OBJECTIVES:
Circulating tumor cells (CTCs) in the blood have been used as diagnostic markers in patients with colorectal cancer (CRC). In this study, we evaluated a CTC detection system based on cell size to assess CTCs and their potential as early diagnostic and prognostic biomarkers for CRC.
METHODS:
From 2014 to 2015, 88 patients with newly diagnosed CRC, who were scheduled for surgery, and 31 healthy volunteers were enrolled and followed up in Pusan National University Hospital. CTCs were enriched using a centrifugal microfluidic system with a new fluid-assisted separation technique (FAST) and detected by cytomorphological evaluation using fluorescence microscopy.
RESULTS:
Two or more CTCs were detected using FAST in 74 patients and 3 healthy volunteers. The number of CTCs in the CRC group was significantly higher than that in the healthy volunteers (P < 0.001). When a receiver operating characteristic curve was created to differentiate patients with CRC from healthy volunteers, the sensitivity and specificity were almost optimized when the critical CTC value was 5/7.5 mL of blood. When this value was used, the sensitivity and specificity in differentiating patients with CRC from the healthy controls were 75% and 100%, respectively. In patients with CRC with ≥5 CTCs, vascular invasion was frequently identified (P = 0.035). All patients with stage IV were positive for CTCs. Patients with ≥5 CTCs showed a trend toward poor overall and progression-free survival.
DISCUSSION:
Our study demonstrated promising results with the use of FAST-based CTC detection for the early diagnosis and prognosis of CRC.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies worldwide and one of the leading causes of cancer-related deaths. Despite tremendous efforts, CRC remains the third most commonly diagnosed cancer in men and the second most commonly diagnosed cancer in women, with 1.65 million new cases and approximately 835,000 deaths per year worldwide (1). Its incidence has been increasing and is expected to increase by 60% with more than 2.2 million new cases and 1.1 million cancer deaths by 2030 (2). In Korea, the CRC incidence rate was the highest in the world according to Global Burden of Disease Cancer 2012 estimates, with an age-standardized incidence rate of 45.0 per 100,000 person-years (1). Despite recent advances in the management of resected CRC and introduction of more effective treatments for metastatic tumors, approximately 30%–50% of patients with CRC who have undergone potentially curative resection experience recurrence in the form of regional lymph node or distant metastasis (3–5). This suggests the presence of potential metastatic cells, which cannot be detected by currently used diagnostic methods.
The tumor cells that shed into the blood circulation from primary or metastatic cancers are referred to as circulating tumor cells (CTCs). Ashworth first reported the presence of CTCs in a metastatic cancer patient in 1869 (6). On 100 years after CTCs were first observed, attempts to detect CTCs were initiated, with immunomagnetic separation as one of the first techniques. Despite several studies reporting on CTC detection, the methodological aspects have not presented a clear appraisal of the clinical impact. Although immune-mediated methods and reverse transcriptase polymerase chain reaction can be sensitive, their specificity remains a critical issue, because CTCs that have undergone epithelial–mesenchymal transitions (EMTs) during cancer dissemination do not express epithelial markers (7). As complementary methods, many researchers have developed new technologies using a size-based isolation method to overcome the disadvantages of immunomagnetic separation. More recently, for the direct detection of CTCs in peripheral blood, we developed a new size-based platform, a centrifugal microfluidic system based on the fluid-assisted separation technique (FAST), and demonstrated that FAST allows for the rapid, highly selective and sensitive, and label-free isolation of CTCs from whole blood without prior sample treatment (8,9).
Although several studies have reported that CTCs can assist in CRC diagnosis and the evaluation of prognoses, the clinicopathological significance of CTCs remains unclear (10,11). Therefore, the aim of this study is to evaluate the efficacy of FAST in assessing CTCs in patients with CRC and to investigate the clinical potential of CTCs as early diagnostic tools and prognostic biomarkers for CRC.
METHODS
Study design (patients and sample)
Ninety-nine patients with CRC before undergoing surgery and 31 healthy controls were prospectively enrolled at the Pusan National University Hospital (Busan, Korea) from August 2014 to December 2015. Among patients with CRC, 5 patients who previously had other concomitant malignancies, such as gastric cancer (1 patient), renal cell carcinoma (1 patient), bladder cancer (1 patient), and laryngeal cancer (2 patients), were sequentially excluded. In addition, 1 patient who underwent palliative resection for CRC recurrence after previous surgery and 1 patient with synchronous CRC from among familial adenomatous polyposis patients were excluded. Furthermore, 4 patients were excluded due to sample clogging. Finally, 88 patients were included in the analysis. The 31 healthy controls included 21 men and 10 women with no current illnesses or history of any neoplastic disease. This study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Pusan National University Hospital (H-1412-011-024), and all participants provided signed informed consent.
Peripheral blood samples were collected before surgery. The evaluation included medical history-taking and physical examination, complete blood count and blood chemistry testing, and preoperative carcinoembryonic antigen (CEA) level measurement, as well as radiography and computed tomography (CT) for tumor staging. To determine the risk factors for CTC positivity after curative CRC surgery, we compared the preoperative patient characteristics (sex, age, and preoperative CEA level), intraoperative findings (tumor location and tumor size), and postoperative pathological characteristics (cell type, lymph node involvement, vascular invasion, lymphatic invasion, perineural invasion, KRAS mutation, and microsatellite instability positivity). The staging evaluation was performed according to the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging for CRC (seventh edition) (12). All patients were followed up for a median period of 19.5 months (range, 1–37.9 months). Overall survival (OS) was defined as the period from surgery to death from any cause, and progression-free survival (PFS) was defined as the period from surgery to the first event of either CRC relapse or death. Patients who did not experience relapse or who died (for PFS) or remained alive (for OS) at the final follow-up were censored at that time. The biospecimens and data used for this study were provided by the Biobank of Pusan National University Hospital, a member of the Korea Biobank Network.
