Abstract
Some patients have poor response to adult-onset Still disease (AOSD) traditional treatment, which easily recurs during the reduction of prednisone. We observed the efficacy and safety of tocilizumab combined with methotrexate (MTX) in the treatment of refractory AOSD, and to explore the possibility of reducing the dosage of tocilizumab after disease control.
A total of 28 refractory AOSD cases who had an inadequate response to corticosteroids combined with at least 1 traditional immunosuppressive agent, and even large-dose prednisone could not relieve their conditions after recurrence, were selected in this study. They were treated with tocilizumab (intravenous 8 mg/kg) combined with MTX (oral 12.5 mg once a week). In detail, tocilizumab was firstly given every 4 weeks and after 6-month remission, it was then given every 8 weeks. Some items including body temperature, skin rash, joint swelling and pain, hepatosplenomegaly, blood routine, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum ferritin, and dosage of prednisone were observed before treatment as well as 2, 4, 8, 12, 24, 36, and 48 weeks after treatment. The adverse reactions occurring during the treatment were recorded.
The body temperature was normal, the skin rash as well as joint swelling and pain disappeared, and laboratory indexes including CRP, ESR, white blood cell, neutrophilic granulocyte, platelet, hemoglobin, and ferritin were significantly improved after 8-week treatment (all P < .05). The clinical symptoms and laboratory indexes above mentioned were continuously improved 12, 24, 36, and 48 weeks after treatment. The mean dosage of prednisone was reduced from 71.4 ± 20.7 mg/day to 55.0 ± 11.1 mg/day after 2-week treatment, and to 3.3 ± 2.1 mg/day after 48-week treatment (all P < .05). Prednisone was discontinued in 5 cases after 36-week treatment and in 7 cases after 48-week treatment. No serious adverse reactions occurred during the treatment.
Tocilizumab can rapidly and markedly improve the clinical symptoms and laboratory indexes and contribute to reduction and discontinuation of prednisone in refractory AOSD. The patients’ conditions are stable after reduction or discontinuation of prednisone and the tocilizumab possesses good safety.
Keywords: adult-onset Still's disease, methotrexate, safety, tocilizumab
1. Introduction
Adult-onset Still disease (AOSD) is a clinical syndrome characterized by fever, transient skin rash, arthritis, or arthralgia, increased neutrophils, hepatosplenomegaly, and lymphadenectasis. Mild AOSD may be treated with nonsteroidal anti-inflammatory drugs (NSAIDs) alone. If patients have no response to NSAIDs, glucocorticoids and/or immunosuppressive agents are necessary. However, long-term use of these drugs can induce many adverse reactions, some patients have poor response to traditional treatment, and AOSD easily recurs during the reduction of glucocorticoids. Therefore, it is necessary to seek more appropriate drugs. At present, the etiology and pathogenesis of the AOSD are still not very clear. The levels of macrophage cytokines such as interleukin (IL)-1, IL-6, IL-18, migration inhibiting factor, and heme oxygenase-1 were significantly increased in the blood of patients with AOSD.[1–3] Therefore, AOSD may be related to the abnormal activation of macrophages. These cytokines play a biologic role by regulating inflammatory response cells such as T cells, NK cells, and granulocytes. Excessive IL-6 can cause acute or chronic inflammatory reactions, hematopoietic disorders, and immune abnormalities, which play an important role in the pathogenesis of AOSD.[1] Therefore, blocking IL-6 is likely to can control AOSD. Tocilizumab is an IL-6 receptor antagonist. The aim of this study was to evaluate the efficacy and safety of tocilizumab combined with methotrexate (MTX) in the treatment of refractory AOSD, and to observe outcomes of patients in whom tocilizumab dosage was reduced.
2. Patients and methods
All study methods were approved by Institutional Review Board and Ethics Committee of the Second Hospital of Lanzhou University, and were performed in accordance with relevant guidelines and regulations. An informed consent was obtained from all subjects.
