INTRODUCTION:
Inhibition of tumor growth factor-β (TGF-β) receptor type I potentiated the activity of sorafenib in preclinical models of hepatocellular carcinoma (HCC). Galunisertib is a small-molecule selective inhibitor of TGF-β1 receptor type I, which demonstrated activity in a phase 2 trial as second-line HCC treatment.
METHODS:
The combination of galunisertib and sorafenib (400 mg BID) was tested in patients with advanced HCC and Child-Pugh A liver function without prior systemic therapy. Galunisertib dose was administered 80 or 150 mg b.i.d. orally for 14 days every 28 days in safety lead-in cohorts; in the expansion cohort, all patients received galunisertib 150 mg b.i.d. Objectives included time-to-tumor progression, changes in circulating alpha fetoprotein and TGF-β1, safety, overall survival (OS), response rate, and pharmacokinetics (PK).
RESULTS:
Patients (n = 47) were enrolled from 5 non-Asian countries; 3 and 44 patients received the 80 mg and 150 mg b.i.d. doses of galunisertib, respectively. The pharmacokinetics and safety profiles were consistent with monotherapy of each drug. For the 150 mg b.i.d. galunisertib cohort, the median time-to-tumor progression was 4.1 months; the median OS was 18.8 months. A partial response was seen in 2 patients, stable disease in 21, and progressive disease in 13. TGF-β1 responders (decrease of >20% from baseline) vs nonresponders had longer OS (22.8 vs 12.0 months, P = 0.038).
DISCUSSION:
The combination of galunisertib and sorafenib showed acceptable safety and a prolonged OS outcome.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer, which was the second most common cause of global cancer mortality in 2012 (1). In the United States, mortality from liver cancer is rising faster than from any other cancer (2). HCC is associated with chronic liver diseases such as hepatitis B or C virus infection, nonalcoholic fatty liver disease, alcoholic liver disease, and cirrhosis. HCC is most often diagnosed at a late stage, when surgical treatment is no longer an option and systemic therapy is minimally effective. Sorafenib, a multikinase inhibitor approved in 2007 for liver cancer, is the first-line standard-of-care for patients with advanced Barcelona Clinic Liver Cancer stage C HCC or intermediate Barcelona Clinic Liver Cancer stage B HCC noneligible or after failure of chemoembolization (3). In randomized studies of advanced HCC patients with Child-Pugh A liver function, sorafenib was associated with improved survival compared to placebo in both a Western population in the SHARP trial (overall survival [OS] 10.7 vs 7.9 months, hazard ratio 0.69) and a predominantly Asian hepatitis B virus-positive population (OS 6.5 vs 4.2 months, hazard ratio 0.68) (4,5). After progression on sorafenib, regorafenib, cabozantinib, and ramucirumab have improved survival in phase 3 trials (6–8). Recent studies of PD-1 immune checkpoint inhibitors in HCC have demonstrated prolonged, durable responses, leading to regulatory approval in the United States (9,10). In the front-line setting for advanced HCC, the multikinase inhibitor, lenvatinib, demonstrated noninferiority to sorafenib in the REFLECT trial (11), but multiple drugs and combination regimens have failed to improve upon the outcomes of sorafenib monotherapy (12).
Tumor growth factor-β (TGF-β) is a secreted cytokine that acts as a paracrine tumor promotor in advanced, metastatic cancer (13). TGF-β is a master regulating molecule triggering (i) the epithelial-mesenchymal transition through E-cadherin downregulation (14), (ii) neoangiogenesis by increasing production of the vascular endothelial growth factor (15), (iii) invasion by β1 integrin activation (16), and (iv) altering the tumor/host interaction upregulating connective tissue growth factor production (17). These activities lead to HCC tumor progression.
Galunisertib (LY2157299) is a small-molecule selective inhibitor of the TGF-β receptor type I (RI), a serine/threonine kinase. TGF-β has been shown to be elevated in both serum and tumor samples from patients with HCC (18,19), and TGF-β signaling is associated with resistance to sorafenib in HCC cell lines (20). In preclinical models of HCC, TGF-β inhibitors including galunisertib reduce growth, invasion, and progression (14–16,21). Galunisertib also has been studied in combination with sorafenib in HCC cell lines and ex vivo tumor samples, resulting in increased growth inhibition and apoptosis, and underscoring a potential role for TGF-β inhibition in overcoming sorafenib resistance (22). This may be explained by a recent report that TGF-β signaling confers sorafenib resistance, and that galunisertib enhanced sorafenib-induced apoptosis (20).
