OBJECTIVES:
Anti-cytolethal distending toxin B (CdtB) and anti-vinculin antibodies have been proposed as biomarkers that discriminate irritable bowel syndrome (IBS) diarrhea from inflammatory bowel disease; however, it is unknown whether they can also discriminate patients with IBS and IBS subtypes and functional dyspepsia (FD) from healthy individuals in the general population. We aimed to determine whether anti-CdtB and anti-vinculin can discriminate IBS and FD from health and from organic gastrointestinal (GI) disease.
METHODS:
Adults were enrolled from 2 Australian studies: (i) a random, population-based study (n = 331) with subjects diagnosed with IBS (n = 63) or FD (n = 61) by modified Rome III criteria or healthy control subjects (n = 246) who did not meet criteria for IBS and/or FD and (ii) an outpatient-based study with subjects diagnosed with IBS (n = 256) and/or FD (n = 55) or organic GI disease (n = 182) by an independent clinician. Serum levels of anti-CdtB/anti-vinculin antibodies were determined by enzyme-linked immunosorbent assay.
RESULTS:
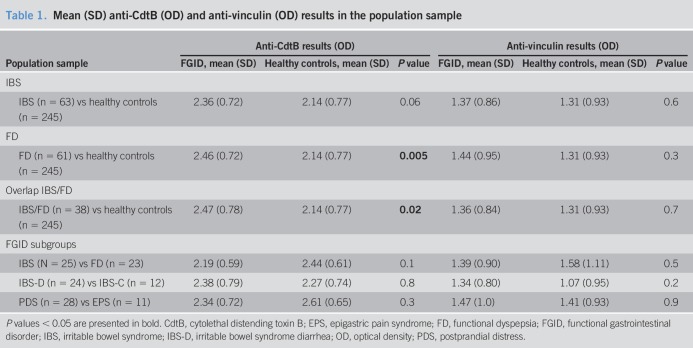
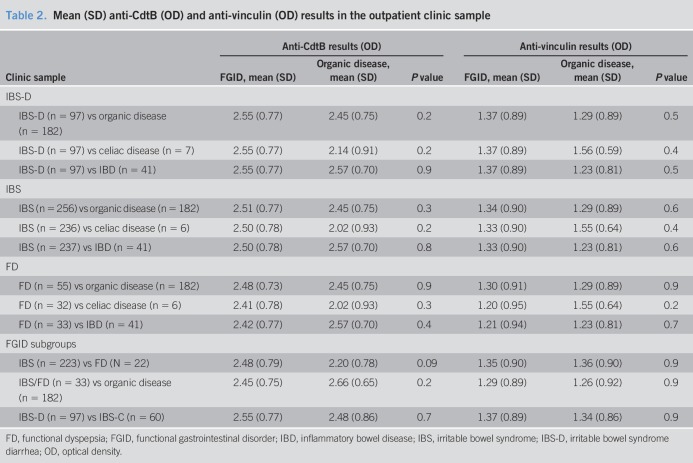
There was a significantly higher mean value of anti-CdtB in FD vs healthy controls (mean = 2.46 [SD = 0.72] vs mean = 2.14 [SD = 0.77]; P = 0.005) and IBS/FD overlap vs healthy controls (mean = 2.47 [SD = 0.78] vs mean = 2.14 [SD = 0.77]; P = 0.02). There were no significant differences in anti-CdtB in IBS and FD outpatients or IBS/FD subgroups compared with patients with organic GI disease. In terms of anti-vinculin, there were no significant differences between IBS and FD and healthy controls or between IBS and FD and organic GI disease controls.
DISCUSSION:
We did not confirm that anti-CdtB/anti-vinculin discriminated IBS diarrhea from organic GI disease in Australian subjects. However, we did find higher anti-CdtB in FD and IBS/FD overlap vs healthy controls. Postinfectious FD may be more common than currently recognized.
INTRODUCTION
Irritable bowel syndrome (IBS) and functional dyspepsia (FD) are the most common functional gastrointestinal (GI) disorders, each affecting up to 15% of the population and overlapping in one-third of cases (1). In the absence of a cure, these disorders are associated with a reduced quality of life (2) and impose a significant burden on the healthcare system (3), due in large part to expensive and unnecessary diagnostic testing (4). Recent breakthroughs in the understanding of the underlying pathophysiology of these disorders in terms of immune activation and the microbiome (5–10) have resulted in an increased interest in the development of cost-effective, reliable, blood-based biomarkers to positively diagnose these conditions.
Currently, the diagnosis of IBS and FD relies on symptom-based Rome criteria (11). The Rome criteria were developed by expert clinical consensus and remain the currently accepted gold standard (albeit an imperfect standard based on symptoms not pathology or biomarkers). The development of a reliable, cost-effective biomarker for IBS and FD has been hindered by the lack of a clear understanding of the pathophysiology of these conditions. Earlier biomarker panels have had some success in differentiating IBS from non-IBS (12,13). Lembo et al. (12) found the sensitivity and specificity of a 10-biomarker algorithm for differentiating IBS from non-IBS to be 50% and 88%, respectively. The positive predictive value was 81%, but the negative predictive value was only 64% at 50% IBS prevalence in the validation cohort. Jones et al. (13) found that the performance of a 35-biomarker panel was good in differentiating IBS from health (area under the receiver operating characteristic curve [AUC] = 0.81) but was markedly improved with the addition of 4 psychological markers (combined AUC = 0.93). However, only patients with IBS seeking health care were assessed.
