OBJECTIVES:
Prouroguanylin (ProUGN) in the intestine is cleaved to form uroguanylin (UGN), which stimulates guanylate cyclase C (GUCY2C), inducing cyclic guanosine monophosphate signaling. Paracrine release regulates fluid secretion, contributing to bowel function, whereas endocrine secretion evoked by eating forms a gut-brain axis, controlling appetite. Whereas hormone insufficiency contributes to hyperphagia in obesity, its contribution to the pathophysiology of constipation syndromes remains unexplored. Here, we compared circulating ProUGN and UGN in healthy subjects and in patients with chronic idiopathic constipation (CIC) and patients with irritable bowel syndrome with constipation (IBS-C).
METHODS:
Circulating ProUGN and UGN levels were measured in 60 healthy subjects, 53 patients with CIC, and 54 patients with IBS-C. After an overnight fast, the participants ingested a standardized meal; blood samples were drawn at fasting and at 30, 60, and 90 minutes thereafter, and hormone levels were quantified by enzyme-linked immunosorbent assay.
RESULTS:
Fasting ProUGN levels were >30% lower in patients with CIC and those with IBS-C compared with healthy subjects regardless of age, sex, or disease state. After eating, ProUGN levels increased compared with fasting levels, although the rate of change was slower and maximum levels were lower in patients with CIC and those with IBS-C. Similarly, fasting UGN levels were lower in patients with CIC and those with IBS-C compared with healthy subjects. However, unlike ProUGN levels, UGN levels did not increase after eating.
DISCUSSION:
These observations support a novel pathophysiologic model in which CIC and IBS-C reflect a contribution of ProUGN insufficiency dysregulating intestinal fluid and electrolyte secretion.
TRANSLATIONAL IMPACT:
This study suggests that CIC and IBS-C can be treated by oral GUCY2C hormone replacement. Indeed, these observations provide a mechanistic framework for the clinical utility of oral GUCY2C ligands like plecanatide (Trulance) and linaclotide (Linzess) to treat CIC and IBS-C.
INTRODUCTION
Functional gastrointestinal (GI) disorders affect a substantial number of patients worldwide, accounting for a significant percentage of healthcare spending globally (1–5). Whereas their clinical and economic impact is considerable, the mechanisms underlying these related disorders remain incompletely defined (1,4). In that context, chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C) are two of the most prevalent functional GI disorders (1). IBS is characterized by recurrent episodes of abdominal pain, with at least 1 episode per week in the last 3 months, on average, of pain related to defecation, associated with a change in the frequency of stool, or associated with a change in the form of stool (3). IBS can be subtyped by the predominant stool form: constipation (IBS-C), diarrhea (IBS-D), or mixed (IBS-M) (3,4). Those within the IBS-C subtype experience hard or lumpy stools more than 25% of the time they defecate and loose or watery stools less than 25% of the time (2). IBS-C affects about 1 in 20 people, representing about 5% of the adult population, in the United States (1,2,5). Similarly, CIC is a complex functional GI disorder defined by symptoms including fewer than 3 bowel movements a week and hard-to-pass or incomplete bowel movements (4). In addition to physical symptoms including abdominal bloating and discomfort, CIC can adversely affect an individual's quality of life, including increasing stress levels and anxiety (6,7). CIC affects approximately 33 million Americans and an estimated 14% of the global population (1,4,5). Although IBS-C and CIC can be discriminated based on clinical criteria, there is substantial overlap in these functional GI disorders (1,2,4,5). These conditions remain prevalent and consume a disproportionate amount of healthcare resources including up to $10B in direct costs and $20B in indirect costs in the United States alone (5). Although their clinical and economic impact is substantial, precise molecular mechanisms underlying the pathophysiology of these related disorders remain incompletely defined (1,4).
Guanylate cyclase C (GUCY2C) is an epithelial cell receptor expressed along the rostral-caudal axis of the intestine from proximal of the duodenum to the distal rectum (8). It was first identified as the receptor for heat-stable enterotoxins (STs) produced by enterotoxigenic bacteria that causes diarrheal diseases (9–11). Bacterial STs represent an example of molecular mimicry with strong structural and functional homologies to endogenous hormones produced segmentally in the intestine. In that context, uroguanylin (UGN) is a 16-amino acid peptide that activates GUCY2C with maximum potency in the slightly acidic (pH 5–6) environments of the duodenum and proximal jejunum (12). UGN is expressed by tuft-like epithelial cells, primarily in the proximal small intestine, with lower levels in the stomach, the distal small intestine, and the colorectum (13,14). By contrast, guanylin is a 15-amino acid peptide that activates GUCY2C in neutral to slightly basic pH environments and is principally expressed in the colorectum by goblet cells (12–14). Both peptides undergo paracrine secretion into the intestinal lumen as unprocessed prohormones that are cleaved to mature forms stabilized by 2 disulfide bonds compatible with structural flexibility and isomerization (8,15–17).
Ligand-receptor interaction activates the intracellular catalytic domain of GUCY2C, which converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP) (8,18). In the proximal small intestine, cGMP accumulation induced by UGN activates signaling intermediates, including protein kinases, whose phosphotransferase activities regulate downstream effectors, including the cystic fibrosis transmembrane conductance regulator and sodium-hydrogen exchanger, producing fluid and electrolyte secretion essential to normal bowel function (18,19). Outside these local paracrine signaling activities in the intestine, there is an emerging paradigm in which a novel GUCY2C endocrine gut-brain axis regulates feeding, energy and metabolic homeostasis, and body weight. In that context in mice and humans, ingestion of food evokes endocrine secretion of prouroguanylin (ProUGN) from the small intestine into the circulation (20–25). Circulating ProUGN, but not proguanylin, is processed by the hypothalamus to the mature UGN hormone (25). UGN binds to and activates GUCY2C expressed in the hypothalamus, inducing cGMP accumulation (23,25). In turn, hormone activation of central GUCY2C-cGMP signaling induces hypothalamic neuronal activity associated with satiety, decreasing feeding (22,23,25,26).
