INTRODUCTION:
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide. Today, a promising treatment strategy is focused on the enhancement of antitumor immune responses by immune checkpoint modification. However, as only 20% of patients with HCC are responders, identification of predictive factors is urgently required. Therefore, for the first time, the features of the intrahepatic and circulating immune system in patients with advanced-stage HCC, before and during the treatment, were analyzed.
METHODS:
We collected fresh HCC biopsies, along with adjacent tumor-free liver tissues and peripheral blood samples, from 21 patients with advanced HCC. Furthermore, we performed an extensive immunomonitoring of patients with HCC treated with sorafenib or programmed death (PD)-1/PD-L1 pathway blockade using multiparametric flow cytometry.
RESULTS:
We observed that regardless of the treatment, low baseline intratumoral CD4+/CD8+ T-cell ratio was associated with better overall survival (P = 0.0002). The baseline frequency of intratumoral PD-1high CD8+ T cells was significantly lower in patients responding to sorafenib treatment than in the nonresponders (P = 0.0117), and the frequency of circulating PD-1high T cells increased with tumor progression (P = 0.0329). By contrast, responders to PD-1/PD-L1 pathway blockade showed a trend of high baseline frequency of intratumoral PD-1high CD8+ T cells. Moreover, we observed a trend of LAG3 and TIM3 upregulation on circulating T cells in nonresponding patients to PD-1/PD-L1 pathway blockade.
DISCUSSION:
Immunosuppressive state, characterized by an enhanced intratumoral CD4+/CD8+ T-cell ratio, was associated with poor prognosis. Additionally, our results suggest that the frequency of intratumoral PD-1high CD8+ T cells may serve as a biomarker to identify which individuals will benefit from which treatment and support the use of combination strategies.
INTRODUCTION
In the last few decades, hepatocellular carcinoma (HCC)-related mortality has increased at a rate faster than mortality related to any other cancer type (1). Primarily for patients with advanced HCC, the available treatment options are extremely limited and the prognosis is very poor. A multi-tyrosine kinase inhibitor, sorafenib, is considered as a gold standard treatment of patient with HCC. However, its efficacy is limited, improved survival time is modest (2), and predictive factors of response are lacking. Therefore, there is an urgent need to determine an effective therapy for the treatment of patients with HCC. At present, an enhancement of antitumor immune responses via immunotherapies serves as a promising treatment strategy in the field of oncology. HCC is an important target for immunotherapy as chronic liver inflammation, which is associated with HCC risk factors (including chronic hepatitis B and C and metabolic disorders), and it promotes an immunosuppressive environment and T-cell exhaustion (3–6). Several inhibitory checkpoint molecules have been associated with this process, including the programmed death (PD)-1/PD-L1 immune checkpoint pathway. In patients suffering from HCC, the expression of PD-1 is constantly increased on CD8+ T cells (7), and the high frequency of circulating and tumor-infiltrating PD-1+ CD8+ T cells was associated with disease progression after curative hepatic resection (8). High PD-L1 expression was also determined as a predictor of tumor recurrence for patients with HCC (9) and was associated with tumor aggressiveness (10).
In September 2017, the Food and Drug Administration granted an accelerated approval to anti-PD-1 antibody nivolumab for the treatment of patients with HCC after a previous sorafenib, regardless of the PD-L1 status, based on the objective response rate observed in the phase I/II CheckMate 040 trial (15% in a dose-escalation cohort and 20% in a dose-expansion cohort (11)). Moreover, pembrolizumab (Keytruda; Merck, Kenilworth, NJ) was also tested in a phase 2 study concerning second-line treatment for advanced HCC after sorafenib failure, and the study confirmed an objective response rate of 17% (12). Based on this finding, in November 2018, the FDA approved pembrolizumab for the treatment of patients with HCC who have been previously treated with sorafenib. Nevertheless, more than 80% of such patients do not respond to this therapy. Therefore, there is an urgent need to better understand the subversion of the immune system during HCC and its modulations during treatment. Although important research has been already conducted in the field of melanoma and other types of cancer, wherein immunotherapies have been used for some time now, almost no data exist for the field of HCC. In fact, limited information is available at present regarding the effect of HCC therapies on the immune system of patients with advanced HCC, including the coexpression and potential compensatory changes of inhibitory and stimulatory checkpoint molecules. A deeper understanding of the mechanisms, functional relevance, and the pattern of coexpression of immune checkpoint molecules in HCC, surrounding liver and in circulation, is mandatory to develop more effective immunotherapeutic strategies and determine a better response for its treatment.
Recently, Zhou et al. (13) analyzed inhibitory immune checkpoint molecules in patients with early-stage HCC and pointed out the importance of PD-1, TIM3, and LAG3 pertaining to the inhibition of tumor-infiltrating lymphocytes' function. Considering the complexity of the receptor network that may have either similar or distinct pathways to the modulate immune system, extensive studies need to be conducted to clarify the expression of both inhibitory and stimulatory checkpoint molecules on several cell subsets. Moreover, extremely limited information is available at present concerning potential treatment-associated modulations of various immune checkpoint molecules during HCC therapy. Additionally, immune checkpoint blockade strategies are currently being tested in cases of advanced HCC. Hence, systematic analysis of immune checkpoint molecules at an advanced stage is required.
In this study, we provide a characterization of immune checkpoint expression in advanced HCC, at the circulating and liver tissue levels, based on fresh blood and liver biopsies. In addition, we provide detailed immunomonitoring of patients with HCC treated by means of the classical treatment (sorafenib) or by immunotherapies and present the link with the clinical evolution of patients.
METHODS
Patients and sample processing
Twenty-one patients (17 men and 4 women) suffering from advanced HCC were included in this study and were selected before the treatment (Department of Gastroenterology and Hepatology, CHU Grenoble-Alpes). The mean age of the patients was 71.6 ± 0.4 years, among which 81% were male individuals, and 52% of the patients had fibrosis (stage F4). Detailed patient characteristics are provided in Supplementary Table 1 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A58). Subsequently, blood samples obtained from 7 healthy donors were used for comparison. Liver biopsies (tumor and nontumor tissues) were divided into 2 parts. One part was used for histologic examination assessed by experienced liver pathologists to define whether biopsy was performed within HCC, whereas the other part was processed within 1 hour after the clinical biopsy to conduct extensive phenotypic and functional immunologic analyses.
