OBJECTIVES:
To investigate the safety profile and diagnostic efficacy of transjugular liver biopsy (TJLB), with a focus on patients with severe coagulopathies and with multiple biopsies.
METHODS:
Clinical, laboratory, and demographic information was collected on 1,321 TJLBs in 932 patients (mean age 43.5 ± 23.2 years) performed between January 2009 and May 2017 to determine the diagnostic success rate and incidence of both major and minor complications in the 3-day and 30-day period post-biopsies. These outcomes were also analyzed for severely coagulopathic patients and a subgroup of patients who underwent multiple biopsies.
RESULTS:
The overall success rate (diagnostic yield) of the TJLB procedure was 97.7% (1,291/1,321). Overall, the major and minor complication rates were 1.0% (13/1,321) and 9.5% (126/1,321), respectively. In patients with multiple biopsies, the overall complication rate was similar to the entire study cohort, which was 10.4% (57/550). Patients were also stratified according to the platelet counts of 0–50, 51–100, 101–200, 201–300 and >300 × 103 platelets/μL. The overall complication rates were 8.0% (10/124), 11.6% (36/310), 9.9% (54/547), 11.9% (28/235), and 14.3% (11/77), respectively, and these were not statistically significant from each other. Patients were also stratified by international normalized ratio into 0–1, 1.1–2, 2.1–3, and >3. The overall complication rates of these patients were 8.0% (19/237), 11.8% (113/954), 16.3% (7/43), and 0% (0/9), respectively, and were not statistically significant from each other.
DISCUSSION:
TJLB is a highly efficacious, well-tolerated and safe procedure. It can be safely performed multiple times in the same patient or in critically ill, severely coagulopathic patients with no significant increase in the rate of complication while maintaining an extremely favorable diagnostic yield.
INTRODUCTION
Transjugular liver biopsy (TJLB) is a safe and efficacious method of obtaining hepatic tissue samples for histopathologic analysis (1–3). First developed in 1964 as an alternative to percutaneous liver biopsy, TJLB can be safely performed in high-risk patients or those requiring simultaneous hepatic hemodynamic measurements (1,2,4–6). By using a vascular access route rather than a percutaneous one, TJLB greatly reduces the risk of hemorrhage secondary to compromise of the hepatic arterial or portal venous system (7,8).
However, TJLB is not without risk. Minor complications including abdominal pain, capsular perforation, neck hematoma, fever, and hypotension have been associated with the procedure (9–11). In addition, extremely rare major complications such as intraperitoneal hemorrhage, retroperitoneal hemorrhage, and death have also been reported (4,8,10,11). However, because any potential post-biopsy bleeding may occur intravascularly, the risk of some of the major complications is minimized (1,6). As such, a transjugular approach is the preferred biopsy method in high-risk patients: those with coagulopathy, coagulation disorders, or high-volume ascites and those not clinically stable enough to tolerate percutaneous procedures (1,4). Diagnostic efficacy and the quality of specimens obtained by TJLB are comparable to those obtained by a percutaneous route, with success rates approaching 87%–98% (4,10,11).
Because of the decreased risk of hemorrhagic complications, TJLB is often performed as an alternative to percutaneous liver biopsy in coagulopathic patients for whom percutaneous biopsy presents an unacceptable risk of bleeding. As bleeding risk may preclude the performance of any biopsy at all, to date, the safety of TJLB has not been studied in a large group of these patients. In addition, the complication rate of TJLB in patients who have undergone multiple biopsies has not previously been studied.
In this retrospective analysis, we aimed at investigating the overall complication rate and diagnostic yield of TJLB, with a particular focus on patients who underwent multiple biopsies during the study period and those with severe coagulopathy.
METHODS
Patient selection and demographic information
This retrospective study was approved by the institutional review board (IRB# 10-000469). We analyzed data from 2 different clinical sites, including a tertiary liver transplant center. The medical records of 952 patients were accessed to obtain demographic, medical, and procedural information on a total of 1,321 transjugular liver biopsies performed between January 2009 and May 2017. Additional data were collected on technical success of the procedure and relevant laboratory data. All data were prepared and statistically analyzed using SPSS 22 and Microsoft Excel and are reported using ranges, percentages, and mean ± SD. Statistical significance is considered when P values are <0.05.