Beyond intestinal fluid and electrolyte secretion and central regulation of appetite and satiety, the GUCY2C-hormone axis has emerged as a key component of physiologic mechanisms modulating intestinal barrier function, inflammation, visceral pain, and tumorigenesis (19,27–36). Furthermore, disruptions in GUCY2C signaling specifically involving hormone insufficiency have been linked to the pathophysiologic mechanisms underlying inflammatory bowel diseases, colorectal cancer, and obesity, and the metabolic syndrome (23,34,36–40). Moreover, genetic mutations that result in loss or gain of function of GUCY2C are associated with constipation or diarrhea, respectively (41–44). Taken together, these observations raise the intriguing possibility that the GUCY2C paracrine signaling axis may play a mechanistic role in chronic constipation syndromes like CIC and IBS-C. Indeed, as in other diseases in which silencing of GUCY2C reflects hormone loss (23,34,36–40), it is tempting to speculate that an insufficiency of ProUGN, at least in part, contributes to the pathophysiology of chronic constipation. Here, we exploit evoked endocrine secretion of ProUGN by eating (20,25) and the direct relationship between circulating and epithelial cell levels of the hormone (20,23) to assess intestinal GUCY2C hormone production in patients with CIC or those with IBS-C.
METHODS
Study design
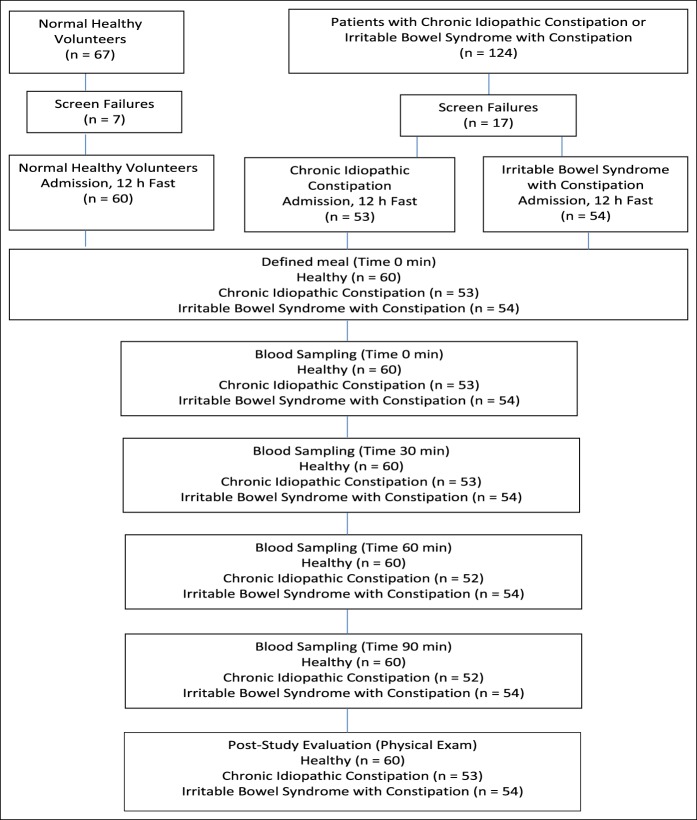
This was a phase 0 exploratory 1-day study to evaluate the effect of a standardized meal on circulating ProUGN and UGN levels in healthy volunteers and in patients with functional GI disorders. The study was designed to test the hypothesis that circulating levels of ProUGN and UGN evoked by feeding were reduced in patients with CIC and those with IBS-C compared with normal, healthy volunteers. The study composed of 3 populations of subjects enrolled at a single site and identified in an investigator's database (Figure 1): normal, healthy men and women, aged 18–80 years, without a history or evidence of chronic disease (n = 60); male and female patients with CIC defined by modified Rome III functional constipation criteria, aged 18–80 years, who had not taken any treatments in the 14 days before enrollment (n = 53); and male and female patients with IBS-C defined by Rome III criteria using the modular questionnaire, aged 18–85 years, who had not taken any treatments in the 14 days before enrollment (n = 54) (45). Previous drug histories including the use of, and responses to, oral GUCY2C agonists were not collected as part of the study. After obtaining informed consent, the participants underwent prescreening with an interview, physical examination, and laboratory assessment to eliminate those with conditions that alter circulating ProUGN levels, including heart failure, renal failure, or the nephrotic syndrome (46–48). Enrolled participants were admitted to the clinical research unit and subjected to an overnight (12 hours) fast. In the morning, the participants consumed a standardized meal of 750 calories (49 g of fat, 25 g of protein, 53 g of carbohydrate, and 1.49 g of sodium) within 15 minutes (25). Blood samples were drawn immediately before (0 minutes) and 30, 60, and 90 minutes after initiating the meal. After collection of the last blood sample, the participants underwent post-study evaluation, including an interview and physical examination, after which they were discharged. The study was approved by the Alpha IRB, 1001 Avenida Pico, Suite C, #497, San Clemente, California.
Figure 1.
Flowchart describing participant recruitment, study flow, assessments, and timing.
Primary endpoint
The primary endpoint of the study is the circulating level of ProUGN and UGN, before and after a standardized meal, in healthy subjects and in patients with CIC and those with IBS-C.
Primary and exploratory analyses
The primary analysis is the comparison of the time course of mean concentration levels of circulating ProUGN and UGN. Exploratory analyses include comparisons between healthy volunteers and patients with CIC and those with IBS-C of: maximum concentration levels of ProUGN and UGN, the rate of change in ProUGN and UGN concentration levels, and the differences in ProUGN and UGN maximum concentration levels and rates of change based on gender and age.
