Abstract
Background.
Liver retransplantation is technically challenging, and historical outcomes are significantly worse than for first transplantations. This study aimed to assess graft and patient survival in all Australian and New Zealand liver transplantation units.
Methods.
A retrospective cohort analysis was performed using data from the Australia and New Zealand Liver Transplant Registry. Graft and patient survival were analyzed according to era. Cox regression was used to determine recipient, donor, or intraoperative variables associated with outcomes.
Results.
Between 1986 and 2017, Australia and New Zealand performed 4514 adult liver transplants, 302 (6.7%) of which were retransplantations (278 with 2, 22 with 3, 2 with 4). The main causes of graft failure were hepatic artery or portal vein thrombosis (29%), disease recurrence (21%), and graft nonfunction (15%). Patients retransplanted after 2000 had a graft survival of 85% at 1 year, 75% at 5 years, and 64% at 10 years. Patient survival was 89%, 81%, and 74%, respectively. This was higher than retransplantations before 2000 (P < 0.001). Univariate analysis found that increased recipient age (P = 0.001), recipient weight (P = 0.019), and donor age (P = 0.011) were associated with decreased graft survival prior to 2000; however, only increased patient weight was significant after 2000 (P = 0.041). Multivariate analysis found only increased recipient weight (P = 0.042) and donor age (P = 0.025) was significant prior to 2000. There was no difference in survival for second and third retransplants or comparing time to retransplant.
Conclusions.
Australia and New Zealand have excellent survival following liver retransplantation. These contemporary results should be utilized for transplant waitlist methods.
INTRODUCTION
Liver transplantation is the definitive treatment for those with end-stage liver disease, with 1-, 5-, and 10-year graft survival for a first transplant of 88%, 79%, and 69%, respectively, achieved in Australia and New Zealand transplant services since 1986.1 All units are either state or national quaternary services and are funded by national government medical systems. Multiple previous studies have shown that there is worse graft and patient survival following retransplantation when compared with the first transplantation.2-6 The Transplantation Society of Australia and New Zealand (TSANZ) guidelines state that the presence or absence of a previous liver transplant should not affect access to transplantation, except where this impacts upon medical suitability.7 This has not always been the case, and liver retransplantation often has a lower priority compared with the first transplant.8,9
Liver registry data from the US reports 1-year graft survival of 75% and 5-year survival of 66% for liver retransplantations performed between 2009 and 2011.10 Retransplantation accounted for 4.3%–8.2% per annum of all transplants.10 Slightly worse outcomes were reported from Europe and the United Kingdom. In Europe, 1-year graft survival following a second transplantation was 58%, and retransplantation accounted for 9.8% of all transplants per annum.11 A single major UK center reported 1-, 5-, and 10-year patient survival rates following second transplantation as 66%, 57%, and 47%, respectively.12 The rate of retransplantations compared with all transplants varied between 12% and 7.6% per annum.
Most of these reports include decades-old data that were collected well before major advances in transplantation medicine. Improved immunosuppression after liver transplantation has drastically changed practice and outcomes,13 as have advances in surgical techniques.14 Direct acting antiviral therapy for hepatitis C virus infection is a more recent example which has had an positive impact on graft survival based on early findings.15 Given this background, there is a need for an up-to-date assessment of liver retransplantation in Australia and New Zealand, particularly as waitlist prioritization guidelines or transplant benefit scores may adversely affect retransplantation.
The aim of this study was to assess graft and patient survival for adult liver retransplantation in all Australian and New Zealand liver transplantation centers and determine what variables impact this survival.
MATERIALS AND METHODS
Cohort
From 1986 to 2017, all patients who underwent liver retransplantation in Australia and New Zealand (ASNZ) over 18 years of age were studied. Inclusion criteria were all patients who received a whole or split liver graft and follow-up at an Australian or New Zealand transplantation center. Liver donation from donors after brain death and donors after circulatory death were both included.
