Abstract
Trial Design:
The aim of this study was to identify independent risk factors for post-pancreatoduodenectomy (post-PD) abdominal fluid collections (AFCs) and evaluate our management protocol on it.
Methods:
A retrospective analysis of consecutive 2064 cases who underwent PD over the past decade in 1 single center was conducted. The patients were divided into AFCs and non-AFCs group. Univariable and multivariate logistic regression analysis was performed to identify independent risk factors of AFCs. The AFCs group was compared with the non-AFCs group with respect to the incidence of postoperative outcomes. The characteristics of AFCs were further analyzed in terms of clinical manifestations.
Results:
Two thousand sixty-four cases with pancreaticoduodenectomy were recruited and 15% of them were found AFCs. Diameter of main pancreatic duct ≤3 mm was found to be an independent predictor of AFCs (P < .001), along with soft pancreatic texture (P = .002), mesenterico-portal vein resection (P < .001), and estimated intraoperative blood loss >800 mL (P < .001). The incidence of mild complications was significantly higher in AFCs group than in non-AFCs group (34% vs 20%, P < .001), whereas no significant differences were noted in the rate of severe complications between these 2 groups (15% vs 15%, P = .939).
Conclusion:
Enhanced drainage is recommended as an effective measure to decrease the incidence of severe complications caused by post-PD AFCs.
Keywords: abdominal fluid collections, management strategy, pancreatoduodenectomy
1. Introduction
Pancreaticoduodenectomy (PD) or pylorus-preserving pancreaticoduodenectomy (PPPD) is the standard surgical method of pancreatic head region tumors.[1] Over the past 3 decades, operative mortality rates after PD have decreased dramatically.[2–4] An increase in experience and continuous improvements in surgical techniques, anesthesia, and perioperative care have led to this decline, and in many high-volume centers, the mortality rate is lower than 4%.[5–9] However, the postoperative morbidity rate is still ranging from 30% to 50%.[10–13]
Abdominal fluid collections (AFCs), as one of the most common complications of post-PD, is a critical trigger of life-threatening complications such as digestive tract fistula, intra-abdominal abscess, and hemorrhage.[14] It leads to prolonged hospitalization, severe morbidity, or even surgical mortality.[15] The incidence of AFCs is reported to be 0% to 17% based on a variety of definitions.[16–18] However, little is known about AFCs, which is partly related to the complexity of operation, difficult detectability, and lack of routine postoperative abdominal image screening.[19] Consequently, it is clear that many aspects of AFCs remain to be clarified to provide useful information for clinical decision.[20–25] Meanwhile, an effective management strategy for post-PD AFCs is needed to be established.
The objective of this study was to earlier identify AFCs and evaluate the effectiveness of our management protocol on post-PD AFCs.
2. Material and methods
2.1. Preoperative work-up
From March 11, 2008, through March 15, 2018, 2064 patients underwent PD in our center. All patients were subjected to thorough history taking, clinical examination, and their clinical information was collected from our institutional database. Computed tomography (CT) scan was done for all patients. Age, gender, body mass index (BMI), medical history of diabetes mellitus, and preoperative serum bilirubin level were identified from the patient medical reports. Preoperative biliary drainage (endoscopic or percutaneous transhepatic) was done to cases with preoperative total bilirubin >400 μmol/L. This study protocol was approved by the ethics committee of our college. All patients signed an informed consent regarding their understanding of the procedure and its potential complications as well as their approval of participation in the research.
All procedures were carried out by 4 senior consultant surgeons experienced in pancreatobiliary surgery and used a similar technique of dissection.[19] Pylorus-preserving PD (PPPD) was performed in 129 cases (6.25%), whereas a Whipple resection was done in 1935 cases (93.75%). If the portal vein and/or superior mesenteric vein was involved, resection of the mesentericoportal vein and an end-to-end anastomosis were carried out as reported previously.[26] The stump of the gastroduodenal artery (GDA) was left around 5 mm long and closed with a suture ligature. In patients with cancer, lymphadenectomy was routinely undertaken with skeletonization of the hepatic artery from the hepatic pedicle to the celiac axis along with removal of the retroportal pancreatic lamina on the right aspect of the superior mesenteric artery.[27] Reconstruction was as follows: pancreatojejunostomy, end-to-side hepatico-jejunostomy, and side-to-side gastrojejunostomy with a single loop.[28] The pancreaticojejunostomy was performed using a duct-to-mucosa anastomosis (n = 1806, 87.5%) or, alternatively, an invaginated anastomosis (n = 258, 12.5%) based on surgeon preference. A pancreatic duct stent was usually used in all pancreaticojejunostomy.[29] There were no pancreaticogastrostomies used. All patients had 2 closed suction drains placed at the time of operation, one behind the pancreatic anastomosis and the other at the level of the biliary anastomosis.[15] Seven intraoperative variables, including diameter of main pancreatic duct, pancreatic texture, type of resection (standard versus pylorus preservation), type of pancreaticojejunostomy (invagination or end-to-side duct to mucosa), mesenterico-portal vein resection, estimated intraoperative blood loss, and number of lymph nodes harvested, were identified from anesthesiologist's records and the operative reports.