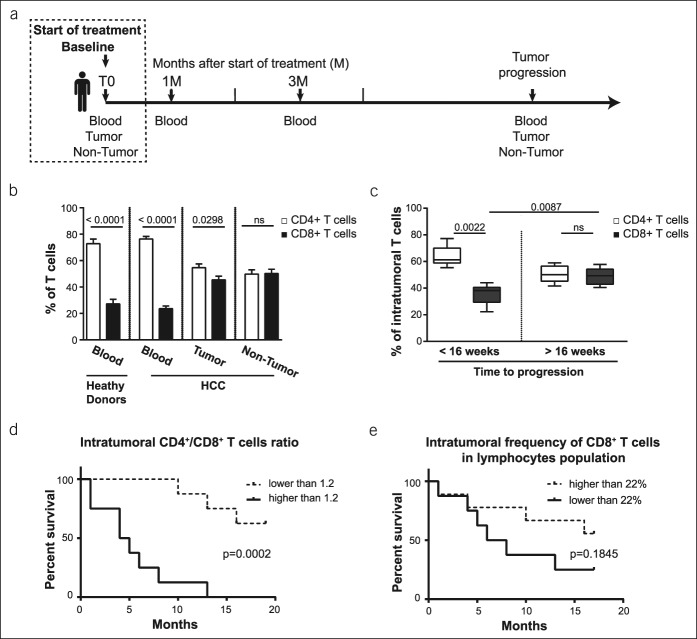
Furthermore, the patients were treated with sorafenib (n = 7), anti-PD-L1/transforming growth factor-β TRAP (n = 4), anti-PD-1 antibodies (nivolumab, n = 1; pembrolizumab, n = 3), c-Met inhibitor Tepotinib (n = 2), or were untreated (n = 4). In addition, anti-PD-L1/TGF-β TRAP and pembrolizumab were the second-line treatments provided after sorafenib, whereas nivolumab was the first-line treatment. For immunomonitoring, blood samples were collected during the time of treatment; the sampling plan is detailed in Figure 1.
Figure 1.
CD4+/CD8+ T-cell ratio at the baseline is related to clinical evolution in advanced HCC. (a) Sampling plan of patients with HCC. Samples were collected only when the condition of the patient allowed it. (b) Baseline frequency of CD4+ vs CD8+ T cells in the blood of healthy donors (n = 7), blood of patients with HCC (n = 21), tumor tissue (n = 16), and nontumoral tissue (n = 13); 2-tailed P value. (c) Frequency of T cells in the tumor tissues before the treatment. Patients were followed up to determine the time to tumor progression, and they were divided into groups with short time to tumor progression (<16 weeks, n = 6) and with progression-free status at 16 weeks (>16 weeks, n = 5); 2-tailed P value. (d) Log-rank (Mantel-Cox) overall survival curves segregating the cohort of patients according to the ratio of CD4+/CD8+ T cells in the tumor tissue at the baseline. (e) Log-rank (Mantel-Cox) overall survival curves segregating the cohort of patients according to the median of the frequency of intratumoral CD8+ T cells per lymphocyte population (per CD45+ cell) at the baseline. HCC, hepatocellular carcinoma.
Based on their radiologic response to therapy, the treated patients were later categorized into the following groups: (i) responders (complete or partial response), (ii) stable disease (SD), and (iii) nonresponders (progression) according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Additionally, the immunomonitoring parameters were analyzed with regard to the progression-free status of patients at 16 weeks as well as their overall survival. This study was performed in accordance with the Declaration of Helsinki and the French legislation based on local sample collection (DC-2014-2295), and all of its participants provided written informed consent.
Flow cytometry analyses
Immediately after the liver biopsy, tumor and nontumor samples were transferred in the RPMI medium and cells were recovered through mechanical disruption. Moreover, fresh peripheral blood and intrahepatic cell suspensions were immunostained without any stimulation, using the antihuman antibodies of surface markers, as described in SI.
Stimulation of immune cells
Peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll-Paque method, and cryopreserved PBMCs were stored in liquid nitrogen. Cells were resuspended (1 × 106/mL), and stimulation was performed by phorbol 12-myristate 13acetate (50 ng/mL; Sigma Aldrich [L'isle-d'Abeau Chesnes, France]) and ionomycin (1 μg/mL, Iono; Sigma) for 16 h at 37 °C in a CO2 incubator.
Cytometric bead array
The supernatant derived from stimulated and nonstimulated PBMC cultures was collected, after which the amount of cytokines produced by immune cells was evaluated using cytometric bead array (BD Bioscience, San Diego, CA), and the data were analyzed by the FCAP Array Software. Subsequently, the following cytokines were determined: interferon gamma, tumor necrosis factor α, granzyme B, IL-4, IL-6, IL-10, IL-13, and IL-17.
Assessment of soluble immune checkpoint molecules by multiplex immunoassays
Serum samples were analyzed via multiprofiling of immune checkpoint molecules using the Luminex MAGPIX system (Research Platform, Département de Biochimie, Toxicologie et Pharmacologie, Institut de Biologie et Pathologie, CHU Grenoble-Alpes) with the following panels: PD-1, PD-L1, PD-L2, CTLA-4, TIM-3, GITR, GITRL, LAG-3, and CD137 (4-1BB).
Statistical analysis
Analyses were performed using the statistical software GraphPad Prism 6 (GraphPad Software, CA). Normal distribution was tested using the D'Agostino-Pearson omnibus normality test. When data derived from both cohorts were normally distributed, the unpaired t test was used to determine significant differences observed between the groups. Contrarily, when data from either cohort were not normally distributed, the Mann-Whitney test was performed. The nonparametric Kruskal-Wallis one-way analysis of variance was used for multiple comparisons. Furthermore, the Spearman correlation nonparametric test was conducted to determine the degree of correlation between 2 variables, and P value of <0.05 was considered significant.
RESULTS
CD4+/CD8+ T-cell ratio is strongly linked to the clinical outcomes
Our cohort was based on 21 patients suffering from advanced HCC. Only liver biopsy samples that were histologically approved by pathologists for sampling within HCC area (tumor samples) and nontumor area (nontumor samples) were included in this study.
For the flow cytometry analysis, fresh samples were stained using 2 panels for the identification of the major lymphocyte populations and the expression of immune checkpoint molecules (refer to Methods). Moreover, we investigated T cells, natural killer (NK) cells, and NKT cells in the blood, in conjunction with nontumor and tumor liver tissue obtained from patients with advanced HCC, by the strategy concerning the principle of gating as previously described (14–16) (Supplementary Figure 1a, see Supplementary Digital Content 1, http://links.lww.com/CTG/A58), whereas isotype controls were used to define the positivity of each marker (Supplementary Figure 1b, see Supplementary Digital Content 1, http://links.lww.com/CTG/A58).
In our cohort of patients with advanced HCC, CD3+CD56− T cells found in the blood accounted for more than 60% of all CD45high lymphocytes, whereas in the liver, their frequency reduced to 50%. Contrarily, CD3+CD56+ cells (NKT and CD3brightCD56+ T cells) and CD3−CD56+ NK cells represented a significantly smaller population in the blood (7 and 11%), whereas their frequency increased up to 21 and 21% in nontumor liver tissues and 19 and 17% in tumor liver tissues, respectively (Supplementary Figures 1b and 2a, see Supplementary Digital Content 1, http://links.lww.com/CTG/A58).
