of the studies. Moreover, to the best of our knowledge, none of the previous studies simultaneously evaluated the influence of ground-level ambient pollutants on the hospitalization rate of AECOPD, stroke, and MI. Therefore, large epidemiologic studies are needed to determine the effects of ground-level ambient pollutants on hospitalization rates of individuals with AECOPD, stroke, and MI in China.
Jinan, the capital city of Shandong Province in China, has been reported to be one of the most polluted cities in China in recent years. Overall, Jinan is a northern inland city with complex natural terrain and poor airflow and ventilation, which ranks it among the top 10 cities with the worst air quality in China. The characteristic terrain in Jinan includes higher elevations in the southern area and lower elevations in the northern area, surrounded by mountains that hinder the management and diffusion of ambient pollutants. Accordingly, the objective of this study is to explore the association between the air pollution and hospitalization rates of individuals with AECOPD, stroke, and MI in Jinan from 2013 to 2015. Results of our study are expected to clarify the influence of ground-level ambient pollutants on hospitalizations attributed to acute respiratory and cardiovascular diseases in China, and to provide pertinent advice and guidance for the prevention of acute respiratory and cardiovascular diseases.
2. Materials
2.1. Study population
Information on 6981 AECOPD, 56,922 stroke, and 11,583 MI patients, including age, gender, date of admission, date of discharge, clinical symptoms, disease classification, workplace, and current residence, were obtained from Jinan Qilu Hospital, the Provincial Hospital of Shandong Province, and the Central Hospital of Shandong Province database, respectively. Patients who did not reside or work in Jinan were excluded from the present study. According to epidemiological studies about AECOPD, stroke, and MI in China,[8–10] patients who met the following criteria were included:
-
(1)
≥18 years of age;
-
(2)
resided and worked in the study area (Jinan City) during study period.
The exclusion criteria were as follows:
-
(1)
<18 years old;
-
(2)
not residing and working in Jinan during the study period;
-
(3)
patients with duplicate records;
-
(4)
more than 1 patient admission per week.
The study was approved by the ethic committees of the 3 hospitals.
2.2. Data source
Data reflecting the daily average concentrations of fine particles (PM2.5), inhalable particles (PM10), sulfur dioxide (SO2), nitrogen dioxide (NO2) for 24-hours, and Ozone (O3) for 8-hours, acquired from the 14 fix-sited monitoring stations in urban areas of Jinan, during 2013 to 2015 were obtained from Jinan Environment Monitoring Center. The expectation-maximization method was used to impute missing data. Daily average air temperatures and relative humidity in the corresponding period were obtained from the Jinan Bureau of Meteorology.
2.3. Methods
The generalized additive model with the link function of Poisson distribution was established to assess the impact of air pollution on the hospitalization rates of individuals with AECOPD, stroke, and MI. Because of the application of link function of Poisson distribution and the emergence of extreme values in the data, the quasi-Poisson regression model was applied to reduce the influence of over-dispersion of the results.[11] Confounding factors such as day of the week, holidays, and long-term trends were included as dummy variables and subsequently adjusted.
2.4. Degree of freedom of basic model
The basic models of AECOPD, stroke, and MI were as follows
For AECOPD:
Log [E(Yt)] = α + β1Ci + ns (Temperature, df = 3) + ns (Humidity, df = 5) + ns (Time, df = 6∗3) + β2 factor (DOW) + β3 factor (Holiday)
For stroke:
Log [E(Yt)] = α + β1Ci + ns (Temperature, df = 4) + ns (Humidity, df = 4) + ns (Time, df = 8∗3) + β2 factor (DOW) + β3 factor (Holiday)
df represents degree freedom
For MI:
Log [E(Yt)] = α + β1Ci + ns (Temperature, df = 3) + ns (Humidity, df = 6) + ns (Time, df = 7∗3) + β2 factor (DOW) + β3 factor (Holiday)
Yt represents the number of hospitalized patients on t days; E (Yt) represents the expected number of hospitalized patients on t days; β1 represents the daily average concentration coefficient of pollutants on t days or lag t days; Ci represents the daily average concentration of pollutants on t days or lag t days; ns represents a natural spline smooth function; Time represents the long-term trend of the date; the dummy variables DOW and Holiday represent the effect of “day of the week” and the statutory holidays, respectively, and the β2 and β3 represent the coefficients of factor variables DOW and Holiday, respectively.
The basic model without pollutants was established, and the natural spline smooth function of time, daily temperature, and relative humidity were included to fit the nonlinear effects of the model. The degree of freedom for time, daily temperature, and relative humidity were determined according to the akaike information criterion.[12]
The single-pollutant composed of basic model and daily concentrations of major ambient pollutants were established to estimate the relative risk (RR) and 95% confidence interval for the associations between major ambient pollutants with an increment of 10 μg/m3 and the risk of hospitalizations of the above diseases. A P < .05 was considered statistically significant. Lag structures (from lag 0 day to lag 7 day) were defined as lag 0 to lag 7, and the multi-day moving averages lag structures (from lag 0–1 day [average] to lag 0–7 day [average]) were defined as lag 01 to lag 07. The time lag and cumulative time lag effects of major ambient pollutants were included in the model.
The 2-pollutant models of pollutants and O3 with multi-day moving averages lag structures (from lag 0–1 day [average] to lag 0–7 day [average]) were established for sensitive analysis and to confirm model stability.
The stratification analyses of pollutants exposure based on gender (male or female) and age (<65 years and ≥65 years) were conducted to explore the influence of age and gender on the associations between air pollution and hospitalization rates of individuals with AECOPD, stroke, and MI.
2.5. Spearman correlation analysis
Spearman correlation was applied to describe the correlation of daily concentrations of air pollutants, temperature, and relative humidity.