Circulating ProUGN and UGN levels
Primary and exploratory analyses focused on the concentration of ProUGN and UGN in the circulation. Blood samples were collected at prespecified time points into tubes containing 3.2% sodium citrate, immediately inverted several times, and then placed on ice. Samples were centrifuged at 1,300 relative centrifugal force at 4 °C for 10 minutes. Supernatant serum was removed to a fresh, precooled, labeled tube for distribution into cryovials and storage at −80 °C. Human ProUGN levels in freshly thawed plasma, assayed in duplicate, were measured by enzyme-linked immunosorbent assay using a quantitative sandwich enzyme immunoassay kit from BioVendor (Brno, Czech Republic), analytically validated previously (20,48,49). Human UGN levels were quantified by enzyme-linked immunosorbent assay using a kit from Biomatik (Wilmington, DE). ProUGN and UGN levels were quantified by Charles River (San Diego, CA). Briefly, standards and samples were added to microplate wells coated with polyclonal antihuman ProUGN antibody. After incubation (60 minutes) and washing, a biotin-labeled polyclonal antihuman ProUGN antibody was added to the wells (60 minutes), followed by another washing step, then by incubation (30 minutes) with streptavidin-horseradish peroxidase conjugate, and a final washing step. The addition of the substrate 3,30,5,50-tetramethylbenzidine allows the enzyme activity proportional to the concentration of ProUGN to be measured spectrophotometrically.
Statistical analyses
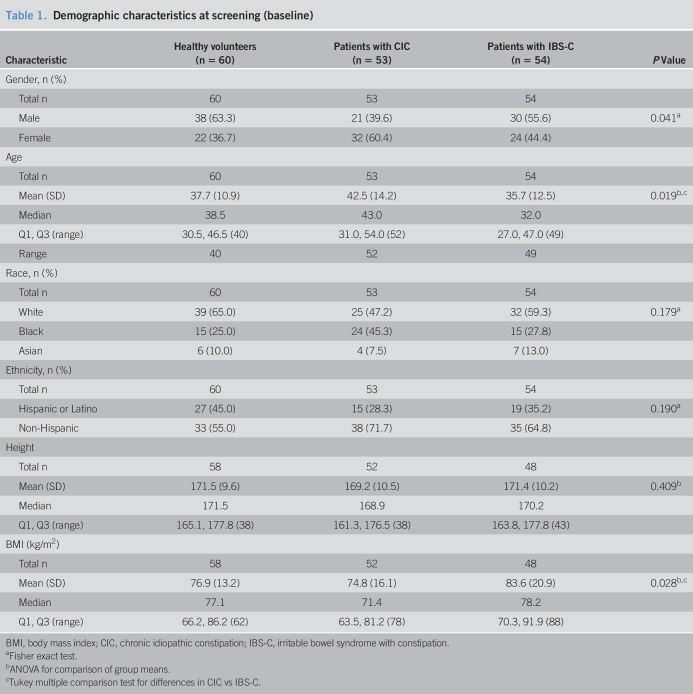
A summary of baseline demographic parameters (age, gender, race, ethnicity, height, weight, and body mass index) is presented in Table 1, by participant group (healthy volunteers, patients with CIC, patients with IBS-C, and CIC and IBS-C patients combined). A summary of baseline clinical laboratory parameters (hematology, serum chemistry, and urinalysis) is presented in Supplementary Table 1 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A66). Statistical summaries and analyses were performed using SAS version 9.4 (SAS Institute). All tests of statistical significance are 2-sided and performed at the 0.05 level of significance. P values presented are intended for informational purposes only; no adjustments have been made for repeated testing.
Table 1.
Demographic characteristics at screening (baseline)
RESULTS
Subject characteristics
For this study, 67 healthy volunteers and 124 patients (diagnosed with either CIC or IBS-C) were screened, with 7 healthy subjects and 17 patients with CIC or IBS-C determined to be screen failures (Figure 1). The enrolled 60 healthy subjects, 53 patients with CIC, and 54 patients with IBS-C did not differ significantly by race, ethnicity, or height (Table 1). There was a greater proportion of women in patient cohorts (P = 0.041), patients with CIC were slightly older (P = 0.019), and patients with IBS-C had a modestly increased body mass index (P = 0.028), consistent with established demographic characteristics for these populations (Table 1) (2–4). Physical examinations and laboratory analyses revealed no clinically significant findings and no evidence of renal or cardiovascular disease (see Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A66).
Fasting hormone levels
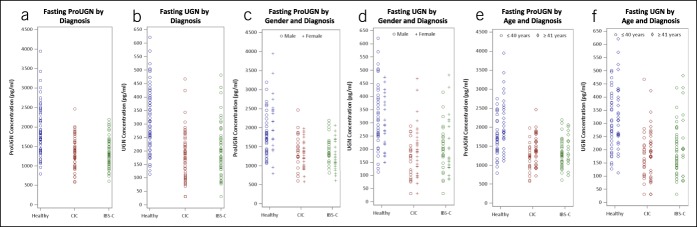
This is the first report of the simultaneous quantification of ProUGN and UGN levels in humans. Mean fasting ProUGN concentration levels were substantially greater than mean fasting UGN concentration levels across all diagnosis, age, and gender subgroups, aligning with the results in rodents (12,28–30). Fasting ProUGN and UGN levels were lower in patients with chronic constipation syndromes, compared with normal subjects (Figure 2). The difference in mean fasting ProUGN levels in patients with chronic constipation was 583 pg/mL (95% confidence interval: 406–761; P < 0.0001). Similarly, the difference in mean fasting UGN levels in patients with chronic constipation was 112 pg/mL (95% confidence interval: 74–150; P < 0.0001). There were no differences in fasting ProUGN or UGN levels in patients with CIC and those with IBS-C (Figure 2a,b). Similarly, there were no differences in fasting ProUGN or UGN levels by sex (Figure 2c,d). Interestingly, there was a difference in fasting ProUGN levels but not UGN levels in healthy subjects, but not constipated patients, by age (Figure 2e,f). Indeed, older healthy subjects (≥41 years) had a mean fasting ProUGN level of 2,159 ± 708, whereas younger healthy subjects (≤40 years) had a mean level of 1741 ± 495 (P < 0.0127).
