Supplemental Digital Content is available in the text.
Abstract
Introduction:
Opioid abuse in the United States is a public health emergency. From 2000 to 2009, prenatal maternal opiate use increased from 1.19 to 5.63 per 1,000 births, with up to 80% of in utero opioid-exposed infants requiring pharmacotherapy. This study aimed to increase the percentage of neonatal abstinence syndrome (NAS) medication orders based on birth weight (BW) in neonates admitted to a neonatal intensive care unit with a principal diagnosis of NAS from 29% to 90%, within 4 months of project initiation, and to sustain this for 6 months.
Methods:
This project occurred at an academic medical center with 5,000 deliveries per year and a 49-bed Level III neonatal intensive care unit. We used the Institute for Healthcare Improvement methodology, largely focusing interventions on clinical decision support (CDS) tools. We plotted all measures on Shewhart charts, and Nelson rules differentiated special versus common cause variation.
Results:
The percent of orders based on BW increased from 29% to 78% after implementing multiple interventions focused primarily on CDS. However, this later decreased to 48% as workarounds began. There was also a significant decrease in the length of stay variability, which persisted throughout the project.
Discussion:
CDS is a helpful tool to guide prescribing behavior; however, workarounds can negate its usefulness. Standardized use of BW for weight-based NAS medication prescribing can decrease the length of stay variability. Further studies are needed using a human factors approach to minimize workarounds in CDS and potentially decrease the length of stay in neonates with NAS.
INTRODUCTION
In 2017, the US Department of Health and Human Services declared opioid abuse as a public health emergency. Opioid use disorder disproportionately affects women of childbearing age, with one presenting to the emergency department every 3 minutes from prescription opioid abuse.1 The illicit opioid abuse rate is also increasing in these women.1 From 2000 to 2009, the prenatal maternal opiate use rate across the United States increased from 1.19 to 5.63 per 1,000 births per year.2 During the same period, the neonatal abstinence syndrome (NAS; ie, neonatal withdrawal) rate, increased from 1.2 to 3.39 per 1,000 births per year.2 Although only 60%–80% of infants born with prenatal opioid exposure develop NAS symptoms, their hospital length of stay is 3.5 times longer than non-NAS infants with costs more than 3 times higher.3 The United States spent an estimated $316 million on NAS care in 2012.3 In Ohio, the opioid abuse rate in pregnant women and resulting NAS surpasses the national rate. In 2015, 1,949 women had an opioid use disorder diagnosis at the time of delivery, and 15.4 of every 1,000 infants born in Ohio developed NAS.4 Across the state, the length of stay for infants with NAS is approximately 3.8 times higher, and hospital charges are almost 4 times higher, than those of infants without NAS.4
All infants with intrauterine opioid exposure (IUOE) should receive treatment to manage symptoms and allow normal newborn activities, including sleep, feeding, appropriate weight gain, and interaction with the environment. Although all these infants should also receive nonpharmacologic management (eg, swaddling, rocking, minimizing stimulation and increased feeding frequency), up to 80% of neonates with IUOE also require pharmacologic therapy.5,6 Pharmacologic therapy for moderate-to-severe NAS symptoms helps prevent complications including fever, excessive weight loss, and seizure while promoting maternal–infant bonding.6,7 Despite the burden of NAS, pharmacologic therapy is variable across providers and institutions. Although first-line medications are frequently opioids, such as morphine, many other medications treat withdrawal including benzodiazepines, barbiturates, alpha-2 adrenergic agonists, and others.6 Center-specific pharmacologic treatment protocols decrease the length of stay and length of treatment without increasing the number of infants requiring pharmacologic therapy.8 However, the superiority of weight-independent fixed dose titration versus weight-based dosing regimens for pharmacologic treatment is not evident.7–9
The Nationwide Children’s Hospital neonatal intensive care unit (NICU) at the Ohio State Wexner Medical Center uses protocol-guided care for patients admitted with NAS due to opiate or polysubstance exposure in utero. The NAS protocol encompasses initiation, escalation, and weaning with weight-based morphine dosed as first-line pharmacologic therapy. Also, weight-based adjunctive therapy (clonidine or phenobarbital) may be added as indicated. Because most infants requiring pharmacologic therapy are below birth weight (BW) at the time of treatment initiation, BW is the infant’s dosing weight (DW) for treatment.