As expected, 24% of the circulating T cells were CD8+; the absolute number of T cells is listed in Supplementary Table 2 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A58). In the liver, CD8+ T cells represented approximately 45% of the T-cell population in the tumor tissue and 50% in the nontumoral part of the tissue (Figure 1b). Notably, in our cohort of patients, the frequency of intratumoral CD8+ T cells before the treatment was associated with clinical outcomes. Moreover, the patients were divided into 2 groups according to their progression-free status at 16 weeks. We observed that irrespective of the following treatment, the frequency of intratumoral CD8+ T cells at the baseline was higher in patients who were free of progression at 16 weeks (P = 0.0087), as depicted in Figure 1c. In fact, in patients with time to tumor progression higher than 16 weeks, the intratumoral T-cell population is composed of 50.7% ± 2.9% of CD4+ cells and 49.3% ± 2.9% of CD8+ cells (not significant), whereas in patients with time to tumor progression shorter than 16 weeks, the intratumoral CD4+ T-cell population was significantly higher (63.8% ± 3.1%) compared with CD8+ T cells (36.2% ± 3.1%; P = 0.0022).
The median intratumoral CD4+/CD8+ T-cell ratio was 1.2. Based on this median, a high intratumoral CD4+/CD8+ T-cell ratio was a negative predictive factor of the patients' overall survival (P = 0.0002) (Figure 1d). The median overall survival of patients with a ratio lower than 1.2 was 16.2 ± 1.6 months in comparison with 4.6 ± 1.4 months for patients with a ratio higher than 1.2 (P = 0.0002) (Figure 1d). Subsequently, we separated the cohort of patients according to the median of the frequency of the intratumoral CD8+ T cells per lymphocyte population (per CD45+ cell) at the baseline. We observed the tendency of association regarding the high frequency of intratumoral CD8+ T cells per lymphocyte population at the baseline with improved survival (P = 0.1845) (Figure 1e).
In addition, cytokine secretion on stimulation of PBMCs, with phorbol 12-myristate 13acetate/Iono analyzed by cytometric bead array, showed that from all cytokines, only secreted IL-10 levels of both unstimulated and stimulated PBMCs were positively correlated to CD4+ T cells and negatively correlated to CD8+ T cells. In fact, we found a significant positive correlation between the IL-10 levels secreted by PBMCs in circulation and CD4+/CD8+ T-cell ratio in both tumoral and nontumoral tissues (Supplementary Table 3, see Supplementary Digital Content 1, http://links.lww.com/CTG/A58). However, there was no direct association with the clinical outcomes. Collectively, our results show that the immunosuppressive state, characterized by enhanced IL-10 levels and high intrahepatic CD4+/CD8+ T-cell ratio, was associated with poor prognosis.
Immune checkpoint distribution on lymphocyte subsets in advanced HCC before therapy
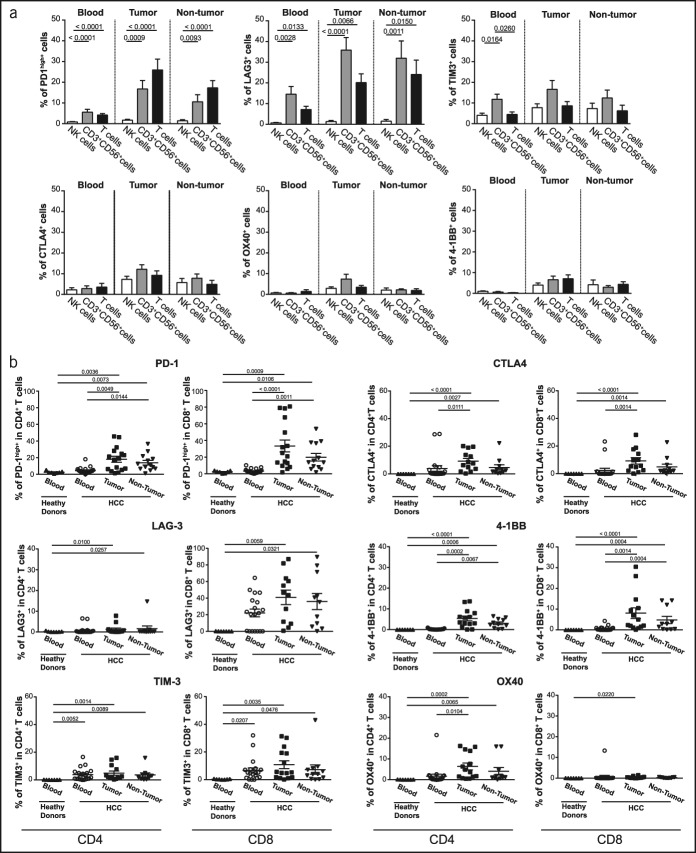
We investigated the frequency of NK cells, CD3+CD56+ cells (NKT and CD3brightCD56+ T cells), and T cells expressing immune checkpoint molecules on the cell surface (PD-1, TIM3, LAG3, CTLA4, 4-1BB, and OX40) in fresh samples of peripheral blood and analyzed tumoral and nontumoral biopsies of patients with advanced HCC. As the expression of PD-1 increases constantly in patients with HCC (7), we focused directly on the PD-1high population (Supplementary Figure 1b, see Supplementary Digital Content 1, http://links.lww.com/CTG/A58), as observed in the recent publications (17,18).
In our cohort, the frequency of PD-1high or LAG3+ NK cells was negligible compared with that of CD3+CD56+ cells or the T-cell population in the blood, tumor tissues, and/or nontumor tissues (Figure 2a). However, in terms of their frequency, NK cells expressing TIM3, CTLA-4, 4-1BB, and OX40 were similar to T cells and CD3+CD56+ cells (Figure 2a).
Figure 2.
Immune checkpoint distribution on lymphocyte subsets in advanced HCC at the baseline. (a) The percentage of immune checkpoint-positive cells among NK, CD3+CD56+ cells (NKT and CD3brightCD56+ T cells), and T cells in the blood (n = 21), tumor tissue (n = 16), and nontumoral tissue (n = 13). (b) The percentage of immune checkpoint-positive cells among CD4+ or CD8+ T cells in the blood (n = 21), tumor tissue (n = 16), and nontumoral tissue (n = 13). Each dot represents a patient. The nonparametric Kruskal-Wallis one-way analysis of variance was used for multiple comparisons. HCC, hepatocellular carcinoma.