The emergence of new evidence for the role of acute gastroenteritis (14,15) and microbiome dysfunction (16) in functional GI disorders (FGIDs), particularly in IBS diarrhea (IBS-D) but also FD, has resulted in the identification of potential new biomarkers. Cytolethal distending toxin B (CdtB), which is produced by Gram-negative bacteria that causes acute gastroenteritis, and vinculin, which cross-reacts with anti-CdtB antibodies in postinfectious animal models of IBS, are 2 biomarkers currently of interest in the field (17,18). Pimentel et al. (18) evaluated circulating anti-CdtB and anti-vinculin antibodies in 2,375 subjects aged between 18 and 65 years with IBS-D derived from a clinical trial cohort, compared with 142 subjects with inflammatory bowel disease (IBD), 121 subjects with celiac disease, and 43 healthy controls. They found that anti-CdtB titers were significantly higher in subjects with IBS-D compared with subjects with IBD, healthy controls, and subjects with celiac disease (P < 0.001). Anti-vinculin titers were also significantly higher in subjects with IBS compared with the other groups. The AUCs were 0.81 and 0.62 for the diagnosis of IBS-D against IBD for anti-CdtB and anti-vinculin, respectively. Limited confirmatory data are available, but these antibodies have been commercialized into a diagnostic test (17).
However, it is unknown whether these biomarkers can also discriminate IBS and IBS subtypes from healthy individuals in the general population and whether these antibodies may also be useful in diagnosing FD.
Thus, the aim of the current study is to extend the novel and important previous biomarker work by testing serum biomarkers anti-CdtB and anti-vinculin to determine whether they can discriminate IBS and FD from both health and organic GI disease in hospital outpatients and community subjects.
METHODS
In a multicenter study, participants were recruited from both the general population and hospital outpatient clinics (Figure 1). The study was approved by the Hunter New England Human Research Ethics Committee (approval reference number 16/11/16/4.04).
Figure 1.
Participant sample recruitment into the biomarkers for functional gut disorders Australian study. FD, functional dyspepsia; IBS, irritable bowel syndrome.
Community population-based study arm
A total of 331 subjects aged 18 years and above who had previously enrolled in a population-based arm of a biobank study were included in this study. Participants were originally randomly recruited from the electorates of Newcastle and Hunter New England, Australia, and are representative of the Australian population sociodemographically. Most subjects provided a fasted blood sample of up to 1 mL for use in the current study. Clinical information on IBS, FD, and organic disease diagnosis, basic demographics, and the presence of anxiety and depression was extracted from validated questionnaires, and where available was confirmed by medical interviews and/or medical records. Participants were diagnosed according to modified Rome III criteria (11) based on their responses to a previously completed validated questionnaire.
Irritable bowel syndrome.
-
• Defined as the presence of pain or discomfort anywhere in the abdomen on at least 1 day per week in the last 3 months, and
• the pain was sometimes/often/most of the time or always made better by having a bowel movement, or
-
• the following features were associated with the pain when it began (sometimes/often/most of the time or always):
• more bowel motions than usual
• less bowel motions than usual
• harder bowel motions than usual, or
• looser bowel motions than usual.
IBS subtypes
All IBS subtypes fulfilled the above criteria for a diagnosis of IBS, in addition to the following symptoms being present in the last 3 months.
Constipation-predominant IBS-C
Lumpy or hard stools sometimes/often/most of the time or always.
Loose or watery stools never or rarely.
Diarrhea-predominant IBS-D
Lumpy or hard stools never or rarely.
Loose or watery stools sometimes/often/most of the time or always.
Mixed constipation and diarrhea IBS-M
Lumpy or hard stools sometimes/often/most of the time or always.
Loose or watery stools sometimes/often/most of the time or always.
Undifferentiated IBS
Lumpy or hard stools never or rarely.
Loose or watery stools never or rarely.
Functional dyspepsia.
Fulfill criteria for either the postprandial distress (PDS) or epigastric pain syndrome (EPS) subtypes as detailed below.
FD—PDS subtype
-
Defined by the presence of one of the following being present on more than 1 day per week in the last 3 months:
◦ inability to finish a regular-sized meal or
◦ feeling uncomfortably full after a regular-sized meal.
FD—EPS subtype
Defined as the presence of pain or burning in the epigastrium being present on 1 or more days per week in the last 3 months.
Healthy controls.
Defined as not meeting criteria for IBS and/or FD.
Psychological markers.