2.2. Postoperative work-up
All patients were admitted to ICU on the night of the operating day. Patients received Octreotide (100 μg/8 h) until postoperative day (POD) 5. Parenteral antibiotic with amoxicillin/clavulanate or piperacillin/tazobactam were administered to all patients prophylactically.[28] Proton pump inhibitor Omeprazole was given in a dosage of 40 mg/12 h for 7 days. Routine blood analysis was performed 3 times a week, and more often if a likely complication was foreseen.[28] The nasogastric tube was left in place until POD 5 to protect the gastrojejunostomy.[15]
As our unit protocol for postoperative monitoring, abdominal image screening (ultrasound and/or CT) was routinely carried out in all patients at least on POD 3, the day before drain removal and before discharge. Ultrasound and/or CT were also performed in any patient with clinical symptoms (fever and persistent abdominal pain), laboratory abnormalities (elevated total white blood cell count and increased C-reactive protein or procalcitonin levels), or suspicion of surgical complications (such as collections, hemorrhage, fistulae, suture dehiscence, or others). Moreover, all patients were subject to routine abdominal ultrasound or CT during follow-up visits.[19] Abdominal drains were typically removed from POD 4 to 5 if daily drainage was <50 mL of unsuspected effluent with low amylase content and negative bacterial culture.[19] Due to the serious consequences that intra-abdominal abscess and fistula might lead to, enhanced drainage would be instituted immediately in all AFCs cases without waiting for microbiology or laboratory results.[26] For the collection adjacent to intraoperatively placing drains, it was most probably due to obstruction of tube tip by fibrins. Sterile saline solution was flushed into the collections or abdominal drain was replaced to maintain adequate drainage and tube patency. For the collection remote from drains, percutaneous drainage was performed as long as it was accessible. Samples from the AFCs were sent for both laboratory test and bacteriological culture after drainage.[26] When the drain fluid turned cloudy with sediment before the abdominal drain was removed, a low-speed intermittent irrigation was added until the drain fluid returned clear.
The management strategy of post-PD AFCs in our unit is shown in Fig. 1. In hemorrhage AFCs patients, basic emergent resuscitative measures would be initiated immediately, which included aggressive fluid therapy, blood product transfusion, octreotide and proton pump inhibitor treatment, and vasoactive agent if hemodynamic instability was indicated. In situations where the hemorrhage has not “really settled,” angiographic embolization would be considered. The failure to control hemorrhage by above measures may necessitate reoperation. In those patients who require reoperation, a thorough exploration of the resection site is required and if necessary, ligation of the arterial stumps (including occasionally the common hepatic artery) and inspection of the anastomosis by enterotomy. After placing drainage tubes again beside these anastomotic stomas, the abdomen was closed. Once hemorrhage is under effective control, management will be transitioned to nonhemorrhage collection protocol. In nonhemorrhage AFCs, no matter pancreatic fistula, bile leakage, enteric fistula, chyle leak, or simple abscess, successful management of this serious complication depends on close clinical examination, which requires a high index of clinical suspicion. Analysis of drainage fluid is the principal diagnostic tool, while CT, ultrasound, and pancreaticography could provide additional information. Conservative management strategies form the cornerstone of management in majority of the patients and include managing fluid balance, ensuring patency of abdominal drains, providing parenteral or enteral nutrition, and administrating antibiotics or octreotide. Repeated image-guided drainage or replacing drains is indicated if new-onset collections are observed. The indication for surgical intervention in AFCs includes worsening clinical parameters, signs of diffuse peritonitis, severe wound infection, continuous bowel dysfunction, and septic shock. The resurgical intervention is generally comprised of abdominal lavage, repositioning of drains, feeding jejunostomy, reinforcing sutures in case of minor-leak from anastomosis, completion pancreatectomy, or reconstruction (if drainage alone could not solve the problem).
Figure 1.
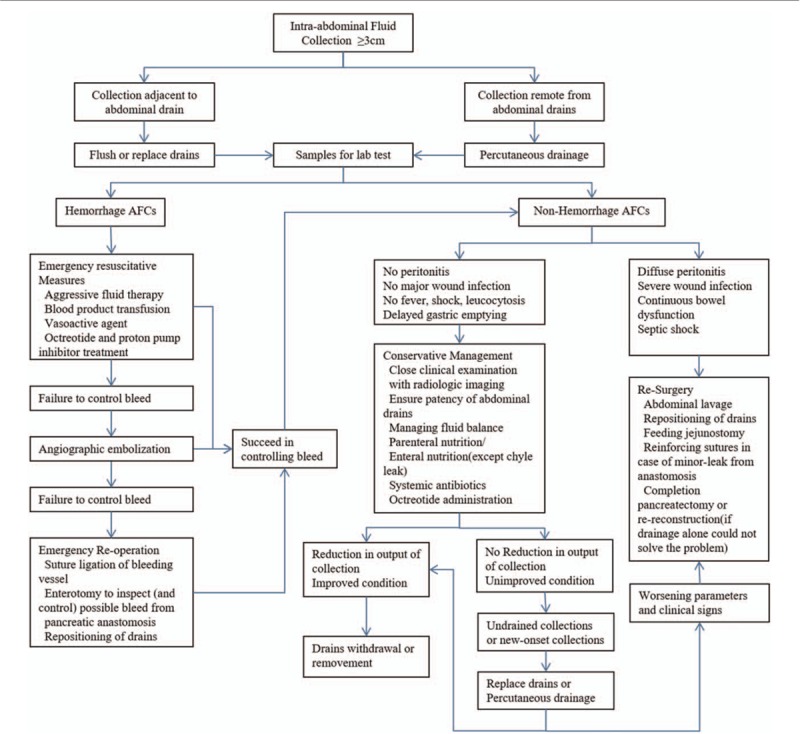
The diagnostic and therapeutic strategies of post-PD AFCs in our unit.
2.3. Inclusion criteria
-
(1)
Age: >18 years, <75 years;
-
(2)
Patients with pancreatic diseases (including tumor and inflammatory disease) or nonpancreatic tumors (biliary duct cancer or ampullary tumor) who underwent pancreatoduodenectomy.