Subsequently, we analyzed the distribution of immune checkpoint molecules in the population of CD4+ and CD8+ T cells (Figure 2b, Supplementary Figure 2b, see Supplementary Digital Content 1, http://links.lww.com/CTG/A58). Furthermore, the inhibitory checkpoint molecules PD-1, LAG3, and TIM3 were expressed primarily by CD8+ T cells, whereas a stimulatory checkpoint molecule, OX40, was expressed preferentially by CD4+ T lymphocytes (Figure 2b, Supplementary Figure 2b, see Supplementary Digital Content 1, http://links.lww.com/CTG/A58). CTLA4 and 4-1BB were equally expressed in CD8+ and CD4+ T cells. Moreover, the expression of immune checkpoint molecules was not statistically different between the tumor and nontumor tissues (Figure 2b).
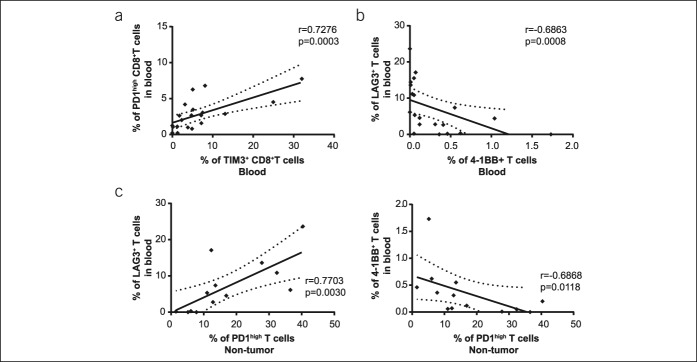
Because we analyzed the markers of immune checkpoint receptors in 2 separated tubes, we cannot provide data pertaining to their coexpression per individual cell for the entire cohort. However, the positive correlation between the percentage of PD-1high CD8+ T cells and TIM3+ CD8+ T cells in circulation suggested frequent coexpression of those receptors (r = 0.7276, P = 0.0003), as shown in Figure 3a. Contrarily, a negative correlation was observed between the cells expressing the inhibitory receptor LAG3 and the stimulatory receptor 4-1BB (r = −0.6863, P = 0.0008) (Figure 3b). Similarly, the frequency of LAG3+ and 4-1BB+ T cells in the blood was associated with PD-1high T cells in nontumoral tissues (Figure 3c).
Figure 3.
Correlations between immune checkpoint-positive cells. (a) Correlation between PD-1high CD8+ T cells and TIM3+ CD8+ T cells in the blood of patients with HCC. (b) Correlation between the frequency of circulating LAG3+ and 4-1BB+ T cells. (c) Correlation between the frequency of circulating LAG3+ T cells (left) or 4-1BB+ T cells (right), with PD-1high T cells in the liver tissue. Each dot represents a patient. HCC, hepatocellular carcinoma; PD, programmed death; r, Spearman correlation coefficient.
As expected, we found positive correlations between the circulating and liver tissue expression of the immune checkpoint receptors (Supplementary Table 4, see Supplementary Digital Content 1, http://links.lww.com/CTG/A58), notably between the circulating and intratumoral frequency of 4-1BB+ T cells (r = 0.6143, P = 0.0255) and PD-1high cells (r = 0.4857, P = 0.0505), indicating that the expression of immune checkpoint molecules in the tumor tissue is partially reflected on the circulating levels.
Interestingly, the frequency of intratumoral PD-1high T cells, LAG3+ T cells, OX40+ T cells, and CD69+ CD4+ T cells was positively correlated to the most widely used biomarker of HCC, circulating alpha-fetoprotein, as shown in Supplementary Table 5 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A58), indicating the possible link between one's immune status and tumor growth.
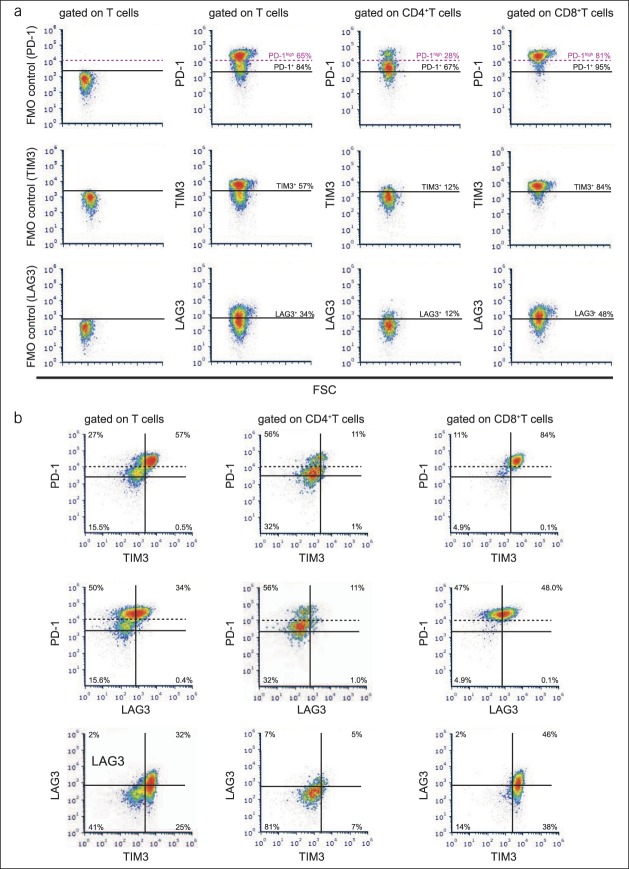
In a small cohort of patients with HCC, we directly determined the coexpression of PD-1, TIM3, and LAG3. Representative flow cytometry plots of patients with advanced HCC with a high frequency of intratumoral PD-1high T cells (Figure 4a) show that TIM3 and LAG3 expressions were predominantly observed in PD1high intratumoral CD8+ T cells. Evidently, most intratumoral PD-1high CD8+ T cells coexpressed TIM3, but only a part of PD-1high CD8+ T cells expressed LAG3 (Figure 4b).
Figure 4.
TIM3 and LAG3 expressions are predominantly observed in PD1high intratumoral CD8+ T cells in advanced HCC. (a) Representative flow cytometry plots of immune checkpoint molecules, including FMO controls. (b) Coexpression patterns of PD-1, TIM3, and LAG3 in the intratumoral T cells of patients with advanced HCC. FSC, forward scatter; FMO, fluorescence minus one; HCC, hepatocellular carcinoma; PD, programmed death.
