OBJECTIVES:
Despite overall reductions in colorectal cancer burden, incidence rates continue to rise among younger patients, and causes remain unknown. We examined differences in clinicopathologic and racial/ethnic characteristics within the adolescent and young adult (AYA) population diagnosed with colorectal cancer in the United States.
METHODS:
Using the National Cancer Institute's Surveillance, Epidemiology, and End Results program data, we identified individuals diagnosed with first primary colorectal cancer between ages 15 and 39 years from 2010 to 2015. Adjusted multivariable logistic regression models were used to quantify clinicopathologic and racial/ethnic differences across age at onset subgroups (15–19, 20–24, 25–29, 30–34, and 35–39 years).
RESULTS:
We identified 5,350 AYA patients diagnosed with colorectal cancer. Of note, 28.6% of AYA cases were diagnosed with right-sided tumors (cecum to transverse colon). The proportion of right-sided colorectal cancers differed significantly by age group at diagnosis (38.3% vs 27.3% of AYAs aged 15–19 vs 35–39 years, respectively; P trend = 0.01). Proportions of cases with mucinous adenocarcinoma and signet ring cell carcinoma histopathologic subtypes significantly increased with younger age at onset (P trends = 0.01 and 0.03, respectively). Differences in clinical stage were observed across AYA age groups, with stage II disease increasing with younger age (P trend = 0.01). The proportion of Hispanic AYAs was higher within younger patients, accounting for 21.0% of the AYA population aged 35–39 years up to 28.3% of 15–19-year-old individuals (P trend = 0.003).
DISCUSSION:
Within the AYA population, colorectal cancers differ by clinicopathologic and racial/ethnic characteristics. Further investigation of the clinical and biologic diversity of colorectal cancers that partially underlie age- and race-related differences in cancer susceptibility and outcomes is warranted.
INTRODUCTION
Colorectal cancer will affect 1 in 22 men and 1 in 24 women in their lifetime (1). Despite overall reductions in incidence over the past 3 decades, colorectal cancer remains the third leading cause of cancer-related deaths among men and women in the United States (1). Notably, colorectal cancer incidence rates have been increasing among young individuals (aged <50 years at diagnosis), as those born circa 1990 are estimated to have double the risk of colon cancer and quadruple the risk of rectal cancer compared with their similarly aged counterparts born circa 1950 (2). The underlying causes of this alarming increase remain unknown, although they may be due to the environment (e.g., diet and physical activity) or reflect gene-environment interactions (2,3).
Although long-term projections suggest that by 2030, 11% of colon cancers and 23% of rectal cancers in the United States will occur among individuals younger than 50 years (4), information on the colorectal cancer burden in this population remains limited. Nearly one quarter of young-onset colorectal cancer cases are diagnosed in individuals younger than 40 years (5). Furthermore, individuals aged 15–39 years at cancer diagnosis—the adolescent and young adult (AYA) population—differ from individuals diagnosed at a later age. Although cases of young-onset colorectal cancer more often have a familial component compared with older counterparts, 5 of every 6 patients with colorectal cancer younger than 50 years do not carry a germline mutation associated with cancer predisposition (6–9). Several studies have also suggested that AYAs experience more aggressive disease (10,11). In contrast, AYAs may have better survival outcomes relative to older counterparts diagnosed with colorectal cancer (12–15). Potential explanations for this survival disparity include underlying differences in tumor biology by patient race/ethnicity (5) or a lesser burden of other comorbidities in AYAs compared with older individuals (16). A 2014 population-based study by Li et al. (17) reported age-related patterns in histopathologic subtype, stage, and disease-specific survival among patients aged 18–40 years at colorectal cancer diagnosis. However, it is important to note that this study was limited to operable, nonmetastatic colon cancer cases and did not evaluate patterns by patient ethnicity or tumor site. No studies to date have examined age-related differences in clinicopathologic and racial/ethnic patterns that may underlie the increasing incidence and burden of colorectal cancer restricted to the AYA population. Furthermore, the small number of AYA patients included in previous studies (18,19) has made it challenging to evaluate subgroups and draw conclusions about the biology of malignancies among individuals within this population (10).
The study of AYA patients diagnosed with colorectal cancer provides an opportunity to examine differences in clinical, pathologic, and racial/ethnic features by age at diagnosis in a population with unique clinical care needs even within their own age group (20) and low accrual rates to clinical trials (21,22). Thus, the purpose of our study was to investigate the clinicopathologic and racial/ethnic patterns of colorectal cancers within the broad population of AYAs, individuals aged 15–39 years at colorectal cancer diagnosis, using data from the population-based Surveillance, Epidemiology, and End Results (SEER) program.
METHODS
Data sources and case selection
Data were obtained from the National Cancer Institute's SEER program. The SEER program collects data on cancer incidence and mortality from 18 population-based cancer registries that cover approximately 28% of the United States population (23).