Data from the TSANZ registry was used with permission from the Australia and New Zealand Liver Transplant Registry (ANZLT). The TSANZ liver transplant registry is a prospectively collected database from the 5 liver transplant units in Australia and the 1 unit in New Zealand. All data in the register are de-identified and the data are updated on a yearly basis. No organs from executed prisoners were used.
Data Collection
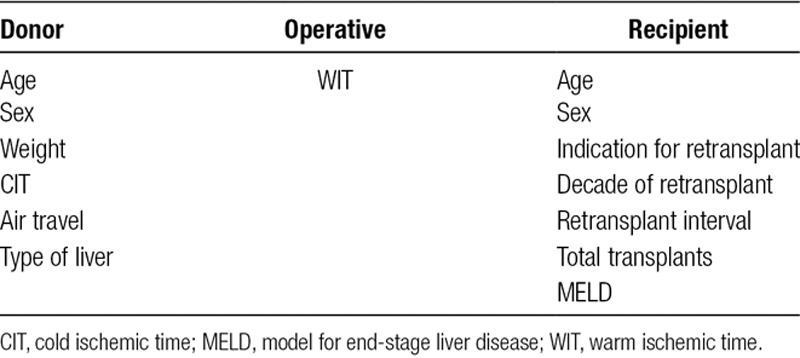
A retrospective cohort analysis was performed using data from the ANZLT.1 Patients who received 2 or more liver grafts were included. Preoperative recipient variables included patient age, sex, number of grafts, decade of transplant, model for end-stage liver disease (MELD) score, and time between transplants. Liver donor information included donor sex, weight, if air travel was required, and cold ischemic time (CIT). Operative variables included warm ischemic time (WIT) (Table 1).
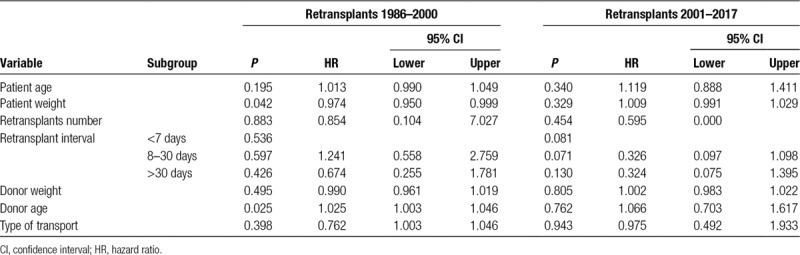
TABLE 1.
Donor and recipient variables
The primary endpoints for this study were graft failure and patient survival. Cause of graft failure was also analyzed and included graft nonfunction (primary and delayed), hepatic artery and portal vein thrombosis, biliary causes, recurrent liver disease, and rejection (acute and chronic).
Statistics
Survival analysis was undertaken using Kaplan-Meier analysis with comparison using the log-rank test. All statistical analysis was completed using the SPSS Statistics software package. For survival analysis, variables were categorized depending on type of variable and using normal ranges. Significance was defined as P < 0.05. Cox Regression was used to determine significant of all variables. Baseline characteristics were compared according to era with comparison made using T-test analysis in Microsoft Excel.
RESULTS
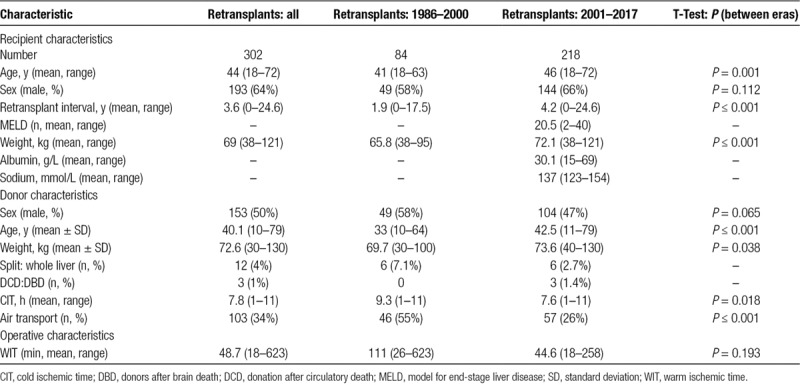
Liver transplantation commenced in ASNZ in 1986, and since then 4514 liver transplants have been performed, 302 (6.7%) of which were retransplanted. Of these, 278 received a second graft, 22 received a third graft, and 2 received a fourth graft. The mean follow-up time was 6.7 years (range: 1 day to 29 y). Baseline characteristics of these patients are found in Table 2. The average time between retransplants was 3.6 years for all cases with an average MELD score of 20.5. Four percent of patients received a split liver graft, and 1% of patients received a donation after circulatory death (DCD).