Surgical procedure
Open or laparoscopy-assisted colorectal resection with lymphadenectomy, resection, and reconstruction were performed. The appropriate surgical technique was selected based on the site of the primary tumor, lymphovascular pathway, histopathological diagnosis of the previous biopsy specimen through colonoscopy, and degree of cancer progression. In cases with right-sided cancer, standard or extended right hemicolectomy was performed. For patients with left-sided cancer above the sigmoid colon, left hemicolectomy was performed. Various types and levels of anterior resection were performed for patients with sigmoid and rectal cancer. Surgical colorectal specimens were obtained for histopathological evaluation and pathological TNM staging.
Isolation, enumeration, and identification of CTCs
From all participants, 3–5 mL of peripheral blood samples were drawn into a buffered ethylenediaminetetraacetic acid tube by venipuncture. To prevent contamination by skin epithelial cells, the first 2 mL of blood were discarded. Blood samples were used within 8 hours to prevent cell damage. The CTC isolation protocol using FAST has been described previously (9). In brief, before CTC isolation, the disk surface was passivated with 1% bovine serum albumin solution. Furthermore, 1 mL of phosphate-buffered saline (PBS) was used to wash the disc surface. After surface passivation, 3 mL of blood without any sample treatment steps (such as red blood cell lysis and dilution) were transferred to the disc. CTCs were isolated on the track-etched polycarbonate membrane with 8-μm pores by rotating the disc using a programmed spin program.
The immunofluorescent technique was performed on the disc to identify the number of isolated CTCs from the blood sample. First, the isolated cells were fixed with 4% formaldehyde for 15 minutes at 37 °C. The isolated cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, MO) in PBS for 5 minutes and then washed with PBS. Samples were then blocked with 20 μg/mL immunoglobulin G, followed by staining with several antibodies. The isolated cells could be differentially identified with the use of an epithelial marker (cytokeratin [CK]) and a hematopoietic white blood cell marker (CD45), as well as the existence of a cell nucleus (4,6-diamidino-2-phenylindole [DAPI]) or surface epithelial cell adhesion molecule (EpCAM), as described in our previous studies (13). The cells were subsequently subjected to image analysis under a fluorescence microscope (Eclipse Ti-E fluorescence microscopy; Nikon, Tokyo, Japan) at ×40 magnification. CTCs were defined as cells with the following characteristics: DAPI-positive, CD45-negative, EpCAM-positive cell, or DAPI-positive and CK-positive cell, with a diameter of >8 μm (Figure 1). The results were quantitatively reported as the number of CTCs per 7.5 mL of whole blood.
Figure 1.
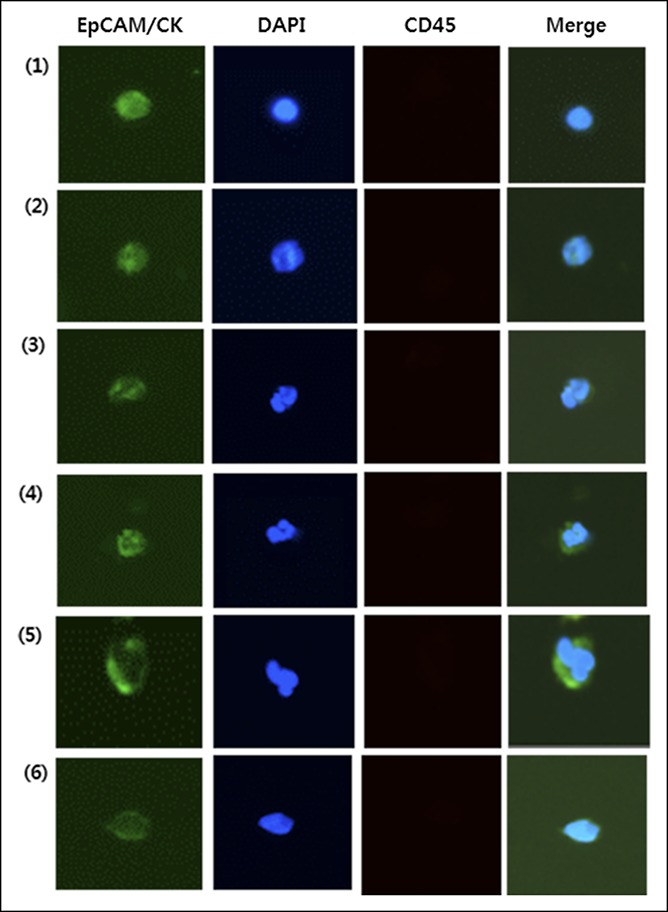
Representative immunofluorescent images of CTCs in patients with CRC. CTCs were defined as captured cells that were CK+ or EpCAM+, CD45−, and DAPI+ and had a diameter of >8 μm. CK, cytokeratin; CRC, colorectal cancer; CTC, circulating tumor cell; DAPI, 4,6-diamidino-2-phenylindole; EpCAM, epithelial cell adhesion molecule.
Statistical analysis
We created a receiver operating characteristic (ROC) curve to identify the best sensitivity and specificity threshold values for CTCs to differentiate patients with CRC from healthy controls. As the area under the curve (AUC) was the largest, this value was set as the cutoff. All data were analyzed using the IBM SPSS software, version 20.0 for Windows (IBM, Armonk, NY). The association between CTC positivity and various clinicopathological characteristics was analyzed using a Fisher exact test or χ2 test for categorical variables and a Student t test for quantitative variables. Kaplan–Meier survival curves for survival outcomes (OS and PFS) according to CTC level were constructed with available clinical data, and the differences were evaluated using a log-rank test. Statistical significance was set at P < 0.05.