Furthermore, DW should not change throughout the infant’s NICU course. The rationale for not adjusting DW is two-fold: (1) consistency, as entering incorrect DW may lead to prolonged suffering, prolonged medication weans, or potential overdose and (2) as the infant grows, continuing medication dosing based on BW allows the infant to “auto-wean” in addition to weaning per protocol. At the outset of this project, review of NAS medication orders over 4 months revealed 43% of all administered doses were at the appropriate amount, whereas 46% and 11% were underdosed and overdosed, respectively (Fig. 2). The specific aim of this quality improvement project was to increase the percentage of NAS medication orders based on BW in neonates admitted to Nationwide Children’s Hospital NICU at the Ohio State Wexner Medical Center with a principal diagnosis of NAS from 29% to 90% by December 31, 2017, 4 months after project initiation, and sustain this increase for 6 months, in hopes of decreasing length of stay.
Fig. 2.
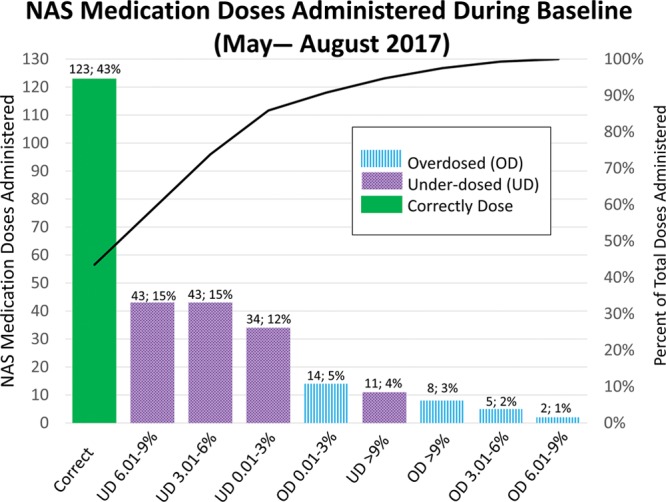
Pareto chart for deviation in NAS medication dosing during the baseline period.
METHODS
Context
This project occurred in a large academic center with approximately 5,000 deliveries per year and a 49-bed level III NICU. The NICU staffing includes at least one in-house neonatologist, neonatal-perinatal medicine fellows, neonatal nurse practitioners along with primarily first- and second-year medical residents. Medical residents spend a total of 2 months in the NICU, one during the first year and one during the second year. Neonatal clinical pharmacists are present for rounds during the weekdays and available for consultation by phone on nights and weekends. If medically stable, all infants with IUOE stay with mothers in the postpartum unit allowing for enhanced bonding and nonpharmacologic treatment of withdrawal symptoms. All infants with IUOE undergo modified Finnegan scoring10 every 3–4 hours with feeds. Those infants meeting criteria for pharmacologic treatment of NAS are transferred to the NICU for enhanced monitoring and increased nurse-to-patient ratios. We treat infants with NAS according to the previously mentioned protocol. A protocol update occurred in February 2018 independent of this project. Both protocols use weight-based morphine dosing as a first-line treatment with clonidine and phenobarbital as adjuncts after discussion with a clinical pharmacist. Our protocol uses weight-based dosing due to the concern for potential higher than necessary medication doses in smaller infants when using a weight-independent approach. Of note, despite being in the same building, the NICU and the postpartum unit are operated by 2 separate entities; therefore, the transition from one location to the other requires a separate electronic health record (EHR) encounter and new orders.
Interventions
This study used the Institute for Healthcare Improvement methodology with multiple rapid cycles of change, or plan, do, study, act cycles.11 A multidisciplinary team, including physicians, nurse practitioners, residents, nursing, nursing leadership and educators, and pharmacists, met and developed the key driver diagram (Fig. 1). Because medical residents (the primary order writers) change every 4 weeks, interventions focused on utilizing the EHR and clinical decision support (CDS) tools. In September 2017, the team rolled out an NAS bundle, consisting of the following: (1) educating providers about DW, the use of BW as DW for infants within the first week of life, and appropriate procedure to update DW for medications not related to NAS treatment; (2) defaulting NAS medications (morphine, clonidine, and phenobarbital) to infant’s DW; (3) improvement of the NAS admission order set and development of an NAS continuation order set for use ordering any NAS medications after the time of admission; and (4) utilizing CDS in the NAS order sets, including red explanation points, for orders without an entered DW.