2.1. Clinical data
The 28 cases were in line with the Yamaguchi criteria and were treated in the Department of Rheumatology and Immunology of Second Hospital of Lanzhou University from January 2014 to January 2017. The 28 cases had an inadequate response to a median dose of 14.6 ± 4.1 mg/day of prednisone combined with at least 1 traditional immunosuppressive agent (3 in the 28 cases also received tumor necrosis factor-alpha [TNF-α] antagonist), and even large-dose prednisone of 1.5 to 2 mg/kg/day could not relieve their conditions after recurrence. The exclusion criteria were potential tuberculosis infection and other infectious diseases detected by T-cell enzyme-linked immunosorbent assay and chest computed tomography; hepatitis B; and pregnant or lactating women.
2.2. Observational items and time points
Observational items and time points were as follows: clinical symptoms including body temperature, skin rash, joint swelling and pain, hepatomegaly, splenomegaly, and dosage of prednisone; laboratory examinations including white blood cell count, neutrophilic granulocyte count, platelet count, hemoglobin level, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), alanine transaminase (ALT), aspartate transaminase (AST), ferritin level, and creatinine; observational time points including before treatment and 2, 4, 8, 12, 24, 36, and 48 weeks after treatment.
2.3. Drug use
Tocilizumab (8 mg/kg, Yamelo; Shanghai Roche Pharmaceutical Co, Ltd, Shanghai, China, 80 mg/ampoule) dissolved in 100 mL of 0.9% sodium chloride was intravenously given every 4 weeks. After 6-month complete remission (body temperature and laboratory indexes became normal, and skin rash as well as joint swelling and pain disappeared), 8 mg/kg of tocilizumab was intravenously given every 8 weeks. MTX (12.5 mg, 2.5 mg/tablet) was orally given after dinner once a week during the whole treatment process and no folic acid was administered. The dosage of prednisone was reduced according to the following protocol. After complete remission (body temperature and laboratory indices became normal, and skin rash as well as joint swelling and pain disappeared), prednisone was immediately decreased to 1 mg/kg/day, then was reduced by 5 mg every week for 4 weeks. If patients’ conditions were stable, the dosage of prednisone was reduced by 2.5 mg every week for 4 to 6 weeks. If patients’ conditions were stable, the dosage of prednisone was reduced by 2.5 mg every 2 weeks until 7.5 to 10 mg of maintenance dose followed by trying to decrease by 2.5 mg every 3 months.
2.4. Safety assessments
Infusion reaction, anaphylaxis, digestive tract reaction, impairment of liver, and kidney function as well as infection were observed.
2.5. Statistical analysis
Statistical treatment was performed using SPSS 18.0 software. Data were expressed as x ± s. The t test was used for continuous data. Chi-squared test was used for comparison of categorical data. Statistical significance was established at P < .05.
3. Results
3.1. General data
Of the 28 cases, 12 were males and 16 females with a mean disease course of 5.6 ± 2.2 years. All patients had fever with an average body temperature of 39.2 ± 0.5°C. Joint swelling and pain occurred in 27 cases. Seventeen cases experienced pharyngodynia and 21 cases had hepatosplenomegaly confirmed by color Doppler ultrasound (Table 1). The 28 cases receive a median dose of 14.6 ± 4.1 mg/day of prednisone combined with 1 or 2 kinds of immunosuppressive agents and 3 cases in the 28 cases also received TNF-α antagonist when AOSD recurred, but their clinical symptoms were still not relieved after 2 to 3 weeks.
Table 1.
Clinical data of the 28 cases with refractory adult-onset Still disease.