2.4. Definitions
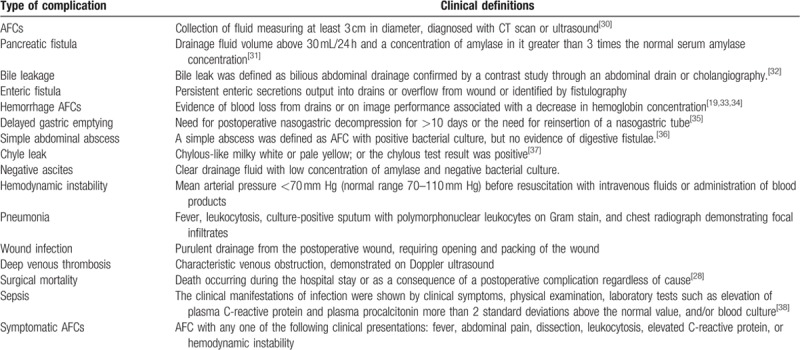
The definitions of the complications are provided in Table 1.[28,30–38] The patients recruited in this study were divided into 2 groups according to the presence of post-PD AFCs: AFCs group and non-AFCs group. AFC patients were further divided into symptomatic and asymptomatic subgroups according to the presence of clinical symptoms, or hemorrhage and nonhemorrhage subgroups according to the nature of fluid collections. We adopted the classification system of postoperative complication proposed by Dindo et al.[30] According to this system, a severe complication was defined as grade IIIb or above and mild complication as grade IIIa or below.[39]
Table 1.
Definitions used in the present study.
2.5. Statistical analysis
Patient characteristics were compared using t tests for continuous variables and χ2 or Fisher exact tests for categorical variables. To select final predictors, all candidate predictors with a P < .1 in univariate analysis were included in a multivariate logistic regression model. Variates with P < .05 in the multivariate analysis were deemed independent predictors.
3. Results
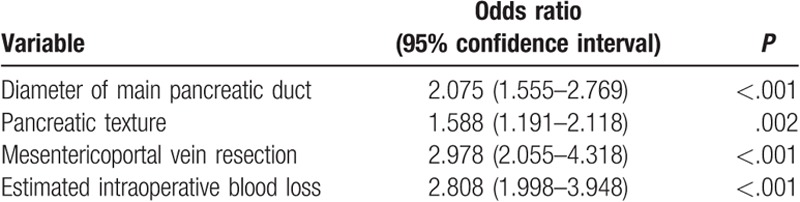
As outlined in Table 2, this study included 2064 consecutive patients [1046 male and 1018 female; mean age 55.8 years (range 14–82)] who underwent PD from March 11, 2008, through March 15, 2018. Postoperative AFCs were found in 309 patients, while non-AFCs in the rest 1755 patients. The results of the univariable logistic regression analysis for AFCs are summarized in Table 3. Diameter of main pancreatic duct ≤3 mm, soft pancreatic texture, mesenterico-portal vein resection, and estimated intraoperative blood loss >800 mL were significant risk factors of AFCs post-PD at the univariable level. When these variables were assessed in the multivariable logistic regression, all remained highly significant (Table 4). Therefore, diameter of main pancreatic duct ≤3 mm was found to be an independent risk factor of AFCs (P < .001), along with soft pancreatic texture (P = .002), mesenterico-portal vein resection (P < .001), and estimated intraoperative blood loss >800 mL (P < .001).
Table 2.
Preoperative characteristic, pathologies, and operative details comparisons between patients undergoing PD with and without AFCs.
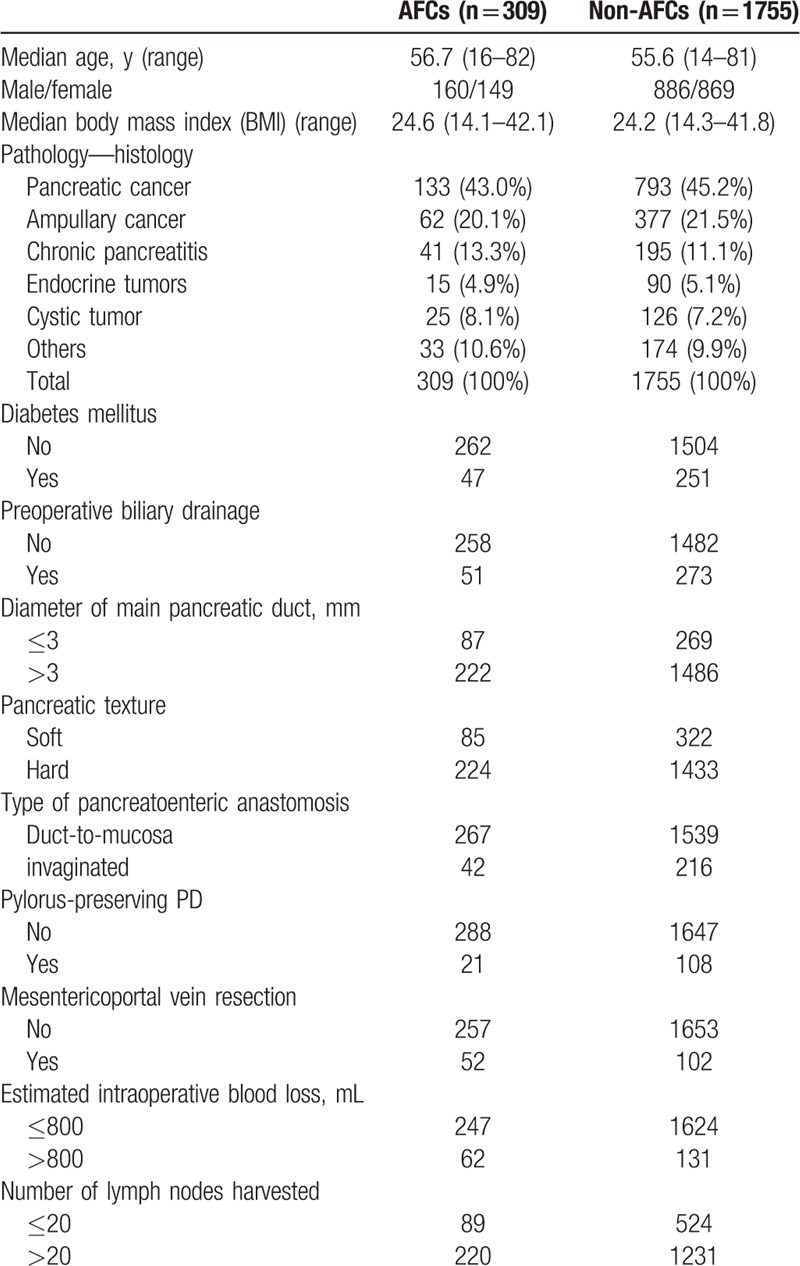
Table 3.