Immunologic features of sorafenib-treated patients
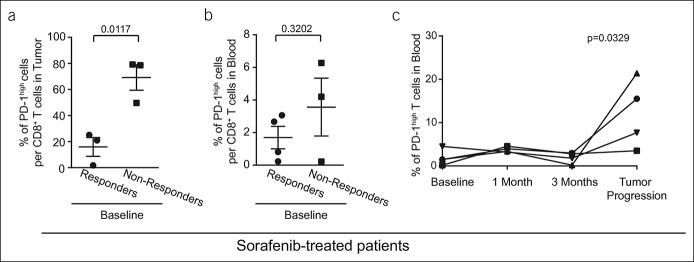
In our cohort, 7 patients were treated with sorafenib, and we collected the results from their blood samples (n = 7) and liver biopsies (n = 6). First, we sought to identify the cell population that best described the differences between the responders and nonresponders with respect to sorafenib at the baseline of treatment. Sorafenib-treated patients were categorized into the following groups based on the radiologic response to therapy: (i) responders (partial response; n = 3) and (ii) nonresponders (tumor progression; n = 4). From all immune checkpoint molecules, only PD-1 expression was found to be different at the baseline when comparing the responders with the nonresponders. In fact, in the tumor tissues, the baseline frequency of PD-1high CD8+ T cells was drastically and significantly lower in responders to sorafenib treatment (15.9% ± 7.2%, n = 3) in comparison with the nonresponders (69.1% ± 9.7%, n = 3; P = 0.0117), as shown in Figure 5a. In the blood, the same tendency was observed, but the difference did not reach the significance (responders: 1.7% ± 0.7%, n = 3; nonresponders: 3.6% ± 1.8%, n = 4; P = 0.3202) (Figure 5b).
Figure 5.
PD-1high CD8+ T-cell status in sorafenib-treated patients. (a) Intratumoral frequency of PD-1high CD8+ T cells at the baseline of patients who later responded to sorafenib (n = 3) or those who do not do so (n = 3). (b) Frequency of PD-1high CD8+ T cells in the blood at the baseline of patients who later responded to sorafenib (n = 4) or those who do not do so (n = 3). Mann-Whitney U test was used to compare responders and nonresponders. (c) Immunomonitoring of patients treated with sorafenib (n = 4). Circulating PD-1high T cells at 4 different time points: (i) before treatment, (ii) 1 month after the commencement of sorafenib treatment, (iii) 3 months after the beginning of treatment, and (iv) in case of tumor progression (based on radiologic evaluation); nonparametric Friedman test. Each dot represents a patient; mean ± SE, 2-tailed P value. PD, programmed death.
In addition, in 4 sorafenib-treated patients, we got the opportunity to perform detailed immunomonitoring. To follow the immunologic changes induced during the treatment, we performed flow cytometry analyses of the blood at 4 different time points: (i) before treatment, (ii) 1 month after the start of sorafenib treatment, (iii) 3 months after the start of the treatment, and (iv) in case of tumor progression (based on radiologic evaluation). We determined that out of all immune checkpoint molecules, only the frequencies of PD-1high cells were modified during the immunomonitoring period. In fact, the mean frequency of the circulating PD-1high T cells was 1.92% ± 0.92% at the baseline and increased to 12.04% ± 3.99% during tumor progression (Figure 5c). This indicates that the expression of PD-1 on circulating levels may reflect the progression of HCC during sorafenib treatment and can serve as a biomarker of response and PD-1 expression on intratumoral T cells. Moreover, it could be used as a predictive factor of response.
Immunomonitoring of PD-1/PD-L1 pathway blockade-treated patients
In our cohort, 8 patients were treated via PD-1/PD-L1 pathway blockade therapies by either anti-PD-L1/TGF-β TRAP (n = 4) or PD-1 antibodies (nivolumab, n = 1; pembrolizumab, n = 3). In 6 of these patients, we had access to their liver biopsies.
To describe the differences between the responders and nonresponders to PD-1/PD-L1 pathway blockade at the baseline of treatment, the patients were divided into the following categories based on their radiologic evaluation: (i) responders (complete or partial response, n = 2), (ii) SD (n = 3), and (iii) nonresponders (tumor progression, n = 3).
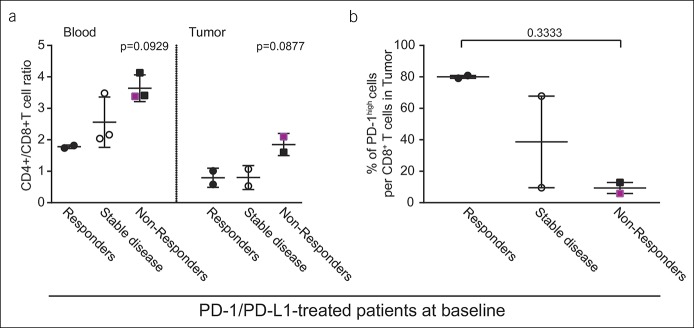
As expected from their response to checkpoint blockade, we found that patients who were classified as responders had lower CD4+/CD8+ ratio and therefore higher frequency of CD8+ T cells per T-cell population in both blood and tumor tissue in comparison with the nonresponders (Figure 6a), but due to the low sample number, this difference is not significant. In contrast to the results obtained from sorafenib-treated patients, responders to PD-1/PD-L1 pathway blockade had 8 times higher baseline frequency of intratumoral PD-1high cells within the CD8+ T-cell population compared with the nonresponders (80.0% ± 0.9%, n = 2 vs 9.4% ± 3.5%, n = 2; P = 0.3333) (Figure 6b). Similarly, slightly higher frequency of intratumoral TIM3+ cells within the CD8+ T-cell population was observed in responders to PD-1/PDL-1 treatment when compared with the nonresponders (23.9% ± 2.5%, n = 2 vs 12.3% ± 4.5%, n = 2; P = 0.4586).
Figure 6.
T-cell status in PD-1/PD-L1 pathway blockade-treated patients at the baseline. (a) CD4+/CD8+ T-cell ratio in the blood and tumor tissues at the baseline and (b) intratumoral frequency of PD-1high CD8+ T cells at the baseline of the patients who later responded to PD-1/PD-L1 therapy (n = 2), display stable disease (n = 3/2), or those who were nonresponders (n = 2). Fuchsia denotes a patient with anti-PD-1 antibody as the first-line treatment, whereas black denotes a patient with anti-PD-1/PD-L1 treatment as the second-line treatment after sorafenib. Each dot represents a patient; mean ± SE. PD, programmed death.