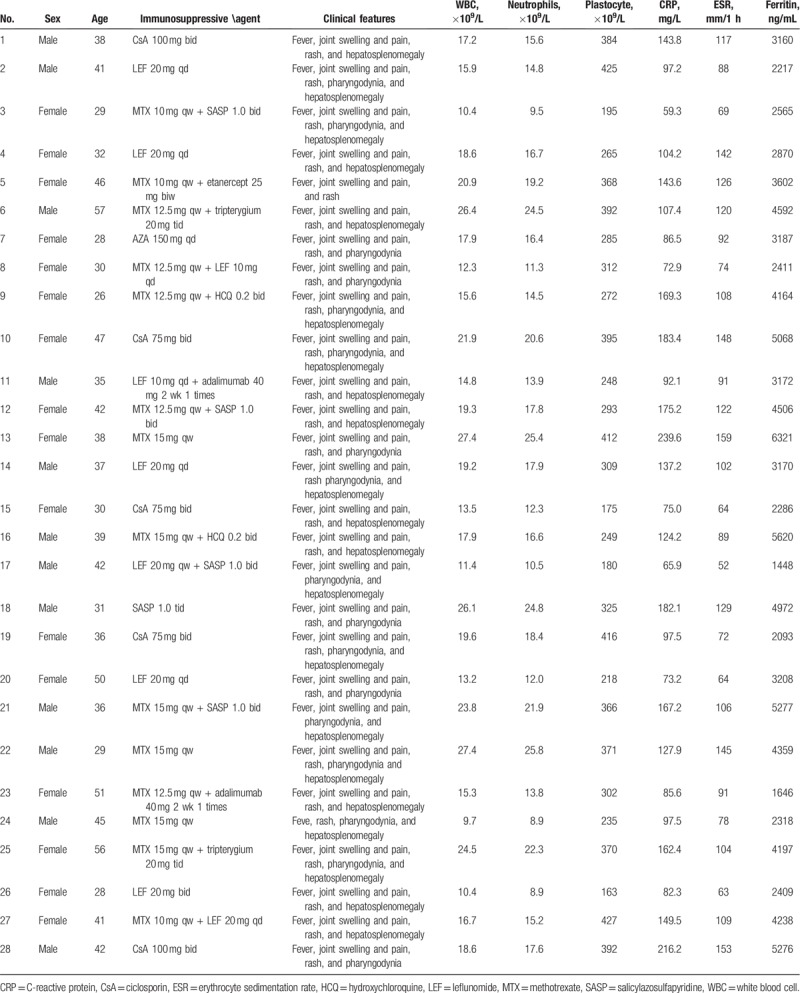
3.2. Comparison of clinical symptoms and signs between pre- and posttreatment with tocilizumab
The improvement rates of body temperature, skin rash as well as joint swelling and pain were 67.9%, 85.7%, and 60.8%, respectively, as compared with pretreatment, there were significant differences (all P < .05); hepatosplenomegaly was also significantly improved after 4-week treatment (P < .05); the body temperature was normal, the skin rash as well as joint swelling and pain disappeared in all cases after 8-week treatment; and patients’ conditions were stable in 12, 24, 36, and 48-week treatment (all P < .05, Table 2).
Table 2.
Comparison of clinical symptoms and signs between pre- and posttreatment with tocilizumab.
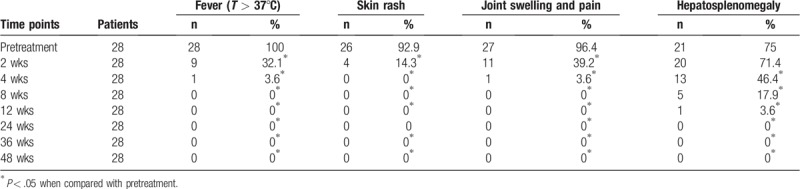
3.3. Comparison of laboratory indexes between pre- and posttreatment with tocilizumab
Compared with pretreatment, CRP was significantly decreased after 2-week treatment (P < .05); white blood cell count, neutrophilic granulocyte count, platelet count, and ESR was significantly reduced after 4-week treatment (all P < .05); hemoglobin level was significantly increased but serum ferritin level was significantly decreased after 8-week treatment (all P < .05); and laboratory indices were stable in 12-, 24-, 36-, and 48-week treatment (all P < .05, Table 3).
Table 3.
Comparison of laboratory indexes between pre- and posttreatment with tocilizumab (x ± standard deviation).
3.4. Reduction of tocilizumab
Intravenous 8 mg/kg of tocilizumab was changed from every 4 weeks to every 8 weeks in 19 cases at the 28th week; and in all cases at the 32nd week.
3.5. Administration of prednisone
The dosage of prednisone was reduced from 71.4 ± 20.7 mg/day to 55.0 ± 11.1 mg/day after 2-week treatment, to 11.5 ± 3.1 mg/day after 12-week treatment, to 7.9 ± 3.4 mg/day after 24-week treatment and to 3.3 ± 2.1 mg/day after 48-week treatment (all P < .05, Fig. 1). The prednisone was discontinued in 5 cases after 36-week treatment and in 7 cases after 48-week treatment.