A subset of people in the population arm of the study completed the valid Hospital Anxiety and Depression Scale (HADS) (19). The questionnaire contains 14 items that assess anxiety (7 items) and depression (7 items). Each item is rated on a 4-point scale ranging from 0 to 3, such that each subscale has a maximum score of 21. Higher scores on the HADS indicate depression or anxiety in a patient, with a score of 7 or less on either subscale indicating a “non-case,” a score of 8–10 as a “doubtful case,” and a score of 11 or more as a “case” of anxiety or depression.
Hospital outpatient clinic study arm
A total of 460 patients aged above 18 years enrolled in previous patient-based studies had provided serum for this study. All patients were recruited from primary care, gastroenterology outpatient clinics, and GI endoscopic procedure facilities from Newcastle and Brisbane, Australia. Most subjects provided a fasted blood sample of up to 1 mL for use in the current study. Clinical information on IBS, FD, and organic disease diagnosis, and basic demographics were based on medical records.
Diagnoses were made by an independent Board-certified physician at each site based on the findings from medical records and diagnostic questionnaire data where available. Gastroenterologists determined the primary diagnosis for the presenting symptoms. Patients were classified into IBS-D predominant, IBS constipation predominant, and FD. GI control groups included an organic GI disease group, including celiac disease (n = 7), IBD (n = 41), and other organic GI diseases (n = 74) that included GERD (n = 45) and diverticular disease (n = 14).
Biomarker testing panel
Anti-CdtB and anti-vinculin were run on the complete set of serum samples (n = 791). Enzyme-linked immunosorbent assays were performed using complete recombinant Campylobacter CdtB protein (Creative Biomart, Shirley, NY) and full-length human vinculin protein (Novoprotein, Short Hills, NJ) as antigens at a concentration of 1.2 μg/mL by Commonwealth laboratories (13). Antigens were immobilized overnight at 4 °C onto high-binding 96-well plates (Grenier Bio-One, Monroe, NC) in borate buffered saline (Medicago, Uppsala, Sweden) at a pH of 8.2. Wells were alternately coated with antigen or left uncoated in borate buffered saline to allow determination of nonspecific binding of serum. Wells were blocked with 3% bovine serum albumin in 1× PBS for 1 hour at room temperature. Coated and uncoated wells were then incubated with a 1:512 dilution of serum for CdtB and a 1:32 dilution of serum for vinculin for 1 hour at room temperature. Antibodies to CdtB and vinculin were used as positive controls. This was followed by 1 hour of incubation with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Each step was followed by a series of washes using 0.05% PBS-Tween 20. Finally, a 3,3′,5,5′-Tetramethylbenzidine substrate solution (Pierce, Rockford, IL) was used for visualization and immediately read on a BioTek Synergy HT plate reader (Winooski, VT). The optical densities (ODs) were read for 90 minutes at 370 nm and will be used to compare levels of anti-CdtB or anti-vinculin. Raw OD values were used for the data analysis.
A “consistent” result signifies that either antibody is above the indicated OD reference ranges of ≥2.80 for anti-CdtB and ≥1.68 for anti-vinculin based on the data from Pimentel et al. (18). An “inconclusive” result signifies that both antibodies are below these ranges.
Statistical analysis
The clinical effect size and statistical significance of the utility of biomarkers in discriminating disease states were based on unconditional logistic regression, with probability of a given disease state as the outcome and biomarkers and psychological markers as the predictive variables. A separate model was fitted to evaluate discrimination of disease states listed in the study primary objectives, using only biomarkers and additionally incorporating psychological markers that have been shown to provide incremental utility in previous studies (13). The magnitude of discrimination of each biomarker (and psychological marker) was characterized by its odds ratio (OR), which is reported with 95% confidence interval. The overall performance of the combination of biomarkers was evaluated using standard methods for the evaluation of diagnostic tests. In particular, overall discrimination performance was measured via the AUC.
Statistical power.
Because this study is based on the sample available, a retrospective power analysis has been undertaken based on these subject counts and clinically meaningful effect sizes.
Aim (a): For discriminating IBS-D from health, assuming an OR of 1.5 per SD of a marker and α = 0.01 statistical power is >0.9 based on the available sample sizes ignoring other markers and 0.8 if the correlation with other markers is as high as 0.5.
For discriminating IBS-D from organic disease, statistical power is 0.89 or greater under the same conditions, although a larger effect size is required (OR = 1.75). Statistical power for smaller effect sizes and/or more conservative statistical significance is generally below 0.8.
Aim (b): For discriminating FD from health and FD from IBS, assuming an OR of 1.5 per SD of a marker and α = 0.01 statistical power is >0.9 based on the available sample sizes ignoring other markers and if the correlation with other markers is as high as 0.5.
For discriminating FD from organic disease, statistical power is 0.9 or greater under the same conditions, although a larger effect size is required (OR = 1.75). Statistical power for smaller effect sizes is generally below 0.8.
Aim (c): The power calculations for aims (a) and (b) are measure independent so also apply to the psychological markers.
All statistical analyses were prespecified, and all P values were 2 tailed.
RESULTS
Disposition of study sample
A total of 791 samples were included in this study. Of these, 331 were population-based samples collected from Newcastle; 460 clinic samples were also included, with 126 from Newcastle and 334 from Brisbane (Figure 1).