RESULTS
Baseline clinicopathological characteristics of the patients with CRC
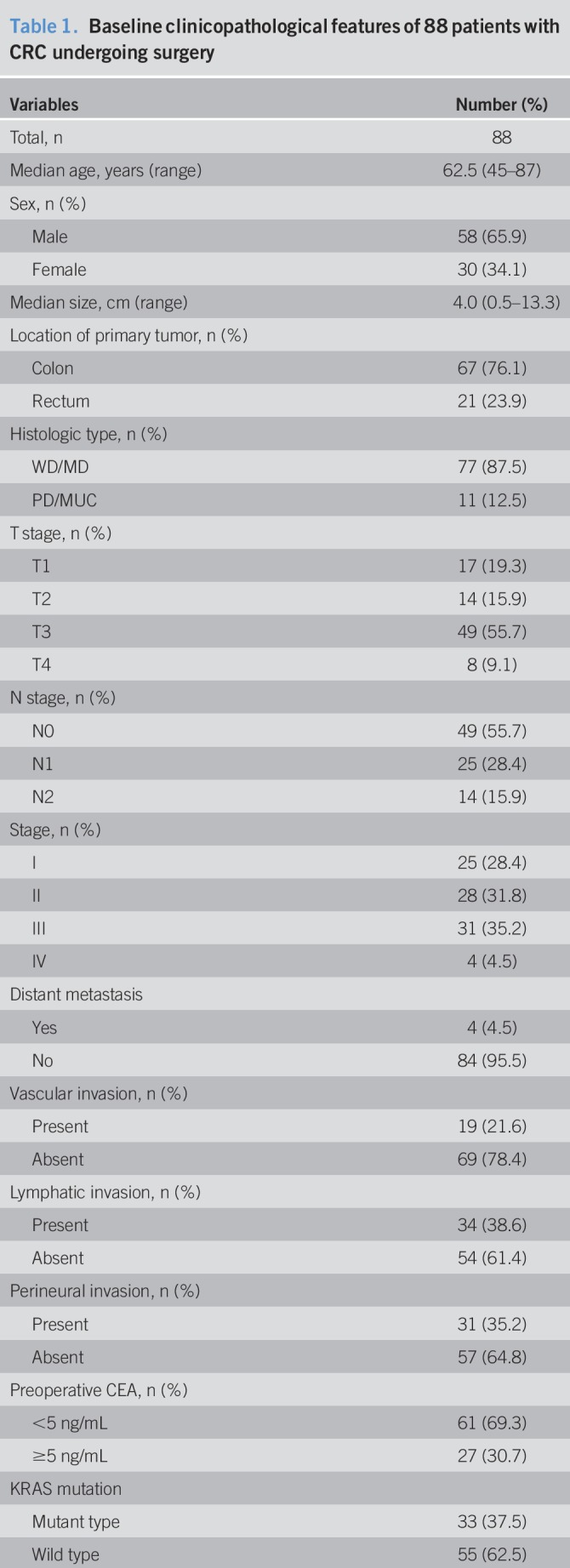
Between August 2014 and December 2015, a total of 88 patients with CRC who underwent potentially curative therapy were enrolled into this study; we detected the presence of CTCs using FAST. The main clinicopathological characteristics of the 88 patients are presented in Table 1. There were 58 men (65.9%) and 30 women (34.1%), and their median age was 62.5 years (range, 45–87 years). All patients were pathologically diagnosed with colorectal adenocarcinoma; 67 patients presented with colon cancer and 21 with rectal cancer. The median tumor size was 4 cm (range, 0.5–13.3 cm). According to histological type, 77 (87.5%) of the tumors were differentiated-type adenocarcinomas and 11 (12.5%) were undifferentiated-type adenocarcinomas. For T staging, there were 17 T1, 14 T2, 49 T3, and 8 T4 stage cases, and for N staging, there were 49 N0, 25 N1, and 14 N2 stage cases. TNM classification revealed the presence of 25 patients with stage I, 28 patients with stage II, 31 patients with stage III, and 4 patients stage IV cancer. All patients with stage IV cancer had liver metastasis. For clinicopathological features, 19 (21.6%) patients had vascular invasion, 34 (38.6%) had lymphatic invasion, and 31 (35.2%) had perineural invasion. For genotyping of the resected surgical specimens, 33 and 11 tumors had KRAS mutation and microsatellite instability-H, respectively.
Table 1.
Baseline clinicopathological features of 88 patients with CRC undergoing surgery
Identification of CTCs in patients with CRC and healthy controls
We first compared the CTC detection rates using FAST. CTCs were identified in 3 of the 31 healthy volunteers (9.7%, CTC counts: 2.5, 5, and 5 in 7.5 mL of blood). In contrast, CTCs presented preoperatively in 74 of 88 patients with CRC (84.1%). The median number of CTCs among these patients was 60 (range, 0–120) per 7.5 mL of blood. The number of CTCs in the CRC group was significantly higher than that in the healthy control group (P < 0.001). The sample size in the control was powered appropriately for this analysis.
We then distinguished patients with CRC from healthy controls using ROC curves (Figure 2). A scatter plot of the CTC values of the healthy controls and patients with CRC are presented in Figure 3. The “optimal” cutoff value was defined by the highest Youden index value. Therefore, we defined the cutoff as 5 CTCs in 7.5 mL of blood sample (AUC = 0.906). Of the 68 participants with a CTC level ≥5 per 7.5 mL of blood, 66 (97.1%) had CRC. However, among those with a CTC level <5 per 7.5 mL of blood, 29 (56.9%) were healthy controls. The corresponding Youden index was 0.750, sensitivity was 75%, specificity was 100%, positive predictive value was 100%, and negative predictive value was 58.5% (Table 2).
Figure 2.