TABLE 2.
Baseline characteristics
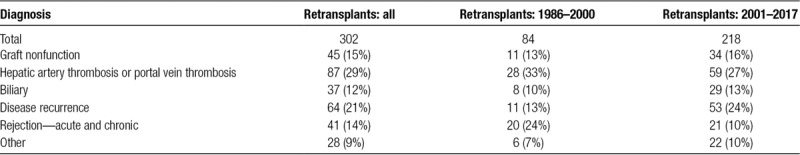
Overall, the most common cause of graft failure post liver retransplantation was hepatic artery or portal vein thrombosis (29%). This was followed by disease recurrence (21%) and graft nonfunction (15%) (Table 3). Over time, the cause of graft failure has changed. Hepatic artery or portal vein thrombosis decreased as an indicator for retransplantation from 33% (1986–2000) to 27% (2001–2017), acute or chronic rejection decreased from 24% to 10%, and disease recurrence increased from 13% to 24%. Other causes have remained relatively stable over this time (Table 3).
TABLE 3.
Cause of graft failure in patients requiring retransplantation
Overall graft survival for all retransplants from 1986 to 2017 was 79% at 1 year, 69% at 5 years, and 59% at 10 years. Overall patient survival for all retransplants was 80% at 1 year, 72% at 5 years, and 66% at 10 years. Analysis of patient, donor, and operative variables found that the era of retransplant was the only variable that was significantly associated with graft and patient survival (P < 0.001) (Figure 1, Figure 2). Retransplantation rate has remained similar between eras, with liver retransplantation accounting for 6.5% of all transplantations between 1986 and 2000, compared with 6.7% from 2001 to 2017.
FIGURE 1.
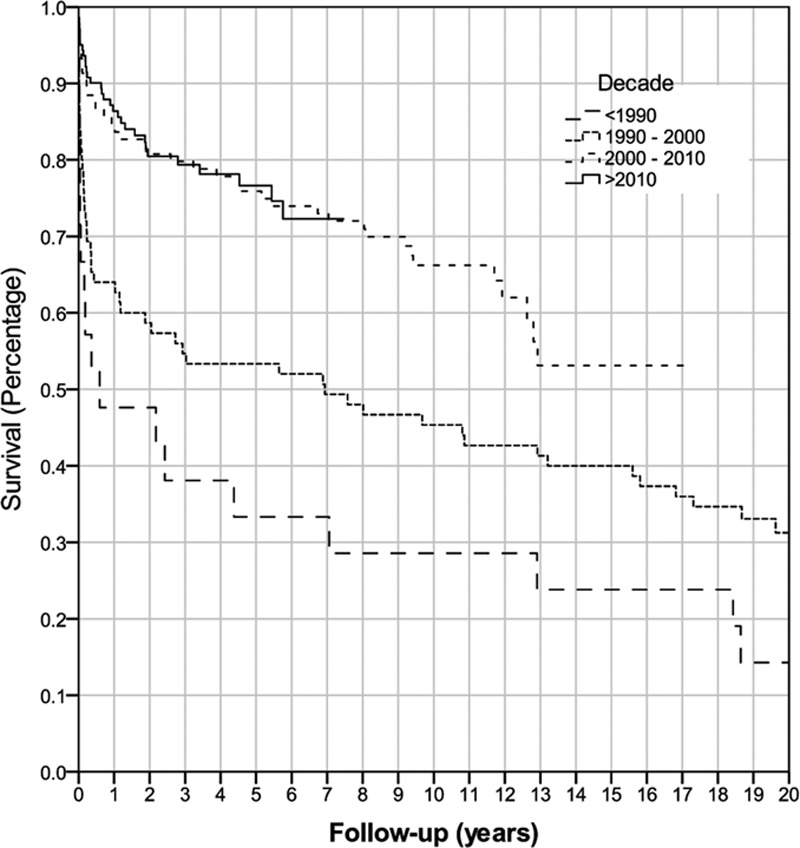
Kaplan-Meier graft survival of all retransplanted patients by decade (P < 0.001).