Univariable logistic regression: risk factors for AFCs.
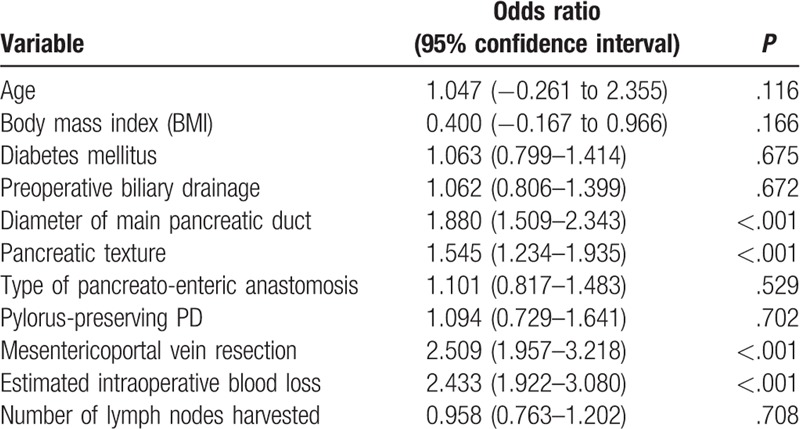
Table 4.
Multivariable logistic regression: independent risk factors for AFCs.
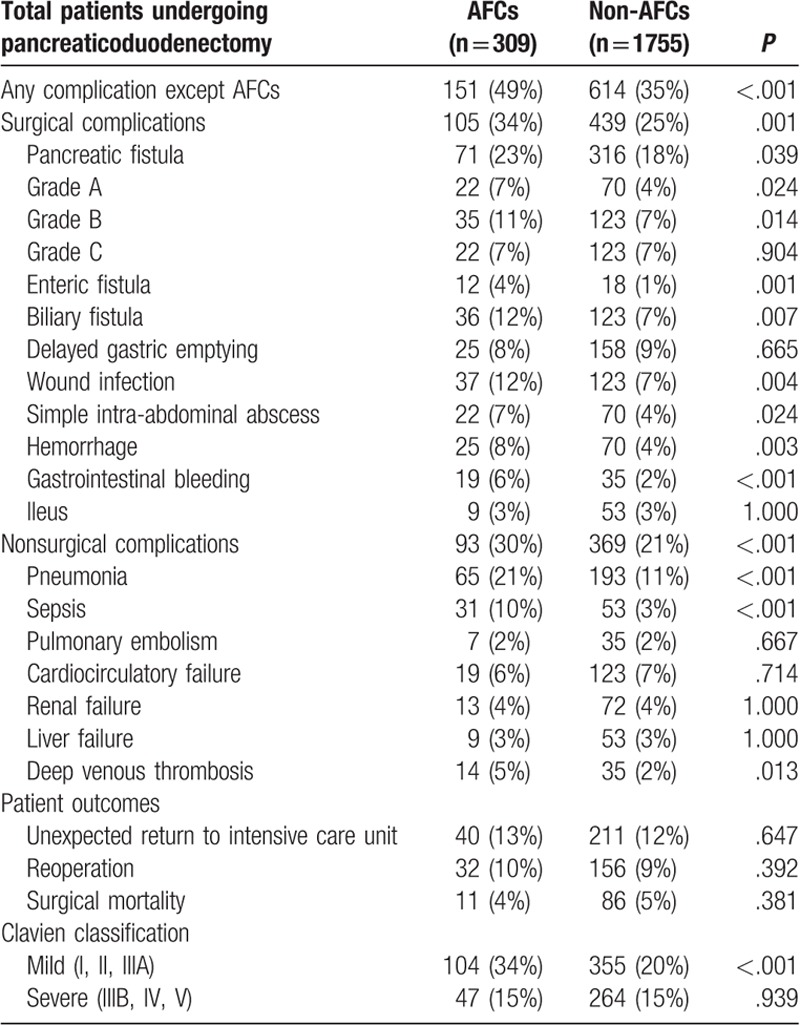
Postoperative outcomes were compared between AFCs and non-AFCs groups in Table 5. Surgical complications were more frequent in AFCs group than non-AFCs [Grade A pancreatic fistula: 22 (7%) cases vs 70 (4%) cases, P = .014; Grade B pancreatic fistula: 35 (11%) cases vs 123 (7%) cases, P = .008; Enteric fistula: 12 (4%) cases vs 18 (1%) cases (P < .001); Biliary fistula 36 (12%) cases vs 123 (7%) cases, P = .005; Wound infection 37 (12%) cases vs 123 (7%) cases, P = .003; Simple intra-abdominal abscess 22 (7%) cases vs 70 (4%) cases, P = .014; Hemorrhage 25 (8%) cases vs 70 (4%) cases, P = .002; Gastrointestinal bleeding: 19 (6%) cases vs 35 (2%) cases, P < .001]. AFCs group became more prone to nonsurgical complications than non-AFCs group [Pneumonia: 65 (21%) cases vs 193 (11%) cases, P < .001; Sepsis: 31 (10%) cases vs 53 (3%) cases, P < .001; Deep venous thrombosis: 14 (5%) cases vs 35 (2%) cases, P = .007]. Broadly, the incidence of mild complication in AFCs group is higher than in non-AFCs group (34% cases vs 20% cases, P < .001), whereas AFCs group after active intervention appeared to have a similar rate of severe complication with non-AFCs group [Clavien Class IIIB, IV, V: 47 (15%) cases vs 264 (15%) cases, P = .939; Unexpected return to intensive care unit: 40 (13%) cases vs 211 (12%) cases, P = .647; Reoperation: 321 (10%) cases vs 156 (9%) cases, P = .409; Surgical mortality: 11 (4%) cases vs 86 (5%) cases, P = .305].