Laboratory assessment
When available via electronic chart review, preprocedure values for international normalized ratio (INR), prothrombin time, and activated partial thromboplastin time were collected. Patient data were also stratified by INR values into 4 groups for subgroup analysis: INR of 0–1, 1.1–2.0, 2.1–3.0, and 3.1 or higher. In addition, platelet counts were used to group the patients into 5 subgroups: platelet counts of 0–50, 51–100, 101–200, 201–300, and 300 × 103 platelets/μL or higher. Separate statistical analyses of complication and success rates were performed on these subgroups.
Multiple biopsies
Following analysis of the data set as a whole, data from patients who underwent multiple transjugular liver biopsies during the study period were isolated and reanalyzed. All analyses and calculations performed on the overall data set, including complication rates, laboratory assessments, and technical outcomes, were investigated within this subgroup.
Technical outcomes
The technical success/diagnostic yield of each biopsy was analyzed based on pathology reports of the specimens obtained during each procedure. A successful diagnostic yield is considered when 11 central portal triads are obtained in cores to make a pathological diagnosis.
Complications
Data were also gathered on both major and minor complication rates and types within 2 periods: those occurring within 3 and 30 days of the procedure. Complications were classified according to the Society of Interventional Radiology guidelines as major or minor (12). Major complications included those requiring major therapy, escalation of care, or prolonged hospitalization and those resulting in permanent adverse sequelae or death. Minor complications were those requiring nominal therapy with no lasting consequences or overnight hospitalization. In our study, minor complications included fever, abdominal pain, and hypotension, and major complications encompassed intraperitoneal bleed, intra-abdominal infection, cardiac arrhythmia, hepatic artery thrombosis, and inadvertent biopsy of an adjacent organ.
Statistical analysis
Continuous variables were reported as mean ± SD, whereas categorical data were expressed as frequencies. The Student t test was used to compare the differences in continuous variables, and the Pearson χ2 test or Fisher exact test was used to compare categorical variables between the 2 groups. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 22.0 (SPSS, Chicago, IL).
RESULTS
Patient demographics
