Abstract
Background:
Healthcare-associated infections are a major focus for quality improvement in hospitals today. Surgical site infections (SSIs), a postoperative complication in cardiac surgery, are associated with increased morbidity, mortality, hospital length of stay, and financial burden.
Methods:
A recent increase in cardiothoracic surgery SSIs (CT-SSIs) at our institution instigated a multidisciplinary team to explore infection prevention, bundle element compliance, and to identify interventions to reduce the CT-SSI rate. Key interventions included preoperative screening and decolonization of methicillin-sensitive Staphylococcus aureus and methicillin-resistant S. aureus with repeated intranasal applications of mupirocin, universal skin prep with chlorhexidine for all patients, and additional antibiotic dosing upon initiating cardiopulmonary bypass.
Results:
In 2014, the CT-SSI rate at our institution was 1.9/100 cases, which increased during the “intervention period” to 3.6 infections/100 cases in 2015 (16 total infections). Postinterventions, the CT-SSI rate decreased to 0.3 infections/100 cases (2 total infections), which was significantly lower than our baseline before the spike in infection rate.
Conclusions:
A comprehensive interdisciplinary approach with multiple interventions was successful in significantly reducing the CT-SSI rate in cardiothoracic surgery at a tertiary care pediatric hospital.
INTRODUCTION
Surgical site infections (SSIs) are common, with approximately 157,500 incident cases reported nationally in 2011.1 As a surgical subset, SSIs among cardiothoracic (CT) patients occur in 0.25%–6.0% of patients and have associated mortality of 7%–20%.2–4 These infections are associated with a significant increase in morbidity, mortality, time in the intensive care unit, and total hospital length of stay.5 Furthermore, a significant infection may almost double the cost of the hospital admission.5–9 Despite the impact of SSIs in the pediatric population, literature related to prevention strategies is limited.10
In 2011, The Heart Center (THC) began work with the Ohio Children’s Hospitals Solutions for Patient Safety (SPS) to prevent cardiothoracic surgery SSIs (CT-SSIs). This collaboration initiated a set of elements that should ensure decreased rates of CT-SSIs if staff complied with all “bundle” elements; these bundle elements, implemented in 2011, are described elsewhere.11–14 THC followed all components of the SPS bundle elements with high reported reliability (>90% for preoperative wiping, 100% for operating room [OR] skin prep, and antimicrobial prophylaxis) (Fig. 1).
Fig. 1.
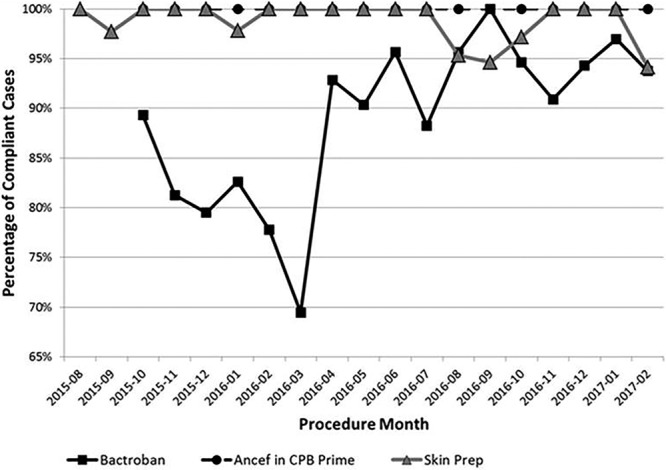
Run chart of compliance with the 3 interventions to reduce cardiothoracic surgery surgical site infection rate.
Following the Centers for Disease Control and Prevention and the Society for Thoracic Surgeons definitions, the baseline CT-SSI rates at our institution for 2013 and 2014 were 2.2 and 1.9 per 100 cases, respectively.15 In the first 5 months of 2015 (January–May), the observed rate increased almost 2-fold to 3.5/100 cases. Additionally, of the 8 infections reported in that period, 7 were deep or organ space, which represented a more aggressive infection profile when compared with prior years (for 2013 and 2014 combined, the number of deep and organ-space infections were only 3 and 0, respectively).