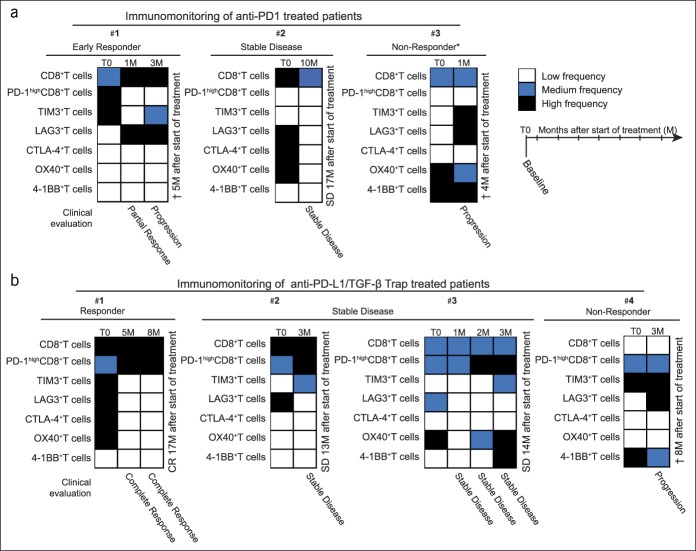
To follow the immunologic changes induced during PD-1/PD-L1 pathway blockade, we performed immunomonitoring on the circulating level. Additionally, we analyzed fresh blood samples taken during different time points from 3 patients treated with anti-PD-1 antibody (Figure 7a) and from 4 patients treated with anti-PD-L1/TGF-β TRAP (Figure 7b). The results of the same are expressed as a heat map-based frequency of the positive cells compared with the mean frequency of a corresponding subpopulation of the entire cohort of patients with advanced HCC before treatment (T0), i.e., low (<mean frequency, −20%), medium (mean frequency, ±20%), and high (>mean frequency, +20%).
Figure 7.
Immunomonitoring of PD-1/PD-L1 pathway blockade-treated patients in the blood. (a) Immunomonitoring of patients treated with anti-PD-1 antibody (n = 3). (b) Immunomonitoring of patients treated with anti-PD-L1/TGF-β Trap (n = 4); T0, baseline; M, month after the start of treatment. Results are expressed as a heat map-based frequency of positive cells compared with the mean frequency of a corresponding subpopulation of the entire cohort at the baseline (T0): low (white; <mean, −20%), medium (blue; mean, ±20%), and high (black; >mean, +20%). *Anti-PD-1 antibody as the first-line treatment; the rest were second-line treatments after sorafenib. PD, programmed death.
Furthermore, we noticed that after anti-PD-1 antibody administration, the expression of PD-1 was hardly detectable in the fresh blood samples (Figure 7a), probably owing to receptor occupancy by the therapeutic antibody. However, the antibodies that specifically detect therapeutic anti-PD-1 antibodies were not used in this study to directly investigate the PD-1 receptor occupancy after anti-PD-1 treatment. As expected, this effect on PD-1 detection was not observed in patients treated with anti-PD-L1/TGF-β TRAP (Figure 7b), as the said treatment directly targets PD-L1.
Interestingly, in patients who did not respond to PD-1/PD-L1 pathway blockade, we observed the compensatory upregulation of LAG3 and TIM3 inhibitory immune checkpoints on circulating T cells. Contrarily, the frequency of inhibitory immune checkpoint-positive T cells decreased in patients who achieved long-term SD or complete response (Figure 7a,b). It is noteworthy that we found no significant correlation between the immune checkpoints on circulating immune cells and the concentration of corresponding soluble immune checkpoints measured by multiplex immunoassays.
Collectively, we provided an evidence of feasibility along with the potential significance of detailed immunomonitoring performed by multiparameter flow cytometry during the treatment of patients suffering from advanced HCC. Importantly, compensatory changes in TIM3 and LAG3 after PD-1/PD-L1 pathway blockade therapy may support the use of immunotherapy combination strategies targeting multiple immune checkpoints in advanced HCC.
DISCUSSION
In this study, we focused on a cohort of patients with advanced-stage HCC. To the best of our knowledge, this is the first study to analyze immunologic intrahepatic and circulating parameters before treatment, which followed the immunologic changes induced during HCC treatment. By performing extensive phenotypic and functional analyses of the immune cells, we highlighted unique clinical correlates. To elaborate, we achieved the following in this study: (i) defined the immune checkpoint expression on both circulating and tumor-infiltrating lymphocytes in advanced HCC, (ii) highlighted prognostic factors of clinical evolution and (iii) pointed out specific immune features, allowing one to distinguish responders and nonresponders to targeted therapy with tyrosine kinase inhibitor and immunotherapy treatments. This study is limited due to the low number of patients receiving each treatment, but it demonstrates the importance of detailed immunomonitoring during HCC therapies and the importance of analyzing fresh biopsies of patients to define predictive factors of response. Moreover, our results suggest that the CD4+/CD8+ T-cell ratio and the frequency of intratumoral PD-1high CD8+ T cells may serve as markers to identify which patients will benefit from which treatment. In our study, the baseline frequency of CD8+ T cells per T-cell population in the tumor tissue was significantly associated with a positive clinical outcome, which is in accordance with the findings of research works conducted on most other cancers (19,20) including HCC (21,22), wherein an increased number of tumor-infiltrating CD8+ T cells predicts a favorable prognosis. Furthermore, CD8+ T cells are the key effector cells for antitumor immunity, mainly tumor-associated, antigen-specific CD8+ T cells, which are known to represent an important component of the host's immune response against a tumor.
By contrast, Tregs, the dominant subset of CD4+ T cells in the late stages of cancer (23), are known to be unfavorable prognostic markers in patients with HCC (24–26). In more advanced stages of HCC, the entire CD4/CD8 T-cell ratio is modified as the CD8+ T-cell population reduces, which is associated with an increase in the frequency of CD4+ T cells (22,27). In this research, we show that intratumoral CD4/CD8 T-cell ratio is clearly associated with tumor progression and overall survival in patients with advanced HCC, independent of the following treatment. This is not surprising as Tregs are preferentially enriched in the CD4+ T-cell population in patients with HCC and repress CD8+ T-cell functions, as demonstrated previously (25,27–29). However, we could not analyze the proportion of Tregs in the CD4+ T-cell population in this study, which limits the interpretation of its results.
We highlighted that PD-1 expression on T cells is another important marker associated with treatment's response. Our results suggest that reduced intratumoral frequency of PD-1high cells within the CD8+ T-cell population at the baseline predicts the patient's response to sorafenib treatment. This is in line with a recent report proposing low intratumoral frequency of PD-1+ CD8+ T cells as a biomarker of response to sorafenib treatment in patients with HCC (30). In addition, reduction in circulating PD-1+ T cells correlates to the survival of patients with HCC after sorafenib therapy (31). Similarly, our results demonstrate that circulating PD-1+ T cells increase during tumor progression, suggesting that the expression of PD-1 on the circulating level reflects the progression of HCC during sorafenib treatment.