Fig. 1.
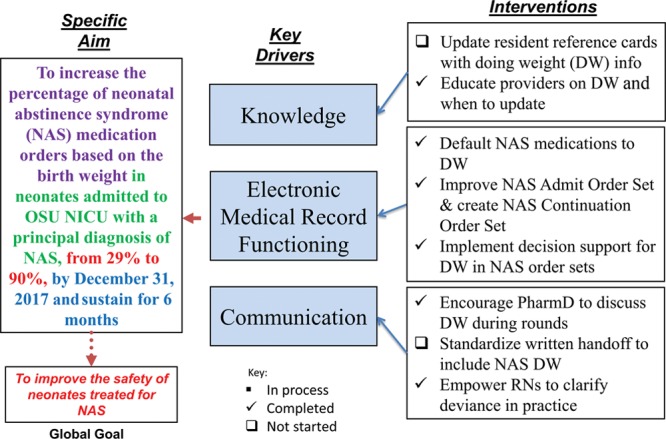
Project key driver diagram.
Additional interventions included utilizing pharmacists and nurses as change agents during the ordering and administration process; comparing the medication order weight against the infant’s BW; and additional education about the importance of DW. Education included presenting the definition and appropriate use of DW at a nurse staff meeting. It also included monthly presentations to residents including the DW definition and how to update this in the EHR, and posting a brief, easy to read DW fact sheet in the resident workroom.
Measures
The outcome measure of this project was the percent of orders for NAS medications based on the BW. This measure was obtained by reviewing all orders placed for morphine, clonidine, and phenobarbital in infants with a diagnosis of NAS. For this initiative, we included medication orders for infants with a primary diagnosis of NAS and excluded those for infants less than 35 weeks and those with a different principal diagnosis, including congenital malformations, respiratory distress syndrome, meconium aspiration syndrome, etc. Percent of orders with a DW entered and the sum of weights per patient were process measures, whereas the length of stay was a balancing measure.
Analysis
All data were manually extracted from the EHR and collected using Microsoft Excel 2016.12 Measures were tracked on control charts over time using a locally developed Microsoft Excel plug-in. Control chart analysis used the Nelson rules to identify common versus special cause variation.
Ethical Considerations
The project was quality improvement research and not human subjects research. Therefore, review and approval by the institutional review board were not required per policy.
RESULTS
We collected baseline data from May through August 2017. The number of NAS medication orders placed on BW during the baseline period was approximately 29% (by weekly average), although the percentage of total orders based on BW during this period was 43% (Figs. 2 and 3). Additionally, during this time, 39% percent of medication doses were underdosed by greater than 3% of the expected amount, whereas only 6% of administered medications were overdosed by greater than 3% (Fig. 2). After implementing the DW bundle, orders placed on BW improved significantly to 78%, according to the Nelson rules (Fig. 3). Concurrently, the percentage of patients with a DW entered increased from 18% to 100% (Fig. 4). This improvement in BW use, however, was not sustained. In December 2017, BW use decreased to 48%. At this time, there were some orders without a DW entered, although these points were still within common cause variation. From February to March 2018, multiple points clustered below the center line for the outcome measure but did not reach statistical significance. With decreased compliance, the average number of weights per patient increased (see figure, Supplemental Digital Content 1: Number of Weights Per Infant U-Chart. Weeks without a data point reflect weeks where there were no infants admitted with NAS. Available at http://links.lww.com/PQ9/A103). Importantly, we noted a significant decrease in the variation of length of stay when the percent of orders based on DW improved (Figs. 3 and 5; figure, Supplemental Digital Content 2: Length of NICU Stay for Infants with NAS MR-Chart. Available at http://links.lww.com/PQ9/A104). Of note, the end of February through March saw a decrease in the percent of orders based on BW despite 100% compliance with DW entry.
Fig. 3.