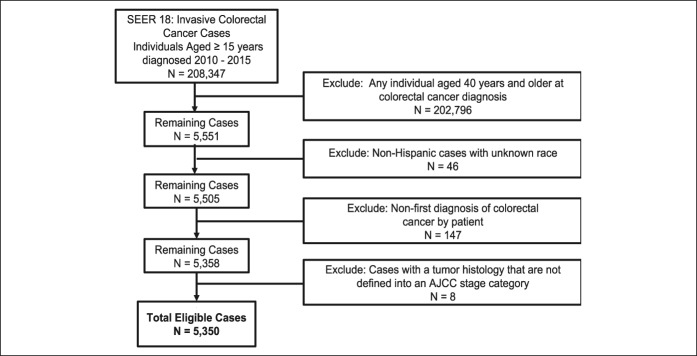
A case listing session in SEER*Stat was run on the SEER 18 incidence data set (24) to obtain demographic and clinical characteristics on colorectal cancers in the AYA population aged 15–39 years at colorectal cancer diagnosis. We restricted our analysis to cases diagnosed during the years 2010–2015 with known age and race/ethnicity classified as non-Hispanic white (NHW), non-Hispanic African American or black (NHB), Hispanic, Asian or Pacific Islander, or American Indian or Alaskan Native. Subjects whose race was coded as unknown were not included in this analysis because of the small sample size (n = 46 cases excluded) (Figure 1). Cases were restricted to first colorectal cancer diagnosis per patient (n = 147 cases excluded). Histopathologic subtypes that the American Joint Commission of Cancer (AJCC) does not define stage for in these groups were not included in this analysis (n = 8 cases excluded). Our final cohort consisted of 5,350 patients aged 15–39 years at first colorectal cancer diagnosis (Figure 1).
Figure 1.
Composition of the study population with exclusion criteria. AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology, and End Results.
Clinical and demographic variables examined by age at diagnosis included patient sex, race/ethnicity, AJCC clinical stage, and histopathologic subtype. Tumor sidedness was grouped by primary site, where C18.0-C18.4 (cecum to transverse colon) was categorized as right-sided colorectal cancer, and C18.5-C18.9, C19.9, C20.9, and C26.0 (splenic flexure to rectum) was categorized as left-sided colorectal cancer per National Comprehensive Cancer Network clinical guidelines (25). We also classified cases by insurance status as available in SEER (privately insured, Medicaid, and uninsured). Follow-up for each case is current through December 31, 2015.
Statistical analysis
To assess patterns of colorectal cancer presentation among adolescents and young adults, we compared differences in clinicopathologic and demographic characteristics by age at disease onset group (15–19, 20–24, 25–29, 30–34, and 35–39 years) (26) and by race/ethnicity among age at disease onset groups using χ2 tests for categorical variables and analysis of variance for continuous variables. Cochran-Armitage trend tests were used to assess trends in the distribution of clinicopathologic and demographic characteristics by age at disease onset groups and by race/ethnicity.