The opposite situation occurs in the patients receiving immunotherapy, blocking the PD-1/PD-L1 inhibitory pathway. The principle of PD-1/PD-L1 pathway blockade therapy is to reinvigorate preexisting intratumoral T cells by removing the inhibition induced by the activation of the PD-1/PD-L1 axis and finally induce tumor rejection. Thus, the accumulation of PD-1+ CD8+ T cells in a tumor at the baseline often defines a subgroup of patients who are able to respond to PD-1/PD-L1 pathway blockade therapy (as reviewed by Simon and Labarriere (20)). Especially, the PD-1high CD8+ T cells seem to be crucial as this subset shows the following: (i) higher capacity for tumor recognition and (ii) markedly different transcriptional and metabolic profiles compared with PD-1neg and PD-1int lymphocytes (18). Significantly, the frequency of intratumoral PD-1high cells was strongly predictive for both the response and survival of patients with non–small cell lung cancer treated with PD-1 blockade (18). Similarly, patients with advanced malignant melanoma, who received anti-PD-1 antibodies and had more than 20% of tumor-infiltrating CTLA-4high PD-1high cells within their CD8+ T-cell population, showed a favorable response to treatment compared with patients with 20% and less CTLA-4high PD-1high cells per CD8+ T-cell population (17). Accordingly, we observed a very high baseline frequency of intratumoral PD-1high cells per CD8+ T cell in patients experiencing tumor response to PD-1/PD-L1 pathway blockade, whereas a low frequency was observed in nonresponders. Importantly, a recent study investigating CD8+ T cells isolated from HCC specimens also demonstrated that tumors with high proportions of PD-1high CD8+ T cells are susceptible to immune checkpoint blockade-based therapies, as this subset expresses multiple immune checkpoint receptors and could be further reinvigorated by immune checkpoint blockade (32). Similarly, Chew et al. recently demonstrated that HCC tissues' resident memory PD-1+ CD8+ T cells constitute the predominant T-cell subset responsive to anti-PD-1 treatment in vitro (29).
Unfortunately, PD-1 expression on CD8+ T cells was not assessed as a part of the CheckMate 040 clinical trial; only the PD-L1 expression on tumor cells was noted. Moreover, objective responses were observed in 26% of the patients with PD-L1 expression on at least 1% of tumor cells and in 19% of patients with PD-L1 on less than 1% of tumor cells, showing no significant difference in this regard (11). However, data pertaining to PD-1 and PD-L1 expression on tumor-infiltrating immune cells are not available for a CheckMate 040 clinical trial.
Today, anti-PD-1 antibody nivolumab is approved in the United States for the treatment of patients with HCC after the first-line treatment of sorafenib. From this perspective, the frequency of intratumoral PD-1high CD8+ T cells may serve as an immune marker to divide patients with HCC to a subgroup with a low frequency of intratumoral PD-1high CD8+ T cells, which may benefit from sorafenib treatment, and a subgroup with a high frequency of intratumoral PD-1high CD8+ T cells that should instead be treated directly via PD-1/PD-L1 pathway blockade.
There is growing evidence that the efficacy of single immunotherapies is often limited by compensatory induction of other immune checkpoint molecules, which contributes to a feedback loop that acts to mediate immune suppression. Compensatory upregulation of inhibitory checkpoint molecules after PD-1 blockade was recently described in mouse models of lung adenocarcinoma (33) and in ovarian tumor (34). Herein, we analyzed the immune changes that take place in peripheral blood during treatment and observed the compensatory upregulation of LAG3 and TIM3 inhibitory immune checkpoints on T cells in nonresponding patients to PD-1/PD-L1 pathway blockade. However, further analyses are needed to validate these findings, preferentially as translational protocols in clinical trial cohorts. Such information will be crucial for the rational design of combinatorial immune checkpoint blockade in advanced HCC, to increase the number of responders and the efficacy of treatment. For instance, simultaneous blockade of the PD-L1 and TGF-β pathways by anti-PD-L1/TGF-β bifunctional immunotherapy fusion protein showed superior antitumor activity in relation to monotherapies (35). Similarly, in our study, we observed that 3 of the 4 patients treated with anti-PD-L1/TGF-β clearly benefited from this therapy. Thus, combination therapies constitute a logical step in this regard, and adequate information to select proper immunotherapy combination partners and the biomarkers of response is required currently. However, the possibility that additional TGF-beta treatment affected the immunomonitoring results also needs to be taken into account.
In this study, we thoroughly characterized immune checkpoint distribution on lymphocyte subsets. Recently, Zhou et al. published the characterization of inhibitory immune checkpoint molecules in the early stages of HCC13 based on samples obtained from surgical resection. The authors reported that the expression of PD-1, TIM3, LAG3, and CTLA4 is significantly higher on T cells isolated from tumor tissue than from nontumoral tissue. In our cohort of patients with advanced HCC, we observed only a slightly higher frequency of immune checkpoint molecules in the tumor tissue compared with nontumoral tissues. This is an important observation reflecting the difference between early-stage and advanced-stage HCC4. Similarly, we observed a very high frequency of LAG3-positive cells, which is probably also related to the advanced stage of HCC. Previously, it has been shown that in lung cancer, LAG3 increases with disease stage (36). Contrarily, the CTLA-4 surface expression was low in our study. The intracellular staining would be needed to characterize CTLA-4 completely as most of the CTLA-4 protein resides intracellularly (37).
In addition, in this study we provided information about the expression of stimulatory immune checkpoint molecules OX40 and 4-1BB. The former is known to be expressed mainly by Treg, promoting immune tolerance (38). High OX40 expression was associated with high serum alpha-fetoprotein levels and shorter survival of patients with HCC (39). Accordingly, we observed that OX40 is expressed primarily by CD4+ T cells and that intratumoral frequency of OX40+ T cells and OX40+ CD4+ T cells strongly correlates to the alpha-fetoprotein levels. The stimulatory immune checkpoint 4-1BB was expressed by both CD4+ and CD8+ T cells, and the expression correlated negatively to the expression of inhibitory immune checkpoints. Interestingly, 4-1BB agonist utomilumab was recently used in a phase I study of patients with advanced cancer (40), and it is currently tested in combination with anti-PD-1 antibodies in solid cancers.
The analysis of immune checkpoint expression highlighted that CD3+CD56+ cells express a high level of immune checkpoint molecules, indicating that NKT and CD3brightCD56+ T cells may also be targeted by immune checkpoint blockers and could be a part of the mechanism of action.
The main limitation of our study is the sample size, which especially hampers subgroup analyses that might be of interest. Further study with larger number of patients, also including patients with different stages of HCC, will be necessary. To conclude, apart from the characterization of immune checkpoint molecules on crucial antitumor effectors in advanced HCC, we also demonstrated the feasibility and potential importance of detailed immunomonitoring during therapies, to define predictive factors of response and the mechanism of immunotherapy resistance.