Demographic characteristics of the sample
In terms of the demographics, the population-based sample from Newcastle had a mean age of 62 years (SD = 12), and 49% were female patients. In clinic samples, in Newcastle, the mean age was 44 years (SD = 17), and 67% were female patients, whereas in Brisbane the mean age was 50 years (SD = 16), and 62% were female patients.
Diagnostic characteristics of the sample
Figure 1 shows the number of people who met modified Rome III criteria for an FGID and healthy controls in the population arm of the study. There was a similar number of people with IBS and FD and approximately 250 healthy controls. In terms of the clinic arm of the study, there were more IBS-D diagnosed in the Brisbane clinic sample, although other diagnoses were evenly spread across the 2 sites. The most common organic GI conditions were gastroesophageal reflux disease and diverticulitis.
Anti-CdtB results (OD)
Community population-based sample.
We found a significantly higher mean value of the anti-CdtB biomarker in FD (P = 0.005) vs healthy controls (Figure 2) and IBS and FD overlap vs healthy controls (P = 0.02) (Figure 3 and Table 1). The effect sizes, however, were quite low. The mean value of the anti-CdtB biomarker was higher in the IBS vs healthy control group, but this just failed to reach significance (P = 0.06). There were no significant differences between other FGID comparisons including IBS from FD, IBS-D from IBS-C, or FD-EPS from FD-PDS.
Figure 2.
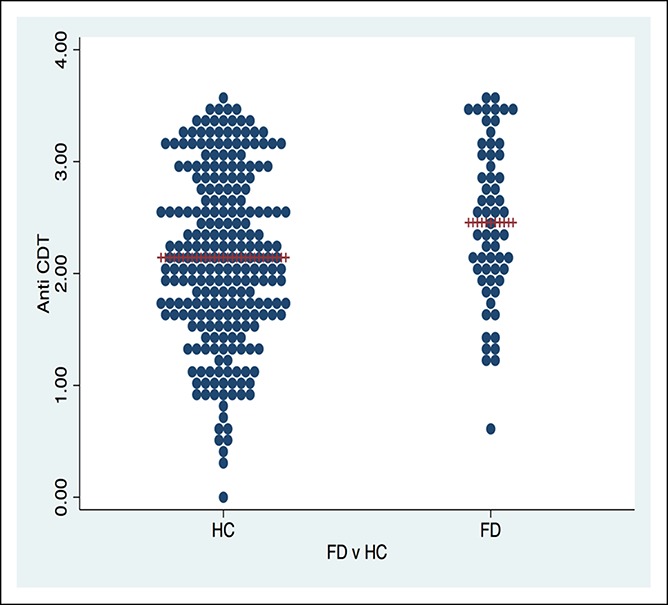
Anti-cytolethal distending toxin B dot plot for FD vs HC in a representative random, population-based sample. CDT, cytolethal distending toxin; FD, functional dyspepsia; HC, healthy control.
Figure 3.
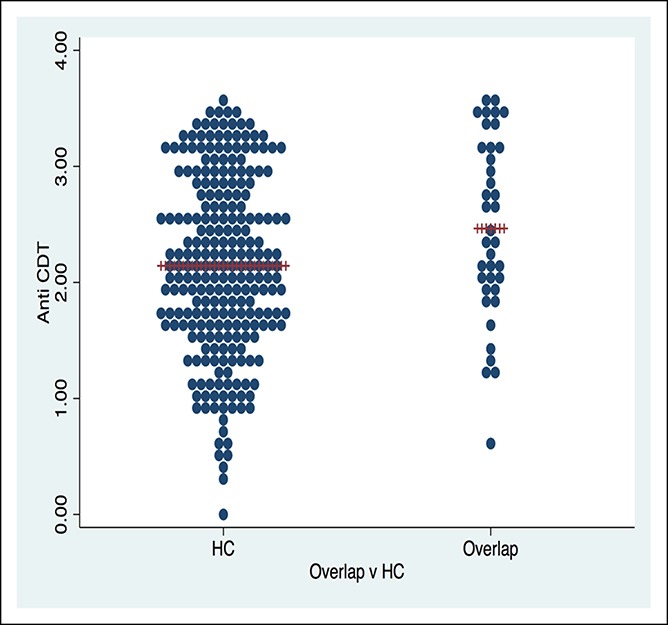
Anti-cytolethal distending toxin B dot plot for irritable bowel syndrome/functional dyspepsia overlap vs HC in a representative random, population-based sample. CDT, cytolethal distending toxin; HC, healthy control.
Table 1.
Mean (SD) anti-CdtB (OD) and anti-vinculin (OD) results in the population sample
Hospital outpatient clinic-based sample.
We did not find any of the comparisons between IBS-D, IBS, FD, and organic disease including celiac and IBD to be significant in terms of the anti-CdtB biomarker. There were also no significant differences with respect to the anti-CdtB biomarker between FGID subgroups including IBS and FD overlap and organic disease, IBS from FD, or IBS-D from IBS-C (Table 2).