Figure 2.
Fasting serum ProUGN (a, c, e) and UGN (b, d, f) concentrations in healthy volunteers, patients with CIC, and patients with IBS-C by (a and b) diagnosis (blue, healthy volunteers; red, patients with CIC; green, patients with IBS-C), (c and d) gender (o, male; +, female), and (e and f) age (○, ≤40 years; ◊, >40 years). CIC, chronic idiopathic constipation; IBS-C, irritable bowel syndrome with constipation; ProUGN, prouroguanylin; UGN, uroguanylin.
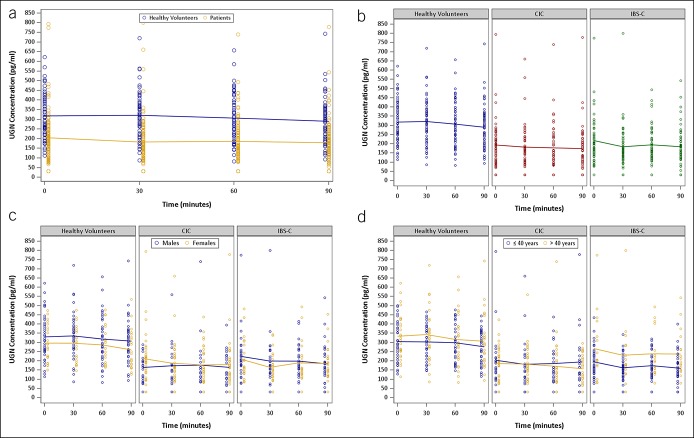
Postprandial ProUGN levels
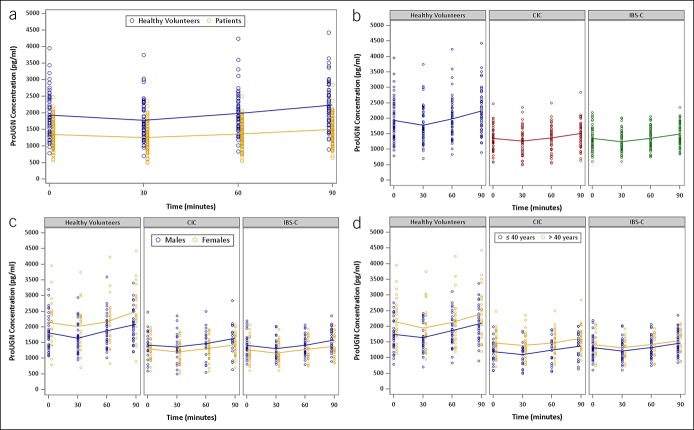
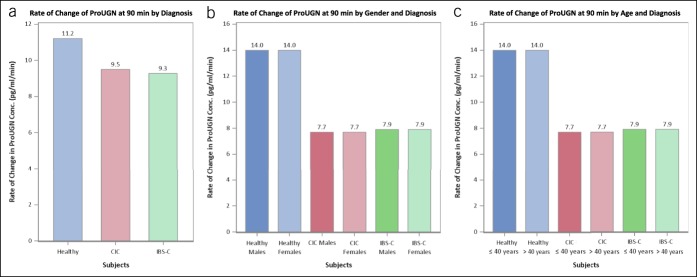
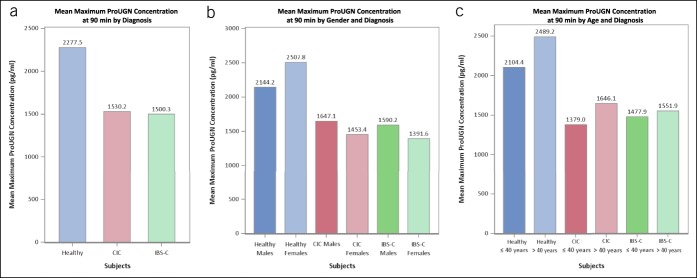
This is the first report of postprandial circulating ProUGN levels in adults. ProUGN levels increased from fasting levels after a standard meal in all cohorts, in close agreement with the results obtained in rodents and in adolescent humans (20,25). ProUGN levels were more than 29% lower (P < 0.0001) at all time points in patients with chronic constipation syndromes, compared with normal subjects (Figure 3a; see Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A66). The rate of change in ProUGN levels was greater in normal subjects compared with patients with chronic constipation (P < 0.0004; Figures 3a, 4a; see Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A66). Moreover, maximum levels of ProUGN were greater in normal subjects compared with patients with chronic constipation (P < 0.0001; Figures 3a, 5a). There were no differences in ProUGN levels (Figure 3b), postprandial rate of change (Figure 4a), or postprandial maximum concentrations (Figure 5a) in patients with CIC and those with IBS-C. Similarly, there were no differences in the postprandial rate of change in ProUGN by sex (Figure 4b) or age (Figure 4c). However, healthy women had higher postprandial levels of ProUGN (P < 0.0193; Figure 3c), compared with healthy men. Moreover, older (≥41 years) healthy subjects and patients with CIC, but not those with IBS-C, had higher postprandial ProUGN levels (P < 0.0049, healthy subjects; P < 0.0016, patients with CIC; Figure 3d), associated with a greater maximum concentration (P < 0.0325, healthy subjects; P < 0.0217, patients with CIC; Figure 5c), compared with younger (≤40 years) healthy subjects or patients with CIC.