3. Results
3.1. Distribution of ambient pollutants and weather data
The annual average concentrations of PM2.5, PM10, SO2, NO2, and O3 were 94 μg/m3, 188 μg/m3, 79 μg/m3, 57 μg/m3, and 104 μg/m3, respectively, during 2013 to 2015, which were 2.7-, 2.7-, 1.3-, 1.4-, and 0.7-fold greater than the Annual Secondary National Ambient Air Quality Standards (GB3095-2013). The distribution of daily data on ambient pollutants and weather parameters is shown in Table 1. The distribution of daily ambient pollutant concentrations and temperature is shown in Figure 1.
Table 1.
Distribution of daily data on airborne pollutants and weather parameters in Jinan, 2013–2015.
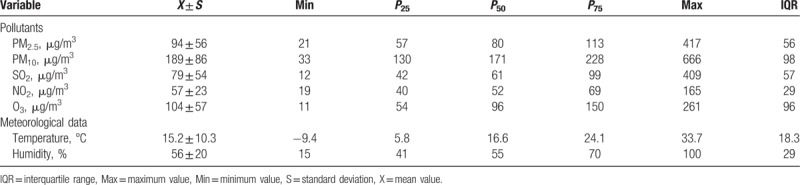
Figure 1.
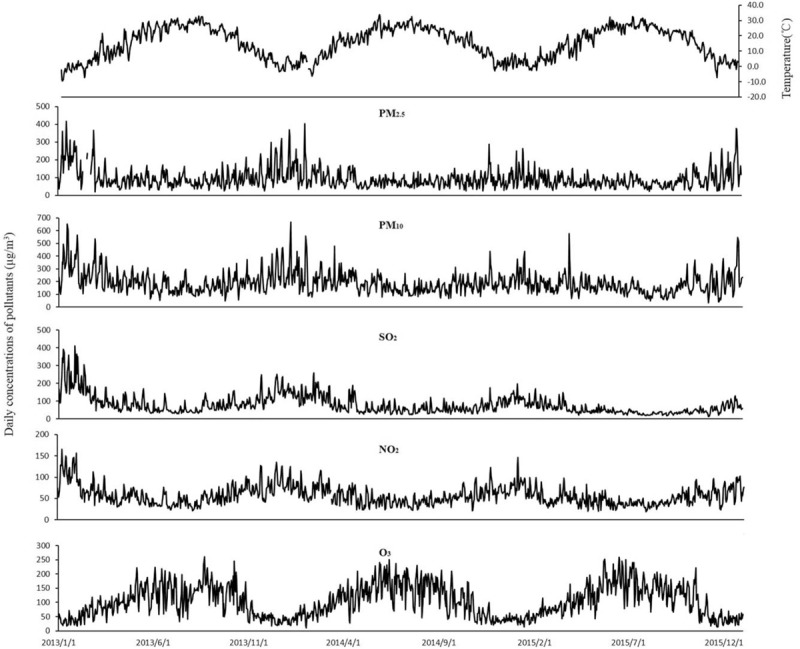
The temporal distribution of daily average ambient pollutant concentrations and daily mean temperature in Jinan during 2013 to 2015.
3.2. Data description
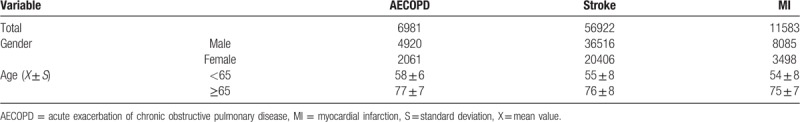
Table 2 shows the demographic characteristic of patients with respiratory and cardiovascular diseases. A total of 6981 AECOPD hospitalizations (4920 males and 2061 females), 56,922 stroke hospitalizations (36,516 males and 20,406 females), and 11,583 MI hospitalizations (8085 males and 3498 females), were obtained from the databases of the primary class 3-A hospitals in Jinan from 2013 to 2015. The percentage of patients aged <65 years was 44.4% (33,492/75,486), while that of patients aged ≥65 years was 55.6% (41,994/75,486).
Table 2.
Demographic characteristics of patients with respiratory and cardiovascular diseases in Jinan, 2013–2015.
3.3. Spearman correlation analysis
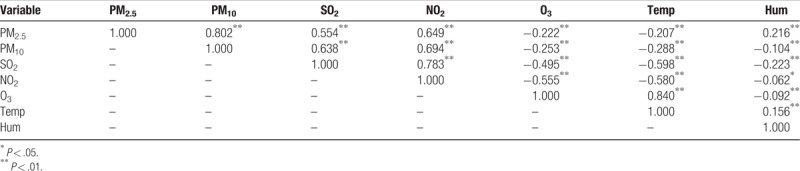
Table 3 shows the Spearman correlation coefficients among daily concentrations of air pollutants, temperature, and relative humidity. Positive correlations were detected among PM2.5, PM10, SO2, and NO2, while there was a significant negative association between these pollutants and O3, temperature, and relative humidity. The Spearman correlations between PM2.5 and PM10 (r = 0.802), SO2 and NO2 (r = 0.783), PM2.5 and NO2 (r = 0.694), and O3 and temperature (r = 0.840) were statistically significant (P < .01).
Table 3.
Spearman correlation coefficients among daily concentrations of air pollutants and meteorological data in Jinan, 2013–2015.