Table 5.
Comparisons on outcomes of PD patients with versus without postoperative AFCs.
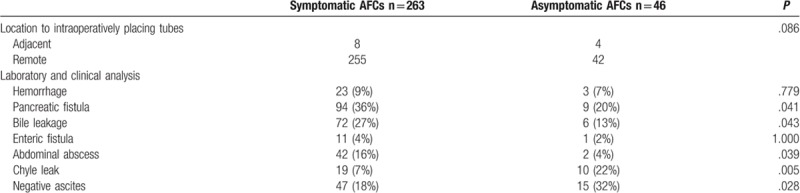
The characteristics of AFCs were further analyzed and compared between symptomatic and asymptomatic subgroup in Table 6. A total of 263 patients was classified into symptomatic group, and the remaining 46 patients into asymptomatic group. There is no significant difference in the distance from intraoperatively placing tubes between 2 groups. Some types of AFCs were significantly higher in symptomatic groups than asymptomatic group, including pancreatic fistula (36% vs 20%; P = .041), bile leakage (27% vs 13%; P = .043), and abdominal abscess (16% vs 4%; P = .039), whereas some were similar in both groups, including enteric fistula (4% vs 2%; P = 1.000) and hemorrhage (9% vs 7%; P = .779). About 67% of asymptomatic AFCs were associated with pancreatic fistula (20%), bile fistula (13%), enteric fistula (2%), hemorrhage (7%), chyle leakage (22%), and abdominal abscess (4%).
Table 6.
Type of post-PD AFCs.
The time from surgery to the diagnosis of AFCs was recorded and the proportion of hemorrhage and nonhemorrhage subgroup is shown in Fig. 2. The median time from surgery to the diagnosis of AFCs was 5 days [interquartile range (IQR), 3–12 days]. The peak time of hemorrhage AFCs and nonhemorrhage AFCs was 24 hours and 3 to 5 days.
Figure 2.
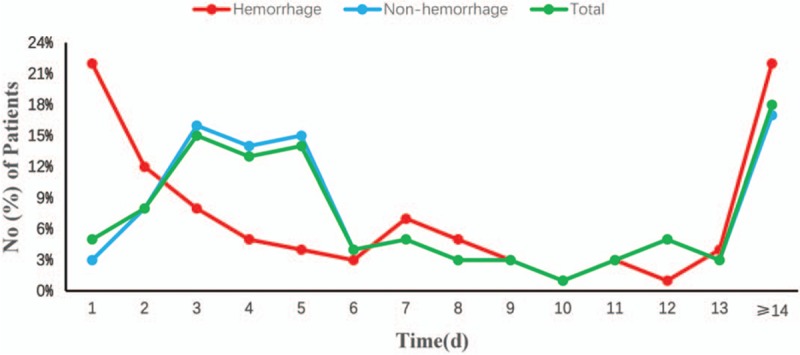
The time from surgery to the diagnosis of AFCs.
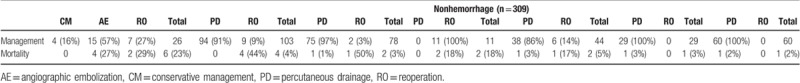
The clinical outcomes of patients through our unit protocol are demonstrated in Table 7. The proportion of hemorrhage AFCs resolved by reoperation and nonoperative intervention was 19.3% and 57.7%, respectively, while 24% finally died. The clinical success of nonhemorrhage AFCs by percutaneous drainage and reoperation was 90.2% and 6.2%, including pancreatic fistula (91.3% vs 4.9%); bile leak (96.2% vs 1.3%); enteric fistula (0 vs 81.8%); abdominal abscess (84.1% vs 11.4%); chyle leak (96.6% vs 0); and negative AFCs (98.3% vs 0). The mortality of nonhemorrhage AFCs is 3.7%, including 3.9% pancreatic fistula, 2.6% bile leak, 18.2% enteric fistula, 4.5% abdominal abscess, 3.4% chyle leak, and 1.7% negative AFCs.
Table 7.
Management and clinical outcomes in patients with AFCs.
4. Discussion
The proper prevention and treatment of the postoperative complications of the patients with PD presents a considerable technical challenge for each pancreatic surgery center.[19] Multiple factors contribute to the formation of post-PD AFCs.[40–47] Some researchers[48] reported that soft pancreatic texture and thin pancreatic duct were risk factors for fluid collections. Yeh et al demonstrated that increased intraoperative blood loss was another independent risk factor for post-PD AFCs by univariate and multivariate analysis.[25,49–51] The factors that might increase blood loss during operation included a more advanced stage of the disease such as portal vein invasion or superior mesenteric vein, adhesions due to prior operations, jaundice-associated coagulopathy, obesity, and concurrent pancreatitis.[25,49–51] A similar conclusion was reached in our study that the diameter of main pancreatic duct ≤3 mm, soft pancreatic texture, mesenterico-portal vein resection, and estimated intraoperative blood loss >800 mL were closely related with the formation of AFCs. Under such circumstances, enhanced drainage strategy might like to be undertaken, which included more intraoperative site drainage, more frequent postoperative image checkup, and longer duration of drainage.