Multivariable logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) to quantify associations between clinicopathologic/demographic factors and age group at colorectal cancer diagnosis as the outcome, where the reference outcome category was individuals aged 35–39 years at diagnosis. Associations between clinicopathologic/demographic factors and age group were assessed in adjusted models that included patient sex, race/ethnicity, AJCC clinical stage, tumor sidedness, histopathologic subtype, and insurance status, based on patients having complete information for these covariates. All data were analyzed using SAS version 9.4 statistical software (SAS Institute, Cary, NC). All tests were 2 sided, and P < 0.05 was considered statistically significant.
RESULTS
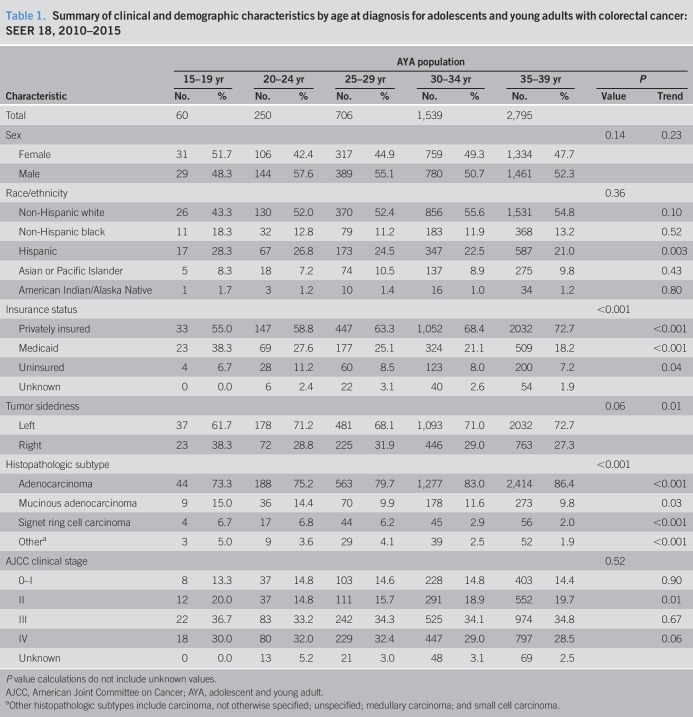
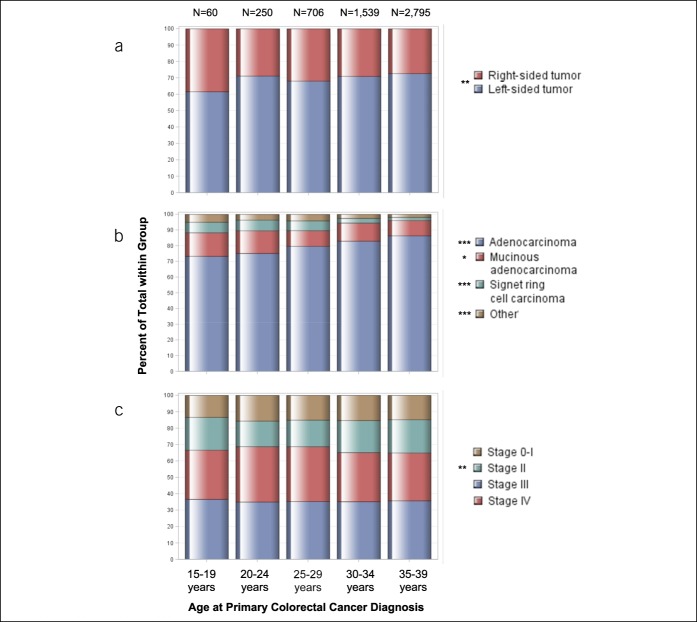
A total of 5,350 colorectal cancers diagnosed among individuals aged 15–39 years were identified from SEER over the study period (Table 1). The distribution of colorectal cancer location, histopathologic subtype, and AJCC clinical stage by age at onset group is illustrated in Figure 2. Overall, 28.6% of colorectal tumors (1,529 of 5,350 cases) were right sided (cecum to transverse colon). The proportion of right-sided colorectal cancers differed significantly by age group at diagnosis. Younger age was statistically significantly associated with increasing proportion of right-sided compared with left-sided tumors (38.3% vs 27.3% of AYAs aged 15–19 vs 35–39 years, respectively; P trend = 0.01). Median age at colorectal cancer diagnosis differed moderately by anatomical location, as AYAs with right-sided tumors were diagnosed at younger ages compared with AYAs with left-sided tumors (34.0 vs 35.0 years, respectively; P = 0.02). Histopathologic subtype of colorectal cancer also differed by age at onset within the AYA population. Similar to trends reported by Li et al. (17) among young patients with operable, nonmetastatic colorectal cancer, the proportion of signet ring cell carcinomas, a