This project aimed to reduce CT-SSI rates to historical rates (<2 infections per 100 cases) and then further reduce infections to zero in future years to eliminate preventable harm.
METHODS
Setting
THC within Nationwide Children’s Hospital (NCH) includes CT surgery services and 2 inpatient units (a 20-bed cardiothoracic intensive care unit [CTICU] and a 24-bed cardiac step-down unit). THC has multiple service line programs including adult congenital, ambulatory clinics, interventional cardiology (catheterization and electrophysiology), heart and lung transplantation, and support services (including perfusion and mechanical support and a dedicated cardiac anesthesia team). Annually, THC performs around 500 surgical cases, of those approximately 300 are cardiopulmonary bypass (CPB) cases.
Workgroup
A CT-SSI workgroup responded to the unacceptable infection rates reported for 2015, an increase from those reported in 2013–2014. The multidisciplinary workgroup involved staff from the CTICU, the CT surgery OR, epidemiology, sterile processing, engineering, and perioperative services. Staff members included physicians, nurses, advanced practice nurses, perfusionists, anesthesiologists, quality improvement experts, and nonclinical experts from the hospital-based facilities and operations unit. CT Surgery Quality and Safety Officer, supported by the hospital administration’s Quality Improvement Services department, led the team. The workgroup used the Associates in Process Improvement Model for Improvement to identify key drivers and associated interventions (Table 1).16
Table 1.
Key Drivers and Associated Interventions in Cardiothoracic Surgical Site Infection Prevention
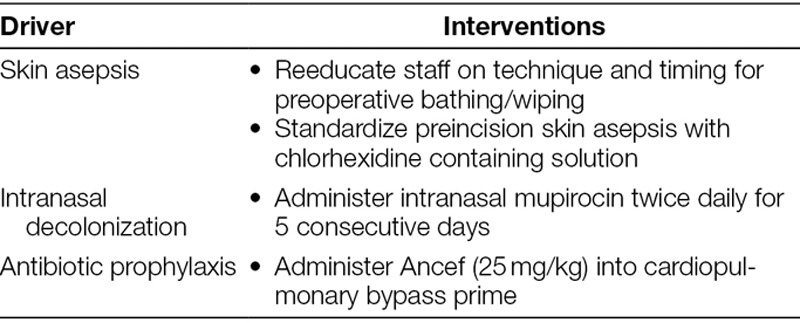
Interventions
The workgroup developed a causal tree to explore all potential causes for infection. We identified multiple potential failure modes, which were used to identify key drivers for the project: awareness and surveillance, infection prevention/control, environment, processes, materials, and people.
The workgroup’s first charge was to assure high reliability to all existing bundle elements. Although we implemented multiple interventions, including OR traffic flow pattern changes and conversion to stainless steel sterile instrument trays, we report more thoroughly on our key interventions of standardized skin asepsis, preoperative nasal decolonization, and enhanced antibiotic prophylaxis.
Skin Asepsis
Our standard of care is to bathe children the night before surgery with soap, water, and hair shampoo. An hour after bathing, the skin should be cleaned with an appropriate wipe (2% chlorhexidine gluconate [CHG] wipe for patients older than 2 months or a non-CHG–containing bath wipe for infants younger than 2 months or those with a CHG allergy). On the morning of surgery, as close to the OR time as possible, the skin is wiped again with the appropriate wipe. During the investigative period, we discovered practice variation among staff when completing preoperative bathing/wiping. Although asepsis was occurring, the timing and technique of application were not standardized, and staff had significant confusion regarding which type of wipe should be used based on patient age. We clarified the protocol and provided staff reeducation.