3.4. Daily hospitalizations: AECOPD, stroke, and MI
3.4.1. AECOPD
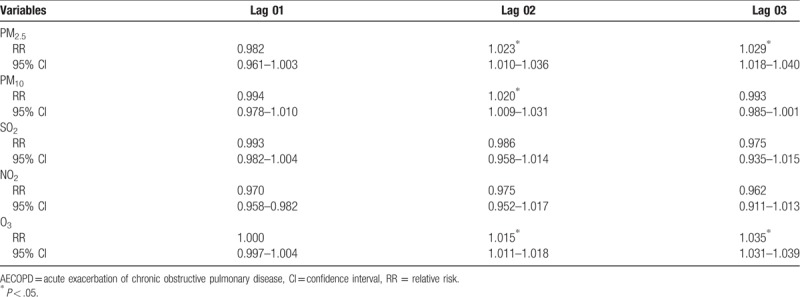
The strongest associations for PM2.5, PM10, and O3 and the risk of AECOPD hospitalizations were observed on lag 3, lag 2, and lag 3 with an increment of 3.1% (1.017–1.044), 1.6% (1.070–1.025), and 2.8% (1.026–1.030), respectively (Table 4). For moving averages lag structures, the strongest associations for PM2.5, PM10, and O3 and the risk of AECOPD hospitalizations were observed in lag 03, lag 02, and lag 03 with an increment of 2.9% (1.018–1.040), 2.0% (1.009–1.031), and 3.5% (1.031–1.039), respectively (Table 5).
Table 4.
Relative risk (RR) with 95% confidence interval (CI) for the association between airborne pollutants concentrations and AECOPD hospitalizations in different lag structures in Jinan, 2013–2015.
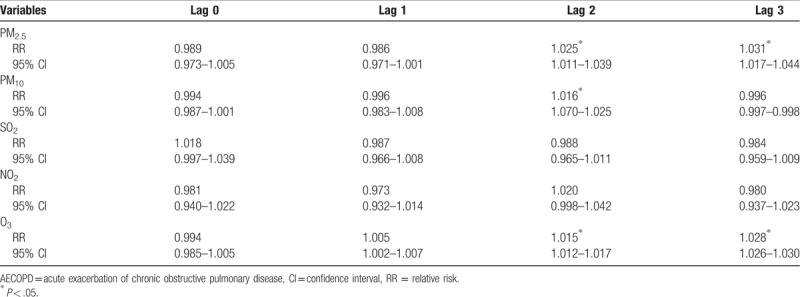
Table 5.
Relative risk (RR) with 95% confidence interval (CI) for the association between airborne pollutants concentrations and AECOPD hospitalizations in different moving averages lag structures for in Jinan, 2013–2015.
Stratified analysis based on gender and age showed the impact of concentrations of PM2.5, PM10, and O3 on the hospitalization risk of AECOPD was stronger for males compared to females and stronger for older participants (≥65 years) compared to younger participants (Fig. 2).
Figure 2.
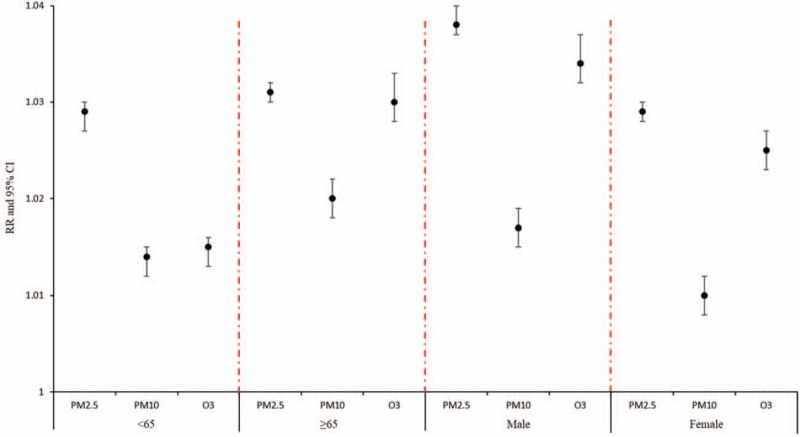
A stratified analysis of the effects of air pollutant concentrations at optimal lag on admission risk in patients with acute exacerbation of chronic obstructive pulmonary disease.
3.5. Stroke
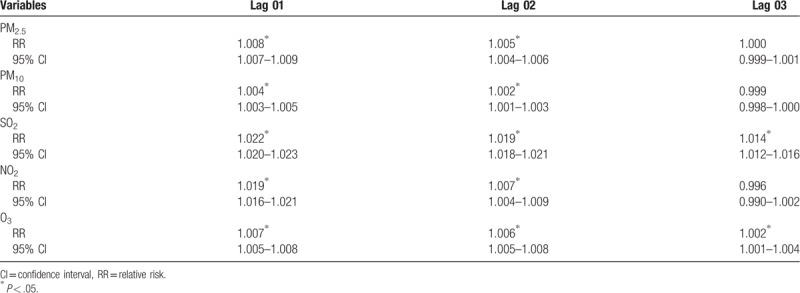
The strongest associations for PM2.5, PM10, SO2, and NO2 and the risk of stroke hospitalizations were all observed on lag 0 with an increment of 1.1% (1.010–1.012), 0.6% (1.005–1.007), 1.8% (1.017–1.020), and 2.5% (1.023–1.027), respectively. The association of O3 and the risk of stroke hospitalizations were statistically significant with an increment 0.2% (1.001–1.003) (Table 6). In addition, the strongest association for O3 was observed on lag 1 with an increment of 0.5% (1.004–1.006) (Table 6). For moving averages lag structures, the strongest associations for PM2.5, PM10, SO2, NO2, and O3 and the risk of stroke hospitalizations were all observed on lag 01 with an increment of 0.8% (1.007–1.009), 0.4% (1.003–1.005), 2.2% (1.020–1.023), 1.9% (1.016–1.021), and 0.7% (1.005–1.008) respectively (Table 7).
Table 6.