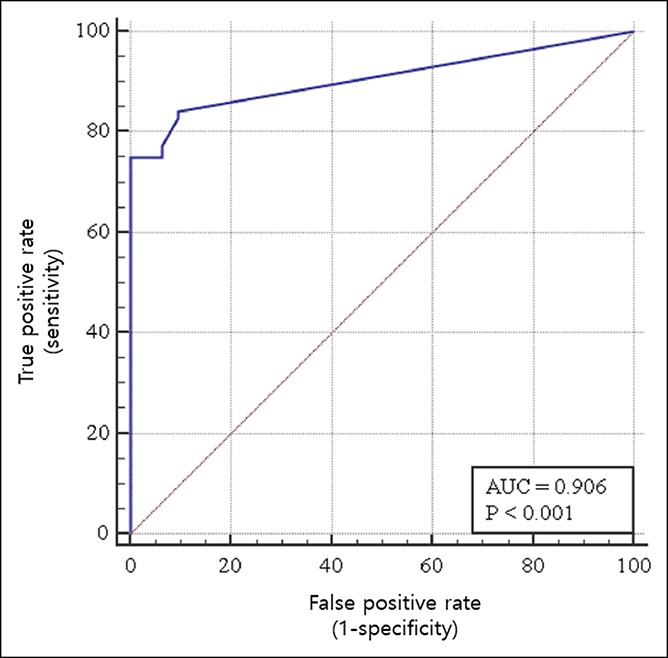
Differentiating patients with CRC from healthy controls using a ROC curve. To identify the optimal CTC threshold value for differentiating patients with CRC from healthy controls, the sensitivity and specificity were optimized using a threshold count of 5 CTCs per 7.5 mL of blood. Red line, reference line; Blue line, ROC curve; CTC, circulating tumor cell; AUC, area under the curve; CRC, colorectal cancer; ROC, receiver operating characteristic.
Figure 3.
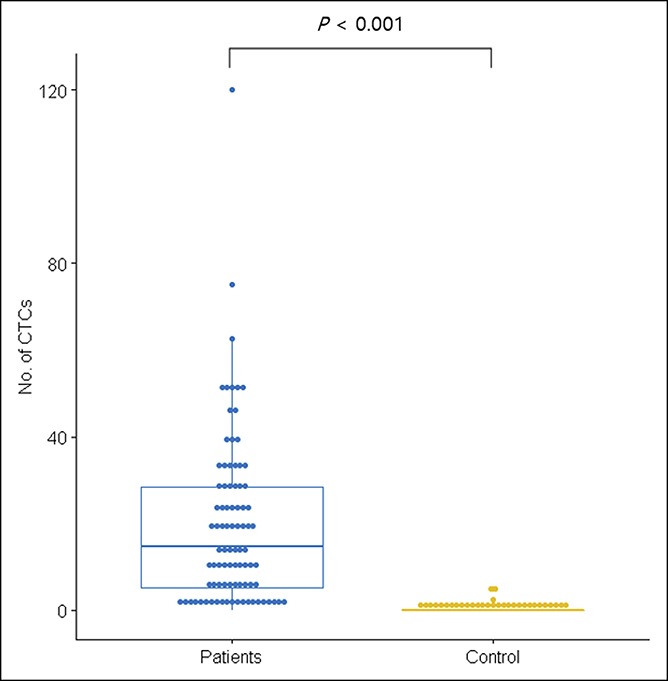
Scatter plot of CTC values of healthy controls and patients with CRC. CRC, colorectal cancer; CTCs, circulating tumor cells.
Table 2.
Values for CTCs to differentiate patients with CRC (n = 88) from healthy controls (n = 31)
Association of CTCs with clinicopathological parameters in CRC
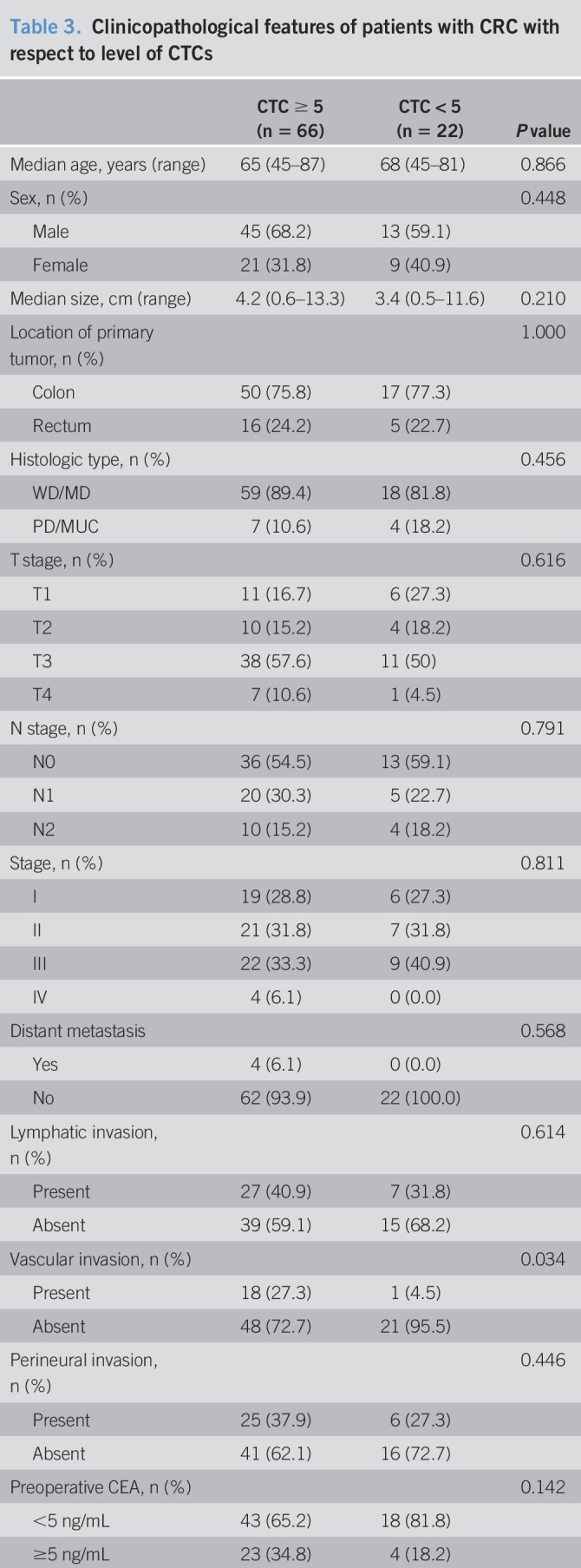
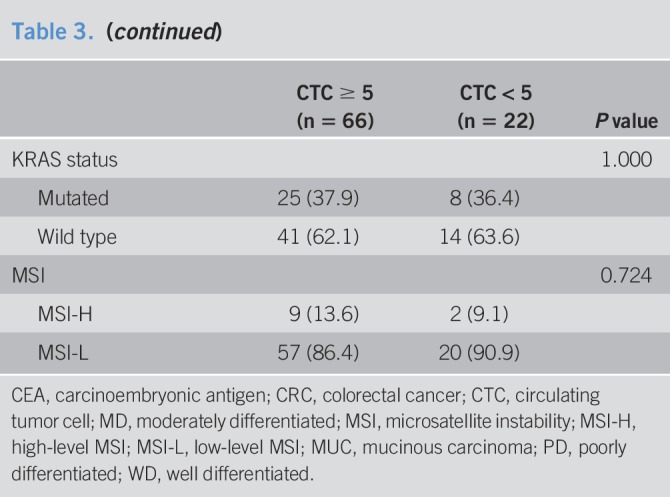
To investigate whether the detection of CTCs was associated with clinicopathological parameters, we first analyzed the correlation according to CTC positivity. A threshold of ≥5 was selected for CTC positivity in our correlation analysis. The patients' characteristics, according to CTC positivity, are presented in Table 3. CTC-positive patients more frequently had vascular invasion (P = 0.034). Except for vascular invasion, there was no significant difference in the other patient characteristics according to CTC positivity. Among the enrolled patients with CRC, 4 were diagnosed with stage IV cancer; all these patients belonged to the CTC-positive group.
Table 3.
Clinicopathological features of patients with CRC with respect to level of CTCs
Survival outcomes according to CTC positivity
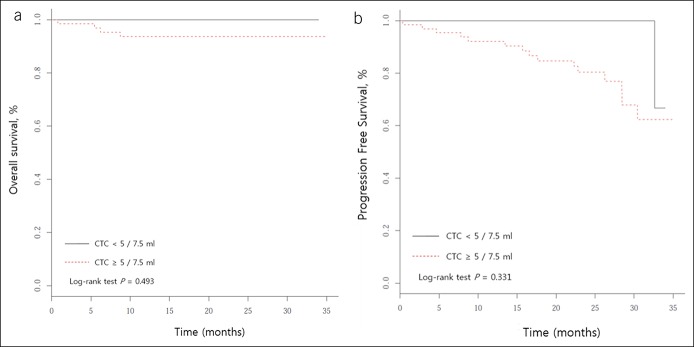
All participants were categorized into CTC-positive and CTC-negative groups during the follow-up period. To investigate the relationship between CTCs and clinical outcomes, we analyzed the survival outcomes (OS and PFS) according to CTC positivity. The median follow-up period was 19.5 months, and there were 15 (17.0%) cases of recurrence and 5 (5.7%) cases of death. CTC positivity in this study demonstrated a trend toward poor OS and PFS, but the differences were not statistically significant (Figure 4).
Figure 4.
Kaplan–Meier survival plots of CTC-positive vs -negative patients. (a) Overall survival. (b) Progression-free survival. CTC, circulating tumor cell.
DISCUSSION
The present study revealed 3 main results for CTC detection in patients with CRC. First, we evaluated the efficacy of FAST in assessing CTCs in patients with CRC. CTCs were detected by FAST in 74 of 88 (84.1%) patients with CRC, which was higher than the positivity rates (10.5–36.2%) previously reported using the CellSearch system. Second, we investigated the role of CTCs in the early diagnosis of CRC. ROC curves and the corresponding AUC values were generated to compare the predictive sensitivity and specificity. A