It is universally accepted that AFCs is often associated with a series of serious complications.[52] Liu et al[53] suggested that intra-abdominal collection correlated with post-PD delayed gastric emptying rates significantly. Zink et al[23] found that 74.7% (62/83) of post-PD fluid collections were proven abscesses, and 61.4% (51/83) were complicated by pancreatic fistula. In the research by Feng et al,[54] intra-AFCs could be independent risk factors for post-PD hemorrhage. In our study, AFCs could produce a higher rate of mild complications compared with non-AFCs, but it got a similar incidence of severe complications through active intervention.
Previous reports suggested that about half of AFCs are asymptomatic and resolve spontaneously.[12,19,21,55–58] According to this stereotyped experience, imaging tests were usually carried out only in patients with symptoms suggestive of intra-abdominal complications (pyrexia, abdominal distension or abdominal pain, and so on), and asymptomatic AFCs patients do not mandate drainage in most centers.[59] However, protocol in our unit could allow for early identification and monitoring of more potential abdominal complications especially in asymptomatic patients. Our study found about 67% of asymptomatic AFCs are associated with pancreatic fistula (20%), bile fistula (13%), enteric fistula (2%), hemorrhage (7%), chyle leakage (22%), and abdominal abscess (4%). We would probably have been misguided by previous stereotyped experience to neglect these cases, which needed to be promptly treated just because of no positive symptom manifested. Moreover, some of AFCs may be partly attributed to displacement or occlusion of drainage tubes, routine postoperative abdominal image, and corresponding remedy could help to prevent the deterioration by dysfunctional tubes. Upon further analyses, we discovered that POD 3 to 5 could be the “peak time” when AFCs was detected. Thus, routine postoperative abdominal image screening was recommended at least earlier than POD 3.
Once an intra-abdominal collection is identified, it is first choice to reposition operatively placed drain or place a percutaneous drainage under image guidance.[51,60] Effective drainage could convert the digestive leakage into controlled ones instead of making intra-abdominal abscess formation around the anastomotic site.[61] Furthermore, drainage will not only help prevent pain and potential complications such as ileus, fever, and sepsis, but also serve as an early warning sign of anastomotic leak and associated hemorrhage.[62–66] Clinical cure of AFCs could be achieved in majority of the patients by enhanced drainage combined with other conservative management strategies, including 91.3% (94/103) pancreatic fistula, 96.2% (75/78) bile leak, 84.1% (37/44) abdominal abscess, 96.6% (28/29) chyle leak, and 98.3% (59/60) negative ascites in our study. Kazanjian et al[67] evaluated 436 patients who underwent PD. A total of 55 (12.6%) developed AFCs; 52 patients (94.5%) had successful conservative management with percutaneous drainage, 4 required repeated percutaneous drainage, and only 3 patients (5.5%) had reoperation. Surgery still plays a crucial role in enteric fistula, failure in controlling intra-abdominal hemorrhage by angioembolization, inaccessible deep abdominal abscesses without any safe image approach, and persistent clinical deterioration.[27] All 11 enteric fistula patients in our study experienced reoperation after percutaneous drainage because of thick viscosity of abscess contents from enteric dehiscence even though the catheter was in proper position.
This study's limitations deserve commentary. First, due to the lack of definite practical guidance for intra-AFCs, the indication and timing of the drainage strategy was made empirically instead of evidence-based. Second, this was a nonrandomized retrospective analysis from a single center, and as such, there were potential biases for comparison. Third, the type of surgical technique used and of the ability and strategies to manage patients’ complications reflect the diversity in our center. However, our data reflect the common practice in our country where post-PD AFCs is diagnosed and treated in both academic and community settings. It shows that diagnostic and therapeutic strategies of this complications have been well standardized and mortality and morbidity are improved over historical data. The large cohort of patient and completeness of the collected data support the strength of this study. The results of the present analysis will hopefully lead to a prospective randomized study with the ultimate goal of a centralized national program for pancreatic surgery.
Author contributions
Ning Zhao, Tao Peng, and Heshui Wu conceived and designed the study. Ning Zhao, Xin Li, Jing Cui, and Jiongxin Xiong collected and analyzed data. Ning Zhao and Jing Cui wrote the paper. Zhiyong Yang, Chunyou Wang, and Tao Peng reviewed and edited the manuscript. All authors read and approved the manuscript.
Conceptualization: Ning Zhao, Heshui Wu, Tao Peng.
Data curation: Jing Cui, Zhiyong Yang, Jiongxin Xiong, Heshui Wu, Chunyou Wang.
Formal analysis: Jiongxin Xiong.
Investigation: Jing Cui, Chunyou Wang.
Methodology: Ning Zhao, Zhiyong Yang, Jiongxin Xiong, Chunyou Wang.
Project administration: Zhiyong Yang, Heshui Wu, Tao Peng.
Software: Ning Zhao, Jing Cui, Heshui Wu.
Supervision: Tao Peng.
Validation: Tao Peng.
Writing – original draft: Ning Zhao.
Writing – review & editing: Tao Peng.
Tao Peng orcid: 0000-0003-1336-1772.
Footnotes
Abbreviations: CT = computed tomography, GDA = gastroduodenal artery, post-PD AFCs = post-pancreatoduodenectomy abdominal fluid collections, PPPD = pylorus-preserving pancreaticoduodenectomy.
The authors certify that they have no conflicts of interest to disclose.