The pharmacokinetics (PK), pharmacodynamics, safety, and efficacy of galunisertib have been evaluated in preclinical and clinical phase 1 studies (23–27). An intermittent dosing strategy of 14 days on, 14 days off, and a therapeutic window of 160–300 mg/d were defined. No dose-limiting toxicities were observed and galunisertib was well tolerated.
In a phase 2 study of second-line patients with HCC, galunisertib showed clinical benefit in a subset of patients (28,29). Median time-to-tumor progression (TTP) was 2.7 months (95% confidence interval [CI]: 1.5–2.9) in patients with higher baseline alpha fetoprotein (AFP, ≥1.5× upper limit of normal) plasma levels and 4.2 months (95% CI: 1.7–5.5) in patients with lower baseline AFP. Median OS was 7.3 months (95% CI: 4.9–10.5) in patients with higher baseline AFP and 16.8 months (95% CI: 10.4–24.1) in patients with lower baseline AFP. The safety profile of galunisertib in HCC was consistent with observations in other tumor types. The most frequently occurring treatment-emergent adverse events overall (>20% of patients) were fatigue (33.6%), anemia (25.5%), peripheral edema (22.8%), and abdominal pain (21.5%). The most common grade 3/4 treatment-related adverse events were neutropenia (2.7%) and fatigue, anemia, increased bilirubin, hypoalbuminemia, and embolism (1.3% each).
Based upon preclinical evidence that galunisertib could augment the efficacy of sorafenib, we conducted a phase 2 trial of galunisertib in combination with sorafenib as the first line of systemic therapy for advanced HCC in patients with Child-Pugh A liver function. Here, we report the efficacy, safety, and AFP and TGF-β biomarker results for this novel combination in advanced HCC.
METHODS
Subjects and study design
This was an open-label, multicenter, phase 2 study of LY2152799 (galunisertib) in patients with HCC (ClinicalTrials.gov: NCT01246986). The study had multiple parts; reported here is part C, which evaluated galunisertib in combination with sorafenib in patients with advanced HCC who had not received prior systemic treatment (see Figure S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A55). Galunisertib was administered orally at a dose of 80 or 150 mg b.i.d. in the safety lead-in cohort and 150 mg b.i.d. in the expansion cohort, on days 1–14 in 28 day cycles. All patients received sorafenib at the standard starting dose of 400 mg b.i.d. Sorafenib was adjusted based on previously published guidelines (4).
Eligible patients were adults with a histologic diagnosis of HCC not amenable to surgery, measurable disease by Response Evaluation Criteria in Solid Tumor (RECIST) 1.1 criteria, performance status ≤1 on the Eastern Cooperative Oncology Group scale, adequate organ function including Child-Pugh A liver function, and who had not received previous systemic therapy. Patients with mixed histology or moderate or severe cardiac disease were excluded. All patients provided informed consent for participation in the study and before any study-specific procedures. The protocol was approved by local ethical review boards and was conducted according to the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki.
OBJECTIVES
The primary objectives were to characterize the TTP by RECISTs v1.1 (30) and the effect of treatment on the TGF-β associated biomarkers AFP and TGF-β1 with the recommended dose of galunisertib in combination with sorafenib. The secondary objectives were to evaluate safety of the combination and the effect of treatment on OS, progression-free survival, overall response rate by RECIST 1.1 as well as modified RECIST (31) (tumor assessment was performed every 6 weeks), and population PK of galunisertib in combination with sorafenib.
PK and biomarker methods
Plasma samples were analyzed for galunisertib using validated liquid chromatography-mass spectrometry/MS methods (BPLY215A and BPLY215B). The lower limit and the upper limit of quantification were 0.050 and 10.0 ng/mL for BPLY215A and 5.00 and 1,000 ng/mL for BPLY215B. AFP and TGF-β1 levels in plasma were analyzed by ELISA (R&D Systems, DB100B, Minneapolis, MN).
Statistical analyses
Part C of this study was designed to enroll approximately 40 first-line patients with HCC into 2 dose cohorts of galunisertib in combination with standard dose sorafenib. Based upon the acceptable safety profile of the higher dose of galunisertib of 150 mg b.i.d. in the safety lead-in cohort, all patients were enrolled to the subsequent expansion cohort, in accordance with the study design and after an interim safety analysis. All patients who received at least 1 dose of study drug were evaluated for safety. Patients with measurable disease at baseline were included in summaries of tumor response. Time-to-event efficacy endpoints (TTP and OS) were analyzed for patients treated at 150 mg b.i.d. For efficacy endpoints, no formal hypothesis testing based on a priori power calculations was planned; rather differences in various endpoints between appropriate subgroups were estimated.