Table 2.
Mean (SD) anti-CdtB (OD) and anti-vinculin (OD) results in the outpatient clinic sample
Anti-vinculin results (OD)
Community population-based sample.
Although there was a trend for higher mean values of anti-vinculin in IBS, FD, and overlap IBS and FD compared with healthy controls, this did not reach significance (Table 1). Similarly, there was no significant difference between other comparisons with respect to anti-vinculin including IBS from FD, IBS-D from IBS-C, and FD-PDS from FD-EPS.
Hospital outpatient clinic-based sample.
There were no significance differences between IBS-D, IBS, FD, and organic disease including celiac and IBD in terms of the anti-vinculin biomarker. The following comparisons between overlap IBS and FD and organic disease, IBS and FD, IBS-D, and IBS-C were also not significantly different with respect to the anti-vinculin biomarker (Table 2).
Overall diagnostic outcome
Applying the commercial cut-off values for the diagnosis of IBS, in terms of the overall diagnostic outcome, there were no significance differences between FGIDs and either healthy controls (Table 3) or organic GI disease controls (Table 4).
Table 3.
Overall diagnostic outcome (consistent) in the population sample
Table 4.
Overall diagnostic outcome (consistent) in the outpatient clinic sample
Addition of psychological markers.
In a subset of 267 samples obtained from the population, we examined the performance of anti-CdtB biomarker and anti-vinculin biomarkers and whether adding psychological markers would improve discrimination.
The best performance of the anti-CdtB and anti-vinculin biomarkers were for IBS and FD overlap differentiating from health (receiver operating characteristic curve [ROC] = 0.62), which improved considerably with the addition of 2 psychological markers (combined ROC = 0.78), indicating good discrimination of IBS/FD overlap from health.
We also found that the performance of anti-CdtB and anti-vinculin biomarkers was modest in differentiating FD from health (ROC = 0.63) but was improved considerably with the addition of 2 psychological markers (combined ROC = 0.76). There was an adequate discrimination (ROC = 0.58) for IBS and health with anti-CdtB and anti-vinculin biomarkers, which was improved to ROC = 0.7 with 2 psychological markers. The differentiation of IBS-D from IBS-C and IBS from FD was only modest (ROC = 0.59 and 0.63, respectively), and this did not improve substantially with psychological markers (ROC = 0.65 and 0.65, respectively).
DISCUSSION
There is an unmet need for cost-effective reliable biomarkers to positively identify FGIDs. Such a biomarker or panel of markers could reduce the substantial costs and patient anxiety associated with unnecessary and expensive diagnostic testing for these conditions such as colonoscopy (3,4) in low-risk patients. This study extended previous biomarker work (12,13,17,18) by assessing 2 biomarkers, anti-CdtB and anti-vinculin in IBS and, for the first time to our knowledge, FD and their respective subgroups. We hypothesized that these biomarkers would usefully discriminate these conditions from both healthy controls in the general population and from patients with organic GI diseases.
Contrary to the previous work, we did not confirm in our clinic-based study Pimentel et al.'s (18) findings that anti-CdtB and anti-vinculin discriminated IBS-D from IBD or other organic GI disease. This may have been due to methodologic differences between the 2 studies. The current study was not specifically powered for differentiating IBS from IBD. In Pimentel et al.’s (18) study, IBS was diagnosed in patients recruited to a clinical trial program aged 18–65 years according to Rome criteria, whereas the current study recruited unselected patients applying clinician diagnoses for both IBS and FD; Rome criteria in outpatients were not routinely applied, and therefore, the population of patients was likely broader and more representative of routine clinical practice. Subjects with IBD and celiac disease were recruited based on the presence of intestinal complaints and histologic confirmation of chronic inflammatory changes in the colon or small intestine. Subjects with celiac disease were also required to have an elevated tissue transglutaminase and biopsy. In the current study, these diseases were similarly diagnosed by a clinician based on the predominant presenting symptoms after a thorough review of medical records was undertaken. Comorbidities in the patient population may also be a confounding factor as a recent study suggests that anti-vinculin antibodies are increased in patients with systemic sclerosis (20), which is commonly associated with bowel dysfunction (21). This same study also identifies higher anti-vinculin antibodies in patients with high body mass index.
Pimentel et al. (18) assessed plasma levels of anti-CdtB and anti-vinculin antibodies, whereas serum levels were assessed in the current study. Blood sample preparation and storage conditions can influence the detection of low-concentration proteins, and while some biomarker proteins such as C-reactive protein show good correlation between serum and plasma (22), other biomarkers such as the tumor marker beta-2-microglobulin (23) and some cytokines show poor agreement, and this may be attributed to the use of anticoagulant or clotting factors present in plasma (24,25). Storage of plasma samples can also be important, and clotting proteins such as fibrin, present in plasma, can polymerize and precipitate, influencing immunoassay sensitivity.