histopathologic subtype more frequently associated with sporadic young-onset colorectal cancers (11,27), and mucinous adenocarcinomas was higher in younger age groups (P trend = 0.03 and P trend < 0.001, respectively). There were no statistically significant differences in AJCC clinical stage between AYA age groups (P = 0.52), although the proportion of AJCC stage II colorectal cancers was found to be the highest among individuals aged 15–19 years and 35–39 years (20.0% and 19.7% of cases, respectively; P trend = 0.01) (Table 1 and Figure 2).
Table 1.
Summary of clinical and demographic characteristics by age at diagnosis for adolescents and young adults with colorectal cancer: SEER 18, 2010–2015
Figure 2.
Age patterns by colorectal cancer clinicopathologic characteristics among adolescents and young adults. Proportions of adolescents and young adults, grouped by age at diagnosis, by (a) colorectal tumor location, (b) histopathologic subtype, and (c) American Joint Committee on Cancer clinical stage. Other histopathologic subtype includes carcinoma, not otherwise specified; unspecified; medullary carcinoma; and small cell carcinoma. *P trend < 0.05; **P trend ≤ 0.01; ***P trend ≤ 0.001.
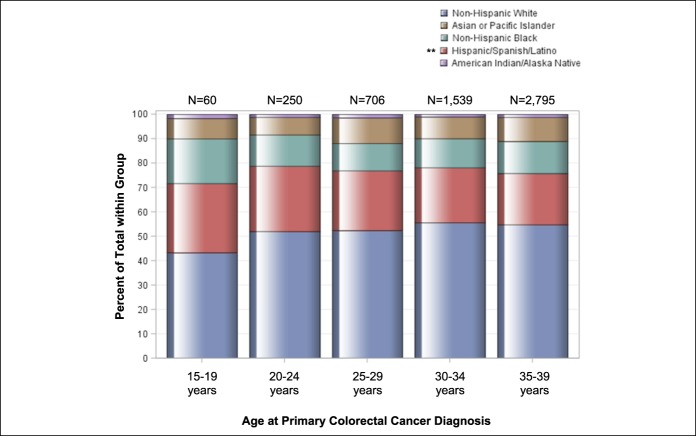
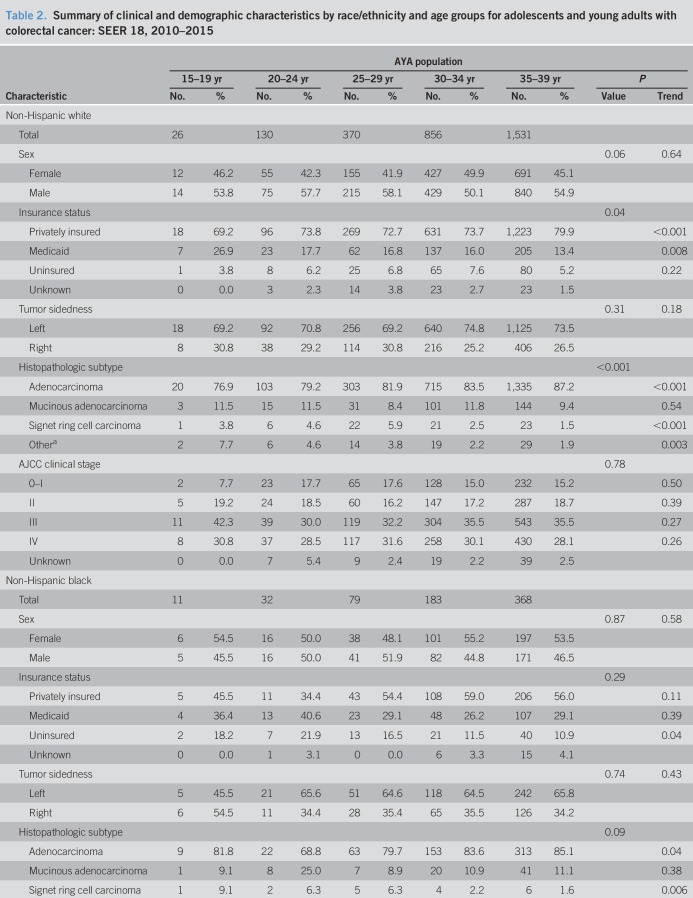
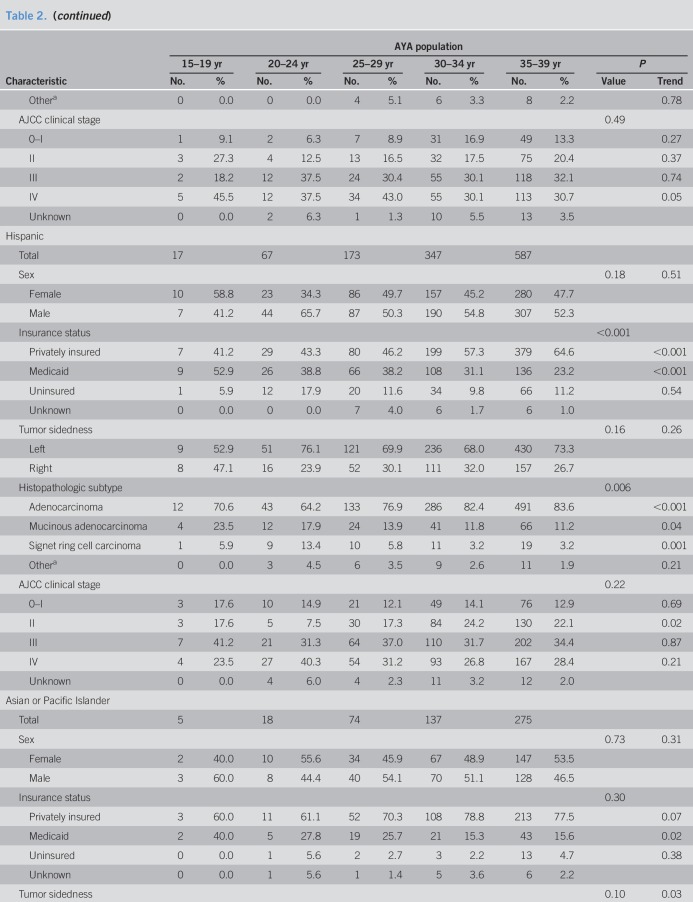
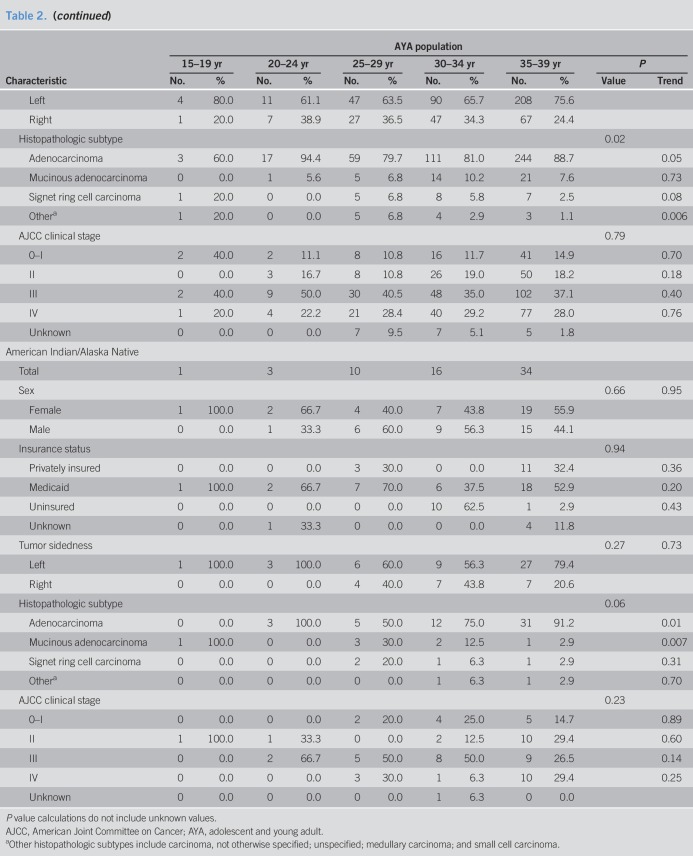
Mean age of AYAs at colorectal cancer diagnosis was 33.6 years (SD, 4.8 years). Men comprised 52.4% of the AYA population (2,803 of 5,350 cases). Racial/ethnic patterns across AYA age groups revealed most individuals self-identified as NHW (Table 1). The proportion of Hispanic individuals was higher in younger age groups (P trend = 0.003)—accounting for 21.0% of the AYA population aged 35–39 years to 28.3% of individuals aged 15 to 19 years (Table 1 and Figure 3)—in concordance with earlier findings by Holowatyj et al. (5) that among all-comers aged <50 years at diagnosis, Hispanic individuals were diagnosed with colorectal cancer at a younger age compared with NHWs or non-Hispanic blacks (NHBs). Given the pronounced racial disparities in young-onset colorectal cancer, patterns of clinicopathologic features by race/ethnicity within AYA age groups are described in Table 2.