Median TTP and the secondary objective of OS were estimated using the Kaplan-Meier method, with the log-rank tests to compare distributions of TTP or OS between subgroups. TTP was measured from the date of first dose to the first date of objective progression of disease. For each patient who was not known to have had a progression of disease as of the data-inclusion cutoff date for this analysis, or who died without progression of disease, TTP was censored for the analysis at the date of the patient's last tumor assessment before that cutoff date. OS was measured from the date of first dose to the date of death from any cause. For each patient who was not known to have died as of the data inclusion cutoff date for this analysis, OS duration was censored for the analysis at the date of last prior contact.
Biomarker response was defined as >20% decrease in the biomarker from baseline values at any time point, based upon thresholds employed in previous studies (32). TTP was compared between biomarker subgroups at baseline (AFP ≥ 400 ng/mL vs AFP < 400 ng/mL; TGF-β1 less than or greater than/equal to median). TTP and OS were compared between biomarker responders and nonresponders postbaseline. All confidence intervals of treatment effects are provided at a 2-sided alpha level of 0.05, unless otherwise stated. Statistical software used was SAS version 9.2 or higher (SAS Institute, Cary, NC).
RESULTS
Twelve sites in 5 non-Asian countries (Germany, France, Italy, New Zealand, and the United States) enrolled a total of 47 patients between March 2013 and June 2015. Three patients in the safety lead-in cohort received galunisertib at the lower dose of galunisertib of 80 mg orally b.i.d. for 14 days every 28 days. After an interim safety analysis of the first 3 patients, 44 patients subsequently received galunisertib at the higher dose of 150 mg b.i.d. At the cutoff date for this analysis of November 21, 2017, 4 patients remained on treatment and 6 patients were in survival follow-up. The complete patient disposition is shown in Figure S2 (see Supplementary Digital Content 2, http://links.lww.com/CTG/A56). The demographic and baseline disease characteristics of the patient population are shown in Table 1. Approximately 50% of patients entered the study less than 6 months from initial HCC diagnosis (range: 0.7–141 months).
Table 1.
Baseline demographics and disease characteristics
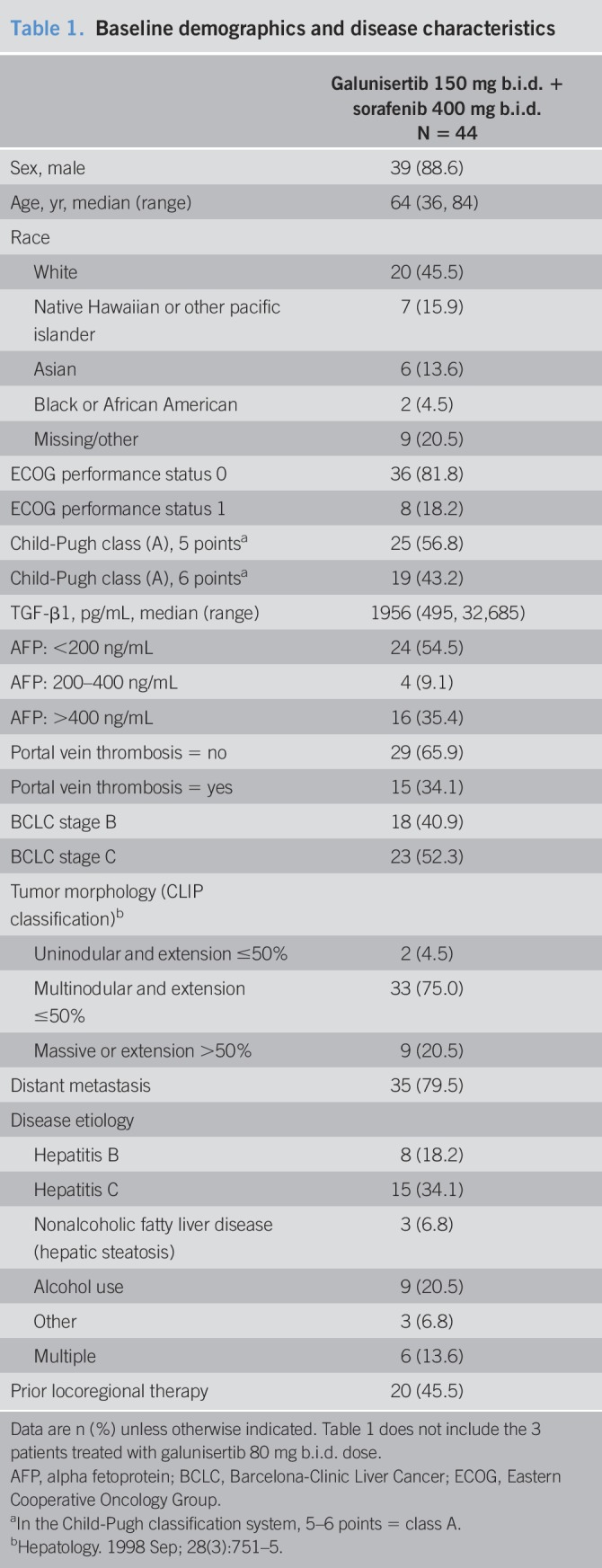
No dose-limiting or significant toxicities were observed during the lead-in period. The combination dose of galunisertib, when administered with sorafenib 400 mg orally b.i.d., was defined as 150 mg orally b.i.d. for 14 days, followed by 14 days off, in a 28-day cycle.
Efficacy
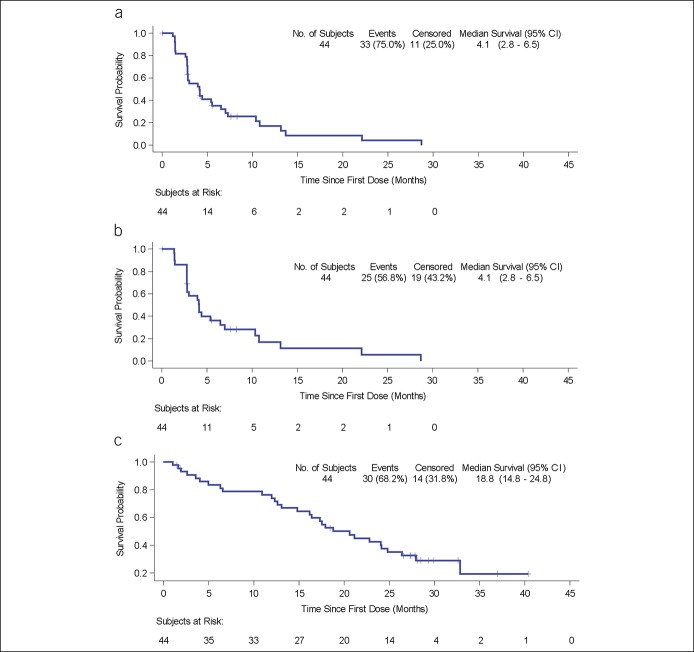
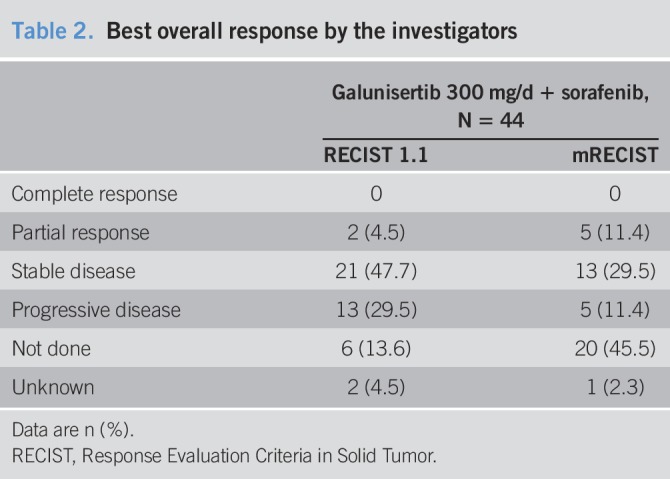
In patients treated with galunisertib at the higher dose of 150 mg b.i.d., the median TTP by RECIST v1.1 was 4.1 months (95% CI: 2.8, 6.5) (Figure 1a); the median TTP by mRECIST was 4.1 months (95% CI: 2.8, 6.5) (Figure 1b); and the median OS was 18.8 months (95% CI: 14.8, 24.8) (Figure 1c). Partial responses occurred in 4.5% by RECIST v1.1 assessed by investigators (Table 2).