Although titers of these antibodies were higher in IBS-D vs IBS-C in the current study, the differences were not significant. This was at odds with the Rezaie et al.'s study (17) that found plasma levels of anti-CdtB and anti-vinculin antibodies to be significantly higher in IBS-D and lowest in IBS-C and healthy controls (P < 0.001), whereas levels in subjects with IBS-C were not statistically different from controls (P > 0.1). Again subgroups of IBS were determined via Rome III criteria vs clinician diagnoses in the current study. However, we also did not find these antibodies to differentiate IBS-C from IBS-D or between FD subgroups defined using Rome III criteria in our population-based sample. Further validation studies of these antibodies as potential biomarkers for discriminating IBS and FD from organic GI disease will now be required.
Notably, we did find anti-CdtB discriminated Rome III FD and IBS/FD overlap from health and this was borderline significant in IBS. The overall risk of developing IBS is 4.2 times higher in individuals exposed to infectious enteritis, particularly bacterial enteritis (26). Cytolethal distending toxin (Cdt) is a powerful bacterial virulence factor that disrupts epithelial barriers, suppresses acquired immunity, and promotes proinflammatory responses, weakening host defenses (17). Cdt allows early infection and contributes to persistence by impairing host elimination of the bacteria (17,27). The most common gut bacterial pathogens are Gram-negative bacilli such as Campylobacter jejuni, Salmonella, Escherichia coli, and Shigella (17). The Cdt toxin is commonly produced by these pathogens, and CdtB is the active subunit (17).
Vinculin, a cytoplasmic actin-binding protein, regulates adhesion by directly binding to actin. If vinculin is reduced or absent, cell-matrix and cell-cell adhesion are severely weakened (17). Vinculin is also present in myenteric ganglia and the interstitial cells of Cajal (ICC), controlling gut motility, and after an infection in rats with C. jejuni, ICC numbers in the small intestine are significantly reduced, which may contribute to dysmotility, bacterial overgrowth, and thus IBS-like symptoms (28). After infectious enteritis, antibodies to CdtB are present, and due to molecular mimicry, these may cross-react with vinculin, leading to anti-vinculin autoantibody production and subsequent damage to ICC and development of IBS symptoms (28).
Postinfectious FD is thought to be uncommon, but where outbreaks of acute gastroenteritis have occurred, the risk developing FD is one in 7 over 12 months (29). Furthermore, some affected subjects developed IBS alone, or both FD and IBS (29). Other studies have identified duodenal microinflammation including eosinophils in FD (9), and in postinfectious FD in Japan also increased CCR2-/CD68-double positive cell compared with healthy volunteers (30). Our data suggest that postinfectious FD may be more common than currently recognized and anti-CdtB serves as a new biomarker for this condition, but the prevalence of duodenal microinflammation and FD with anti-CdtB remains to be established.
In line with a previous study by Jones et al. (13), we found that the performance of anti-CdtB and anti-vinculin was only modest but was improved when 2 psychological markers (HADS-anxiety and HADS-depression) were added, especially for discriminating IBS/FD overlap from health (ROC = 0.78) and FD from health (ROC = 0.76). We speculate that low-grade intestinal inflammation after bacterial gastroenteritis and an altered microbiome may drive psychological distress in a subset with FGIDs (gut-brain disease), explaining the findings observed (31,32). However, as psychological distress is known to be comorbid with FGIDs, it is possible that psychological markers would improve discrimination of any biomarker panel no matter how poorly the markers perform. There were several strengths of this study. The inclusion of both people with IBS and FD from the general population and those seeking health care is an extension of previous work that only examined people seeking health care for these conditions which may be subject to selection biases. The limitations include Rome III was not specifically used for the diagnosis among outpatients, although this is generally consistent with clinical practice where Rome criteria are rarely used (33), and in our hands Rome diagnoses significantly correlate with clinical diagnostic measures in patient studies (34).
In conclusion, we did not confirm that anti-CdtB or anti-vinculin discriminated IBS-D from organic GI disease in Australian subjects using either the commercially applied cut-offs or raw OD values. This may relate to the use of serum over plasma in our study; it is unclear whether plasma induces false-positive findings. However, we did observe higher anti-CdtB in FD and IBS/FD overlap vs healthy controls in serum. We conclude that postinfectious FD may be more common than currently recognized. Both antibodies tested are commercially available for clinical use. Based on the results from this study, we conclude that the diagnostic utility of testing is currently insufficient to guide clinical practice. However, with further refinement, serologic testing in our view is likely to have a place in the diagnostic algorithm in the future, especially if testing is found to influence treatment decisions (e.g., if specific therapy becomes available for postinfectious IBS or FD).
CONFLICTS OF INTEREST
Guarantor of the article: Nicholas J. Talley, AC, MD, PhD.
Specific author contributions: N.J.T.: Study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. G.H. and M.M.W.: Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. G.B., M.P. and A.S.: Acquisition of data; interpretation of data; critical revision of the manuscript for important intellectual content. M.P.J.: Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis. N.A.K.: Acquisition of data; interpretation of data; critical revision of the manuscript for important intellectual content. S.K.: Study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Financial support: This study was supported by an investigator-initiated grant by the Commonwealth Diagnostic Laboratories.