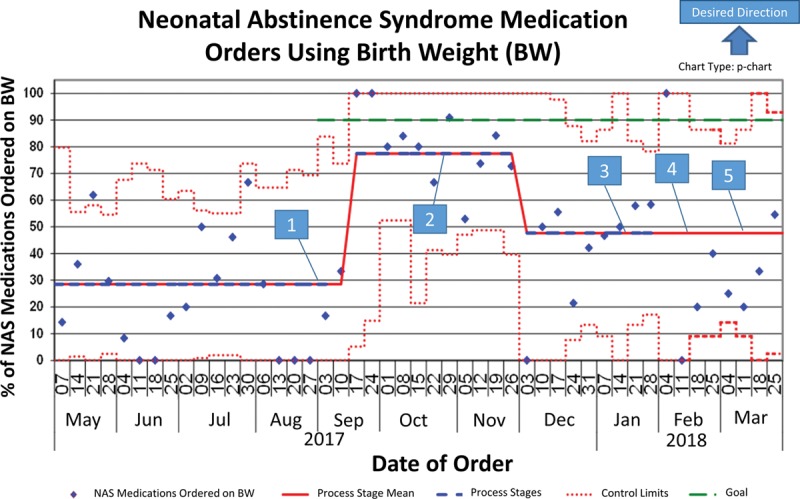
Outcome measure—NAS medication orders using BW. 1. Bundle initiated±. 2. DW fast fact sheets posted. 3. PharmD encouraged to discuss DW during rounds. 4. NAS protocol update. 5. RNs empowered to clarify deviance in practice. ±Bundle: Education for providers on DW and when to update; NAD medications defaulted to DW; NAS Admission order set improved; NAS continuation order set created; Alert generated at the time of order when no DW present.
Fig. 4.
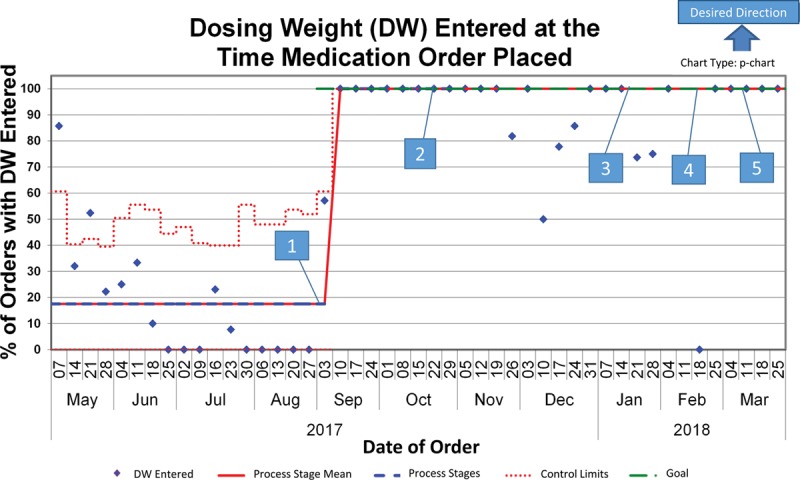
Process measure—percent of Infants with NAS with a DW entered in the EHR. 1. Bundle initiated±. 2. DW fast fact sheets posted. 3. PharmD encouraged to discuss DW during rounds. 4. NAS protocol update. 5. RNs empowered to clarify deviance in practice. ±Bundle: Education for providers on DW and when to update; NAD medications defaulted to DW; NAS admission order set improved; NAS continuation order set created; Alert generated at the time of order when no DW present.
DISCUSSION
Summary and Interpretation
Due to the rapid turnover of the primary ordering providers, medical residents, we focused this project’s interventions on utilizing CDS support tools within the EHR. The combination of education about DW with the other CDS bundle components led to a significant improvement in the outcome measure—NAS medications ordered based on DW. Notably, we did not sustain this result. In December 2017, there was a decrease in the percent of the time a DW was entered, concurrently with a decrease in the outcome measure. This initial decrease in the medication orders based on BW was likely secondary to not having the BW entered as the DW. At the end of February, however, there was a return to 100% compliance of DW entry, with a persistently decreased number of medication orders based on BW. This finding was likely secondary to the development of workarounds as ordering providers clicked “current weight” when ordering NAS medications or entered a “medication-specific weight” instead of using DW. Supporting this is the fact that NICU pharmacists entered the majority of DW during this period instead of allowing this to be performed by the ordering provider at the time of medication entry. With the implementation of the CDS tools, the group focused on updating the order sets and appropriate support within the order sets. Initially, we purposefully did not change the medication ordering outside the order set, as these medications, particularly morphine and phenobarbital, are ordered for other indications. We have since begun discussion with a psychologist specializing in behavioral economics to help decrease the use of workarounds and improve the outcome. We speculate that indication-based ordering for these medications may allow improved CDS for ad hoc orders and, therefore, improved outcomes. Also, this NICU is looking toward using DW for all medication orders, which would allow for additional EHR enhancements, particularly defaulting medication weight to DW, which could also improve appropriate dosing when ordering medications outside the order set.