CTC level of ≥5 per 7.5 mL of blood was determined as the cutoff value in the ROC curves. This model had sensitivity of 75%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 58.5%. Third, the preoperative detection of CTCs can serve as a prognostic biomarker in patients with CRC. OS and PFS tended to be lower in CTC-positive patients than CTC-negative patients. These results suggest the potential of FAST-based CTC detection in the early diagnosis of CRC and prognosis.
The use of CTCs in peripheral blood was recognized as “real-time liquid biopsy” in solid tumors because it could be performed frequently, easily, and less invasively. The field of CTC research is active (14). However, CTCs are rarely found in blood, at levels typically >1 in a billion cells, and they are fragile. Therefore, the enrichment of CTCs with purity and high recovery has been a great challenge (15). There are various assays with different principles that can be used for the enrichment and identification of CTCs (16–18). Many of them are not yet standardized and are confusing to the cancer research community. EpCAMs are the most widely used in CTC detection (19–22). The CellSearch system, which immunologically identifies the EpCAM semiautomatically, is the first and only standardized system approved by the US Food and Drug Administration for CTC detection in patients with metastatic CRC (23). However, carcinoma cells that have passed through a partial or complete EMT process are no longer detectable by epithelial phenotype (EpCAM) in peripheral blood. Recent studies reported that a significant portion of CTCs are EpCAM negative (24,25). Thus, it is evident that the CellSearch system does have decisive limitations in the detection of CTCs to identify EpCAM-negative CTCs. Many researchers have developed new technologies using a size-based isolation method (isolation by size of epithelial tumor cells) to overcome the disadvantages of the CellSearch system. Isolation by size of epithelial tumor cell technology could isolate a higher number of CTCs by capturing tumor cells using differences in the deformability and diameter of hemocytes and abnormal cells (26–28). We previously reported a dramatic increase in recovery rate with the use of FAST (9). CTC detection through FAST provides rapid, clog-free, uniform, and efficient filtration with high throughput under a lower pressure drop from whole blood without prior sample treatment compared to conventional separation. The cost of one FAST disc used in this study is approximately USD 220, and the total process of CTC isolation from whole blood takes less than 3 hours, which can be potentially useful in clinical settings. In this study, we applied FAST for CTC detection in patients with CRC. Among the 88 patients with CRC, 74 (84.1%) presented one or more CTCs; this is higher than the positivity rates (10.5%–36.2%) reported previously using the CellSearch system (29–31).