FIGURE 2.
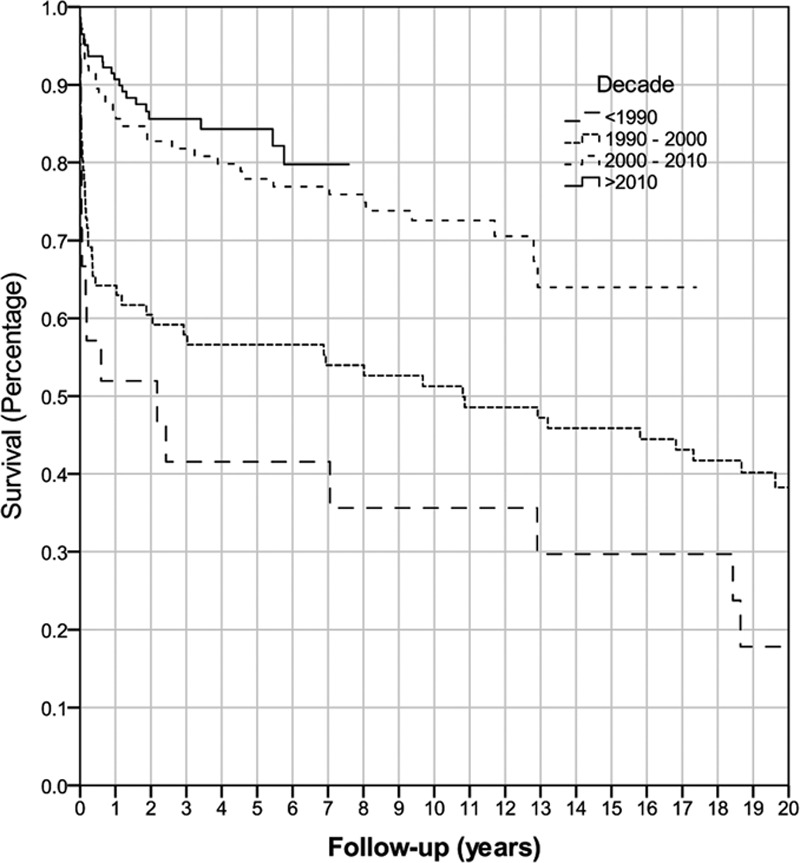
Kaplan-Meier overall patient survival by decade (P < 0.001).
Subsequent analysis was performed on the 218 recipients who had a retransplantation from 2001 to 2017. The graft survival for a second liver transplantation was 82% at 1 year, 76% at 5 years, and 65% at 10 years (Figure 3). The graft survival for a third transplantation was 96% at 1 year, 70% at 5 years, and 70% at 10 years. The 2 cases of a fourth transplantation have yet to meet an endpoint. Overall, graft survival from 2001 to 2017 for retransplantation was 85% at 1 year, 75% at 5 years, and 64% at 10 years. Patient survival for all retransplants from 2001 to 2017 was 89% at 1 year, 81% at 5 years, and 74% at 10 years (Figure 2).
FIGURE 3.