Figure 1.
Kaplan-Meier plots of TTP and OS: 150 mg b.i.d. cohort. (a) TTP by RECIST v1.1. (b) TTP by mRECIST. (c) OS. CI, confidence interval; OS, overall survival; RECIST, Response Evaluation Criteria in Solid Tumor; TTP, time-to-tumor progression.
Table 2.
Best overall response by the investigators
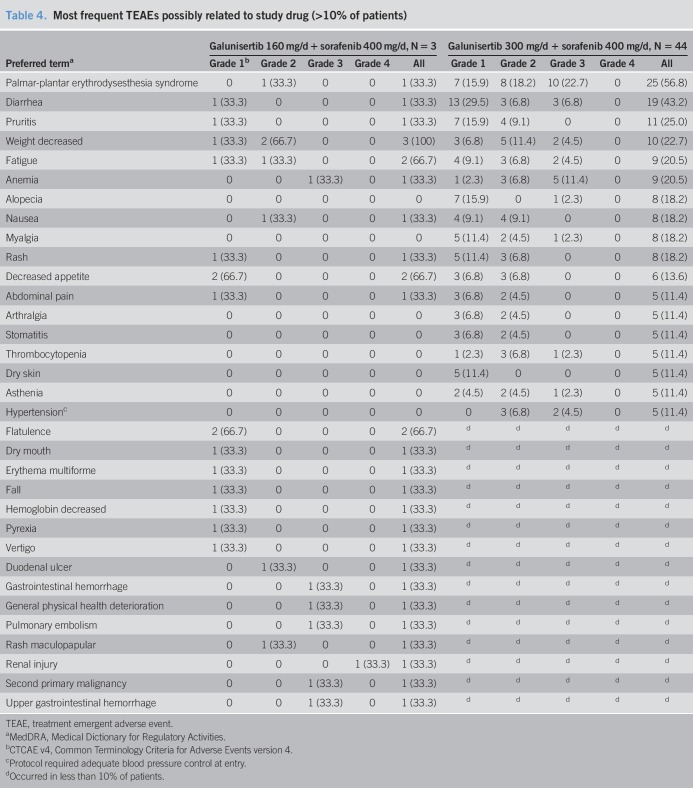
Twelve patients (25.5%) received at least one postdiscontinuation therapy (Tables 3 and 4). Half of these patients received chemotherapy, whereas the other half received targeted agents, including regorafenib, sorafenib, tivantinib, and cabozantinib.
Table 3.
Postdiscontinuation treatments
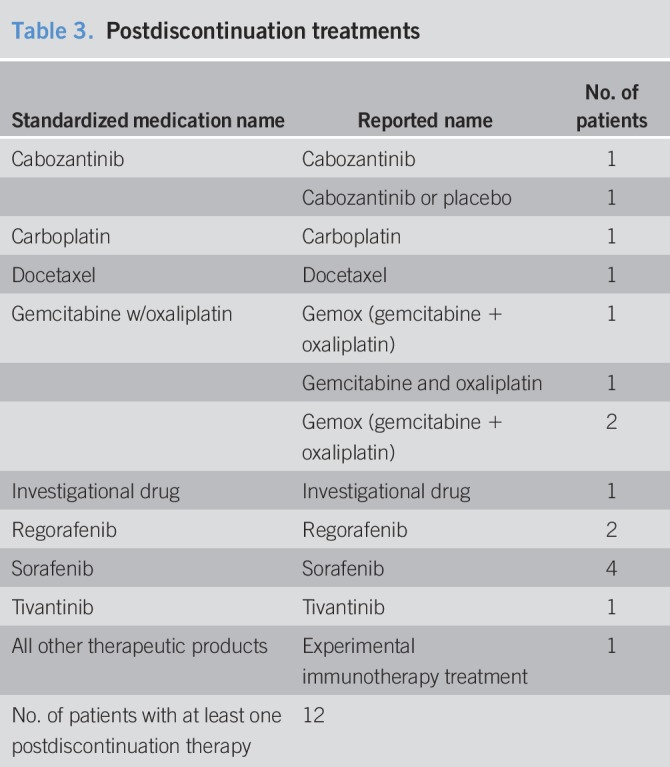
Table 4.