Figure 1.
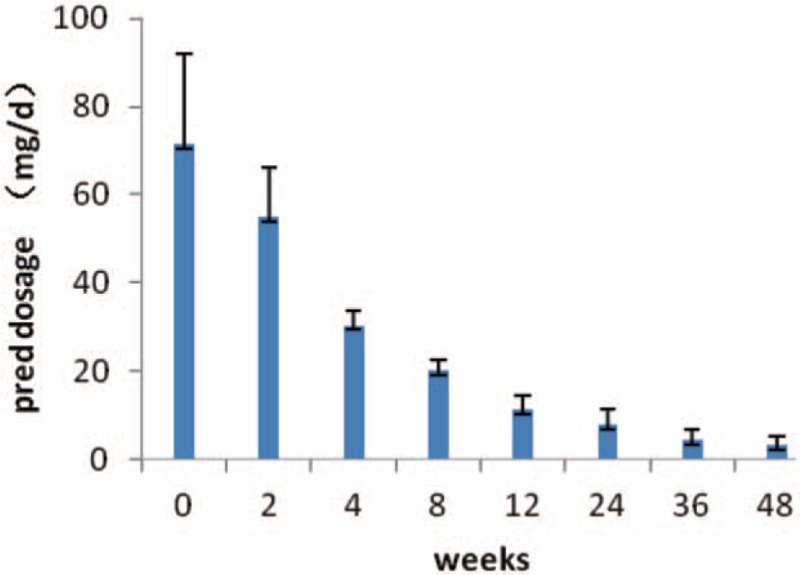
Trend in reducing dosage of prednisone after treatment using tocilizumab. pred = prednisone.
3.6. Adverse reactions
During the treatment, 3 cases had drug-related elevation of ALT and AST, which were within 3 times of the normal value and completely returned to normal levels after taking compound glycyrrhizin (25 mg, tid). Allergic reactions occurred in 2 cases during infusion and were relieved after intravenous 5 mg of dexamethasone; after this, dexamethasone was routinely given before infusion to prevent allergic reactions and no allergic reactions occurred again in the 2 cases. Two cases with upper respiratory tract infection and 1 case with urinary tract infection improved after treatment. Four cases had oral ulcer followed by self-limited improvement. There were no serious adverse reactions such as severe infection, tumor, heart failure, and death.
4. Discussion
In 1971, Bywaters[4] first described AOSD which was characterized by fever, transient skin rash, joint swelling and pain, and increased inflammatory markers such as white blood cell, neutrophilic granulocyte, ESR, and CRP. The pathogenesis of AOSD is still unclear. Currently, the main drugs for treatment of AOSD include NSAIDs, glucocorticoids, disease-modifying anti-rheumatic drugs (DMARDs). These drugs can improve symptoms and relieve inflammation in most patients, but there are still some refractory patients with AOSD who require large dose of glucocorticoids which easily allows AOSD to recur during the reduction of glucocorticoids, and/or have poor response to traditional drugs such as MTX, leflunomide, and ciclosporin.