Before the project start, we used a wipe moistened with 2% CHG skin preparation solution for patients older than 2 months (based on manufacturer’s instructions for use). For all infants under 2 months, we used wipes containing a povidone–iodine solution. Because surgical skin preparation using 2% CHG in 70% alcohol significantly reduces SSIs, the workgroup recommended universal use of CHG-containing skin preparation solution for all patients (except in the case of documented CHG allergy).15 We also standardized the technique for application and dry time for the skin preparation solution.
Nasal Decolonization
Studies have shown improvement in the rates of methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistance S. aureus (MRSA) SSIs with the preoperative intranasal application of mupirocin.17,18 During our peak CT-SSI rate period (January–August 2015), 92% of our CT-SSI cases were culture positive for S. aureus. Therefore, to decolonize our patients, we implemented a protocol to administer 10 doses of intranasal mupirocin (2 doses daily for 5 days) for all CT surgery patients. Inpatients begin their course as soon as the surgical need is identified; prescribing was facilitated by adding mupirocin to the CTICU admitting and preoperative order sets. Outpatients receive their first dose of mupirocin, application instructions, and the medication for home use during their preadmission testing visit with the CT surgery nurse practitioners. Although we consider the application of all 10 doses in the perioperative period compliant, optimal is for all 10 doses to be administered before surgery, if possible. We also added nasal swabs to the preoperative order set for all patients, before administering the first dose of mupirocin; this allowed our team to create antibiotic prophylaxis plans based on patient results positive for MSSA or MRSA detected by polymerase chain reaction technique. Results were available to the team within 24 hours.
Antibiotic Prophylaxis
Cefazolin is the standard antibiotic used during pediatric cardiac surgery. Our compliance with preincision dosing (50 mg/kg up to 2,000 mg) and dosing every 3 hours intraoperatively (25 mg/kg) was high at baseline. However, based on literature supporting a subtherapeutic decrease in the subcutaneous concentration of cefazolin after initiation of CPB, the workgroup elected to add 25 mg/kg to the CPB prime.19 If a patient tested positive for MRSA, we used vancomycin intraoperatively without the additional pump prime dose.
Measures
The main outcome measure for this project was the CT-SSI rate (infections per 100 cases) where the numerator represents incident infections, and the denominator is the total number of CT surgical procedures. Outcome and process measures were all collected by the perfusionist and quality improvement specialist. Compliance with the 3 key interventions was a process measure, where the numerator was the number of cases compliant with an intervention, and the denominator was all CT surgeries eligible for that intervention.
Analysis
Infections were counted in the month of the associated CT procedure. Process and outcome measures were plotted on control and run charts, and special versus common cause variations were differentiated using established rules.20 Statistical significance was determined using the test for 2 proportions with statistical software (Minitab 17.1.0; Minitab LLC, State College, PA). A P value of < 0.05 was considered significant.
Ethical Considerations
As a quality improvement project and not human subject’s research, the project did not require review and approval by the Institutional Review Board.
RESULTS
Intervention Compliance
Monitored compliance rates are shown for the 3 major interventions (Fig. 1). Immediately after implementation of the updated OR skin preparation practice and use of cefazolin in the CPB prime, we were able to achieve and sustain high reliability (>90% compliance) throughout the project. Initial compliance with the full course of intranasal mupirocin was low, but compliance improved to highly reliable levels after the implementation of multiple Plan-Do-Study-Act (PDSA) cycles between October 2015 and February 2016.
CT-SSI Rate
During the baseline period (January 2013 to November 2014), we had a stabilized infection rate of 1.9 infections per 100 cases. Between January 2015 and August 2015, the “trigger period” we experienced an upward shift in the infection rate to 3.5 infections per 100 cases, which triggered the interventions reported herein. After we implemented all interventions, the infection rate declined to a new baseline of 0.3 infections per 100 cases (Fig. 2). This final rate was significantly lower (P < 0.01) than both the peak rate in 2015 and the preproject baseline.
Fig. 2.