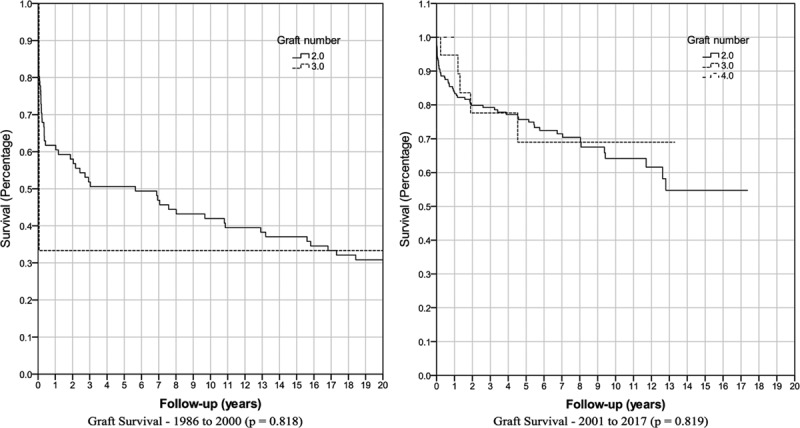
Kaplan-Meier graft survival by number of grafts received.
Baseline characteristics were collected for both the overall cohort and eras from 1986 to 2000 and from 2001 to 2017. There are several notable differences between these 2 groups (Table 2). The mean time between transplant and retransplant has increased over time, from 1.9 to 4.2 years (P < 0.001). Patient age (P = 0.001) and weight (P < 0.001) have also increased significantly. There has been a reduction in air travel over time, with 55% of donor livers requiring air transport from 1986 to 2000 compared with 26% from 2001 to 2017 (P < 0.001). CIT has decreased from a mean of 9.3 to 7.6 hours (P = 0.018), to which the decrease in air travel may have contributed. The donor age (P < 0.001) has also increased. WIT has decreased from a mean of 111 to 44.6 minutes (P = 0.193); however, this was not found to be significant due to limited intraoperative data prior to 2000.
Univariate analysis found no significant difference in graft survival for the number of retransplants, use of whole or split donor livers, or pretransplant MELD score (Table 4). Increased patient age and low patient weight were found to be significantly associated with decreased graft survival from 1986 to 2000 (P = 0.001 and P = 0.019), while only low patient weight remained significant from 2001 to 2017 (P = 0.041). Similarly, increased donor age was significantly associated with decreased graft survival from 1986 to 2000 (P = 0.011), but this was not significant from 2001 to 2017 (P = 0.828). There was no significant difference in graft survival for other donor factors including sex, weight, CIT, and air travel. Similarly, there was no significant difference for recipient or operative factors including, WIT, or sex. There was no significant difference in graft or patient survival when comparing time with retransplant of <7 days, between 8 and 30 days, or >30 days (Figure 4). Multivariate Cox regression was performed using the data from 1986 to 2000 and only low patient weight (P = 0.042) and increased donor age (P = 0.025) were significantly associated with decreased graft survival (Table 5). Cox regression found that no variables were significantly associated with graft survival using data from 2001 to 2017 era.
TABLE 4.
Graft survival after retransplantation
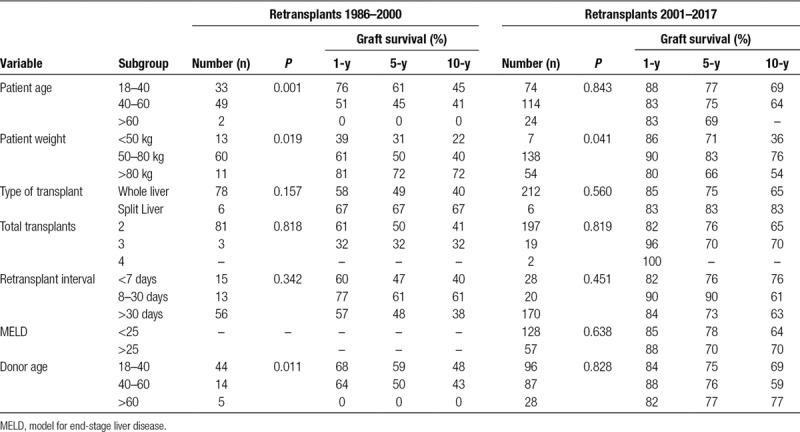
FIGURE 4.