The most important outcome indicator after CRC resection is the pathological stage at diagnosis (12). The stage-dependent CRC survival rates are 94%, 82%, 67%, and 11% for stages I, II, III, and IV, respectively (32). Therefore, screening and the early detection of CRC are critical for the improvement of long-term survival, as well as to ensure an approximately 50% decrease in CRC incidence and mortality (33). Several approaches involving serum CEA (34), fecal occult blood tests (35,36), radiological examinations (such as CT and MRI) (37), and colonoscopy are commonly used to screen or diagnose CRC. However, serum CEA alone is not useful as a diagnostic marker with its low sensitivity and specificity (38). In addition, CT or MRI may miss some early tumor dissemination or micrometastases. Although colonoscopy remains the most effective method to screen and diagnose CRC, colonoscopy is the most invasive CRC screening method and is associated with adverse events such as bleeding and perforation (8 and 4 in 10,000 colonoscopies, respectively), and adverse cardiovascular events (4.9 in 1,000 colonoscopies) (39,40). Therefore, it is necessary to identify biomarkers that can be used for early diagnosis. In this study, we demonstrated the high accuracy of a novel CTC assay in the detection of CRC. CTC detection through FAST has 75% sensitivity in detecting cancer, which is comparable to the sensitivity of guideline-recommended screening tests: 62%–79% for guaiac-based fecal occult blood tests, 73%–88% for fecal immunochemistry alone, 92% for stool DNA plus fecal immunochemistry, and 75%–93% for colonoscopy (41). To our knowledge, this is the first report to demonstrate the high sensitivity and specificity values through CTC detection using FAST as an early diagnostic tool for CRC. This finding is important because there is still some reticence in the use of stool-based tests or invasive examinations such as colonoscopy. Therefore, CTC detection using FAST is advantageous for the early detection of CRC because it can be performed noninvasively and easily with high compliance at any time. In the future, we plan to validate the use of CTC testing using FAST for the early detection of CRC in the general population in Korea.
One of the most intriguing findings of our study is that only vascular invasion was associated with CTC positivity (P = 0.034). Vascular invasion and lymphatic invasion are generally evaluated and diagnosed as single events, i.e., as lymphovascular invasion. However, vascular invasion and lymphatic invasion in CRC are diagnosed and reported separately in our hospital. According to Fujii et al. (42), the advantage of such separate diagnoses is that vascular invasions have a stronger association with the development of visceral metastasis. Although vascular invasion is considered a valid prognostic factor for CRC, it is unclear whether it is associated with poor outcomes. Vascular invasion may be a result of overshooting angiogenesis; all modes of tumor angiogenesis include the shedding of cancer cells into the circulation in the form of CTCs (43). Metastasis is generally hypothesized to reflect that primary cancers locally invade the surrounding tissue through basement membranes and undergo a process known as EMT to achieve their invasive and migratory properties (44,45); after EMT, the cancer cells enter the peripheral blood by a process called intravasation (these cells are termed CTCs), survive during their translocation to the microvessels of distant tissues, exit from the bloodstream (extravasation), adapt to a favorable secondary site, and subsequently form tumors (46–48). The spread to anatomically distant sites appears to occur through the blood vessels, and the phenomenon in which cancer cells invade blood vessels is a critical step in metastasis (49,50). It is also important to note that, in this study, all patients with stage IV disease were positive for CTCs. This finding is consistent with the results of a recent meta-analysis, which stated that overt distant metastases indicate a more pronounced association between CTC detection and poor prognosis (10). These results suggest that the process of cancer metastasis is mediated through CTCs. In summary, in the presence of vascular invasion, the cancer can undergo hematogenous metastasis in the form of CTCs, which may be associated with poor prognoses.
The pathological TNM staging system established by the American Joint Committee on Cancer is widely used to predict the prognosis of patients with CRC (12). In recent times, many studies have indicated that the detection of CTCs in patients with CRC is of strong prognostic significance (29,51–53). However, for clinical applications, the prognostic utility of CTCs detected in patients with CRC has not yet been consistently determined (51,53–55). In addition, the detection methods vary across laboratories and the optimal cutoff value for CTCs has not yet been confirmed. In this study, we assessed the efficacy of CTCs detected by FAST as prognostic biomarkers by categorizing participants into 2 groups (CTC-positive and CTC-negative). OS and PFS of CTC-positive patients were inferior to those of CTC-negative patients (Figure 4), although the differences were not statistically significant. These results are consistent with those of another study that compared CTC positivity using the size-based platform (56). A recent meta-analysis that applied the CellSearch system used a cutoff value of CTCs of ≥3 per 7.5 mL of blood (57). Although our results indicated a cutoff value of ≥5 CTCs per 7.5 mL of blood, the optimal cutoff value defining CTC positivity using a size-based platform in patients with CRC remains unclear. There is a need for further studies with various cutoff values using a size-based platform to assess the prognostic usefulness of CTCs.