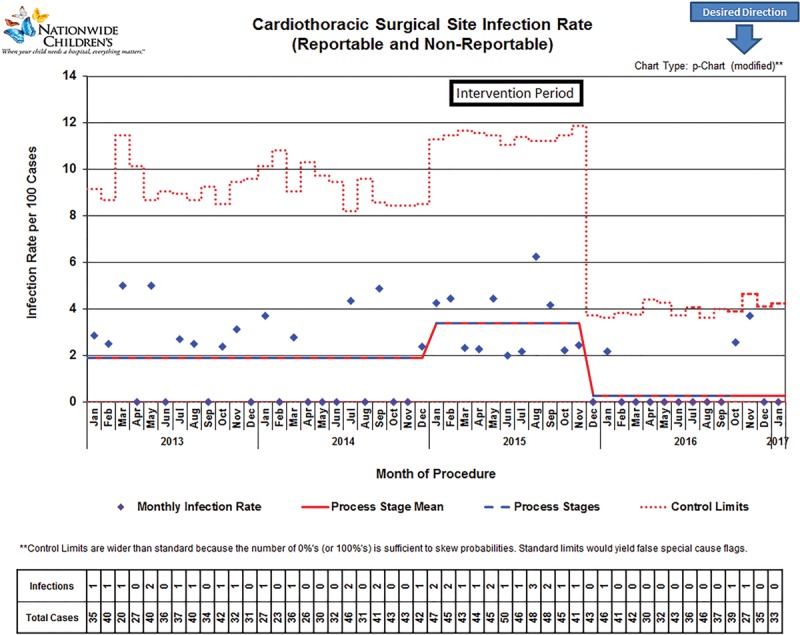
Control chart of reportable and nonreportable cardiothoracic surgery surgical site infections over time.
DISCUSSION
Beginning in 2011, THC at NCH collaborated with the Ohio Children’s Hospitals SPS to reduce SSI rates. The CT-SSI rates after implementation for 2013 and 2014 were steady at 2.2 and 1.9 per 100 cases, respectively. Although our pediatric rates were below the rates for all CT patients, which range from 2.3% to 5.0%,2–4 a sudden increase in the annual CT-SSI rate in 2015 to 3.5/100 cases (Fig. 2) triggered concerns. The motivating factor for THC staff to embrace the PDSA cycles and eventual large-scale deployment of system changes was the unacceptable increase in the CT-SSI rate—a “burning platform.” Additional process optimization measures and interventions using 5 PDSA cycles reported here reduced the preproject and peak CT-SSI rates by 84% and 92%, respectively, by the end of 2016.
Based on the calculated reduction in affected patients (3.5–0.3/100 cases), we estimated that 16 potential patients per year did not experience the risk, complications, inconvenience, and expense of a CT-SSI. Also, at a median cost of $136,950 per SSI ($2.19 million total), implementation of the described measures can significantly impact not only preventable harm rates and patient outcomes but also the financial burden that hospital-acquired infections add to aggregate healthcare costs.21
Using the Associates in Process Improvement Model for Healthcare Improvement,16 multiple PDSA interventions were tested and implemented concurrently, which allowed for rapid improvement over a concise period. However, due to the swift implementation of multiple interventions over a short timeframe, pinpointing the specific intervention that most dramatically influenced the CT-SSI rate is difficult. However, we hypothesize that the reduction in infections was mainly coupled with the initiation of preoperative repeated intranasal applications of mupirocin, which is consistent with other published SSI reduction reports.16,17 During our peak CT-SSI rate period (January–August 2015), most CT-SSI cases (12 of 13; 92%) were culture positive for S. aureus, which is carried in the nostrils of 20%–30% of healthy humans and has been associated with MSSA and MRSA SSIs.22 Although during the first month of protocol implementation, compliance with mupirocin administration was almost 90%, compliance began dropping over the next 5 months to about 70% (Fig. 1). Therefore, during PDSA cycles 3 and beyond (Table 2), we focused interventions on noncompliant patient groups, which included those receiving a transplanted organ, those with preadmission testing the day before or the same day of surgery, and those from out-of-state. To further illustrate the impact of mupirocin, both infections in 2017 thus far were superficial MSSA infections in patients who did not complete their mupirocin courses preoperatively (Fig. 2). To facilitate administration of all 10 doses before surgery, we altered the timing of preadmission testing to at least 5 days before surgery (from 3 days previously). When 5 days were not feasible for some patients, we contacted the patient’s primary with a request to order the mupirocin. Patients in need of a transplant were started on mupirocin as soon as they were listed for transplant. Also, the transplant team administered a repeat course of mupirocin every 2 months the patient remained on the waitlist.