Relative risk (RR) with 95% confidence interval (CI) for the association between airborne pollutants concentrations and stroke hospitalizations in different lag structures for in Jinan, 2013–2015.
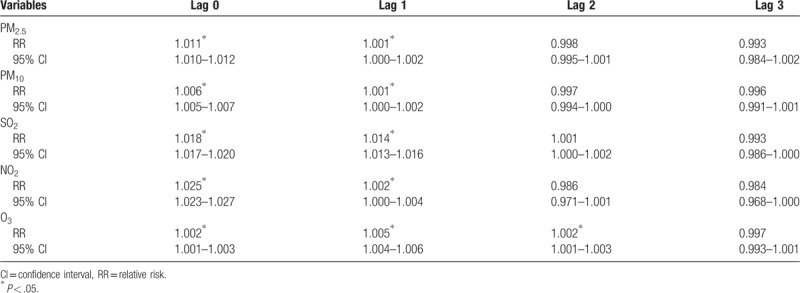
Table 7.
Relative risk (RR) with 95% confidence interval (CI) for the association between airborne pollutants concentrations and stroke hospitalizations in different moving averages lag structures for in Jinan, 2013–2015.
Stratified analysis based on gender and age showed that the impact of concentrations of PM2.5, PM10, and O3 on stroke hospitalization was significantly stronger in patients <65 years of age compared to patients ≥65 years of age, while the impact of concentrations of SO2 and NO2 on stroke hospitalization was significantly stronger in patients ≥65 years of age compared to patients <65 years of age. Stratified analysis based on gender showed that the impact of concentrations of PM2.5, PM10, and SO2 on stroke hospitalization was significantly stronger in males, while the impact of concentrations of NO2 and O3 on stroke hospitalization was significantly stronger in females (Fig. 3).
Figure 3.
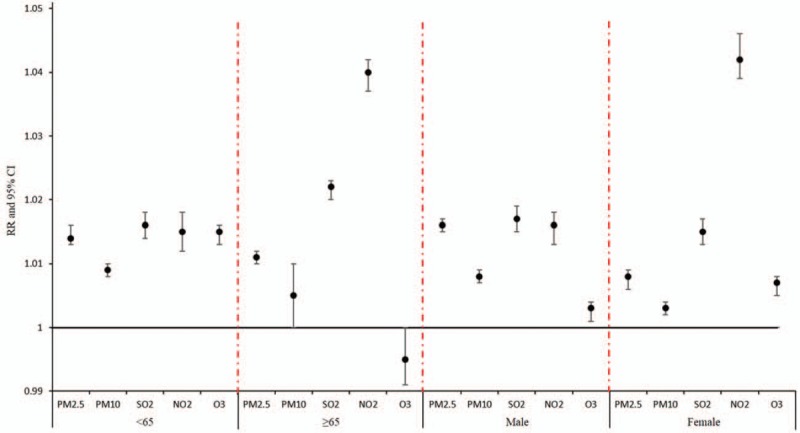
Stratified analysis of the effects of air pollutants concentration on the risk of admission in patients with stroke.
3.6. MI
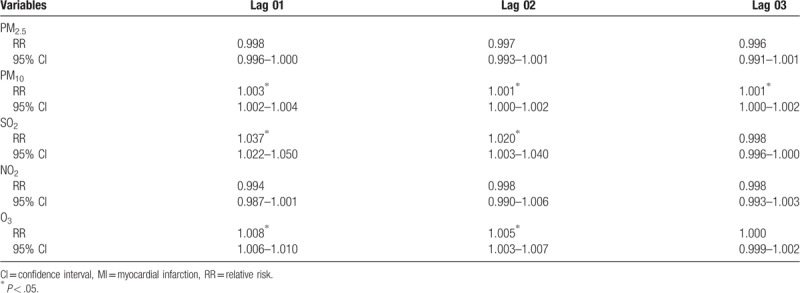
The strongest associations between PM2.5 and PM10 and the risk of MI hospitalizations were both observed in lag 0 with an increment of 1.8% (1.009–1.028) and 4.7% (1.041–1.052), respectively. In addition, the strongest associations for SO2 and O3 and the risk of MI hospitalizations were both observed in lag 1 with an increment of 4.8% (1.035–1.060) and 0.8% (1.007–1.009) (Table 8). For moving averages lag structures, the strongest associations for PM10, SO2, and O3 and the risk of MI hospitalizations were all observed in lag 01 with an increment of 0.3% (1.002–1.004), 3.7% (1.022–1.050), and 0.8% (1.006–1.010), respectively (Table 9).
Table 8.
Relative risk (RR) with 95% confidence interval (CI) for the association between airborne pollutants concentrations and MI hospitalizations in different lag structures for in Jinan, 2013–2015.
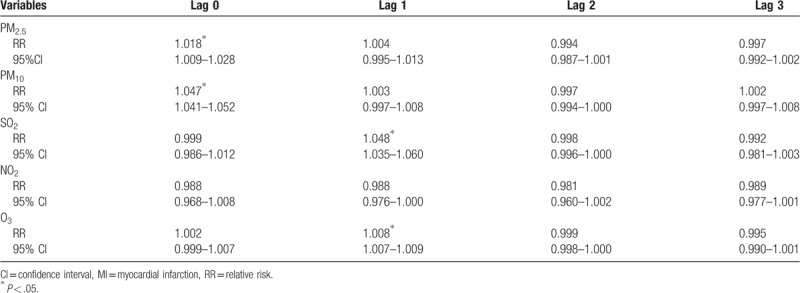
Table 9.
Relative risk (RR) with 95% confidence interval (CI) for the association between airborne pollutants concentrations and MI hospitalizations in different moving averages lag structures for in Jinan, 2013–2015.