Figure 3.
Age patterns by race/ethnicity among adolescents and young adults. Proportions of adolescents and young adults, grouped by age at diagnosis and by race/ethnicity. *P trend < 0.05; **P trend ≤ 0.01; ***P trend ≤ 0.001.
Table 2.
Summary of clinical and demographic characteristics by race/ethnicity and age groups for adolescents and young adults with colorectal cancer: SEER 18, 2010–2015
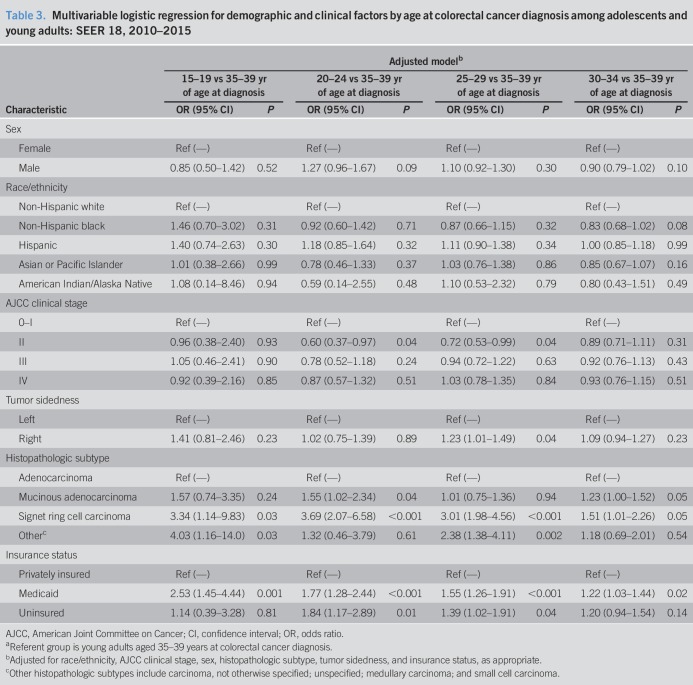
To evaluate associations between clinicopathologic and demographic characteristics and age groups at colorectal cancer diagnosis within the AYA population, we modeled adjusted multivariable logistic regressions (Table 3). Individuals aged 25–29 years at colorectal cancer diagnosis were 23% more likely to be diagnosed with a right-sided tumor (OR = 1.23, 95% CI 1.01–1.49, P = 0.04) or have signet ring cell carcinoma histopathology (OR = 3.01, 95% CI 1.98–4.56, P < 0.001) compared with 35- to 39-year-old counterparts after adjustment for patient sex, race/ethnicity, AJCC stage, and individual insurance status. Similarly, individuals aged 20–24 years were also 3.69-fold more likely to be diagnosed with colorectal cancer with signet ring cell carcinoma histopathology (OR = 3.69, 95% CI 2.08–6.58, P < 0.001), as well as 55% more likely to be diagnosed with mucinous adenocarcinoma (OR = 1.55, 95% CI 1.02–2.34, P = 0.04) histopathology as compared to individuals aged 35–39 years at diagnosis. Individuals aged 20–24 and 25–29 years at colorectal cancer diagnosis were less likely to be diagnosed with AJCC stage II disease (OR = 0.60, 95% CI 0.37–0.97, P = 0.04, and OR = 0.72, 95% CI 0.52–0.99, P = 0.04, respectively) compared with 35- to 39-year-old counterparts (Table 3).
Table 3.
Multivariable logistic regression for demographic and clinical factors by age at colorectal cancer diagnosis among adolescents and young adults: SEER 18, 2010–2015
DISCUSSION
Our investigation of 5,350 individuals diagnosed with colorectal cancer between ages 15 and 39 years in the United States from 2010 to 2015 identified clinicopathologic and racial/ethnic differences of colorectal cancers by age at disease onset. We observed that among AYAs, primary colorectal cancers diagnosed in younger individuals have a significantly higher propensity for right-sided location and for mucinous adenocarcinoma and signet ring cell carcinoma histopathologic subtypes. Differences in tumor stage was also observed across AYA age groups, particularly for AJCC stage II disease. Furthermore, the proportion of Hispanic individuals diagnosed with colorectal cancer was higher in younger age groups. These findings are novel, as our study is the first to stratify the population of AYAs into more specific age groups to characterize the clinicopathologic and racial/ethnic features of colorectal cancer by age at diagnosis within this population.