Most frequent TEAEs possibly related to study drug (>10% of patients)
Subgroup analysis
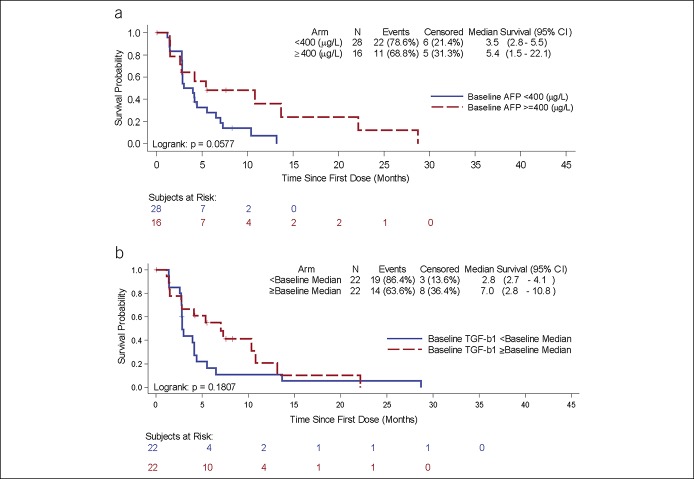
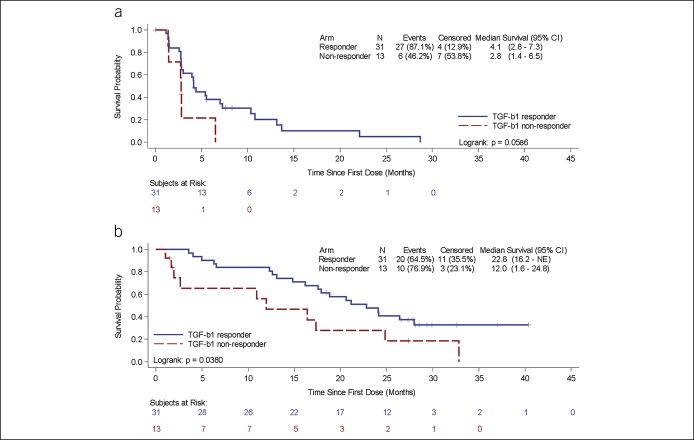
TTP and OS were analyzed by baseline biomarker values: AFP <400 ng/mL and ≥400 ng/mL (Figure 2a) and TGF-β1 less than or greater than/equal to the baseline median (1956 pg/mL) (Figure 2b). Patients with baseline AFP ≥ 400 ng/mL or patients with baseline TGF-β1 ≥ baseline median (1956 pg/mL) demonstrated trends toward longer TTP. TGF-β1 responders (at least 20% decrease from baseline at any time point) had significantly longer OS (22.8 vs 12 months, P = 0.038) (Figure 3). For AFP responders and nonresponders, OS was 17.9 months and 20.6 months, P = 0.49.
Figure 2.
Kaplan-Meier plots of time-to-tumor progression by baseline biomarkers: 150 mg b.i.d. cohort. (a) Subgroups by baseline AFP <400 (blue) and AFP ≥400 ng/mL (red). (b) Subgroups by baseline TGF-β1 <median (blue) and TGF-β1 ≥median (red) (median=1956 pg/mL). AFP, alpha fetoprotein; CI, confidence interval; TGF-β1, tumor growth factor-β.
Figure 3.
Kaplan-Meier plots of time-to-tumor progression (a) and overall survival (b) by TGF-β1 responders (blue, >20% maximum reduction from baseline) and TGF-β1 nonresponders (red, ≤20% maximum reduction from baseline). CI, confidence interval; TGF-β1, tumor growth factor-β.
Limited archival tumor samples (n = 24) were available for analysis. Because of the limited amount of tissue, a comprehensive evaluation of all targets was not possible, including for gene expression studies. Immunohistochemistry data were obtained for expression of TGF-β RII and pSMAD2. Among the 24 patients, 3 patients had tumors positive for TGF-β RII (3/24; 12.5%), and 15 were positive for pSMAD2 (15/24; 62.5%). There was no correlation between tumor pSMAD2 and circulating TGF-β1 (R2 = 0.05). The limited sample size was insufficient for determination of any statistical relationship between tumor tissue TGF-β RII or pSMAD2 expression with TTP or OS.