Table 2.
PDSA Cycles During the Implementation of a Protocol for Repeated Intranasal Applications of Mupirocin for Prevention of Cardiothoracic Surgical Site Infections

To improve adherence to documentation, we have worked to standardize the location for documentation of CT-SSI prevention measures. Although inadequate documentation in the medical record may lead us to erroneously believe that a bundle element was not completed, for nurses who are not part of THC, this documentation is often not a part of the workflow. Also, some noncompliance is related to the patient or parent noncompliance with preoperative instructions for night-before-surgery bathing, wiping, and application of mupirocin. Patients and/or parents may forget to complete the prevention measures or may not understand the importance of the instructions. Although adherence to enhanced and novel prevention protocols was high during the data collection period, compliance was not 100%. Multiple factors likely contribute to these discrepancies between work-as-imagined (protocols) and work-as-done (actual practice).
Limitations
This project was conducted within a single pediatric CTICU. Other patient care procedures may have been variable and were not controlled during the intervention phase. The quality of perioperative bathing and skin antisepsis was not measured. This could have affected the effectiveness of these interventions. Additionally, NCH’s well-developed and well-staffed quality improvement department can support multisystem projects. Other institutions without this resource may find supporting the investigation and data needs, necessary to complete a project of this size, challenging.
Compliance with many of the basic infection prevention techniques and bundle elements was reported as high before and during the spike (Fig. 1). Only after further investigation did we conclude compliance was not as high as reported after auditing a few cases. This gap highlighted an intervention target. One possible explanation was that the float pool nurse, whose primary assignment was not in the CTICU, had less education and awareness surrounding bundle elements, which may have resulted in a less reliable completion. When we brought attention to the situation, and the inherent risk to our patients explicitly clarified, compliance improved. Also, due to our institutional focus on preventable harm, most staff were concerned about the rising CT-SSI rate and may have consciously or subconsciously altered their work habits to be more attentive to the prescribed interventional infection control measures. Additionally, the change in OR traffic and conversion to stainless steel instrument trays were not measured; therefore, the impact of these interventions is unknown.
CONCLUSIONS
Through multiple, concurrent, quality-improvement interventions using plan-do-study-act (PDSA) cycles, we were able to achieve a significant reduction in our CT-SSIs rate. Our goal was to reach our previous baseline after an unexpected and disturbing rise in the annual SSI rate. However, by a thorough review of all processes and tightening compliance on targeted process improvements, we achieved an even greater reduction in the CT-SSI rate at a sustainable 0.3 infections per 100 cases. We implemented key interventions of standardized skin asepsis, preoperative nasal decolonization, enhanced antibiotic prophylaxis, and attribute the greatest improvement to monitored compliance of preoperative nasal decolonization, especially among patient groups not normally inhouse 5 days before surgery.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online July 22, 2019.
To cite: Hodge AB, Thornton BA, Gajarski R, Hersey D, Cannon M, Naguib AN, Joy BF, McConnell PI. Quality Improvement Project in Congenital Cardiothoracic Surgery Patients: Reducing Surgical Site Infections. Pediatr Qual Saf 2019;4:e188.