Tumor sidedness has recently emerged as a prognostic indicator for patients with colorectal cancer, particularly among those with metastatic disease. Tumors located in the cecum to transverse colon (right side) are associated with faster disease progression, poorer prognosis, and distinct pathways to tumorigenesis in contrast to left-sided tumors (splenic flexure to rectum) (28–30). However, conflicting evidence on the prognostic value of tumor sidedness has been reported among all-comers diagnosed with early-stage cancers of the colon and rectum (31–33). Differences in patient outcomes by tumor sidedness are partly attributed to a continuum of molecular variations associated with anatomical location in the colorectum (34,35), variations in therapeutic efficacy (36,37), and lead time bias for left-sided colorectal cancers (38,39). In the last decade, studies from single institutions have found that colorectal cancer cases diagnosed in individuals younger than 40 years tended to present with left-sided tumors (e.g., distal colon) more often compared with older counterparts (11,40). In 2016, Teng et al. (18) summarized the presentation of colorectal cancers among AYAs as a single group, finding that most individuals presented with distal tumors.
Unique to our study, we focused on age groups specifically within the AYA population to compare clinicopathologic characteristics of colorectal cancer, including tumor sidedness per National Comprehensive Cancer Network clinical guidelines. Consistent with earlier findings, we also observed that most AYAs presented with left-sided tumors using data from the population-based national SEER program. Strikingly, within this population, we identified heterogeneity in histopathologic subtype, clinical stage, and tumor location among AYAs, including increasing trends in right-sided colorectal cancer among cases diagnosed at younger age (25–29 years) compared with older counterparts (aged 35–39 year). Potential explanations for the clinicopathologic heterogeneity observed by age at onset groups among AYAs include differences in the prevalence of Lynch syndrome by age at onset groups, as Pearlman et al. (9) recently demonstrated that among patients diagnosed with young-onset colorectal cancer, the prevalence of Lynch syndrome was higher among patients aged 40–49 years at diagnosis compared with 30- to 39-year-old, and especially 20- to 29-year-old, counterparts. Additional reasons for differences in tumor sidedness and clinicopathologic characteristics among AYAs remain unknown, but could include a combination of genetic predispositions and biological factors (10) such as mutational burden (e.g., KRAS and BRAF), environmental exposures, and race/ethnicity.
Among individuals diagnosed with colorectal cancer younger than 50 years, racial and ethnic disparities in colorectal cancer outcomes are amplified (5). Specifically within the AYA population, we identified racial/ethnic patterns by age of colorectal cancer diagnosis. The proportion of Hispanic AYAs diagnosed with colorectal cancer was strikingly higher among younger age groups. A recent examination of ethnic differences in colorectal cancer incidence and mortality among all-comers at a tertiary university center noted that Hispanics presented with colorectal cancer at a younger age and with more advanced disease compared with NHWs (41). However, similar estimates of mismatch repair (MMR)-deficient tumors have been reported among Latinos and NHWs (42). Stern et al. (43) reported differences in colorectal cancer incidence patterns and tumor characteristics among Latino subpopulations by country of origin, suggesting that genetic ancestral heritage, lifestyle changes, environmental risk factors, and gene-environment interactions may contribute to this ethnic heterogeneity. Parental exposures and epigenetic mechanisms may also play a role for cases in the youngest age groups. Together with the changing landscape of age and race/ethnicity across the United States—where Hispanics are a growing population expected to represent nearly one-third of the population by 2060 (44)—further studies of the clinicopathologic characteristics and cultural, epigenetic, lifestyle, and environmental factors associated with age of colorectal cancer onset by race/ethnicity are warranted.
Although our findings suggest that there are clinicopathologic and racial/ethnic differences in colorectal cancer among AYAs, we acknowledge the limitations of our study. Our analyses were conducted using high-quality data from SEER that allowed for a large number of pathologically verified colorectal cancer cases among AYAs to be identified. SEER does not yet routinely collect data on specific and clinically relevant colorectal cancer molecular characteristics (e.g., somatic mutations and microsatellite instability) or detailed information on familial history of disease. As such, we were unable to separate sporadic vs hereditary cases of colorectal cancer among AYAs in our analyses. As colorectal cancer diagnosis before age 50 years remains one of the primary indications for genetic evaluation (25), the prevalence of genetic mutations associated with hereditary cancer syndromes is higher among patients diagnosed with young-onset colorectal cancer (9). Germline mutations in DNA MMR genes associated with Lynch syndrome, the most common cause of hereditary colorectal cancer (7), predispose to hypermutated cancers with a general predominance for right-sided tumors, mucinous histology, and disease presentation at a younger age (8). With increased use of universal microsatellite instability/MMR screening (45), a larger proportion of colorectal cancers with hereditary etiology may be captured within this study population. We were unable to examine differences in environmental exposures, behavioral factors, and comorbidities by age group and to discern whether these factors contribute to differences in the clinicopathologic and racial/ethnic characteristics of colorectal cancers within the AYA population. We also acknowledge the possibility that differences in racial/ethnic age structures could also contribute to our findings. Thus, our findings raise the possibility that colorectal cancer diversity among AYAs may partially underlie age- and race-related differences in cancer susceptibility and outcomes. However, our study does not present any data about the spectrum of germline genetic mutations nor the prevalence of hereditary syndromes, which contribute to the molecular phenotypes of colorectal cancer.
Notwithstanding these limitations, our findings yield important clinical implications. With advancements in therapies leading to an overall reduction in cancer burden, a better understanding of the molecular complexities of young-onset colorectal cancer by clinicopathologic features remains a prerequisite to the optimization of therapies and to meet the unique clinical care needs of the AYA population (22,46). Equal-access, long-term side effects of overtreatment on AYA fertility and therapies designed for patients older than 50 years present a clinical environment mismatch for AYAs (12,13,47–50). Additional research is needed to guide the development of colorectal cancer screening programs targeting high-risk young individuals. Increasing the representation and participation of AYAs in clinical trials (46,51) is also needed for the advancement of therapies and treatment algorithms designed for young individuals.