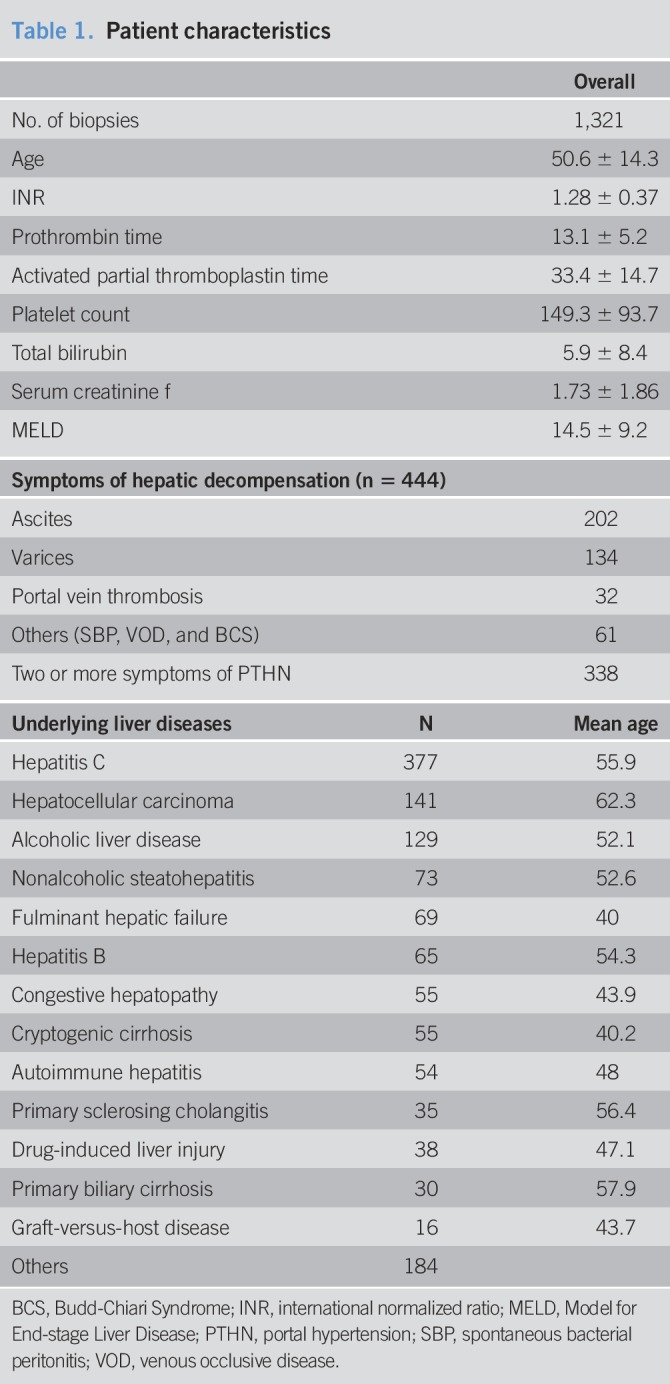
A total of 1,321 biopsies were performed in 952 patients (Table 1). The mean age of this cohort was 50.6 ± 14.3 years. Of note, 58.6% (n = 774) of the biopsies were performed in male patients. One hundred seventy-nine patients (18.8%) underwent multiple biopsies during the study period. The most frequent pathologically proven primary liver diseases included hepatitis C (n = 377), hepatocellular carcinoma (n = 141), alcoholic liver disease (n = 129), nonalcoholic steatohepatitis (n = 73), fulminant hepatic failure (n = 69), hepatitis B infection (n = 65), congestive hepatopathy (n = 55), cryptogenic cirrhosis (n = 55), and autoimmune hepatitis (n = 54). Others including primary biliary cirrhosis, graft-versus-host disease, primary sclerosing cholangitis, and drug-induced liver injury are noted in Table 1. Other diagnoses included Wilson disease, Total Parenteral Nutrition-induced liver injury, metastatic disease, abetalipoproteinemia, amyloidosis, Alagille syndrome, Budd-Chiari disease, biliary atresia, and alpha-1 antitrypsin disease.
Table 1.
Patient characteristics
Laboratory assessment
Preprocedure laboratory values were gathered on all patients, and Model for End-stage Liver Disease (MELD) scores were calculated (Table 1). The mean (±SD) platelet count was 149.3 (±94) × 103 platelets/μL among all patients. The mean (±SD) INR was 1.28 (±0.4), mean (±SD) total serum bilirubin was 5.9 (±8) mg/dL, and mean (±SD) serum creatinine was 1.7 (±2) mg/dL. The mean (±SD) MELD score was 14.5 (±9) among all patients.
In the overall data set, significant differences were noted in the platelet count, serum creatinine values, and MELD scores between male and female patients. Male patients had a mean (±SD) platelet count of 140.3 (±81) platelets/μL, whereas female patients had a mean (±SD) value of 161.9 (±108) platelets/μL (P = 0.0001). MELD scores also differed significantly between male and female patients, with male patients having an average (±SD) score of 15.4 (±9) and females with an average (±SD) score of 13.2 (±9) (P < 0.0001).
Patients who underwent multiple biopsies had a mean (±SD) platelet count of 142.3 (±82) × 103 platelets/μL, mean (±SD) INR of 1.27 (±0.4), mean total bilirubin of 5.6 (±8) mg/dL, and mean (±SD) serum creatinine of 1.5 (±1) mg/dL. In this group, a mean (±SD) MELD score was 13.9 (±9). Among patients who underwent multiple biopsies, only serum creatinine significantly differed between male 1.6 (±1) and female patients 1.3 (±1) (P = 0.0006).