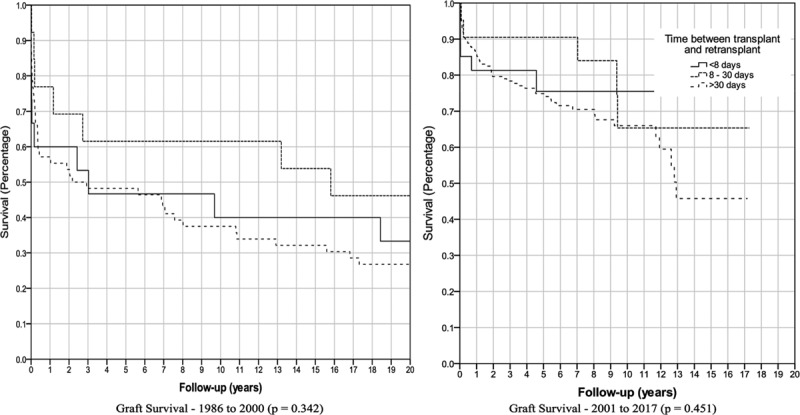
Kaplan-Meier graft survival by time between transplantation and retransplantion.
TABLE 5.
Multivariate analysis
DISCUSSION
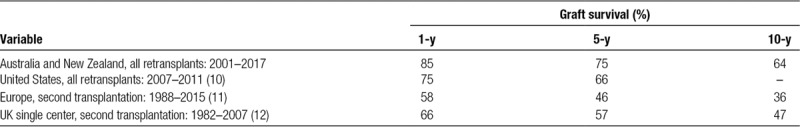
This study shows that liver retransplantation is a valid and definitive treatment for both early hepatic graft failure and end-stage chronic liver disease occurring after primary liver transplantation. Importantly, it was demonstrated that graft survival for retransplantation in ASNZ has greatly improved over past 30 years. The most notable improvement occurred after 2000, where the 1-, 5-, and 10-year graft survival for retransplantation was 85%, 75%, and 64%, respectively, and the 1-, 5-, and 10-year patient survival for retransplantation was 89%, 81%, and 74%, respectively. This compares favorably with graft survival reported by the ANZLT liver transplantation registry from 2000 to 2017 for 4514 patients undergoing a first liver transplantation with a 1-, 5-, and 10-year graft survival of 88%, 79%, and 69%, respectively, and a 1-, 5-, and 10-year patient survival of 93%, 83%, and 74%, respectively.1 Similar trends of improved graft survival for retransplantation have been reported by other international studies5; however, graft survival for retransplantation remains worse than for a first transplant.2 Overall, graft and patient survival rates in ASNZ are excellent when compared with other countries around the world (Table 6).
TABLE 6.
Graft survival for retransplantation by region
Valuable lessons have been learned from the previous landmark studies of liver retransplantation. A number of models that predict survival following liver retransplantation have been developed; however, there is no consensus on which variables contribute to a worse outcome.16 Notable variables that have historically been shown to result in poorer outcomes include a time to retransplantation between 8 and 30 days, multiple liver transplants, a recipient MELD score >25, and advanced patient age.16 Donor characteristics, such as increased donor age and CIT, have been associated with poorer outcomes. The use of DCD donors has also been shown to have higher rates of graft failure17; however, only 3 of our retransplant recipients received a DCD organ. Of these 2 died, one at 10 days and the other at 255 days after retransplantation, but the limited number of cases precludes us from making any comment on the significance of this. This present study found no significant difference in survival when analyzing these key recipient and donor variables.
There are notable differences in the donor and recipient characteristics in this study when compared with other reports. From 2001 to 2017, the average age of ASNZ retransplantation recipients was 46 years, similar to the United States; however, the average donor age was 42.5 years compared with 34 years in the United States.18 ASNZ data also show a mean retransplant interval of well over 1 year compared with an average interval between 1 and 6 months in the United States. ASNZ donor livers had a mean CIT of 7.6 hours compared with 8.45 hours from a major US center.3 This same US study showed that a CIT of >12 hours was of borderline significance in predicting survival.