In summary, we observed clinicopathologic, particularly in tumor location, histopathologic subtype, clinical stage, and racial/ethnic differences of colorectal cancers among individuals aged 15–39 years at disease diagnosis. Younger age at disease diagnosis was associated with higher proportions of right-sided tumors and mucinous adenocarcinoma and signet ring cell carcinoma histopathologic subtypes within the AYA population. By race/ethnicity, the proportion of Hispanic individuals diagnosed with colorectal cancer significantly increased with younger age groups. Together, these data underscore the need for studies to characterize the molecular phenotype of colorectal cancers, particularly among AYAs, to unravel the underlying colorectal cancer etiologies within this population and reduce the global burden of cancer for AYAs (48).
CONFLICTS OF INTEREST
Guarantor of the article: Andreana N. Holowatyj, PhD, MS.
Specific author contributions: A.N.H. and C.M.U. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A.N.H, M.A.L., and C.M.U. contributed to the conception and design of the study. All authors participated in the acquisition, analysis, and interpretation of data and drafting and critical revision of the manuscript for important intellectual content. A.N.H. participated in creating figures and in the statistical analysis. A.N.H. and C.M.U. obtained funding and provided support and supervision for this study.
Financial support: A.N.H. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HG008962 from the National Human Genome Research Institute. This work was also supported by grants from the National Institutes of Health/National Cancer Institute (R01 CA189184 and R01 CA207371 to C.M.U.; K07 CA222060 to S.H.; and U01 CA206110 to C.M.U. and J.C.F.) and the Huntsman Cancer Foundation. The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P30 CA042014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential competing interests: The authors declare no conflicts of interest with this work. C.M.U. has as cancer center director oversight over research funded by several pharmaceutical companies, but has not received funding directly herself.
Study Highlights.
WHAT IS KNOWN
✓ Colorectal cancer incidence rates have been increasing among young individuals, including among the AYA population (individuals aged 15–39 years at diagnosis).
✓ The underlying causes of this alarming increase in young-onset colorectal cancer remain unknown.
WHAT IS NEW HERE
✓ Among AYAs, primary colorectal cancers diagnosed in younger individuals have a significantly higher propensity for right-sided tumor location.
✓ The proportion of mucinous adenocarcinoma and signet ring cell carcinoma histopathologic subtypes was higher in younger AYA age groups.
✓ Differences in tumor stage were observed across AYA age groups, particularly for AJCC stage II disease.
✓ The proportion of Hispanic individuals diagnosed with colorectal cancer was higher in younger age groups.
TRANSLATIONAL IMPACT
✓ Understanding the molecular complexities of young-onset colorectal cancer by clinicopathologic features is a prerequisite to optimize clinical therapies and to meet unique clinical care needs of the AYA population.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer JE, Narang T, Schnoll-Sussman FH, et al. Increasing incidence of rectal cancer in patients aged younger than 40 years: An analysis of the surveillance, epidemiology, and end results database. Cancer 2010;116(18):4354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holowatyj AN, Ruterbusch JJ, Rozek LS, et al. Racial/ethnic disparities in survival among patients with young-onset colorectal cancer. J Clin Oncol 2016;34(18):2148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silla IO, Rueda D, Rodriguez Y, et al. Early-onset colorectal cancer: A separate subset of colorectal cancer. World J Gastroenterol 2014;20(46):17288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerber RA, Neklason DW, Samowitz WS, et al. Frequency of familial colon cancer and hereditary nonpolyposis colorectal cancer (Lynch syndrome) in a large population database. Fam Cancer 2005;4(3):239–44. [DOI] [PubMed] [Google Scholar]
- 8.Stoffel EM, Koeppe E, Everett J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology 2018;154:897–905,e891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol 2017;3(4):464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer 2008;8(4):288–98. [DOI] [PubMed] [Google Scholar]
- 11.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: An adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 2012;25(8):1128–39. [DOI] [PubMed] [Google Scholar]
- 12.Quah HM, Joseph R, Schrag D, et al. Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol 2007;14(10):2759–65. [DOI] [PubMed] [Google Scholar]
- 13.Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: More intense treatments with unmatched survival gains. JAMA Surg 2015;150(5):402–9. [DOI] [PubMed] [Google Scholar]
- 14.McMillan DC, McArdle CS. The impact of young age on cancer-specific and non-cancer-related survival after surgery for colorectal cancer: 10-year follow-up. Br J Cancer 2009;101(4):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer 2016;122(6):929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnett-Hartman A, Powers JD, Chubak J, et al. Tumor Characteristics and Treatment in Early-Onset Colorectal cancer. ASCO 2018 Gastrointestinal Cancers Symposium; 2018; San Francisco, California.