Figure 3.
Time course of postprandial changes in serum ProUGN concentrations by (a) cohorts (blue, healthy volunteers; yellow, all patients with constipation), (b) diagnosis (blue, healthy volunteers; red, patients with CIC; green, patients with IBS-C), (c) gender (blue, male; yellow, female), and (d) age (blue, ≤40 years; yellow, >40 years). CIC, chronic idiopathic constipation; IBS-C, irritable bowel syndrome with constipation; ProUGN, prouroguanylin.
Figure 4.
Rate of change in postprandial ProUGN at 90 minutes by (a) diagnosis (blue, healthy volunteers; red, patients with CIC; green, patients with IBS-C), (b) gender (dark shade, male; light shade, female), and (c) age (dark shade, ≤40 years; light shade, >40 years). CIC, chronic idiopathic constipation; IBS-C, irritable bowel syndrome with constipation; ProUGN, prouroguanylin.
Figure 5.
Maximum concentration of postprandial ProUGN at 90 minutes by (a) diagnosis (blue, healthy volunteers; red, patients with CIC; green, patients with IBS-C), (b) gender (dark shade, male; light shade, female), and (c) age (dark shade, ≤40 years; light shade, >40 years). CIC, chronic idiopathic constipation; IBS-C, irritable bowel syndrome with constipation; ProUGN, prouroguanylin.
Postprandial UGN levels
In contrast to ProUGN levels, UGN levels did not increase from fasting levels after a standard meal in any cohort (Figure 6a). UGN levels were more than 35% lower (P < 0.0001) at all time points in patients with chronic constipation syndromes, compared with normal subjects (Figure 6a). There were no differences in postprandial UGN levels in patients with CIC and IBS-C (Figure 6b). Similarly, there were no differences in postprandial UGN levels by sex (Figure 6c). However, older patients with IBS-C (≥41 years) had higher postprandial UGN levels (P = 0.0081) compared with younger patients with IBS-C (≤40 years) (Figure 6d).
Figure 6.
Time course of postprandial changes in serum UGN concentrations by (a) cohorts (blue, healthy volunteers; yellow, all patients with constipation), (b) diagnosis (blue, healthy volunteers; red, patients with CIC; green, patients with IBS-C), (c) gender (blue, male; yellow, female), and (d) age (blue, ≤40 years; yellow, >40 years). CIC, chronic idiopathic constipation; IBS-C, irritable bowel syndrome with constipation; UGN, uroguanylin.
DISCUSSION
Here, we reveal for the first time that patients with CIC and those with IBS-C have lower baseline levels of circulating ProUGN and UGN. Moreover, they have lower evoked increases in circulating ProUGN and UGN induced by feeding. These observations suggest that patients with CIC and those with IBS-C produce lower levels of ProUGN in the epithelial cells in the small intestine, the primary source of this hormone in the circulation (8,20,25,50). In that context, the intestinal GUCY2C paracrine hormone axis regulates fluid and electrolyte secretion, maintaining intestinal function, including bowel movements (8,18,19). Taken together, these observations suggest a pathophysiologic paradigm in which functional GI disorders of chronic constipation, including CIC and IBS-C, may reflect ProUGN insufficiency that, in turn, produces inadequate stimulation of GUCY2C, resulting in incomplete fluid and electrolyte secretion disrupting normal bowel movements. Indeed, this novel pathophysiologic paradigm suggests that CIC and IBS-C may, in part, represent a paracrine hormone insufficiency syndrome.
A role for GUCY2C in disorders of intestinal secretion has been described. Indeed, a gain-of-function mutation in GUCY2C in a Norwegian family has been associated with chronic mild diarrhea (41). This autosomal dominant mutation in the extracellular domain increases the binding affinity of GC-C for all ligands. Moreover, the affected family members were more susceptible to IBS, IBD, small bowel obstruction, and esophagitis. Another gain-of-function group of mutations was linked to congenital sodium diarrhea, in which GUCY2C is constitutively active, elevating cGMP levels in the absence of ligand binding (42). Of note, the other major cause of congenital sodium diarrhea is loss-of-function autosomal recessive mutations in the Na+/H+ exchanger, which is a key downstream target of GUCY2C-cGMP signaling (51). Conversely, inactivating mutations of GUCY2C associated with decreased cGMP production were discovered in newborns born to families with a history of meconium ileus (43). Though commonly associated with cystic fibrosis, meconium ileus is a distinct diagnosis characterized by neonatal intestinal obstruction. A recent study reported 2 separate autosomal recessive GUCY2C loss-of-function mutations that cause meconium ileus in unrelated Bedouin families (43). In 1 family, a missense mutation produced a change in the extracellular ligand binding domain associated with a 40% decrease in ligand-induced cGMP production. In the second family, a nonsense mutation inactivated the catalytic domain of GUCY2C, preventing cGMP production. More recently, loss-of-function mutations in GUCY2C were identified in a Lebanese family demonstrating altered affinity in receptor ligand binding (44). Ultimately, these mutations were associated with decreased fluid and electrolyte secretion, resulting in meconium ileus (43,44). Interestingly, this loss-of-function mutation in GC-C provides protection against enterotoxigenic diarrhea, demonstrating the structural specificity required for the receptor to respond to the relatively rigid molecular structure of ST, with the extra intramolecular disulfide bridge. Indeed, mice homozygous or heterozygous for inactivating mutations in GC-C exhibited lower mortality rates in response to ST-induced secretory diarrhea compared with wild-type animals (52,53). Moreover, UGN mRNA is elevated in mucosal biopsies in patients with IBS with diarrhea, compared with healthy subjects (54,55). Taken together, these observations highlight the role of the GUCY2C paracrine hormone axis, and its regulation of fluid and electrolyte secretion, in the pathophysiologic mechanisms underlying dysfunctional bowel habits in patients. In that context, they support the results of the present study suggesting a role for the GUCY2C paracrine hormone axis in the pathophysiologic mechanisms underlying CIC and IBS-C.