The indications for retransplantation in this study are different compared with previous studies, and this may have contributed to the difference in outcomes. In 1 major US study, from 1999 to 2003 the majority of retransplants were due to graft nonfunction (37.5%), hepatic artery thrombosis (20%), disease recurrence (17.5%), and graft rejection (12.5%).5 The data from this ASNZ study show that from 2001 to 2017 retransplantation commonly resulted from hepatic artery or portal vein thrombosis (27%) and disease recurrence (24%) and graft nonfunction only accounted for 16% of retransplantations. The notable decrease in graft nonfunction in ASNZ may be contributing to improved survival, as less grafts are failing in the short term following retransplantation.
The difference in graft survival comparing ASNZ data and other regions may also be related to other factors. Transplantation services around the world are different, and each has its own method of patient and donor selection. We hypothesize that graft survival following retransplantation may be associated with local factors, such as quality of the surgical and theater team, physician experience, hospital services including Intensive Care Unit access, interventional radiology, medication availability, pathology services, other support staff, and efficient donor services. These unreported factors clearly may influence which variables are significantly related to graft survival across different centers.
This study has shown that changes in recipient and donor characteristics have occurred over time while graft survival has also increased. Advanced donor age was shown previously to predict poor outcome; however, this ASNZ registry study found that this was no longer the case. This was despite the fact that older recipients were transplanted and older donor livers were used. Only 8% of liver donors were older than 60 years in the 1986–2000 era, and this increased to 13% in the 2001–2017 era. Previous studies found that recipient’s weight of <50 kg was associated with poor survival and likely reflected poor outcomes due to frailty. This relationship was not found in the present study and is likely due to a change in recipient selection and earlier waitlisting over time. In the early era, 15% of recipients had a weight <50 kg compared with only 3.5% in the 2001–2017 era. The cause of graft failure has also changed over time, with a large decrease in frequency of acute or chronic rejection and a comparative increase in liver disease recurrence. These changes in recipient and donor selection are likely due to improvements in surgical and medical treatment. Moreover, liver transplantation recipients are living longer after the first transplant, and the resulting longer retransplantation interval likely accounts for the increased number of retransplants for liver disease recurrence. While we have no data on patients who were denied retransplantation due to increased risk of mortality, this data suggest that the selection criteria being used is contributing towards the improved outcomes we have found.
As this is a multicenter registry study, the data for some variables was incomplete. Only those variables which were collected consistently in each center were used for the final analysis. A detailed analysis of additional variables of interest was not able to be computed due to insufficient data. There is scope for further analysis of variables associated with graft survival that have not been included in this study. These include some specific variables previously shown to be significant, including renal function, requirements of ventilator support, and the use of marginal donor livers.19
In addition to graft and patient survival, other ethical and financial factors must be considered when allocating donor livers. The question remains: will patients, donor families, and society accept repeated use of scarce donor livers in one individual? Existing ethical guidelines recommended that the previous use of a resource, such as a liver allograft, should not impact current patient needs.8 The reality is not as clear, however, as society preferences for fairness in accessing indivisible healthcare resources must also be considered.20 There needs to be further consideration going forward on how to allocate organs when outcomes are comparable for retransplantation.
In summary, this study has confirmed a notable improvement in graft survival for liver retransplantation from 2001 to 2017 when compared with 1986 to 2000. ASNZ has excellent survival rates when compared with other countries. Survival after retransplantation is comparable to a first transplant and should not be a barrier for organ allocation. Several key variables that were previously associated with poorer outcomes are not shown to impact outcomes in ASNZ. These contemporary results should be utilized by liver transplant waitlist prioritization methods to avoid discrimination against those who need a life-saving retransplant.
Footnotes
Published online 23 July, 2019.