Stratified analysis based on gender and age showed that the impact of concentrations of PM2.5, PM10, SO2, and O3 and MI hospitalizations in females was stronger compared to males, and stronger in older participants (≥65 years) compared to younger (<65 years) participants (Fig. 4).
Figure 4.

Stratified analysis of the effects of air pollutants concentration on the risk of admission in patients with myocardial infarction.
3.7. Sensitive analysis
Sensitive analysis showed no significant changes in RR due to concentrations of airborne particulates for AECOPD, stroke, and MI hospitalizations after inclusion of O3 in multi-day moving averages lag structures, which indicated that the effect of the single-pollutant model was robust (Figs. 5–7).
Figure 5.
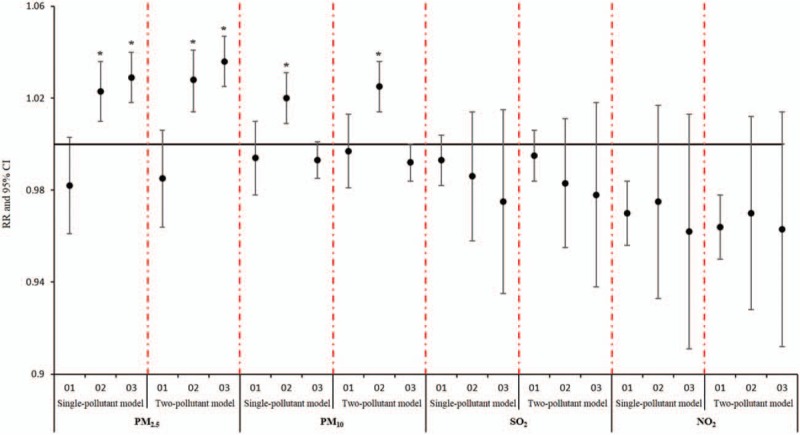
Comparison of cumulative lag effect between single pollutant model and sensitivity analysis model for chronic obstructive pulmonary disease.
Figure 7.
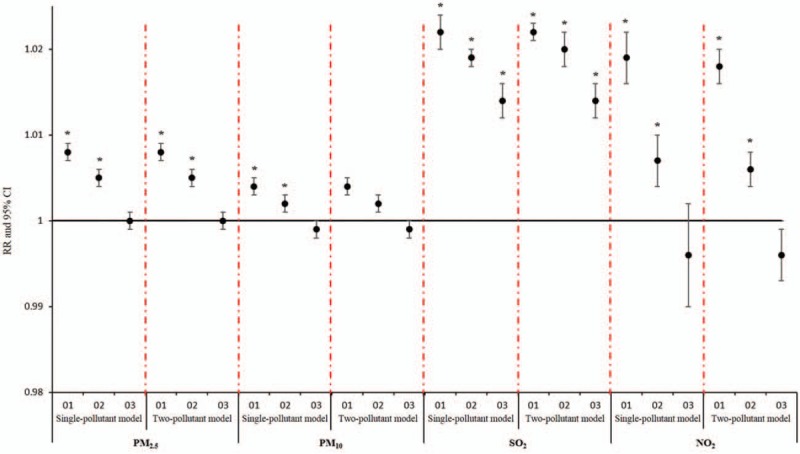
Comparison of cumulative lag effect between single pollutant model and sensitivity analysis model for myocardial infarction.
Figure 6.
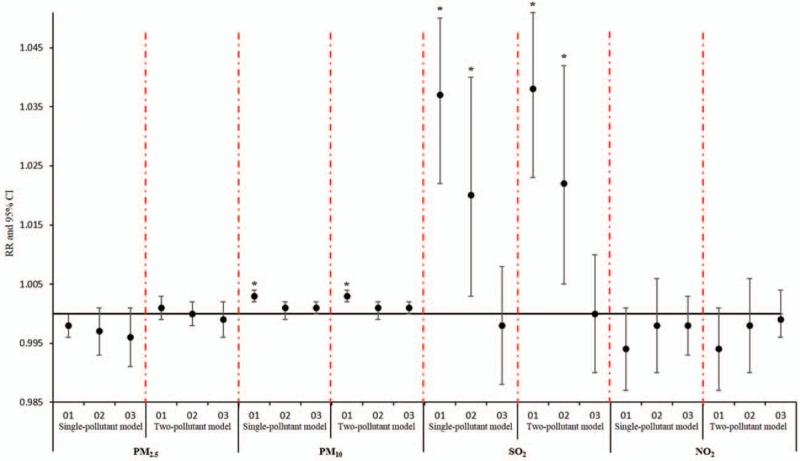
Comparison of cumulative lag effect between single pollutant model and sensitivity analysis model for stroke.
4. Discussion
In this epidemiological study from Jinan, China, we found that air pollution has significant adverse effects on the hospitalization rates of individuals with AECOPD, stroke, and MI. These results suggest that air pollution has become a major contributor to the incidence and exacerbation of acute respiratory and cardiovascular diseases in developing countries. These results highlight the importance of management of air pollution for the prevention of acute respiratory and cardiovascular diseases.
4.1. AECOPD
Our findings are consistent with previous reports. Xu et al included patients with respiratory disease who visited emergency hospitals in Beijing and showed that the severest adverse effect of PM2.5 on the hospitalization of patients with AECOPD was the cumulative lag 0 to 3 (Lag 03) day with an increment of 3.15% in the emergency hospital admission rate of AECOPD.[13] Ko et al also found significant associations between hospital admissions for COPD with PM2.5; the RR for admission for every 10 mg/m3 increase in PM2.5 was 1.031, at a lag day ranging from lag 0 to cumulative lag 0–5.[14]
In our study, although no immediate effects were observed between PM2.5, PM10, O3, and hospitalizations of AECOPD, the effect on different lag days (lag 2, lag 3, lag 02, and lag 03) were statistically significant. As highly active oxidants with poor solubility, these airborne particulates can reach the depths of the lungs after being inhaled and subsequently cause inflammation of the epithelial cells of the respiratory tract. The certain reaction time of airway inflammation caused by oxidants could result in a cumulative effect, rather than an immediate effect[15] as previously indicated.