Potential competing interests: N.J.T.: Grant / Research Support: HVN National Science Challenge NZ (no financial support); Rome Foundation; Abbott Pharmaceuticals; Datapharm; Pfizer; Salix (irritable bowel syndrome); Prometheus Laboratories Inc (Irritable bowel syndrome [IBS] Diagnostic); Janssen (constipation). Patents: Biomarkers of irritable bowel syndrome (#12735358.9 -1405/2710383 and (#12735358.9 -1405/2710384); Licensing Questionnaires (Mayo Clinic) Talley Bowel Disease Questionnaire, Mayo Dysphagia Questionnaire; Nestec European Patent Application No. 12735358.9; Singapore ‘Provisional’ Patent NTU Ref: TD/129/17 “Microbiota Modulation of BDNF Tissue Repair Pathway.” Editorial: Medical Journal of Australia (Editor in Chief) (2015-current); Up to Date (Section Editor) (current); Precision and Future Medicine, Sungkyunkwan University School of Medicine, South Korea (2017-present). Boards: GESA Board Member. Gastroenterology Society of Australia (2017- 2019). Committees: Australian Medical Council (AMC) Council Member (2016-2019); MBS Review Taskforce (current); NHMRC Principal Committee, Research Committee (2016-2021); Asia Pacific Association of Medical Journal Editors (APAME) (2018-). Consultancies: Adelphi values (functional dyspepsia working group to develop a symptom based PRO instrument) 2017; GI therapies (non-invasive device company, consultant and options) 2017; Viscera Labs, USA; Progenity Inc. San Diego, USA; Sanofi-aventis, Sydney; Censa, Wellesley, MA, USA; Anatara Life Sciences, Brisbane; Takeda, Japan (gastroparesis) Allakos (IBS); Allergens PLC; Napo Pharmaceutical; Outpost Medicine; Samsung Bioepis; Yuhan (IBS); Synergy (IBS); Theravance (gastroparesis). Miscellaneous: Avant Foundation (judging of research grants) (2017-2019); Community and patient advocacy groups; Advisory Board, IFFGD (International Foundation for Functional GI Disorders). G.H.: Unrestricted educational support from Bayer Ptd, Ltd, and the Falk Foundation. Research support was provided via the Princess Alexandra Hospital, Brisbane, by GI Therapies Pty Limited, Takeda Development Center Asia Pty Ltd, Eli Lilly Australia Pty Limited, F. Hoffmann-La Roche Limited, MedImmune Ltd, Celgene Pty Limited, Celgene International II Sarl, Gilead Sciences Pty Limited, Quintiles Pty Limited, Vital Food Processors Ltd, Datapharm Australia Pty Ltd, Commonwealth Laboratories Pty Limited, Prometheus Laboratories, FalkGmbHandCoKg, Nestle Pty Ltd, Mylan. Patentholder: A biopsy device to take aseptic biopsies (US 20150320407 A1). M.M.W.: Grant/research support: Prometheus Laboratories Inc (Irritable bowel syndrome [IBS] Diagnostic), Commonwealth Diagnostics International (biomarkers for FGIDs). G.B.: None to disclose. M.P.: None to disclose. A.S.: None to disclose. M.P.J.: Consultancies with GI Therapies (abdominal stimulation in constipation), SFI (prokinetics). N.A.K.: None to disclose. S.K.: Grant/research support: Cancer Institute NSW (Career Development Fellowship), National Health and Medical Research Council (Project Grant APP1128487), Commonwealth Diagnostics International (biomarkers for FGIDs), Syntrix Biosystems (contract research—drug delivery). Anatara Lifesciences (Advisory Board/Funded research). Gossamer Bio (Advisory Board/Funded research).
Study Highlights.
WHAT IS KNOWN
✓ Current diagnosis of IBS and FD relies on symptom-based Rome criteria.
✓ Anti-CdtB and anti-vinculin antibodies have been found in a study to discriminate IBS diarrhea from IBD.
WHAT IS NEW HERE
✓ Higher anti-CdtB in FD and IBS/FD overlap vs healthy controls.
✓ Anti-CdtB/anti-vinculin did not discriminate IBS-D from organic GI disease in Australian subjects.
TRANSLATIONAL IMPACT
✓ Further study is needed to determine if anti-CdtB and anti-vinculin antibodies can be translated to clinically useful diagnostic tests.