It is noteworthy that silencing the GUCY2C axis by suppression of the hormone, but preservation of the GUCY2C receptor, expression is a common molecular mechanism that bridges a number of pathophysiologic mechanisms. Elimination of guanylin expression, silencing the GUCY2C-cGMP signaling, is an early and universal event in colorectal tumorigenesis that is conserved across species. Indeed, oral hormone replacement therapy stimulating GUCY2C-cGMP signaling prevents tumorigenesis in mice, and this paradigm is being extended to colorectal cancer chemoprevention in humans (31,32,34,36,38–40,56,57). Similarly, hyperphagia associated with obesity induces endoplasmic reticulum stress in the epithelial cells along the rostral-caudal axis of the intestine, which, in turn, suppresses GUCY2C hormone expression (23,56). In the colorectum, suppression of guanylin expression by consumption of excess calories is one mechanism contributing to the relationship between obesity and colorectal cancer, and oral GUCY2C hormone replacement blocks intestinal tumorigenesis in the context of diet-induced obesity (56). Similarly, consumption of excess calories suppresses expression of UGN in the small intestine, which eliminates endocrine secretion of ProUGN into the circulation, silencing hypothalamic GUCY2C regulating appetite and satiety, in animals and humans (20,23–25,50). Replacement of the lost endocrine hormone by transgenic expression of UGN in the brain reduces appetite and weight gain associated with diet-induced obesity (23). Together, these observations underscore the role of hormone loss, but preservation of GUCY2C receptor, expression as a key molecular mechanism underlying a number of pathophysiologic conditions. Indeed, they support the present studies defining a role for hormone insufficiency silencing GUCY2C as a novel pathophysiologic mechanism contributing to functional GI disorders like CIC and IBS-C.
There is a paucity of information regarding circulating levels of GUCY2C pro- and mature hormones in humans. Limited previous studies reveal basal levels of ProUGN in adults that were comparable with the levels reported herein, establishing confidence in these determinations (25,48). Furthermore, they are similar to basal circulating ProUGN levels quantified in healthy adolescents, consistent with the uniformity of ProUGN expression across the age continuum in the present study (20). Evoked increases in ProUGN induced by feeding in the present study recapitulate those observed in healthy adults in a limited series previously and in healthy adolescents (20,25). Interestingly, there are no reports quantifying the circulating levels of the mature peptide, UGN, in humans. Here, we report for the first time that circulating levels of UGN are about 10-fold lower than the circulating levels of ProUGN in healthy subjects, recapitulating results obtained in animals (8,12,28–30). Furthermore, while feeding evoked increases in ProUGN (20,25), eating did not alter the circulating levels of UGN in humans. These observations are consistent with a hypothesis that endocrine (circulating) levels of ProUGN responsive to feeding reflect synthesis and evoked secretion of this hormone by the small intestine (20,23,25,50). By contrast, circulating levels of UGN may not be evoked products of intestinal epithelial cells but, rather, reflect end-organ proteolysis of the pro-peptide to the mature hormone.
Although these observations offer potential insights into the pathophysiologic mechanisms underlying CIC and IBS-C, the results should be interpreted within the context of the limitations of this study. In that regard, this was an exploratory phase 0 study, and a sample of convenience was used, because there were no previous data in these patient populations that permitted power analyses to define cohort sizes. Similarly, investigators were not blinded to clinical diagnoses. Furthermore, this study was conducted at a single site, with participants from a well-defined geography, potentially limiting its generalizability. Moreover, inclusion criteria required patients to be free from pharmacotherapy for at least 14 days, potentially biasing recruitment to patients least affected by their condition. These considerations underscore the importance of a future validation study in which cohort sizes are adequately powered based on the data reported herein, investigators are blinded to patient diagnoses, patients across the continuum of disease severity are included, and multiple centers distributed geographically participate.
In conclusion, these studies reveal for the first time that under fasting and fed conditions, circulating levels of ProUGN are reduced in patients with CIC and those with IBS-C. They support a novel pathophysiologic mechanism, suggesting that the expression of ProUGN is reduced in the small intestine, creating a paracrine hormone insufficiency that reduces the activation of intestinal GUCY2C and epithelial cell cGMP signaling, attenuating fluid and electrolyte secretion required for a normal bowel function. These studies also suggest a previously unanticipated diagnostic paradigm in which evoked circulating levels of ProUGN induced by a standardized meal might be used as an objective biologic test to identify patients with CIC and those with IBS-C who could benefit from the treatment. Moreover, these novel observations support the therapeutic paradigm that treatment of chronic constipation using oral GUCY2C ligands, like plecanatide and linaclotide (18,19), represents replacement therapy for a hormone insufficiency syndrome.
CONFLICTS OF INTEREST
Guarantor of the article: Scott A. Waldman, MD, PhD.
Specific author contributions: S.A.W. initiated the concept, interpreted the data, and wrote the manuscript. R.T. designed the study, provided oversight of the clinical trial, and reviewed the data. H.C.F. performed statistical analyses, interpreted the data, and wrote the manuscript. P.W. performed the study and acquired the data. P.G. initiated the concept, designed the study, interpreted the data, and wrote the manuscript. Synergy Pharmaceuticals provided funding to support the work. All authors reviewed and contributed to the final version of the manuscript.
Financial support: This work was sponsored by the Synergy Pharmaceuticals Inc.