We struggled to maintain consistent use of these order sets, which likely resulted in the faltering outcomes. At this institution, medical residents infrequently use order sets. Also, the EHR used by this offsite NICU is different than the EHR utilized by the same medical residents at the pediatric hospital where they primarily rotate. As a result, each medical resident must be educated and add the unit-specific order sets to their profile for use during initial orientation to the NICU. Currently, we cannot automatically add these to each person’s preference list who logs in under the NICU environment, although we are looking at the possibility of doing this in the future. This issue highlights the importance of minimizing the opportunity for workarounds when implementing CDS. These results are consistent with prior literature suggesting CDS has the potential to improve practitioner performance and is most effective when it is integrated into the workflow of the provider, prompting users as opposed to requiring the system to be activated.13,14
Importantly, with the improvement in the percent of orders placed on DW, we saw a decrease in the variation in the length of stay for these infants. This decrease is likely due to gaining initial control of these infants’ symptoms faster, given that before the interventions the majority of the children were underdosed. It is likely that we did not yet see an increase in variation in the length of stay after decreased compliance with DW use as there is a delay in data acquisition due to the length of stay being plotted based on admission data.
Limitations
This study had various limitations. First, a weight was determined to be “inappropriate” if not based on BW. It is possible that there are instances where older infants have a prolonged course and require a rescue or loading dose of clonidine or phenobarbital. In these cases, the team may decide to dose the medication base on the infant’s current weight, resulting in an intentional higher dose of medication. Because we extracted these data from the EHR, it is not possible to determine when aberrancies in the weight used was intentional versus unintentional. We would expect that throughout the project, this practice would have been relatively rare, and would have remained consistent.
Also, it is possible that the team decided to continue with the weight used for treatment initiation throughout the infant’s course. Again, this is difficult to discern. For this reason, we collected data for the total number weights used per patient throughout the project, with a goal of one weight per patient, that is, the same weight used throughout the infant’s NICU stay (see figure, Supplemental Digital Content 1, http://links.lww.com/PQ9/A103). The number of weights per patient mirrored the outcome measure control chart (Fig. 3; figure, Supplemental Digital Content 1, http://links.lww.com/PQ9/A103). At baseline, there was an average of 2 weights per infant, which decreased to one when there was an improvement in the percent of orders based on DW. Although there was not a significant increase, the number of weights trended up starting in December 2018 with a special cause point outside of the control limits in March 2018 as the percent of orders based on DW decreased.
CONCLUSIONS
This study demonstrates the ability to decrease variation in the length of stay utilizing appropriate weights for weight-based dosing of NAS medications. It also highlights the potential detrimental effect that workarounds can have on outcomes and the need to utilize a human factors engineering approach. Utilizing this approach when designing CDS will make it “easier” for staff to do the “right” thing and more “difficult” to do the “wrong” through automation. The next steps in this project are working toward enhancing the use of order sets through behavioral economic principles and moving toward indication-based ordering.
Fig. 5.