- 17.Li Q, Zhuo C, Cai G, et al. Pathological features and survival outcomes of young patients with operable colon cancer: Are they homogeneous? PLoS One 2014;9(7):e102004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng A, Lee DY, Cai J, et al. Patterns and outcomes of colorectal cancer in adolescents and young adults. J Surg Res 2016;205(1):19–27. [DOI] [PubMed] [Google Scholar]
- 19.Datta RV, LaQuaglia MP, Paty PB. Genetic and phenotypic correlates of colorectal cancer in young patients. N Engl J Med 2000;342(2):137–8. [DOI] [PubMed] [Google Scholar]
- 20.Pannier ST, Warner EL, Fowler B, et al. Age-specific patient navigation preferences among adolescents and young adults with cancer. J Cancer Educ 2019;34(2):242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanford SD, Beaumont JL, Snyder MA, et al. Clinical research participation among adolescent and young adults at an NCI-designated Comprehensive Cancer Center and affiliated pediatric hospital. Support Care Cancer 2017;25(5):1579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adolescent and Young Adult Oncology Progress Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, and the LiveStrong Young Adult Alliance; https://www.cancer.gov/types/aya/research/ayao-august-2006.pdf. Accessed on March 3, 2019. [Google Scholar]
- 23.Noone AM, Howlader N, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975-2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 24.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov). SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Novomber 2017 Sub (1973-2015 varying)—Linked To County Attributes—Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
- 25.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Colon Cancer. Version 2. Plymouth Meeting, Pennsylvania: National Comprehensive Cancer Network; 2017. [Google Scholar]
- 26.World Population Monitoring Adolescents and Youth: A Concise Report . New York, NY: Department of Economic and Social Affairs, United Nations Secretariat; 2012. [Google Scholar]
- 27.Kam MH, Eu KW, Barben CP, et al. Colorectal cancer in the young: A 12-year review of patients 30 years or less. Colorectal Dis 2004;6(3):191–4. [DOI] [PubMed] [Google Scholar]
- 28.Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 2018;33(1):125–36 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meguid RA, Slidell MB, Wolfgang CL, et al. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol 2008;15(9):2388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncol 2017;3(2):211–9. [DOI] [PubMed] [Google Scholar]
- 31.Karim S, Brennan K, Nanji S, et al. Association between prognosis and tumor laterality in early-stage colon cancer. JAMA Oncol 2017;3(10):1386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss JM, Pfau PR, O'Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: Analysis of surveillance, epidemiology, and end results—Medicare data. J Clin Oncol 2011;29(33):4401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warschkow R, Sulz MC, Marti L, et al. Better survival in right-sided versus left-sided stage I–III colon cancer patients. BMC Cancer 2016;16:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delattre O, Olschwang S, Law DJ, et al. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet 1989;2(8659):353–6. [DOI] [PubMed] [Google Scholar]
- 35.Kim SR, Song N, Yothers G, et al. Tumour sidedness and intrinsic subtypes in patients with stage II/III colon cancer: Analysis of NSABP C-07 (NRG oncology). Br J Cancer 2018;118(5):629–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boeckx N, Koukakis R, Op de Beeck K, et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: Results from two randomized first-line panitumumab studies. Ann Oncol 2017;28(8):1862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28(8):1713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrag D, Weng S, Brooks G, et al. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol 2016;34(15_Suppl):3505. [Google Scholar]
- 39.Salem ME, Weinberg BA, Xiu J, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017;8(49):86356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yantiss RK, Goodarzi M, Zhou XK, et al. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol 2009;33(4):572–82. [DOI] [PubMed] [Google Scholar]
- 41.Stefanidis D, Pollock BH, Miranda J, et al. Colorectal cancer in Hispanics: A population at risk for earlier onset, advanced disease, and decreased survival. Am J Clin Oncol 2006;29(2):123–6. [DOI] [PubMed] [Google Scholar]
- 42.Ricker CN, Hanna DL, Peng C, et al. DNA mismatch repair deficiency and hereditary syndromes in Latino patients with colorectal cancer. Cancer 2017;123(19):3732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stern MC, Zhang J, Lee E, et al. Disparities in colorectal cancer incidence among Latino subpopulations in California defined by country of origin. Cancer Causes Control 2016;27(2):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos . Vol 2012 American Cancer Society: Atlanta, 2012. [Google Scholar]
- 45.Evaluation of Genomic Applications in Practice Prevention Working Group. Recommendations from the EGAPP working group: Genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 2009;11(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bleyer A. Adolescents and young adult cancer trial participation: Will the national community oncology research program also fail? And what about the rest of us? J Oncol Pract 2016;12(5):398–402. [DOI] [PubMed] [Google Scholar]
- 47.Kolarich A, George TJ, Jr., Hughes SJ, et al. Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline-directed treatment for stage II and III disease. Cancer 2018;124(17):3510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bleyer A, Ferrari A, Whelan J, et al. Global assessment of cancer incidence and survival in adolescents and young adults. Pediatr Blood Cancer 2017;64(9). [DOI] [PubMed] [Google Scholar]
- 49.Hubbard JM, Grothey A. Adolescent and young adult colorectal cancer. J Natl Compr Canc Netw 2013;11(10):1219–25. [DOI] [PubMed] [Google Scholar]
- 50.Manjelievskaia J, Brown D, McGlynn KA, et al. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg 2017;152(5):452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrari A, Montello M, Budd T, et al. The challenges of clinical trials for adolescents and young adults with cancer. Pediatr Blood Cancer 2008;50(5 Suppl):1101–4. [DOI] [PubMed] [Google Scholar]