REFERENCES
- 1.Control CDC. Surgical Site Infection (SI) Event. Procedure-Associated Module: SSI. January 2015. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. Accessed June 26 2019. [Google Scholar]
- 2.Allpress AL, Rosenthal GL, Goodrich KM, et al. Risk factors for surgical site infections after pediatric cardiovascular surgery. Pediatr Infect Dis J. 2004;23:231–234. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ami E, Levy I, Katz J, et al. Risk factors for sternal wound infection in children undergoing cardiac surgery: a case-control study. J Hosp Infect. 2008;70:335–340. [DOI] [PubMed] [Google Scholar]
- 4.Mehta PA, Cunningham CK, Colella CB, et al. Risk factors for sternal wound and other infections in pediatric cardiac surgery patients. Pediatr Infect Dis J. 2000;19:1000–1004. [DOI] [PubMed] [Google Scholar]
- 5.Sochet AA, Cartron AM, Nyhan A, et al. Surgical site infection after pediatric cardiothoracic surgery. World J Pediatr Congenit Heart Surg. 2017;8:7–12. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DJ, Kaye KS, Classen D, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S51–S61. [DOI] [PubMed] [Google Scholar]
- 7.Diaz V, Newman J. Surgical site infection and prevention guidelines: a primer for certified registered nurse anesthetists. AANA J. 2015;83:63–68. [PubMed] [Google Scholar]
- 8.Schweizer ML, Cullen JJ, Perencevich EN, et al. Costs associated with surgical site infections in veterans affairs hospitals. JAMA Surg. 2014;149:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NBCo. H. Health Care Purchaser Toolkit: Hospital-Acquired Condition Payment Policy. 2009Baltimore, MD: Discern Consulting. [Google Scholar]
- 10.Toltzis P, O’Riordan M, Cunningham DJ, et al. A statewide collaborative to reduce pediatric surgical site infections. Pediatrics. 2014;134:e1174–e1180. [DOI] [PubMed] [Google Scholar]
- 11.Cannon M, Hersey D, Harrison S, et al. Improving surveillance and prevention of surgical site infection in pediatric cardiac surgery. Am J Crit Care. 2016;25:e30–e37. [DOI] [PubMed] [Google Scholar]
- 12.Schaffzin J, Harte L, Marquette S, et al. Surgical site infection reduction by the solutions for patient safety hospital engagement network. Pediatrics. 2015;136:1353–1360. [DOI] [PubMed] [Google Scholar]
- 13.Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA. 2015;313:2162–2171. [DOI] [PubMed] [Google Scholar]
- 14.Malani PN. Bundled approaches for surgical site infection prevention: the continuing quest to get to zero. JAMA. 2015;313:2131–2132. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. [DOI] [PubMed] [Google Scholar]
- 16.Langley GL, Moen RD, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 20092nd ed San Francisco, CA: Jossey-Bass Publishers. [Google Scholar]
- 17.Hannan MM, O’Sullivan KE, Higgins AM, et al. The combined impact of surgical team education and chlorhexidine 2% alcohol on the reduction of surgical site infection following cardiac surgery. Surg Infect (Larchmt). 2015;16:799–805. [DOI] [PubMed] [Google Scholar]
- 18.George S, Leasure AR, Horstmanshof D. Effectiveness of decolonization with chlorhexidine and mupirocin in reducing surgical site infections: a systematic review. Dimens Crit Care Nurs. 2016;35:204–222. [DOI] [PubMed] [Google Scholar]
- 19.Himebauch AS, Nicolson SC, Sisko M, et al. Skeletal muscle and plasma concentrations of cefazolin during cardiac surgery in infants. J Thorac Cardiovasc Surg. 2014;148:2634–2641. [DOI] [PubMed] [Google Scholar]
- 20.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. 1999;27:97–132; quiz 133. [PubMed] [Google Scholar]
- 21.Provost LP, Murray S. The Health Care Data Guide: Learning from Data for Improvement. 2011San Francisco, CA: Jossey-Bass. [Google Scholar]
- 22.Perl TM, Golub JE. New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S. aureus nasal carriage. Ann Pharmacother. 1998;32:S7–S16. [DOI] [PubMed] [Google Scholar]