Diagnostic and technical outcomes
Overall, TJLB had a diagnostic yield of 97.7% (1,291/1,321) of cases. In technical details, the hepatic venous system was accessed via the right hepatic vein in 89.9% (n = 1,187) of cases and via the middle hepatic vein in 7.0% (n = 92) of cases.
Complications
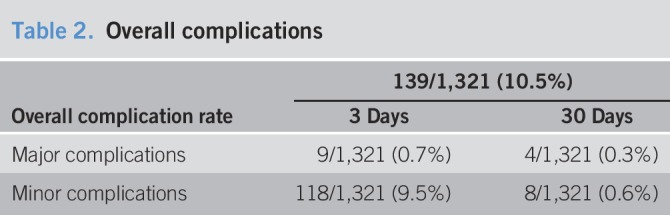
The overall complication rate for TJLB was 10.5% (n = 139), with a mean of 1.8 ± 2 days from the time of the TJLB procedure to the time of the complication (Table 2). Major complications occurred in 1.0% (n = 13) of patients. These complications included intraperitoneal bleeding, intra-abdominal infection, inadvertent renal biopsy, hepatic artery thrombosis, a retained guidewire, cardiac arrhythmias, and massive hemorrhage from the jugular access site. The overall minor complication rate was 10.1%, occurred in 126 patients. The most common minor complication was abdominal pain, which occurred in 61 patients (4.6%). Other minor complications included fever, hematemesis, neck pain, and hypotension. The vast majority of complications occurred within 3 days of the procedure, with the exception of 12 complications that occurred between days 4 and 30 post-biopsy. These included abdominal pain, fever, hemoperitoneum, and peritonitis. No significant differences were observed between the complication rates between male and female patients.
Table 2.
Overall complications
Complications in coagulopathies
Low platelet counts.
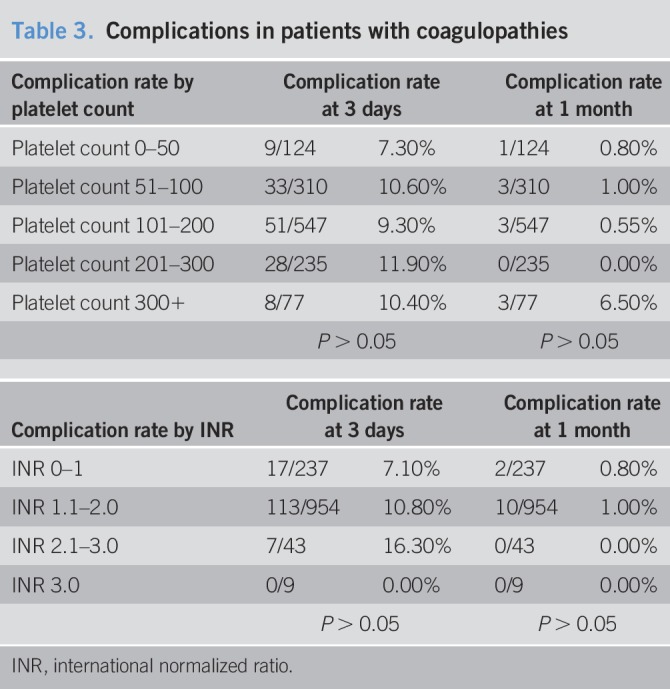
Complication rates were further stratified by preprocedure platelet count and INR (Table 3). Overall, the complication rate in patients with a platelet count of 0–50 × 103 platelets/μL was 7.3% (9/124) within the first 3 days of biopsy and 0.8% (n = 1) in the delayed period. The rate was 10.6% (33/310) on days 1–3 for a platelet count of 51–100 × 103 platelets/μL and 1.0% (3/310) on days 4–30. Patients with platelet counts of 101–200 × 103 platelets/μL had a complication rate of 9.3% (51/547) within the first 3 days and 0.5% (3/547) thereafter. For patients with a platelet count of 201–300 × 103 platelets/μL, the complication rate was 11.9% (28/235), all within the first 3 days. Those with a platelet count of 301 × 103 platelets/μL or more had a complication rate of 10.4% (8/77) in the first 3 days and 6.5% (3/77) at the 1-month mark. The complication rates were not significantly different between each group.