The present study had several limitations. First, FAST-based CTC detection has a potential limitation in the form of its sensitivity. CTCs that have undergone EMT do not express epithelial markers. However, the addition of CK-staining to the protocol overcame the shortage to detect CTCs without EpCAM expression. Second, the number of cases was relatively small for the use of FAST-based CTC detection in differentiating patients with CRC from healthy controls. Third, the markers used for detection of CTCs are mainly focused on detection of carcinoma and are not specific for CRC. To overcome this limitation, we excluded patients who had cancer in other organs. Furthermore, for the healthy control group, the 31 volunteers who agreed to participate in this study had no past or current history of medical illness. Also, during the study period, the volunteers had no specific findings in the national cancer screening program. Finally, because FAST-based CTC detection was performed only preoperatively and we did not check for changes after adjuvant therapy (not only chemotherapy alone but also chemoradiotherapy), CTC detection may not accurately reflect the long-term follow-up results as suggested by current guidelines. Although there are some limitations to this study, we found that a compact standalone instrument (FAST system) provides great potential for establishing a user-friendly, ultrafast, highly sensitive, and cost-effective CTC detection technology from the unprocessed whole blood of patients with CRC, which is critical for rare cell-based diagnosis and prediction of prognosis. Beyond immunostaining and counting, molecular analyses are increasingly being practiced in CTC characterization. The CTCs isolated by the FAST disc are not fixed but are instead alive, allowing them to be readily used for standard analyses, such as mutation analyses (e.g., epidermal growth factor receptor and RAS gene), which are particularly important for personalized therapy (e.g., cetuximab or bevacizumab).
In a high percentage (84.1%) of patients with CRC, preoperative detection of CTCs was possible with the use of FAST. In addition, FAST-based CTC detection correlated to vascular invasion but not to other clinicopathological variables in the patients with CRC. Finally, our study provides promising results for the use of CTCs as early diagnostic tools and prognostic biomarkers in patients with CRC. However, larger trial validation studies with longer follow-up periods are mandatory for the application of FAST-based CTC detection.
CONFLICTS OF INTEREST
Guarantor of the article: Gwang Ha Kim, MD, PhD.
Specific author contributions: D.H.B., G.H.K., H.S.K., Y.-K.C.: designed the study, analyzed clinical and CTCs data, and drafted the manuscript. H.J.J., I.S.H., and D.Y.P. revised clinical data. G.A.S., H.J.J., S.H.K., and E.Y.P. revised and helped to draft the manuscript. All authors approved the final manuscript.
Financial support: This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI12C1845).
Potential competing interests: The authors declare that they have no competing interests.
Study Highlights.
WHAT IS KNOWN
✓ Increasing evidence indicates that CTC detection in peripheral blood can act as a possible biomarker for cancer diagnosis and prognosis in clinical practice.
✓ Despite several studies reporting on CTC detection, the methodological aspects have not presented a clear appraisal of the clinical impact.
WHAT IS NEW HERE
✓ This work is the first report to demonstrate the high sensitivity and specificity values through CTC detection using FAST as an early diagnostic tool for CRC.
✓ CTC detection suggests prognostic value and may be a potential prognostic candidate for predicting overall and PFS for CRC.
TRANSLATIONAL IMPACT
✓ CTC detection using FAST could be used by clinical workers and other healthcare providers, which might greatly augment the ability of early diagnostic tools and prognostic biomarkers in colorectal patients.
ACKNOWLEDGEMENTS
We thank the Department of Biostatistics, Clinical Trial Center, Biomedical Research Institute, Pusan National University Hospital for their excellent assistance in the statistical analysis. The biospecimens and data used for this study were provided by the Biobank of Pusan National University Hospital, a member of the Korea Biobank Network.
REFERENCES
- 1.Global Burden of Disease Cancer C; Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold M, Laversanne M, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson U, Lasson A, Ekelund G. Recurrence rates after curative surgery for rectal carcinoma, with special reference to their accuracy. Dis Colon Rectum 1987;30:431–4. [DOI] [PubMed] [Google Scholar]
- 4.Galandiuk S, Wieand HS, Moertel CG, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet 1992;174:27–32. [PubMed] [Google Scholar]
- 5.Stipa S, Botti C, Cosimelli M, et al. Local recurrence after curative resection for colorectal cancer: frequency, risk factors and treatment. J Surg Oncol Suppl 1991;2:155–60. [DOI] [PubMed] [Google Scholar]
- 6.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 1869;14:146–7. [Google Scholar]
- 7.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 2007;253:180–204. [DOI] [PubMed] [Google Scholar]
- 8.Lee A, Park J, Lim M, et al. All-in-one centrifugal microfluidic device for size-selective circulating tumor cell isolation with high purity. Anal Chem 2014;86:11349–56. [DOI] [PubMed] [Google Scholar]
- 9.Kim TH, Lim M, Park J, et al. FAST: size-selective, clog-free isolation of rare cancer cells from whole blood at a liquid-liquid interface. Anal Chem 2017;89:1155–62. [DOI] [PubMed] [Google Scholar]
- 10.Rahbari NN, Thorlund K, Mollberg N, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 2010;138:1714–26. [DOI] [PubMed] [Google Scholar]
- 11.Liberko M, Kolostova K, Bobek V. Essentials of circulating tumor cells for clinical research and practice. Crit Rev Oncol Hematol 2013;88:338–56. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Compton CC. The American Joint Committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- 13.Kang HM, Jeon HK, Kim DH, et al. Circulating tumor cells detected by lab-on-a-disc: Role in early diagnosis of gastric cancer. PLoS One 2017;12:e0180251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lianidou ES, Markou A, Strati A. The Role of CTCs as tumor biomarkers. Adv Exp Med Biol 2015;867:341–67. [DOI] [PubMed] [Google Scholar]