As a significant risk factor for AECOPD, SO2 dissolves easily in the upper respiratory tract and produces immediate stimulation to the mucosa.[16,17] However, the significant effects of SO2 on hospitalization risk in patients with AECOPD were not observed in this study. The annual average concentrations of SO2 in Jinan during 2013 to 2015 were 102 μg/m3, 79 μg/m3, and 55 μg/m3, respectively, and the average annual concentration in 2015 was below the 2-level standard limit (60 μg/m3) of the national ambient air quality standard (GB 3095-2012) for the first time. The control of coal-fired volume and the improvement of technical treatment methods, such as desulphurization and denitrification, during the “13th 5-Year Plan” period in Jinan were highly effective.
The results of stratified analysis in gender and age showed that male patients aged ≥65 years were more sensitive to air pollutants and were at a consequently higher risk of hospitalization for AECOPD compared to women aged <65 years. It has been shown that the smoking rate is 74% among men aged >35 years in China. As China is one of the largest tobacco producing and consuming countries, the prevalence of chronic bronchitis among smokers is 2- to 8-fold higher than that of non-smokers. Meanwhile, the proportion of smoking in males has been significantly higher than that of females in underdeveloped and developing countries.[16] Moreover, a previous report revealed that male patients with a history of smoking had a higher hospital admission risk and were more sensitive to varied concentrations of pollutants.[18] Stratified analysis by age showed that patients ≥65 years of age were more sensitive to air pollutants exposure and had higher risk of hospital admission for AECOPD compared to patients <65 years of age. The weakness of the immune system, the higher prevalence of chronic respiratory diseases, and the increased sensitivities to the particles in the elderly could be the underlying causes for the stronger association between air pollution and risk of AECOPD hospitalization in those older than 65 years of age.[19–21]
4.2. Stroke
The strongest associations between particulate matter (PM2.5 and PM10) and hospitalizations of stroke were found on the day of admission with an increment of 1.1% and 0.6%, respectively. Our results were supplemented by experimental studies that indicated that exposure to high concentrations of PM2.5 can lead to pathophysiological changes related to the onset of cardiovascular diseases, including increased blood pressure and heart rate, reduced heart rate variability within 12 hours, changes in coagulation factors, systemic inflammation, damaged endothelial cells, and vascular dysfunction.[22] Our study also showed that the impact of PM2.5 on the admission risk of stroke patients seemed to be stronger than PM10. This is consistent with a previous meta-analysis that also indicated that stroke hospitalization increased by 1.011 (95% CI = 1.010–1.012) and 1.002 (95% CI = 1.000–1.003), following exposure to PM2.5 and PM10, respectively.[23] Based on previous reports, it has been estimated that fine particles, ultrafine particles, and gaseous pollutants primarily cause additional systemic cerebrovascular responses,[24] compared to partial lung effects which are usually caused by particulate matter with larger aerodynamics. Moreover, particles with smaller aerodynamics can penetrate the alveolar space of the lungs, and even circulate systemically to reach other organs.[25]
Our results showed that daily exposure to concentrations of SO2 and NO2 may have the strongest impact on the risk of hospitalizations of stroke. A previous study by Joubert et al demonstrated that SO2 can penetrate the protective blood–brain barrier via its own biochemical pathway to elicit specific pathophysiological effects and cause cerebral ischemia.[26] Results of another meta-analysis also showed that hospitalization for ischemic stroke increased by 1.79% (95% CI = 1.0054–1.0306) with an incremental increase of SO2, which was in accordance with our findings.[20] In addition, epidemiological studies from Denmark, Italy, Canada, and other countries consistently showed that variations in NO2 concentration were significantly associated with the incidence of acute ischemic stroke.[27–29] All of the above evidence supports a strong association between air pollution and the risk of stroke.
Moreover, we found that the association between SO2 (RR = 1.018; 95% CI = 1.017–1.020) and NO2 (RR = 1.014; 95% CI = 1.013–1.016) and stroke hospitalizations were stronger than the association between PM2.5 and stroke admissions (RR = 1.011; 95% CI = 1.010–1.012) and PM10 (RR = 1.006; 95% CI = 1.005–1.007). This finding is consistent with previous studies, which suggested that traffic pollution may cause ischemic stroke.[30–32] While the potential reasons for this association remains unclear, we hypothesize that gaseous pollutants exhausted from traffic in Jinan may have become one of the main sources of air pollutants.
Stratified analysis by age showed that stroke patients ≥65 years of age were more sensitive to gaseous pollutants (SO2 and NO2) exposure and were at higher risk of stroke-related hospitalizations. Similar results were supported by a previous case-crossover study from Beijing, which also showed that the increased daily concentrations of SO2 and NO2 were associated with a more significant increased risk of stroke hospitalizations of patients ≥65 years of age.[33] Compared with the younger participants, the elderly are more sensitive to air pollution and have higher incidences of cardiovascular and cerebrovascular diseases. These findings are also supported by previous studies that showed that recurrent attacks of stroke were more frequent in elderly patients.[34]
Stratified analysis by gender showed that female patients were more sensitive to gaseous pollutants (NO2 and O3) exposure, while male patients were more sensitive to particulate matter (PM2.5 and PM10) exposure. Qin et al collected admission data of stroke patients from 3 cities in northern China in a case-crossover study and found that the significant association between air pollution and stroke hospitalization was only observed in females.[35] Moreover, a study in Beijing demonstrated that the impacts of PM2.5 on the first admission due to ischemic stroke did not different by gender.[36] In addition, a study from Shanghai showed that male stroke patients were more sensitive to changes in concentrations of PM10, SO2, and NO2 than female patients.[37] In summary, the potential gender differences underlying the association between air pollution and stroke remain to be determined.