A.W.J. participated in research design, the writing of the paper, the performance of the research, and data analysis. L.D., G.M., M.C., P.A., R.J., G.A.M., J.F., A.W., J.C., E.G., and S.M. participated in data collection and peer review. G.P.J. participated in research design, the writing of the paper, and peer review.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Lynch SV, Balderson GA. ANZLT Registry Report Aust N Z Liver Transpl Regist. 201729Available at https://www3.anzltr.org. Published 2018. Accessed March 20, 2019. [Google Scholar]
- 2.Kitchens WH, Yeh H, Markmann JF. Hepatic retransplant: what have we learned? Clin Liver Dis. 2014;18:731–751. [DOI] [PubMed] [Google Scholar]
- 3.Markmann JF, Markowitz JS, Yersiz H, et al. Long-term survival after retransplantation of the liver. Ann Surg. 1997;226:408–18; discussion 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abt PL, Olthoff KM. The role of retransplantation Schiff’s Diseases of the Liver. 2017:12th ed Hoboken, NJ: John Wiley and Sons; 1143–1155. [Google Scholar]
- 5.Pfitzmann R, Benscheidt B, Langrehr JM, et al. Trends and experiences in liver retransplantation over 15 years Liver Transpl. 2007;13:248–257. [DOI] [PubMed] [Google Scholar]
- 6.Morel P, Rilo HL, Tzakis AG, et al. Liver retransplantation in adults: overall results and determinant factors affecting the outcome. Transplant Proc. 1991;23:3029–3031. [PMC free article] [PubMed] [Google Scholar]
- 7.The Transplantation Society of Australia and New Zealand Clinical Guidelines for Organ Transplantation from Deceased Donors Donate Life. 2016version 1.0. Available at http://www.tsanz.com.au/organallocationguidelines/documents/ClinicalGuidelinesV1.1May2017.pdf. Published May 1, 2017. Accessed March 20, 2019.
- 8.MacDonald P. Ethical guidelines for organ transplantation from deceased donors. Heart Lung Circ. 2015;24:633–634. [DOI] [PubMed] [Google Scholar]
- 9.Ubel PA, Arnold RM, Caplan AL. Rationing failure. The ethical lessons of the retransplantation of scarce vital organs. JAMA. 1993;270:2469–2474. [DOI] [PubMed] [Google Scholar]
- 10.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 Annual Data Report: Liver Am J Transplant. 2018;1818 Suppl 1172–253. [DOI] [PubMed] [Google Scholar]
- 11.European Liver Transplant Registry; Available at http://www.eltr.org/Mortality-and-retransplantation.html. Accessed July 30, 2018. [Google Scholar]
- 12.Marudanayagam R, Shanmugam V, Sandhu B, et al. Liver retransplantation in adults: a single-centre, 25-year experience. HPB (Oxford). 2010;12:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Multicenter FK506 Liver Study Group A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation N Engl J Med. 1994;331:1110–1115. [DOI] [PubMed] [Google Scholar]
- 14.Magliocca JF, Hanish SI, Page EK, et al. Kirk AD, Knechtle SJ, Larsen CP, Madsen JC, Pearson TC, Webber SA. Liver transplantation procedure and surgical technique. In: Textbook of Organ Transplantation. 2014:Hoboken, NJ: Wiley-Blackwell; 631–638. [Google Scholar]
- 15.Ghabril M, Dickson R, Wiesner R. Improving outcomes of liver retransplantation: an analysis of trends and the impact of hepatitis C infection. Am J Transplant. 2008;8:404–411. [DOI] [PubMed] [Google Scholar]
- 16.Berumen J, Hemming A. Liver retransplantation: how much is too much? Clin Liver Dis. 2017;21:435–447. [DOI] [PubMed] [Google Scholar]
- 17.Allen AM, Kim WR, Xiong H, et al. Survival of recipients of livers from donation after circulatory death who are relisted and undergo retransplant for graft failure. Am J Transplant. 2014;14:1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen HR, Madden JP, Martin P. A model to predict survival following liver retransplantation Hepatology. 2003;29:365–370. [DOI] [PubMed] [Google Scholar]
- 19.Tisone G, Manzia TM, Zazza S, et al. Marginal donors in liver transplantation. Transplant Proc. 2004;36:525–526. [DOI] [PubMed] [Google Scholar]
- 20.Huesch MD. One and done? Equality of opportunity and repeated access to scarce, indivisible medical resources. BMC Med Ethics. 2012;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]