Table 3.
Complications in patients with coagulopathies
High INR.
Patients with an INR of 0–1 experienced complications at a rate of 7.1% (17/237) within the first 3 days and 0.8% (2/237) in the 30-day period. Those with an INR of 1.1–2.0 had a complication rate of 10.8% (103/954) on days 1–3 and 1.0% (10/954) on days 4–30. Patients with an INR of 2.1–3 had complications at a rate of 16.3% (7/43) within the first 3 days only. No complications occurred in those with an INR over 3. The complication rates were not significantly different between each group.
Complications in multiple biopsies
Of the 179 patients who underwent a total of 550 biopsies during the study period, 58.9% (n = 324) were performed in males and with an average patient age of 50.4 ± 14 years. The overall complication rate in this group was 10.4% (n = 57/550), with only 3 major complications (0.5%, 3/550) (Table 4). The overall complication and major complication rates in this cohort are not significantly different compared with the overall cohorts (P = 0.9487 and P = 0.2841, respectively). Fifty-four minor complications were noted in the first 3 days after biopsy, with 3 delayed complications recorded.
Table 4.
Complications in patients undergone multiple biopsies
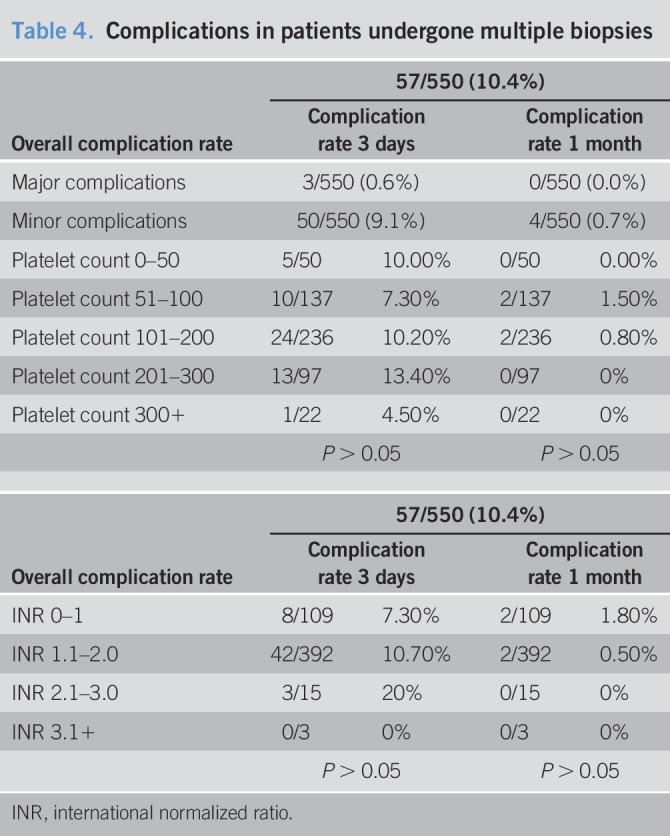
In patients with multiple biopsies, 10.0% (5/50) with a platelet count of 0–50 × 103 platelets/μL had a complication within the first 3 days, and there were no complications on days 4–30. Of note, 7.3% (10/137) of patients with a platelet count of 51–100 × 103 platelets/μL experienced complications within 3 days of the procedure, and 1.5% (2/137) experienced a complication within 4–30 days. In patients with a platelet count of 101–200 × 103 platelets/μL, 10.2% (24/236) had a complication on days 1–3 and 0.8% (2/236) on days 4–30. Patients with a platelet count of 201–300 × 103 platelets/μL had a complication rate of 13.4% (13/97), and those with platelets over 300 × 103 platelets/μL had a complication rate of 4.5% (1/22), all within the first 3 days of biopsy. The complication rates were not significantly different between each group.