At present, the pathogenesis of AOSD is not very clear. IL-1, TNF-α, IL-8, and IL-6 may be all involved in the pathogenesis of AOSD. Therefore, blocking these cytokines has become a new strategy for the treatment of AOSD. At present, the biologic agents such as anakinra, infliximab, and etanercept can markedly improve or completely relieve clinical symptoms in some patients with AOSD who did not respond to conventional therapy.[5,6] These results demonstrate that these cytokines play a key role in the pathogenesis of AOSD. However, macrophage-related cytokines are various and complex, so some patients still do not respond to anti-TNF-α or anti-IL-1. Therefore, it is necessary to find new therapeutic targets. IL-6 plays an important role in the pathogenesis of AOSD because IL-6 can induce T- and B-cell differentiation, stimulate hepatocytes to produce a variety of acute proteins, and promote thrombocytopoiesis. IL-6 also activates osteoclast precursor cells to differentiate into mature osteoclasts by binding to soluble IL-6 receptors, leading to joint damage and osteoporosis.[7] Therefore, blocking IL-6 is expected to become a new target for treatment of AOSD. Tocilizumab, a recombinant humanized monoclonal antibody against human IL-6 receptor, specifically binds to transmembrane and soluble IL-6 receptors, inhibiting the biologic activity of IL-6. In recent years, some studies have indicated that AOSD has good efficacy and safety in the treatment of AOSD.[8,9]
In the 28 cases with refractory AOSD of this study, tocilizumab markedly improved patient's condition. The body temperature, skin rash, joint swelling, and pain as well as hepatosplenomegaly were significantly relieved; and CRP, white blood cell count, neutrophilic granulocyte count, platelet count, and ESR were significantly decreased after 2-week treatment. Clinical symptoms completely disappeared in all cases, and CRP, ESR, and serum ferritin were significantly decreased after 8-week treatment. Our results were similar to the results reported by Iwamoto et al.[10] A case report showed that clinical symptoms and CRP were rapidly improved after 1-week tocilizumab treatment.[10] Our results and other reports all suggest that tocilizumab can quickly control the inflammatory state and stabilize the patient's condition in AOSD. It has been reported that the level of serum IL-6 in patients with AOSD is significantly higher than that in healthy people, high IL-6 is closely related to fever, rash, hepatosplenomegaly, and elevations of CRP and ferritin, and IL-6 is an important marker of disease activity.[7] Therefore, tocilizumab rapidly improves the clinical symptoms because it can decrease IL-6 level.
The dosage of drugs varies according to different diseases. For rheumatoid arthritis, tocilizumab is given 8 mg/kg every 4 weeks,[11] but for systemic juvenile idiopathic arthritis, the tocilizumab is given 12 mg/kg in the patients with <30 kg of body weight and 8 mg/kg in the patients with more than 30 kg of body weight once every 2 weeks.[12] How to use tocilizumab in the treatment of AOSD is still uncertain. It was reported that 8 mg/kg of tocilizumab was given every 4 weeks, or 4 mg/kg of tocilizumab every 4 weeks in the treatment of AOSD.[13] In this study, patients 1st received 8 mg/kg of tocilizumab every 4 weeks, and then patients’ symptoms rapidly improved and the levels of CRP, ESR, and serum ferritin were significantly decreased. After 6-month stable condition, the 8 mg/kg of tocilizumab was changed from every 4 weeks to every 8 weeks, and subsequent follow-up indicated that patients’ conditions were stable and laboratory indexes were normal. After remission, all physicians confront the problem to reduce the dosage of biologic agent. In details, it includes the time to reduce dosage and the approaches to reduce dosage. The approaches to reduce dosage include prolonging the interval of administration or reduction of the single dose. Our results suggest that in the refractory patients with AOSD, the strategy of prolonging the interval of administration is feasible after patients’ conditions are stable, where the strategy remains to be confirmed by large-scale clinical randomized controlled trials.
It is difficult to reduce the dosage of glucocorticoids in refractory AOSD, because relapse easily occurs during the reduction of glucocorticoids. In this study, the dosage of prednisone was significantly reduced after 2-week tocilizumab, and then was reduced to 11.5 ± 3.1 mg/day at the 12th weeks lower than 14.6 ± 4.1 mg/day before treatment, suggesting that tocilizumab could not only control AOSD rapidly, but also stabilize patients’ conditions. Prednisone was discontinued in 5 patients after 36-week tocilizumab, and in 7 patients after 48-week tocilizumab, suggesting that tocilizumab can rapidly reduce or even stop the use of glucocorticoids in the treatment of AOSD, which is consistent with the literature.[8] In most literatures, tocilizumab was not combined with traditional DMARDs in the treatment of AOSD, and there were reports of successful treatment of AOSD with tocilizumab alone.[9,14] Kadavath et al[15] reported that a case with AOSD and pulmonary hypertension was successfully treated using tocilizumab combined with MTX. In this study, we used MTX, and under prolonging the interval of administration of tocilizumab, prednisone was successfully discontinued in 25% patients after 48-week treatment. However, it was reported that prednisone was successfully discontinued in 20% patients after 19-month tocilizumab without using MTX.[8] This suggests that MTX may be conducive to reducing and discontinuing prednisone as well as to reducing tocilizumab.