Potential competing interests: S.A.W. is the chair of the Scientific Advisory Board and a member of the Board of Directors (both uncompensated) of Targeted Diagnostics & Therapeutics, Inc. He is a member of the Scientific Advisory Boards of Therapeutic Architects, Inc., and MLH Exploration, LLC (both uncompensated). He is a member of the Board of Directors of Feelux, Inc. (compensated). He is the Samuel MV Hamilton endowed professor of medicine in Thomas Jefferson University. He receives research funding from Targeted Diagnostics & Therapeutics, Inc., Synergy Pharmaceuticals Inc., and the National Institutes of Health (R01 CA170533, R01 CA206026, P30 CA56036). P.G. is an employee of Synergy Pharmaceuticals Inc. R.T., P.W., and H.C.F. are consultants for Synergy Pharmaceuticals Inc.
Study Highlights.
WHAT IS KNOWN
✓ Although CIC and IBS-C are common GI disorders, molecular mechanisms contributing to their pathogenesis remain unknown.
✓ The GUCY2C receptor regulates intestinal secretion. Overactivation produces diarrhea, whereas signaling insufficiency produces constipation.
✓ ProUGN produced in the small bowel is secreted into the intestinal lumen where it is cleaved to the mature UGN peptide and binds to GUCY2C to stimulate secretion.
✓ Additionally, eating induces ProUGN secretion into the blood, which circulates to the hypothalamus to activate neuronal GUCY2C controlling feeding.
✓ We tested the novel hypothesis that chronic constipation syndromes reflect GUCY2C hormone insufficiency.
WHAT IS NEW HERE
✓ Fasting circulating ProUGN and UGN levels were lower in patients with constipation compared with healthy subjects.
✓ After eating, evoked ProUGN levels increased in the circulation at a slower rate, with maximum levels that were lower in patients with constipation.
✓ In contrast to ProUGN levels, UGN levels did not increase from fasting levels after eating.
TRANSLATIONAL IMPACT
✓ CIC and IBS-C reflect a contribution of ProUGN hormone insufficiency to intestinal hyposecretion.
✓ Evoked circulating levels of ProUGN induced by a standardized meal could be used as an objective test to identify patients with CIC and IBS-C with hormone insufficiency who could benefit from the treatment.
✓ CIC and IBS-C can be treated by oral GUCY2C ligand replacement to mitigate endogenous hormone insufficiency.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A66
REFERENCES
- 1.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2012;367:1626–35. [DOI] [PubMed] [Google Scholar]
- 2.Heidelbaugh JJ, Stelwagon M, Miller SA, et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol 2015;110:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–21.e4. [DOI] [PubMed] [Google Scholar]
- 4.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016. [Epub ahead of print February 18, 2016.] [DOI] [PubMed] [Google Scholar]
- 5.Nellesen D, Yee K, Chawla A, et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013;19:755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johanson JF, Kralstein J. Chronic constipation: A survey of the patient perspective. Aliment Pharmacol Ther 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- 7.Koch A, Voderholzer WA, Klauser AG, et al. Symptoms in chronic constipation. Dis Colon Rectum 1997;40:902–6. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 2016;96:751–804. [DOI] [PubMed] [Google Scholar]
- 9.Field M. Mechanisms of action of cholera and Escherichia coli enterotoxins. Am J Clin Nutr 1979;32:189–96. [DOI] [PubMed] [Google Scholar]
- 10.Guerrant RL, Hughes JM, Chang B, et al. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: Studies of tissue specificity, potential receptors, and intermediates. J Infect Dis 1980;142:220–8. [DOI] [PubMed] [Google Scholar]
- 11.Guarino A, Cohen M, Thompson M, et al. T84 cell receptor binding and guanyl cyclase activation by Escherichia coli heat-stable toxin. Am J Physiol 1987;253:G775–80. [DOI] [PubMed] [Google Scholar]
- 12.Hamra FK, Eber SL, Chin DT, et al. Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc Natl Acad Sci USA 1997;94:2705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenna Ø, Furnes MW, Munkvold B, et al. Cellular localization of guanylin and uroguanylin mRNAs in human and rat duodenal and colonic mucosa. Cell Tissue Res 2016;365:331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian X, Prabhakar S, Nandi A, et al. Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology 2000;141:3210–24. [DOI] [PubMed] [Google Scholar]
- 15.Klodt J, Kuhn M, Marx UC, et al. Synthesis, biological activity and isomerism of guanylate cyclase C-activating peptides guanylin and uroguanylin. J Pept Res 1997;50:222–30. [DOI] [PubMed] [Google Scholar]
- 16.Marx UC, Klodt J, Meyer M, et al. One peptide, two topologies: Structure and interconversion dynamics of human uroguanylin isomers. J Peptide Res 1998;52:229–40. [DOI] [PubMed] [Google Scholar]
- 17.Schulz A, Marx UC, Tidten N, et al. Side chain contributions to the interconversion of the topological isomers of guanylin-like peptides. J Pept Sci 2005;11:319–30. [DOI] [PubMed] [Google Scholar]
- 18.Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut 2018;67:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camilleri M. Guanylate cyclase C agonists: Emerging gastrointestinal therapies and actions. Gastroenterology 2015;148:483–7. [DOI] [PubMed] [Google Scholar]
- 20.Di Guglielmo MD, Tonb D, He Z, et al. Pilot study measuring the novel satiety hormone, pro-uroguanylin, in adolescents with and without obesity. J Pediatr Gastroenterol Nutr 2018;66:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folgueira C, Barja-Fernandez S, Gonzalez-Saenz P, et al. Uroguanylin: A new actor in the energy balance movie. J Mol Endocrinol 2018;60:R31–8. [DOI] [PubMed] [Google Scholar]
- 22.Folgueira C, Sanchez-Rebordelo E, Barja-Fernandez S, et al. Uroguanylin levels in intestine and plasma are regulated by nutritional status in a leptin-dependent manner. Eur J Nutr 2016;55:529–36. [DOI] [PubMed] [Google Scholar]