CONFLICTS OF INTEREST
Guarantor of the article: Thomas Decaens, MD, PhD.
Specific author contributions: Z.M.J., C.A., P.N.M., T.D.: conception and design of the study. C.S., T.D.: sample collection. Z.M.J., C.A., K.K., A.G., N.S., T.D.: acquisition of data. Z.M.J., C.A., K.K., A.G., N.S., T.D.: analysis and interpretation of the data. Z.M.J.: drafting of the article. Z.M.J., C.A., P.N.M., T.D.: critical revision of the article for important intellectual content. Z.M.J., C.A., K.K., A.G., C.S., N.S., P.N.M., T.D.: approval of the final version.
Financial support: This work is funded by la Ligue Nationale Contre le Cancer under Grant 2016-R16145CC—Le comité de Haute-Savoie de La Ligue Contre le Cancer (CD74).
Potential competing interests: None.
Study Highlights.
WHAT ISKNOWN
✓ Immunity is a major player in HCC.
✓ Immune checkpoint therapy is a promising treatment of HCC, but less than 20% of patients respond to this treatment.
✓Predictors of tumor response to HCC treatment are missing.
WHAT IS NEWHERE
✓ Intratumoral CD4+/CD8+T-cell ratio at the baseline negatively correlates with the overall survival.
✓ A high baseline frequency of intratumoral PD-1high CD8+T cells is negatively associated with tumor response to sorafenib but positively associated with tumor response to PD-L1/PD-1 pathway blockade.
TRANSLATIONAL IMPACT
✓ Immunomonitoring helps identify the best combination strategies for HCC treatment.
✓ The frequency of intratumoral PD-1high CD8+ T cells may serve as a biomarker to identify which individuals will benefit from which treatment.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A58
REFERENCES
- 1.Sawyers CL, Abate-Shen C, Anderson KC, et al. AACR cancer progress report 2013. Clin Cancer Res 2013;19:S4–98. [DOI] [PubMed] [Google Scholar]
- 2.Sangiovanni A, Colombo M. Treatment of hepatocellular carcinoma: Beyond international guidelines. Liver Int 2016;36(Suppl 1):124–9. [DOI] [PubMed] [Google Scholar]
- 3.Ringelhan M, Pfister D, O'Connor T, et al. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19:222–32. [DOI] [PubMed] [Google Scholar]
- 4.Elsegood CL, Tirnitz-Parker JE, Olynyk JK, et al. Immune checkpoint inhibition: Prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunol 2017;6:e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth GS, Decaens T. Liver immunotolerance and hepatocellular carcinoma: Patho-physiological mechanisms and therapeutic perspectives. Eur J Cancer 2017;87:101–12. [DOI] [PubMed] [Google Scholar]
- 6.Ramzan M, Sturm N, Decaens T, et al. Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int 2016;36:434–44. [DOI] [PubMed] [Google Scholar]
- 7.Wang BJ, Bao JJ, Wang JZ, et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol 2011;17:3322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011;128:887–96. [DOI] [PubMed] [Google Scholar]
- 9.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971–9. [DOI] [PubMed] [Google Scholar]
- 10.Calderaro J, Rousseau B, Amaddeo G, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship with clinical and pathological features. Hepatology 2016;64:2038–46. [DOI] [PubMed] [Google Scholar]
- 11.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–52. [DOI] [PubMed] [Google Scholar]
- 13.Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology 2017;153:1107–19.e10. [DOI] [PubMed] [Google Scholar]
- 14.Jouvin-Marche E, Macek Jilkova Z, Thelu MA, et al. Lymphocytes degranulation in liver in hepatitis C virus carriers is associated with IFNL4 polymorphisms and ALT levels. J Infect Dis 2014;209:1907–15. [DOI] [PubMed] [Google Scholar]
- 15.Macek Jilkova Z, Decaens T, Marlu A, et al. Sex differences in spontaneous degranulation activity of intrahepatic natural killer cells during chronic hepatitis B: Association with estradiol levels. Mediators Inflamm 2017;2017:3214917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macek Jilkova Z, Afzal S, Marche H, et al. Progression of fibrosis in patients with chronic viral hepatitis is associated with IL-17(+) neutrophils. Liver Int 2016;36:1116–24. [DOI] [PubMed] [Google Scholar]
- 17.Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 2016;126:3447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thommen DS, Koelzer VH, Herzig P, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 2018;24:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Zhang C, Li Q, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer 2014;110:2560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology 2017;7:e1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabrielson A, Wu Y, Wang H, et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res 2016;4:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foerster F, Hess M, Gerhold-Ay A, et al. The immune contexture of hepatocellular carcinoma predicts clinical outcome. Sci Rep 2018;8:5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Ma C, Zhang Q, et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015;6:17462–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007;25:2586–93. [DOI] [PubMed] [Google Scholar]
- 25.Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014;59:1415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao W, He JC, Yang Y, et al. The prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: A systematic review and meta-analysis. Sci Rep 2017;7:7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen KJ, Lin SZ, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One 2011;6:e24671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Zheng L, Yoo JK, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell 2017;169:1342–56.e16. [DOI] [PubMed] [Google Scholar]
- 29.Chew V, Lai L, Pan L, et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci USA 2017;114:E5900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Ji T, Zhao J, et al. Sorafenib-resistant hepatocellular carcinoma stratified by phosphorylated ERK activates PD-1 immune checkpoint. Oncotarget 2016;7:41274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalathil SG, Lugade AA, Miller A, et al. PD-1+ and Foxp3+ T cell reduction correlates with survival of HCC patients after sorafenib therapy. JCI Insight 2016;1:e86182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HD, Song GW, Park S, et al. Association between expression level of PD1 by tumor-infiltrating CD8(+) T cells and features of hepatocellular carcinoma. Gastroenterology 2018;155:1936–50.e17. [DOI] [PubMed] [Google Scholar]
- 33.Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang RY, Francois A, McGray AR, et al. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2017;6:e1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci Transl Med 2018;10:eaan5488. [DOI] [PubMed] [Google Scholar]
- 36.Thommen DS, Schreiner J, Muller P, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 2015;3:1344–55. [DOI] [PubMed] [Google Scholar]
- 37.Walker LS, Sansom DM. Confusing signals: Recent progress in CTLA-4 biology. Trends Immunol 2015;36:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao X, Gong W, Demirci G, et al. New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. J Immunol 2012;188:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie K, Xu L, Wu H, et al. OX40 expression in hepatocellular carcinoma is associated with a distinct immune microenvironment, specific mutation signature, and poor prognosis. Oncoimmunology 2018;7:e1404214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segal NH, He AR, Doi T, et al. Phase I study of single-agent utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in patients with advanced cancer. Clin Cancer Res 2018;24:1816–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.