4.3. MI
According to the results of this study, SO2 has the strongest adverse impact on the admission of individuals with MI (RR = 1.048; 95% CI = 1.035–1.060), which is consistent with the result of a similar study performed in Shanghai Pudong District during 2013 to 2014.[37] This study showed that the incidence of acute MI increased by 5% with an incremental 10 μg/m3 increase in SO2.[36]
However, we failed to detect a significant effect of NO2 on admission rate of MI. Compared to the 4-quartile range of other pollutants (PM2.5 = 56 μg/m3, PM10 = 98 μg/m3, SO2 = 57 μg/m3, and O3 = 96 μg/m3), the quartile range of NO2 only reached 29 μg/m3. The small variance in daily average concentrations of NO2 in Jinan might be the potential reason for the insignificant association between NO2 and admission rate of MI.
Most previous studies have shown that there is no significant association between short-term exposure to O3 and morbidity and mortality of MI.[13,14,38,39] In contrast to previous studies performed in Western countries, our study demonstrated a 0.8% increased risk of hospitalizations for MI per 1 0 μg/m3 increase in O3 concentration on lag 1 and a 0.8%, 0.5% increment on lag 01 and lag 02, respectively. The relatively high concentrations of O3 in Jinan could be one of the potential reasons underlying the variations between our results and the previous findings.
Although the inhalable particles (PM2.5 and PM10) and O3 exposure could induce inflammatory effects, the results of this study showed that the adverse effects of particulate matter (especially for PM10 [RR = 1.047; 95% CI = 1.041–1.052]) were stronger than that of O3 (RR = 1.008; 95% CI = 1.007–1.009). This is consistent with the study conducted by Rosenthal et al, who showed that, compared to O3, PM10 plays a greater role in thrombosis.[7]
The results of the stratified analysis showed that female patients ≥65 years of age were more sensitive to air pollutants exposure and suffered a higher risk of MI admissions than males <65 years of age. As a vulnerable group, the elderly often have atherosclerotic plaques and other cardiovascular diseases and are therefore more susceptible to the risks of air pollution exposure. Exposure to short-term air pollution is an important trigger of cardiovascular diseases, probably due to its ability to increase plaque vulnerability, platelet activation, and coagulation.[11] Some studies suggested that many factors may underlying the potential gender differences regarding the association between air pollution and MI, such secretion of hormones, the physical build, and the range of activities[36] The exact mechanisms deserve further investigation.
The limitation of our study was that we did not include patients <18 years of age.
5. Conclusions
In conclusion, results of our study indicate that air pollution is significantly associated with an increased risk of hospitalization of individuals with AECOPD, stroke, and MI in Jinan, China. Future studies are needed to determine the potential mechanisms involved and explore the potential importance of management of air pollution for the prevention of acute respiratory and cardiovascular diseases.
Author contributions
Conceptualization: Xianfeng Wang, Wei Li, Qi Zhang.
Data curation: Chenguang Lv, Wei Li.
Formal analysis: Cai Chen, Chenguang Lv, Wei Li.
Funding acquisition: Cai Chen, Chenguang Lv, Leilei Dong.
Investigation: Cai Chen, Dedong Ma, Leilei Dong.
Methodology: Cai Chen, Xianfeng Wang, Dedong Ma, Leilei Dong.
Project administration: Xianfeng Wang, Leilei Dong.
Resources: Xianfeng Wang, Dedong Ma, Qi Zhang.
Software: Xianfeng Wang, Qi Zhang.
Supervision: Xianfeng Wang, Qi Zhang.
Validation: Dedong Ma, Qi Zhang.
Visualization: Qi Zhang.
Wei Li orcid: 0000-0001-7367-4178.
Supplementary Material
Footnotes
Abbreviations: AECOPD = acute exacerbation of chronic obstructive pulmonary disease, CI = confidence interval, MI = myocardial infarction, NO2 = nitrogen dioxide, O3 = Ozone, RR = relative risk, SO2 = sulfur dioxide.
This study was approved by the ethics committee of Biomedical Engineering Institute, School of Control Science and Engineering, Shandong University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This study was supported in part by grants from the National Natural Science Foundation (#21728701), the Ministry of Education Postdoctoral Fund (#2015M572044), Shandong Province Science and Technology Development Project (#GG201709260070).