References
- [1].Kim SC, Kim YH, Park KM, et al. Pancreatic cancer surgery: the state of the art. Curr Drug Targets 2012;13:764–71. [DOI] [PubMed] [Google Scholar]
- [2].Allema JH, Reinders ME, van Gulik TM, et al. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer 1995;75:2069–76. [DOI] [PubMed] [Google Scholar]
- [3].Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993;217:430–5. discussion 435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miedema BW, Sarr MG, van Heerden JA, et al. Complications following pancreaticoduodenectomy. Current management. Arch Surg 1992;127:945–9. discussion 949-950. [DOI] [PubMed] [Google Scholar]
- [5].Yeo CJ, Barry MK, Sauter PK, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg 1993;218:229–37. discussion 237-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221:721–31. discussion 731-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990;211:447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cullen JJ, Sarr MG, Ilstrup DM. Pancreatic anastomotic leak after pancreaticoduodenectomy: incidence, significance, and management. Am J Surg 1994;168:295–8. [DOI] [PubMed] [Google Scholar]
- [9].Fernandez-del CC, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990 s. Arch Surg 1995;130:295–9. discussion 299-300. [DOI] [PubMed] [Google Scholar]
- [10].Balcom JT, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg 2001;136:391–8. [DOI] [PubMed] [Google Scholar]
- [11].Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg 2004;139:718–25. discussion 725-727. [DOI] [PubMed] [Google Scholar]
- [12].Bassi C, Falconi M, Salvia R, et al. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg 2001;18:453–7. discussion 458. [DOI] [PubMed] [Google Scholar]
- [13].Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990 s: pathology, complications, and outcomes. Ann Surg 1997;226:248–57. discussion 257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Osada S, Imai H, Sasaki Y, et al. Reconstruction method after pancreaticoduodenectomy. Idea to prevent serious complications. JOP 2012;13:1–6. [PubMed] [Google Scholar]
- [15].Osman MM, Abd EMW. Evaluation of a new modification of pancreaticogastrostomy after pancreaticoduodenectomy: anastomosis of the pancreatic duct to the gastric mucosa with invagination of the pancreatic remnant end into the posterior gastric wall for patients with cancer head of pancreas and periampullary carcinoma in terms of postoperative pancreatic fistula formation. Int J Surg Oncol 2014;2014:490386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kajiwara T, Sakamoto Y, Morofuji N, et al. An analysis of risk factors for pancreatic fistula after pancreaticoduodenectomy: clinical impact of bile juice infection on day 1. Langenbecks Arch Surg 2010;395:707–12. [DOI] [PubMed] [Google Scholar]
- [17].Veillette G, Dominguez I, Ferrone C, et al. Implications and management of pancreatic fistulas following pancreaticoduodenectomy: the Massachusetts General Hospital experience. Arch Surg 2008;143:476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gervais DA, Fernandez-del CC, O’Neill MJ, et al. Complications after pancreatoduodenectomy: imaging and imaging-guided interventional procedures. Radiographics 2001;21:673–90. [DOI] [PubMed] [Google Scholar]
- [19].Sierzega M, Kulig P, Kolodziejczyk P, et al. Natural history of intra-abdominal fluid collections following pancreatic surgery. J Gastrointest Surg 2013;17:1406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].AAssar OS, LaBerge JM, Gordon RL, et al. Percutaneous management of abscess and fistula following pancreaticoduodenectomy. Cardiovasc Intervent Radiol 1999;22:25–8. [DOI] [PubMed] [Google Scholar]
- [21].Barreto G, D'Souza MA, Shukla PJ, et al. The gray zone between postpancreaticoduodenectomy collections and pancreatic fistula. Pancreas 2008;37:422–5. [DOI] [PubMed] [Google Scholar]
- [22].Sohn TA, Yeo CJ, Cameron JL, et al. Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointest Surg 2003;7:209–19. [DOI] [PubMed] [Google Scholar]
- [23].Zink SI, Soloff EV, White RR, et al. Pancreaticoduodenectomy: frequency and outcome of post-operative imaging-guided percutaneous drainage. Abdom Imaging 2009;34:767–71. [DOI] [PubMed] [Google Scholar]
- [24].Vin Y, Sima CS, Getrajdman GI, et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg 2008;207:490–8. [DOI] [PubMed] [Google Scholar]
- [25].Shrikhande SV, D'Souza MA. Pancreatic fistula after pancreatectomy: evolving definitions, preventive strategies and modern management. World J Gastroenterol 2008;14:5789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang J, Qian HG, Leng JH, et al. Long mesentericoportal vein resection and end-to-end anastomosis without graft in pancreaticoduodenectomy. J Gastrointest Surg 2009;13:1524–8. [DOI] [PubMed] [Google Scholar]
- [27].Zhang J, Zhu X, Chen H, et al. Management of delayed post-pancreaticoduodenectomy arterial bleeding: interventional radiological treatment first. Pancreatology 2011;11:455–63. [DOI] [PubMed] [Google Scholar]
- [28].Manas-Gomez MJ, Rodriguez-Revuelto R, Balsells-Valls J, et al. Post-pancreaticoduodenectomy hemorrhage. Incidence, diagnosis, and treatment. World J Surg 2011;35:2543–8. [DOI] [PubMed] [Google Scholar]
- [29].Schmidt CM, Choi J, Powell ES, et al. Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg 2009;2009:404520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Molinari E, Bassi C, Salvia R, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg 2007;246:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burkhart RA, Relles D, Pineda DM, et al. Defining treatment and outcomes of hepaticojejunostomy failure following pancreaticoduodenectomy. J Gastrointest Surg 2013;17:451–60. [DOI] [PubMed] [Google Scholar]
- [33].Machado NO. Pancreatic fistula after pancreatectomy: definitions, risk factors, preventive measures, and management-review. Int J Surg Oncol 2012;2012:602478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen JY, Feng J, Wang XQ, et al. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol 2015;21:5926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Panwar R, Pal S. The International Study Group of Pancreatic Surgery definition of delayed gastric emptying and the effects of various surgical modifications on the occurrence of delayed gastric emptying after pancreatoduodenectomy. Hepatobiliary Pancreat Dis Int 2017;16:353–63. [DOI] [PubMed] [Google Scholar]