Patients with an INR of 0–1 had complications at a rate of 7.3% (8/109) in the first 3 days and 1.8% (2/109) in the delayed period. Those with an INR of 1.1–2 had a complication rate of 10.7% (42/392) in the first 3 days and 0.5% (2/392) on days 4–30. Among patients with an INR of 2.1–3, the complication rate was 20% (3/15), with all complications occurring in the first 3 days. No complications were reported in this group of patients with INR over 3. The complication rates were not significantly different between each group.
DISCUSSION
TJLB is a safe and effective means for obtaining tissue for histopathological evaluation in patients with severe liver disease. For those patients in which percutaneous liver biopsy presents an unacceptable risk, TJLB can be used to obtain specimens while minimizing the risk of major complications such as intraperitoneal bleeding or death. These may include patients with severe coagulopathy or high-volume ascites, abdominal obesity, post-transplant patients, or fulminant hepatic failure (4,7). Overall complication rates as high as 20% have been reported with percutaneous liver biopsies (1,4,7,10,13–15).
The purpose of our eight-year retrospective study was to determine the safety profile of TJLB in critical clinical comorbidities/conditions including severe coagulopathy, and patients undergone multiple biopsies. The overall complication rate in our study was 10.5%, which is higher than the rates reported in the literature, which vary from 2.4% (11) to 7.1% (5). This may be due to the different reporting systems that were used in different studies as our study followed the guideline and descriptions of complications provided by the Society of Interventional Radiology (12) and the American Association for Studying Liver Diseases (16). One of the major risks of TJLB is intraperitoneal hemorrhage following liver capsule perforation during tissue sampling. However, this complication is exceedingly rare. In our study, 8 patients (0.6%) experienced intraperitoneal hemorrhage. This is consistent with published findings from Dohan et al., who reported a rate of 0.59% in 341 biopsies and Gamble et al., who found a bleeding rate of 0.87% in 461 biopsies (1,17). Other studies including those by Bruzzi et al. and Steadman et al. found no incidence of intraperitoneal hemorrhage in studies of 50 and 67 patients, respectively, likely because of the small sample size (1,13,18). Of note, although other published studies have reported mortality due to intraperitoneal bleeding, all 5 cases of bleeding were nonfatal in our patient population.
Additional major complications found in our study include cardiac arrhythmias in 2 patients (0.2%), inadvertent renal biopsy in 3 patients, and hepatic artery thrombosis and intra-abdominal infection in 1 patient each.
The overall major complication rate in our analysis was 1.0%, which is comparable to the rate of 1.5% reported by Mammen et al. (19). In our study, self-resolving minor complications occurred at a rate of 9.5%. The rates reported in the literature range anywhere from 0.5 to 15% by Miraglia et al. and as high as 20.53% as reported by Dohan et al. (5,20). However, it is somewhat higher than the 7.1% reported by Kalambokis et al. (10). Unfortunately (1), because of difference in reporting system (2), difference in inclusion criteria of “minor complications” between different institution and different guidelines, and (3) because of subjectivity of these symptoms, this discrepancy in minor complication rates between studies is difficult to validate.
Many patients included in our study underwent multiple biopsies during the study period. Complication rates in this group were 10.4%, which was very similar to our overall complication rate. However, only 3 patients (0.5%) experienced a major complication on days 1–3, and none of these patients had a major complication at 30 days. Because these rates are not significantly different from the entire cohort, it is safe to state that multiple biopsies do not increase the risk of complications. In contrast, it has been shown that multiple percutaneous biopsies are associated with an increased risk of complications (14,15). Therefore, if multiple biopsies are needed in patients with liver disease, TJLB should be considered first.
Complication rates of those patients with established coagulopathy at the time of the procedure were also investigated as a distinct subgroup. Despite the increased risk of bleeding in patients with thrombocytopenia, there was no statistical difference between each subgroup in both the platelet and INR subgroups. Similar findings were noted in patients with multiple biopsies when they were stratified into different subgroups of platelet counts or INR. No significant differences in complication rates were noted between the different subgroups. As a whole, the complication rates in our study demonstrated and validated the safety of the TJLB procedure, even in those patients with extremely high or low platelet counts or those with supratherapeutic INRs. The complication rate of the procedure was not significantly different no matter how low the platelet count or how high the INR. The procedure also does not have a significantly increased complication rate in patients who underwent multiple biopsies.