- 15.Witzig TE, Kimlinger T, Roche PC, et al. Detection of circulating cytokeratin-positive cells in the blood of breast cancer patients using immunomagnetic enrichment and digital microscopy. Clin Cancer Res 2002;8:1085–91. [PubMed] [Google Scholar]
- 16.Steinert G, Scholch S, Koch M, et al. Biology and significance of circulating and disseminated tumour cells in colorectal cancer. Langenbeck's Arch Surg 2012;397:535–42. [DOI] [PubMed] [Google Scholar]
- 17.Thorsteinsson M, Jess P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer: A review. Eur J Surg Oncol 2011;37:459–65. [DOI] [PubMed] [Google Scholar]
- 18.Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods 2010;50:289–97. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix M. Significance, detection and markers of disseminated breast cancer cells. Endocr Relat Cancer 2006;13:1033–67. [DOI] [PubMed] [Google Scholar]
- 20.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 2008;8:329–40. [DOI] [PubMed] [Google Scholar]
- 21.Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancer: Clinical applications. Nat Rev Clin Oncol 2010;7:693–701. [DOI] [PubMed] [Google Scholar]
- 22.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol 2009;6:339–51. [DOI] [PubMed] [Google Scholar]
- 23.CELLSEARCH® Circulating Tumor Cell Kit (Epithelial) Instructions for Use. Janssen Diagnostics, LLC; 2014. https://www.cellsearchctc.com. Accessed April 20, 2014. [Google Scholar]
- 24.Giordano A, Anfossi S, Cohen E, et al. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther 2012;11:2526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorges TM, Drosch M, Röse L, et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012;12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farace F, Vimond N, Drusch F, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer 2011;105:847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofman VJ, Bonnetaud C, Selva E, et al. Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol 2011;135:146–56. [DOI] [PubMed] [Google Scholar]
- 28.De Giorgi V, Salvianti F, Panelos J, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol 2010;130:2440–7. [DOI] [PubMed] [Google Scholar]
- 29.Bork U, Schölch S, Reissfelder C, et al. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer 2015;112:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sastre J, Maestro ML, Puente J, et al. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol 2008;19:935–8. [DOI] [PubMed] [Google Scholar]
- 31.van Dalum G, Scholten LF, Mastboom WJ, et al. Importance of circulating tumor cells in newly diagnosed colorectal cancer. Int J Oncol 2015;46:1361–8. [DOI] [PubMed] [Google Scholar]
- 32.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst 2009;101:1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welch HG, Robertson DJ. Colorectal cancer on the decline: Why screening can't explain it all. N Engl J Med 2016;374:1605–7. [DOI] [PubMed] [Google Scholar]
- 34.Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, et al. Cancer antigens (CEA and CA 19-9) as markers of advanced stage of colorectal carcinoma. Med Arch 2013;67:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 36.Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging 2016;11:967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Wu H, Guan YS. Colonography by CT, MRI and PET/CT combined with conventional colonoscopy in colorectal cancer screening and staging. World J Gastroenterol 2008;14:853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 2005;5:845–56. [DOI] [PubMed] [Google Scholar]
- 39.Force USPST; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 40.Lin JS, Perdue LA, Rutter CM, et al. Screening for colorectal cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;315:2576–94. [DOI] [PubMed] [Google Scholar]
- 41.Choi Y, Sateia HF, Peairs KS, et al. Screening for colorectal cancer. Semin Oncol 2017;44:34–44. [DOI] [PubMed] [Google Scholar]
- 42.Fujii T, Sutoh T, Morita H, et al. Vascular invasion, but not lymphatic invasion, of the primary tumor is a strong prognostic factor in patients with colorectal cancer. Anticancer Res 2014;34:3147–51. [PubMed] [Google Scholar]
- 43.Arslan B, Hazar İ, Aydın M, et al. Prognostic value of endocan in prostate cancer: Clinicopathologic association between serum endocan levels and biochemical recurrence after radical prostatectomy. Tumori 2017;103:204–8. [DOI] [PubMed] [Google Scholar]
- 44.Schroeder A, Winslow MM, Dahlman JE, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer 2011;12:39–50. [DOI] [PubMed] [Google Scholar]
- 45.Mocellin S, Keilholz U, Rossi CR, et al. Circulating tumor cells: The ‘leukemic phase’ of solid cancers. Trends Mol Med 2006;12:130–9. [DOI] [PubMed] [Google Scholar]
- 46.Thiery JP, Lim CT. Tumor dissemination: an EMT affair. Cancer Cell 2013;23:272–3. [DOI] [PubMed] [Google Scholar]
- 47.Satelli A, Brownlee Z, Xia X, et al. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin Cancer Res 2015;21:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cristofanilli M, Ellis MJ, Stopeck A, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781–91. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 50.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563–72. [DOI] [PubMed] [Google Scholar]
- 51.Cohen SJ, Iannotti N, Saidman BH, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213–21. [DOI] [PubMed] [Google Scholar]
- 52.Aggarwal C, Punt CJ, Iannotti N, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol 2013;24:420–8. [DOI] [PubMed] [Google Scholar]
- 53.Denève E, Ramos J, Nocca D, et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem 2013;59:1384–92. [DOI] [PubMed] [Google Scholar]
- 54.Kuboki Y, Minowa S, Shibata H, et al. Circulating tumor cell (CTC) count and epithelial growth factor receptor expression on CTCs as biomarkers for cetuximab efficacy in advanced colorectal cancer. Anticancer Res 2013;33:3905–10. [PubMed] [Google Scholar]
- 55.Hiraiwa K, Hasegawa H, Saikawa Y, et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol 2008;15:3092–100. [DOI] [PubMed] [Google Scholar]
- 56.Oh BY, Kim J, Lee WY, et al. A new size-based platform for circulating tumor cell detection in colorectal cancer patients. Clin Colorectal Cancer 2017;16:214–9. [DOI] [PubMed] [Google Scholar]
- 57.Huang X, Song Y, Sun J, et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]