- 23.Kim GW, Lin JE, Snook AE, et al. Calorie-induced ER stress suppresses uroguanylin satiety signaling in diet-induced obesity. Nutr Diabetes 2016;6:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez A, Gómez-Ambrosi J, Catalán V, et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int J Obes (Lond) 2016;40:1405–15. [DOI] [PubMed] [Google Scholar]
- 25.Valentino MA, Lin JE, Snook AE, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest 2011;121:3578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folgueira C, Beiroa D, Callon A, et al. Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes 2016;65:421–32. [DOI] [PubMed] [Google Scholar]
- 27.Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3',5'-monophosphate. Gastroenterology 2013;145:1334–46. [DOI] [PubMed] [Google Scholar]
- 28.Currie MG, Fok KF, Kato J, et al. Guanylin: An endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA 1992;89:947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamra FK, Fan X, Krause WJ, et al. Prouroguanylin and proguanylin: Purification from colon, structure, and modulation of bioactivity by proteases. Endocrinology 1996;137:257–65. [DOI] [PubMed] [Google Scholar]
- 30.Hamra FK, Forte LR, Eber SL, et al. Uroguanylin: Structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci USA 1993;90:10464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Schulz S, Bombonati A, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology 2007;133:599–607. [DOI] [PubMed] [Google Scholar]
- 32.Lin JE, Li P, Snook AE, et al. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology 2010;138:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin JE, Snook AE, Li P, et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One 2012;7:e31686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shailubhai K, Yu HH, Karunanandaa K, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res 2000;60:5151–7. [PubMed] [Google Scholar]
- 35.Silos-Santiago I, Hannig G, Eutamene H, et al. Gastrointestinal pain: Unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain 2013;154:1820–30. [DOI] [PubMed] [Google Scholar]
- 36.Steinbrecher KA, Harmel-Laws E, Garin-Laflam MP, et al. Murine guanylate cyclase C regulates colonic injury and inflammation. J Immunol 2011;186:7205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenna Ø, Bruland T, Furnes MW, et al. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand J Gastroenterol 2015;50:1241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharman SK, Islam BN, Hou Y, et al. Cyclic-GMP-elevating agents suppress polyposis in Apc(Min) mice by targeting the preneoplastic epithelium. Cancer Prev Res (Phila) 2018;11:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinbrecher KA, Tuohy TM, Heppner Goss K, et al. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun 2000;273:225–30. [DOI] [PubMed] [Google Scholar]
- 40.Wilson C, Lin JE, Li P, et al. The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prev 2014;23:2328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiskerstrand T, Arshad N, Haukanes BI, et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med 2012;366:1586–95. [DOI] [PubMed] [Google Scholar]
- 42.Muller T, Rasool I, Heinz-Erian P, et al. Congenital secretory diarrhoea caused by activating germline mutations in GUCY2C. Gut 2015;65:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romi H, Cohen I, Landau D, et al. Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet 2012;90:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith A, Bulman DE, Goldsmith C, et al. Meconium ileus in a Lebanese family secondary to mutations in the GUCY2C gene. Eur J Hum Genet 2015;23:990–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377–90. [DOI] [PubMed] [Google Scholar]
- 46.Fukae H, Kinoshita H, Fujimoto S, et al. Plasma concentration of uroguanylin in patients on maintenance dialysis therapy. Nephron 2000;84:206–10. [DOI] [PubMed] [Google Scholar]
- 47.Kinoshita H, Fujimoto S, Fukae H, et al. Plasma and urine levels of uroguanylin, a new natriuretic peptide, in nephrotic syndrome. Nephron 1999;81:160–4. [DOI] [PubMed] [Google Scholar]
- 48.Narayan H, Mohammed N, Quinn PA, et al. Activation of a novel natriuretic endocrine system in humans with heart failure. Clin Sci 2010;118:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaar G, Dieplinger B, Gabriel C, et al. Proguanylin and prouroguanylin: Assay evaluation and clinical analyte characterization. Clin Chim Acta 2011;412:2277–83. [DOI] [PubMed] [Google Scholar]
- 50.Di Guglielmo MD, Perdue L, Adeyemi A, et al. Immunohistochemical staining for uroguanylin, a satiety hormone, is decreased in intestinal tissue specimens from female adolescents with obesity. Pediatr Dev Pathol 2018;21:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janecke AR, Heinz-Erian P, Yin J, et al. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum Mol Genet 2015;24:6614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mann EA, Jump ML, Wu J, et al. Mice lacking the guanylyl cyclase C receptor are resistant to STa-induced intestinal secretion. Biochem Biophys Res Commun 1997;239:463–6. [DOI] [PubMed] [Google Scholar]
- 53.Schulz S, Lopez MJ, Kuhn M, et al. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J Clin Invest 1997;100:1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camilleri M, Carlson P, Acosta A, et al. Colonic mucosal gene expression and genotype in irritable bowel syndrome patients with normal or elevated fecal bile acid excretion. Am J Physiol Gastrointest Liver Physiol 2015;309:G10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Videlock EJ, Mahurkar-Joshi S, Hoffman JM, et al. Sigmoid colon mucosal gene expression supports alterations of neuronal signaling in irritable bowel syndrome with constipation. Am J Physiol Gastrointest Liver Physiol 2018;315:G140–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin JE, Colon-Gonzalez F, Blomain E, et al. Obesity-induced colorectal cancer is driven by caloric silencing of the guanylin-GUCY2C paracrine signaling axis. Cancer Res 2016;76:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinberg DS, Lin JE, Foster NR, et al. Bioactivity of oral linaclotide in human colorectum for cancer chemoprevention. Cancer Prev Res (Phila) 2017;10:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.