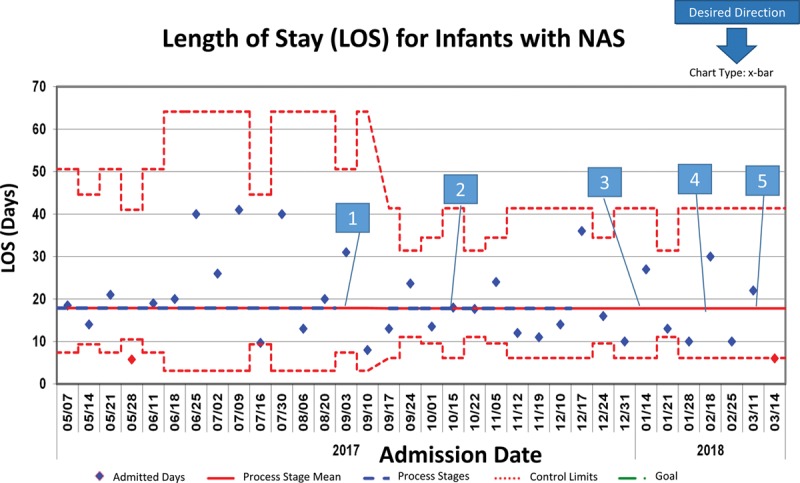
Length of NICU Stay for Infants with NAS—X-bar chart demonstrating shrinking variation in the length of stay based on supporting MR chart in figure, Supplemental Digital Content 2, http://links.lww.com/PQ9/A104. 1. Bundle initiated±. 2. DW fast fact sheets posted. 3. PharmD encouraged to discuss DW during rounds. 4. NAS protocol update. 5. RNs empowered to clarify deviance in practice. ±Bundle: Education for providers on DW and when to update; NAD medications defaulted to DW; NAS admission order set improved; NAS continuation order set created; Alert generated at the time of order when no DW present. A (a+bx)^1/3 transformation to correct for right skew was used to determine control limits. Limits were then reverse transformed to reflect original data metrics.
ACKNOWLEDGMENTS
The authors thank Gregory Ryshen, MS, CQE, MBA; Jill Jones, MSN, NNP, NNP-BC; Jennifer Thompson, MBA, BSN, RN; and Michele Sweet, MS, RN, CCNS for assistance with the study.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online June 28, 2019.
Presented at the Pediatric Academic Societies 2018 Annual Meeting, May 2018. Toronto, Canada; and at the 2018 Neonatal/Perinatal Conference Poster, May 2018, Columbus, Ohio.
To cite: Bertoni CB, Prusakov P, Merandi J, Bartman T. Implementation of Clinical Decision Support to Improve Appropriate Dosing Weight Use in Infants with Neonatal Abstinence Syndrome. Pediatr Qual Saf 2019;4:e184.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Centers for Disease Control and Prevention. Vital Signs: Prescription Painkiller Overdoses: A Growing Epidemic, Especially Among Women. 2016. https://www.cdc.gov/vitalsigns/prescriptionpainkilleroverdoses/index.html. Accessed July 20, 2018.
- 2.Patrick SW, Schumacher RE, Benneyworth BD, et al. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307:1934–1940. [DOI] [PubMed] [Google Scholar]
- 3.Corr TE, Hollenbeak CS. The economic burden of neonatal abstinence syndrome in the United States. Addiction. 2017;112:1590–1599. [DOI] [PubMed] [Google Scholar]
- 4.Ohio Department of Health: Violence and Injury Prevention Program. Neonatal Abstinence Syndrome (NAS) in Ohio. Available at https://odh.ohio.gov/wps/portal/gov/odh/know-our-programs/violence-injury-prevention-program/media/Ohio_NAS_Report_2006-2015. Accessed June 11, 2019.
- 5.Siu A, Robinson CA. Neonatal abstinence syndrome: essentials for the practitioner. J Pediatr Pharmacol Ther. 2014;19:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129:e540–e560. [DOI] [PubMed] [Google Scholar]
- 7.Kraft WK, van den Anker JN. Pharmacologic management of the opioid neonatal abstinence syndrome. Pediatr Clin North Am. 2012;59:1147–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patrick SW, Schumacher RE, Horbar JD, et al. Improving care for neonatal abstinence syndrome. Pediatrics. 2016;137:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh MC, Crowley M, Wexelblatt S, et al. Ohio perinatal quality collaborative improves care of neonatal narcotic abstinence syndrome. Pediatrics. 2018;141:10. [DOI] [PubMed] [Google Scholar]
- 10.Finnegan LP. Nelson N. Neonatal abstinence syndrome: assessment and pharmacotherapy. In: Current Therapy in Neonatal-Perinatal Medicine- 2. 1990Ontario: BC Decker. [Google Scholar]
- 11.Langley G, Moen R, Nolan K, Norman C, Provost L. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 20092nd ed San Francisco, Calif.: Jossey-Bass Publishers. [Google Scholar]
- 12.Microsoft Excel 2016 [computer program]. Version v16.0 2016Santa Rosa, CA: Microsoft Corporation. [Google Scholar]
- 13.Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–1238. [DOI] [PubMed] [Google Scholar]