Although the study population was large, this retrospective study relied solely on patient charts in an electronic health record as its information source. As with any retrospective study, there is a risk of misclassification bias during the data collection process. This risk was somewhat mitigated by the fact that the data were primarily gathered by a single reviewer. In addition, the data were sourced from 2 clinical sites of the same institution, with uniform clinical practice across both sites. For example, as the pre-test probability for bleeding is low for the TJLB procedure, many markers of bleeding risk, including fibrinogen levels, thromboelastography, and rotational thromboelastometry, are not routinely measured as a part of the preprocedure workup. Therefore, nearly all the patients in the study lacked this information, which may inform bleeding risk. These coagulation tests, particularly fibrinogen levels, are an excellent marker for bleeding risk, particularly in patients with cirrhosis with a high risk of periprocedure bleeding, and consideration should be given to gathering this information in the future (21).
This study found the overall rate of bleeding complications associated with TJLB to be quite low. Although the study population was large, a low event rate could falsely depress the overall bleeding rate. Further testing with larger patient populations in a multicenter study should be considered. In addition, as some of these patients underwent surgical interventions or other procedures soon after their TJLB, it is impossible to attribute some complications to a specific procedure or determine whether it was sequelae of active disease. Future directions may include stratifying the patient population by acuity of care. In this study, no distinction was made between inpatients, outpatients, and those being cared for in an intensive care unit, which could have a significant bearing on the impact of potential complications and the recording of stated complications. The future study should be performed in a randomized controlled trial to obtain objective scoring of complications and a better stratification of patients based on their severity of illnesses and comorbidities.
In conclusion, our study validates that TJLB can be safely performed in a wide range of patients with excellent results. This includes patients with extremely high or low platelet counts, a wide range of INR values, severe liver disease, and patients who undergo multiple biopsies. Success rates are high, and overall complication rates are low, particularly major complications, making this a safe procedure for most patients with liver disease.
CONFLICTS OF INTEREST
Guarantor of the article: Edward W. Lee, MD, PhD.
Specific author contributions: Megan Sue, MD, is a trainee of Dr. Edward W. Lee, MD, PhD. Conception and design of the study: M.J.S., S.S., S.T.K., and E.W.L. Collecting and interpreting data: M.J.S. and E.W.L. Drafting the manuscript: M.J.S., S.S., J.P.M., F.K., S.T.K., and E.W.L. Critical revision and editing: M.J.S., S.S., J.P.M., F.D., M.E.-K., F.K., R.W.B., S.T.K., and E.W.L. Final approval of the final version: M.J.S., S.S., J.P.M., F.D., M.E.-K., F.K., R.W.B., S.T.K., and E.W.L.
Financial support: No financial support.
Potential competing interests: None.
Study Highlights.
WHAT IS KNOWN
✓ TJLB is considered a relatively safe method of obtaining liver tissue for diagnostic and molecular analysis in patients with liver disease.
✓ It is still unclear how safe TJLB is in severely ill, severe coagulapathic patients.
✓ Several studies with smaller number of patients have been demonstrating the safety of TJLB.
WHAT IS NEW HERE
✓ TJLB is completely safe and feasible in patients with severe coagulopathy (very high INR and low platelet counts).
✓ Multiple TJLB seems a safe procedure in patients with severe coagulopathy.
TRANSLATIONAL IMPACT
✓ TJLB should be the first choice of biopsy method in liver diseases, even in patients with severe coagulopathy.