- [36].Peng T, Dong L, Zhu Z, et al. CT-guided drainage of deep pelvic abscesses via a percutaneous presacral space approach: a clinical report and review of the literature. Acad Radiol 2016;23:1553–8. [DOI] [PubMed] [Google Scholar]
- [37].Ji W, Wang J, Song B, et al. Cause analysis and therapeutic methods of chylous leakage after pancreaticoduodenectomy. Saudi Med J 2014;35:1396–9. [PMC free article] [PubMed] [Google Scholar]
- [38].Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- [39].Addeo P, Delpero JR, Paye F, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford) 2014;16:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg 1995;222:580–8. discussion 588-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Aranha GV, Hodul P, Golts E, et al. A comparison of pancreaticogastrostomy and pancreaticojejunostomy following pancreaticoduodenectomy. J Gastrointest Surg 2003;7:672–82. [DOI] [PubMed] [Google Scholar]
- [42].Suc B, Msika S, Fingerhut A, et al. Temporary fibrin glue occlusion of the main pancreatic duct in the prevention of intra-abdominal complications after pancreatic resection: prospective randomized trial. Ann Surg 2003;237:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Martin I, Au K. Does fibrin glue sealant decrease the rate of anastomotic leak after a pancreaticoduodenectomy? Results of a prospective randomized trial. HPB (Oxford) 2013;15:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Buchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg 1992;163:125–30. discussion 130-131. [DOI] [PubMed] [Google Scholar]
- [45].Ramesh H, Varghese CJ. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Br J Surg 1994;81:1693–4. [DOI] [PubMed] [Google Scholar]
- [46].Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 2000;232:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lowy AM, Lee JE, Pisters PW, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg 1997;226:632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Q, Jiang YJ, Li J, et al. Is routine drainage necessary after pancreaticoduodenectomy? World J Gastroenterol 2014;20:8110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fu SJ, Shen SL, Li SQ, et al. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: an audit of 532 consecutive cases. BMC Surg 2015;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Seo KW, Yoon KY, Lee SH, et al. Amylase, lipase, and volume of drainage fluid in gastrectomy for the early detection of complications caused by pancreatic leakage. J Korean Surg Soc 2011;81:402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mauri G, Mattiuz C, Sconfienza LM, et al. Role of interventional radiology in the management of complications after pancreatic surgery: a pictorial review. Insights Imaging 2015;6:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Aranha GV, Aaron JM, Shoup M, et al. Current management of pancreatic fistula after pancreaticoduodenectomy. Surgery 2006;140:561–8. discussion 568-569. [DOI] [PubMed] [Google Scholar]
- [53].Liu QY, Li L, Xia HT, et al. Risk factors of delayed gastric emptying following pancreaticoduodenectomy. Anz J Surg 2016;86:69–73. [DOI] [PubMed] [Google Scholar]
- [54].Feng J, Chen YL, Dong JH, et al. Post-pancreaticoduodenectomy hemorrhage: risk factors, managements and outcomes. Hepatobiliary Pancreat Dis Int 2014;13:513–22. [DOI] [PubMed] [Google Scholar]
- [55].Lermite E, Pessaux P, Brehant O, et al. Risk factors of pancreatic fistula and delayed gastric emptying after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg 2007;204:588–96. [DOI] [PubMed] [Google Scholar]
- [56].Malleo G, Crippa S, Butturini G, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. HPB (Oxford) 2010;12:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mortele KJ, Lemmerling M, de Hemptinne B, et al. Postoperative findings following the Whipple procedure: determination of prevalence and morphologic abdominal CT features. Eur Radiol 2000;10:123–8. [DOI] [PubMed] [Google Scholar]
- [58].Pisters PW, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg 2001;234:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McEvoy SH, Lavelle LP, Hoare SM, et al. Pancreaticoduodenectomy: expected post-operative anatomy and complications. Br J Radiol 2014;87:20140050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology 2013;266:930–5. [DOI] [PubMed] [Google Scholar]
- [61].Yoon YI, Hwang S, Cho YJ, et al. Therapeutic effect of trans-drain administration of antibiotics in patients showing intractable pancreatic leak-associated pus drainage after pancreaticoduodenectomy. Korean J Hepatobiliary Pancreat Surg 2015;19:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gonzalez-Pinto I, Gonzalez EM. Optimising the treatment of upper gastrointestinal fistulae. Gut 2001;49suppl 4:iv22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fisher WE, Hodges SE, Silberfein EJ, et al. Pancreatic resection without routine intraperitoneal drainage. HPB (Oxford) 2011;13:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shrikhande SV, Barreto SG, Shetty G, et al. Post-operative abdominal drainage following major upper gastrointestinal surgery: single drain versus two drains. J Cancer Res Ther 2013;9:267–71. [DOI] [PubMed] [Google Scholar]
- [65].Iwata N, Kodera Y, Eguchi T, et al. Amylase concentration of the drainage fluid as a risk factor for intra-abdominal abscess following gastrectomy for gastric cancer. World J Surg 2010;34:1534–9. [DOI] [PubMed] [Google Scholar]
- [66].Kyoden Y, Imamura H, Sano K, et al. Value of prophylactic abdominal drainage in 1269 consecutive cases of elective liver resection. J Hepatobiliary Pancreat Sci 2010;17:186–92. [DOI] [PubMed] [Google Scholar]
- [67].Sutcliffe RP, Battula N, Haque A, et al. Utility of drain fluid amylase measurement on the first postoperative day after pancreaticoduodenectomy. World J Surg 2012;36:879–83. [DOI] [PubMed] [Google Scholar]