In summary, tocilizumab combined with MTX has marked therapeutic effects for refractory AOSD, is conducive to reduction and discontinuation of prednisone, and can allow patients’ conditions to be stable after reducing the dosage of tocilizumab with good safety.
4.1. Limitations
In this study, the observation time was short, the sample size was not large, subgroup was not performed according to the use of tocilizumab alone or combined with MTX, and the level of serum IL-6 was not detected. These will be improved in our future studies.
Author contributions
Conceptualization: Hai-li Shen.
Data curation: Shao-hua Guo.
Formal analysis: Li-ping Wang.
Writing – original draft: Chun-yan Wang.
Footnotes
Abbreviations: ALT = alanine transaminase, AOSD = adult-onset Still disease, AST = aspartate transaminase, CRP = C-reactive protein, DMARDs = disease-modifying anti-rheumatic drugs, ESR = erythrocyte sedimentation rate, MTX = methotrexate, NSAIDs = nonsteroidal anti-inflammatory drugs.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Mavragani CP, Spyridakis EG, Koutsilieri SM. Adult-onset Still's disease: from pathophysiology to targeted therapies. Int J Inflam 2012;2012:879020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Colafrancesco S, Priori R, Alessandri C, et al. IL-18 serum level in adult onset Still's disease: a marker of disease activity. Int J Inflam 2012;2012:156890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kirino Y, Takeno M, Iwasaki M, et al. Increased serum HO-1 in hemophagocytic syndrome and adult-onset Still's disease: use in the differential diagnosis of hyperferritinemia. Arthritis Res Ther 2005;7:R616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bywaters EG. Still′s disease in the adult. Ann Rheum Dis 1971;30:121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Katherine A, Lyseng-Williamson Anakinra in Still's disease: a profile of its use. Drugs Ther Perspect 2018;34:543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fautrel B, Sibilia J, Mariette X, et al. Tumour necrosis factor alpha blocking agents in refractory adult Still's disease: an observational study of 20 cases. Ann Rheum Dis 2005;64:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choy E. Clinical experience with inhibition of interleukin-6. Rheum Dis Clin North Am 2004;30:405–15. [DOI] [PubMed] [Google Scholar]
- [8].De Boysson H, Février J, Nicolle A, et al. Tocilizumab in the treatment of the adult-onset Still′s disease: current clinical evidence. Clin Rheumatol 2013;32:141–7. [DOI] [PubMed] [Google Scholar]
- [9].Ortiz-Sanjuán F, Blanco R, Calvo-Rio V, et al. Efficacy of tocilizumab in refractory adult-onset still′s disease: multicenter retrospective open-label study of 34 patients. Arthritis Rheum 2014;66:1659–65. [DOI] [PubMed] [Google Scholar]
- [10].Iwamoto M, Nara H, Hirata D, et al. Humanized monoclonal anti-interleukin-6 receptor antibody for treatment of intractable adult-onset Still′s disease. Arthritis Rheum 2002;46:3388–9. [DOI] [PubMed] [Google Scholar]
- [11].Hashizume M, Tan SL, Takano J, et al. Tocilizumab, a humanized anti-IL-6R antibody, as an emerging therapeutic option for rheumatoid arthritis: molecular and cellular mechanistic insights. Int Rev Immunol 2015;34:265–79. [DOI] [PubMed] [Google Scholar]
- [12].Yokota S, Imagawa T, MoIi M, et al. Efficacy and safety of toeilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 2008;371:998–1006. [DOI] [PubMed] [Google Scholar]
- [13].Li T, Gu L, Wang XD, et al. A pilot study on tocilizumab for treating refractory adult-onset Still's disease. Sci Rep 2017;18:13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sakai R, Nagasawa H, Nishi E, et al. Successful treatment of adult-onset Still′ s disease with tocilizumab monotherapy: two case reports and literature review. Clin Rheumatol 2012;31:569–74. [DOI] [PubMed] [Google Scholar]
- [15].Kadavath S, Zapantis E, Zolty R, et al. A novel therapeutic approach in pulmonary arterial hypertension as a complication of adult-onset Still′s disease: targeting IL-6. Int J Rheum Dis 2014;17:336–40. [DOI] [PubMed] [Google Scholar]