REFERENCES
- 1.Dohan A, Guerrache Y, Dautry R, et al. Major complications due to transjugular liver biopsy: Incidence, management and outcome. Diagn Interv Imaging 2015;96:571–7. [DOI] [PubMed] [Google Scholar]
- 2.Pathak K, Gopinath M, Salgotra KR. Transjugular liver biopsy. Med J Armed Forces India 2013;69:384–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito AA, Nicolini A, Meregaglia D, et al. Role of transjugular liver biopsy in the diagnostic and therapeutic management of patients with severe liver disease. Radiol Med 2008;113:1008–17. [DOI] [PubMed] [Google Scholar]
- 4.Behrens G, Ferral H. Transjugular liver biopsy. Semin Intervent Radiol 2012;29:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dohan A, Guerrache Y, Boudiaf M, et al. Transjugular liver biopsy: Indications, technique and results. Diagn Interv Imaging 2014;95:11–5. [DOI] [PubMed] [Google Scholar]
- 6.Jana M, Gamanagatti S. Transjugular liver biopsy: Tips and tricks. Trop Gastroenterol 2012;33:168–72. [PubMed] [Google Scholar]
- 7.Atar E, Ben Ari Z, Bachar GN, et al. A comparison of transjugular and plugged-percutaneous liver biopsy in patients with contraindications to ordinary percutaneous liver biopsy and an “in-house” protocol for selecting the procedure of choice. Cardiovasc Intervent Radiol 2010;33:560–4. [DOI] [PubMed] [Google Scholar]
- 8.McGill DB, Rakela J, Zinsmeister AR, et al. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990;99:1396–400. [DOI] [PubMed] [Google Scholar]
- 9.DiMichele DM, Mirani G, Wilfredo Canchis P, et al. Transjugular liver biopsy is safe and diagnostic for patients with congenital bleeding disorders and hepatitis C infection. Haemophilia 2003;9:613–8. [DOI] [PubMed] [Google Scholar]
- 10.Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—Indications, adequacy, quality of specimens, and complications—A systematic review. J Hepatol 2007;47:284–94. [DOI] [PubMed] [Google Scholar]
- 11.Smith TP, Presson TL, Heneghan MA, et al. Transjugular biopsy of the liver in pediatric and adult patients using an 18-gauge automated core biopsy needle: A retrospective review of 410 consecutive procedures. AJR Am J Roentgenol 2003;180:167–72. [DOI] [PubMed] [Google Scholar]
- 12.Omary RA, Bettmann MA, Cardella JF, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol 2003;14:S293–5. [DOI] [PubMed] [Google Scholar]
- 13.Bruzzi JF, O'Connell MJ, Thakore H, et al. Transjugular liver biopsy: Assessment of safety and efficacy of the quick-core biopsy needle. Abdom Imaging 2002;27:711–5. [DOI] [PubMed] [Google Scholar]
- 14.Chi H, Hansen BE, Tang WY, et al. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur J Gastroenterol Hepatol 2017;29:36–41. [DOI] [PubMed] [Google Scholar]
- 15.van der Poorten D, Kwok A, Lam T, et al. Twenty-year audit of percutaneous liver biopsy in a major Australian teaching hospital. Intern Med J 2006;36:692–9. [DOI] [PubMed] [Google Scholar]
- 16.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology 2009;49:1017–44. [DOI] [PubMed] [Google Scholar]
- 17.Steadman C, Teague C, Harper J, et al. Transjugular liver biopsy—An Australian experience. Aust N Z J Med 1988;18:836–40. [DOI] [PubMed] [Google Scholar]
- 18.Gamble P, Colapinto RF, Stronell RD, et al. Transjugular liver biopsy: A review of 461 biopsies. Radiology 1985;157:589–93. [DOI] [PubMed] [Google Scholar]
- 19.Mammen T, Keshava SN, Eapen CE, et al. Transjugular liver biopsy: A retrospective analysis of 601 cases. J Vasc Interv Radiol 2008;19:351–8. [DOI] [PubMed] [Google Scholar]
- 20.Miraglia R, Maruzzelli L, Minervini MI, et al. Transjugular liver biopsy in liver transplant patients using an 18-gauge automated core biopsy needle. Eur J Radiol 2011;80:e269–72. [DOI] [PubMed] [Google Scholar]
- 21.Intagliata NM, Argo CK, Stine JG, et al. Concepts and controversies in haemostasis and thrombosis associated with liver disease: Proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb Haemost 2018;118(8):1491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]