The authors declare that they have no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Villeneuve PJ, Johnson JY, Pasichnyk D, et al. Short-term effects of ambient air pollution on stroke: who is most vulnerable? Sci Total Environ 2012;430:193–201. [DOI] [PubMed] [Google Scholar]
- [2].Belleudi V, Faustini A, Stafoggia M, et al. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology 2010;21:414–23. [DOI] [PubMed] [Google Scholar]
- [3].R Sciaraffa AB, Montuschi P, Gerosa GA, et al. Impact of air pollution on respiratory diseases in urban areas: a systematic review: Daniele Ignazio La Milia. Eur J Public Health 2017;27.28355639 [Google Scholar]
- [4].Mishra S. Is smog innocuous? Air pollution and cardiovascular disease. Indian Heart J 2017;69:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mantovani KC, Nascimento LF, Moreira DS, et al. Air pollutants and hospital admissions due to cardiovascular diseases in Sao Jose do Rio Preto, Brazil. Cien Saude Colet 2016;21:509–15. [DOI] [PubMed] [Google Scholar]
- [6].Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res 2014;30:71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rosenthal FS, Kuisma M, Lanki T, et al. Association of ozone and particulate air pollution with out-of-hospital cardiac arrest in Helsinki, Finland: evidence for two different etiologies. J Expo Sci Environ Epidemiol 2013;23:281–8. [DOI] [PubMed] [Google Scholar]
- [8].Xu T, Li W, Teo K, et al. Association of psychological risk factors and acute myocardial infarction in China: the INTER-HEART China study. Chinese Med J-Peking 2011;124:2083–8. [PubMed] [Google Scholar]
- [9].Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China. Circulation 2017;135:759. [DOI] [PubMed] [Google Scholar]
- [10].Chan KY, Li X, Chen W, et al. Prevalence of chronic obstructive pulmonary disease (COPD) in China in 1990 and 2010. J Glob Health 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gardner B, Ling F, Hopke PK, et al. Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: a case-crossover study. Part Fibre Toxicol 2014;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hurvich CM, Simonoff JS, Tsai CL. Smoothing parameter selection in nonparametric regressiousing an improved Akaike information criterion. J R Stat Soc 1998;60:271–93. [Google Scholar]
- [13].Xu Q, Li X, Wang S, et al. Fine particulate air pollution and hospital emergency room visits for respiratory disease in urban areas in Beijing, China, in 2013. PLoS One 2016;11:e0153099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ko FW, Tam W, Wong TW, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 2007;62:780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gilmour PS, Rahman I, Donaldson K, et al. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol 2003;284:L533–40. [DOI] [PubMed] [Google Scholar]
- [16].Novović M, Topić V. Correlation between arterial and venous blood gas analysis parameters in patients with acute exacerbation of chronic obstructive pulmonary disease. Srp Arh Celok Lek 2012;140:436–40. [DOI] [PubMed] [Google Scholar]
- [17].Wong TW, Lau TS, Yu TS, et al. Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occup Environ Med 1999;56:679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang X, Kindzierski W, Kaul P. Air pollution and acute myocardial infarction hospital admission in Alberta, Canada: a three-step procedure case-crossover study. PLoS One 2015;10:e0132769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mustafic H, Jabre P, Caussin C, et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 2012;307:713–21. [DOI] [PubMed] [Google Scholar]
- [20].Hurvich CMSJ, Tsai CL. Smoothing parameter selection in nonparametric regressiousing an improved Akaike information criterion. J R Stat Soc 1998;60:271–93. [Google Scholar]
- [21].Sant’Anna Neto JLadA VS. Uma reflexão do espaço urbano da cidade de Campo Grande/MS na perspectiva climática. Rev Pantaneira 2003;3: [Google Scholar]
- [22].Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 2009;54:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Andersen ZJ, Olsen TS, Andersen KK, et al. Association between short-term exposure to ultrafine particles and hospital admissions for stroke in Copenhagen, Denmark. Eur Heart J 2010;31:2034–40. [DOI] [PubMed] [Google Scholar]
- [24].Shah AS, Lee KK, McAllister DA, et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ 2015;350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Donaldson K, Duffin R, Langrish JP, et al. Nanoparticles and the cardiovascular system: a critical review. Nanomedicine (Lond) 2013;8:403–23. [DOI] [PubMed] [Google Scholar]
- [26].Joubert J, Cumming TB, McLean AJ. Diversity of risk factors for stroke: the putative roles and mechanisms of depression and air pollution. J Neurol Sci 2007;262:71–6. [DOI] [PubMed] [Google Scholar]
- [27].Kan H, London SJ, Chen G, et al. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: the public health and air pollution in Asia (PAPA) study. Environ Health Perspect 2008;116:1183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miller MR, Raftis JB, Langrish JP, et al. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 2017;11:4542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Andersen ZJ, Kristiansen LC, Andersen KK, et al. Stroke and long-term exposure to outdoor air pollution from nitrogen dioxide: a cohort study. Stroke 2012;43:320–5. [DOI] [PubMed] [Google Scholar]
- [30].Vidale S, Bonanomi A, Guidotti M, et al. Air pollution positively correlates with daily stroke admission and in hospital mortality: a study in the urban area of Como. Italy Neurol Sci 2010;31:179–82. [DOI] [PubMed] [Google Scholar]
- [31].Villeneuve PJ, Chen L, Stieb D, et al. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton. Canada Eur J Epidemiol 2006;21:689–700. [DOI] [PubMed] [Google Scholar]
- [32].Wellenius GA, Burger MR, Coull BA, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med 2012;172:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang F, Luo Y, Tan P, et al. Gaseous air pollution and the risk for stroke admissions: a case-crossover study in Beijing, China. Int J Environ Res Public Health 2017;14: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zanobetti A, Schwartz J, Dockery DW. Airborne particles are a risk factor for hospital admissions for heart and lung disease. Environ Health Perspect 2000;108:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Qin XD, Qian Z, Vaughn MG, et al. Gender-specific differences of interaction between obesity and air pollution on stroke and cardiovascular diseases in Chinese adults from a high pollution range area: a large population based cross sectional study. Sci Total Environ 2015;529:243–8. [DOI] [PubMed] [Google Scholar]
- [36].Qian Y, Zhu M, Cai B, et al. Epidemiological evidence on association between ambient air pollution and stroke mortality. J Epidemiol Community Health 2013;67:635–40. [DOI] [PubMed] [Google Scholar]
- [37].Wang XD, Zhang XM, Zhuang SW, et al. Short-term effects of air pollution on acute myocardial infarctions in Shanghai, China, 2013-2014. J Geriatr Cardiol 2016;13:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Milojevic A, Wilkinson P, Armstrong B, et al. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart 2014;100:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Cien Saude Colet 2011;16:2221–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