REFERENCES
- 1.Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: A population-based study. Am J Gastroenterol 2002;97(9):2290–9. [DOI] [PubMed] [Google Scholar]
- 2.Koloski NA, Talley NJ, Boyce PM. The impact of functional gastrointestinal disorders on quality of life. Am J Gastroenterol 2000;95(1):67–71. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil 2008;20(Suppl 1):121–9. [DOI] [PubMed] [Google Scholar]
- 4.Burbige EJ. Irritable bowel syndrome: Diagnostic approaches in clinical practice. Clin Exp Gastroenterol 2010;3:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keely S, Walker MM, Marks E, et al. Immune dysregulation in the functional gastrointestinal disorders. Eur J Clin Invest 2015;45(12):1350–9. [DOI] [PubMed] [Google Scholar]
- 6.Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol 2011;106(6):1089–98. [DOI] [PubMed] [Google Scholar]
- 7.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007;132(3):913–20. [DOI] [PubMed] [Google Scholar]
- 8.Tornblom H, Abrahamsson H, Barbara G, et al. Inflammation as a cause of functional bowel disorders. Scand J Gastroenterol 2005;40(10):1140–8. [DOI] [PubMed] [Google Scholar]
- 9.Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: An adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol 2007;5(10):1175–83. [DOI] [PubMed] [Google Scholar]
- 10.Walker MM, Talley NJ. Review article: Bacteria and pathogenesis of disease in the upper gastrointestinal tract—Beyond the era of Helicobacter pylori. Aliment Pharmacol Ther 2014;39(8):767–79. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 12.Lembo AJ, Neri B, Tolley J, et al. Use of serum biomarkers in a diagnostic test for irritable bowel syndrome. Aliment Pharmacol Ther 2009;29(8):834–42. [DOI] [PubMed] [Google Scholar]
- 13.Jones MP, Chey WD, Singh S, et al. A biomarker panel and psychological morbidity differentiates the irritable bowel syndrome from health and provides novel pathophysiological leads. Aliment Pharmacol Ther 2014;39(4):426–37. [DOI] [PubMed] [Google Scholar]
- 14.Gwee KA. Post-infectious irritable bowel syndrome, an inflammation-immunological model with relevance for other IBS and functional dyspepsia. J neurogastroenterology Motil 2010;16(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fock KM. Functional dyspepsia, H. pylori and post infectious FD. J Gastroenterol Hepatol 2011;26:39–41. [DOI] [PubMed] [Google Scholar]
- 16.Mayer EA, Savidge T, Shulman RJ. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology 2014;146(6):1500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezaie A, Park SC, Morales W, et al. Assessment of anti-vinculin and anti-cytolethal distending toxin B antibodies in subtypes of irritable bowel syndrome. Dig Dis Sci 2017;62(6):1480–5. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One 2015;10(5):e0126438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 2003;1(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suliman YA, Kafaja S, Aleman M, et al. Anti-vinculin antibodies: A novel biomarker in systemic sclerosis, and its association with vascular invovement [abstract]. Arthritis Rheumatol 2016;68(Suppl 10). [Google Scholar]
- 21.Nagaraja V, McMahan ZH, Getzug T, et al. Management of gastrointestinal involvement in scleroderma. Curr Treat Options Rheumatol 2015;1(1):82–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dossus L, Becker S, Achaintre D, et al. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: Comparison with ELISA. J Immunol Methods 2009;350(1–2):125–32. [DOI] [PubMed] [Google Scholar]
- 23.Bjerrum OW, Birgens HS. Measurement of beta-2-microglobulin in serum and plasma by an enzyme-linked immunosorbent assay (ELISA). Clin Chim Acta 1986;155:69–76. [DOI] [PubMed] [Google Scholar]
- 24.de Jager W, Bourcier K, Rijkers GT, et al. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol 2009;10(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selby C. Interference in immunoassay. Ann Clin Biochem 1999;36(6):704–21. [DOI] [PubMed] [Google Scholar]
- 26.Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: A systematic review and meta-analysis. Gastroenterology 2017;152(5):1042–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scuron MD, Boesze-Battaglia K, Dlakić M, et al. The cytolethal distending toxin contributes to microbial virulence and disease pathogenesis by acting as a tri-perditious toxin. Front Cel Infect Microbiol 2016;6:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pimentel M, Morales W, Pokkunuri V, et al. Autoimmunity links vinculin to the pathophysiology of chronic functional bowel changes following Campylobacter jejuni infection in a rat model. Dig Dis Sci 2015;60:1195–205. [DOI] [PubMed] [Google Scholar]
- 29.Mearin F, Pérez-Oliveras M, Perelló A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: One-year follow-up cohort study. Gastroenterology 2005;129(1):98–104. [DOI] [PubMed] [Google Scholar]
- 30.Futagami S, Shindo T, Kawagoe T, et al. Migration of eosinophils and CCR2-/CD68-double positive cells into the duodenal mucosa of patients with postinfectious functional dyspepsia. Am J Gastroenterol 2010;105(8):1835. [DOI] [PubMed] [Google Scholar]
- 31.Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017;376(26):2566–78. [DOI] [PubMed] [Google Scholar]
- 32.Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med 2015;373(19):1853–63. [DOI] [PubMed] [Google Scholar]
- 33.Lea R, Hopkins V, Hastleton J, et al. Diagnostic criteria for irritable bowel syndrome: Utility and applicability in clinical practice. Digestion 2004;70(4):210–3. [DOI] [PubMed] [Google Scholar]
- 34.Koloski NA, Jones M, Hammer J, et al. The validity of a new structured assessment of gastrointestinal symptoms scale (SAGIS) for evaluating symptoms in the clinical setting. Dig Dis Sci 2017;62(8):1913–22. [DOI] [PubMed] [Google Scholar]