Safety
Most treatment emergent adverse events assessed as at least possibly related to study drug were of low grade (CTCAE v4 grades 1–3); one patient had a grade 4 renal injury (see Table 4). The most frequent treatment emergent adverse events of any grade (% of patients) were palmar-plantar erythrodysesthesia (56.8%), diarrhea (43.2%), and pruritis (25.0%). Twenty-eight (59.6%) patients had a serious adverse event; only the serious adverse event of anemia was reported in >2 patients (7 [14.9%]). One patient died while on study treatment due to hepatic failure (considered unrelated to study treatment). Dose reductions in galunisertib were required in 2 (4.3%) patients, whereas dose reductions in sorafenib were required in 27 (57.4%) patients. Treatment discontinuation for toxicity occurred in 5 (10.6%) patients.
Pharmacokinetics
The PK of galunisertib (see Figure S3, a–b, Supplementary Digital Content 3, http://links.lww.com/CTG/A57) for 80 mg and 150 mg b.i.d. oral doses, coadministered with sorafenib, was similar to that observed in previous studies of galunisertib treatment as monotherapy: absorption within 2–3 hours and an elimination half-life of 8 hours (33). In addition, similar sorafenib plasma concentrations were observed in combination with galunisertib (day 14) and in the absence of galunisertib (day 22) (see Figure S3c, Supplementary Digital Content 3, http://links.lww.com/CTG/A57).
DISCUSSION
Part C of this phase 2 study tested galunisertib, a small molecule that selectively inhibits the serine/threonine kinase of the TGF-β RI, in combination with sorafenib in patients with advanced HCC who had not received prior systemic therapy. In the 150 mg b.i.d. galunisertib cohort, the median TTP of 4.1 months for the combination did not exceed the historical TTP with monotherapy sorafenib in similar populations (4,5,11). The median OS of 18.8 months in this cohort was longer than historical outcomes for this population, however, suggesting that the inhibition of TGF-β signaling may have delayed the resistance of tumors to sorafenib (4,11). Since sorafenib has been reported to reduce regulatory T cells in patient samples and galunisertib has shown a similar effect in preclinical models (34), the assumption was that both agents would have an additive effect in removing regulatory T cells associated with immune escape in HCC. Because there was no control arm and no evaluation of peripheral regulatory T-cell counts in this study, it cannot be concluded whether the combination of agents had an additive effect on improving the immune response in HCC by reducing peripheral T regulatory cells.
Unlike previous studies of other novel agents in combination with sorafenib confounded by additive toxicity (35–37), the combination of sorafenib plus galunisertib showed manageable toxicity and tolerability, without apparent increase of sorafenib toxicities, and without impact on the sorafenib PK profile. The favorable safety profile of galunisertib coupled with its mechanism of TGF-β RI signaling inhibition supports its potential benefit in other combinations, including an ongoing study in combination with nivolumab (NCT02423343) based upon preclinical synergistic activity of galunisertib and checkpoint inhibitors (38).
We monitored plasma TGF-β1 levels at baseline and during treatment and analyzed its effect on outcomes. Patients with TGF-β1 levels greater than median at baseline had a longer TTP than those below the median (not statistically significant). Patients who were classified as TGF-β1 responders, with a decline in circulating TGF-β1 on treatment, also demonstrated a trend toward prolonged TTP, although cut-points for defining response have not been validated. Furthermore, OS was significantly longer in TGF-β1 responders compared to nonresponders (22.8 vs 12 months, P = 0.038). These data are consistent with previous findings suggesting TGF-β1 plasma levels as a marker for response to targeted therapies (28,39).
We investigated factors that may have contributed to the OS and found that, as expected, patients receiving additional treatments after they stopped the experimental treatment (12/47; 25.5%) had a longer median OS (28.8 months) compared to those who did not receive additional therapies (35/47; 74.5% and median OS of 16.4 months, log rank P = 0.0571).
The promising survival and safety outcomes of the combination of galunisertib plus sorafenib in this study support further exploration of TGF-β RI inhibition with galunisertib as a combination strategy with other agents whose activity may be augmented by inhibition of TGF-β signaling, including potential combination with PD1/PDL1 inhibitors.
CONFLICTS OF INTEREST
Guarantor of the article: Karim A. Benhadji, MD.
Specific author contributions: R.K.K.: collected the data, interpreted the data, critically revised the manuscript. E.G.: collected the data, interpreted the data, critically revised the manuscript. E.A.: collected the data, interpreted the data, critically revised the manuscript. J.S.: collected the data, interpreted the data, critically revised the manuscript. P.R.G.: collected the data, interpreted the data, critically revised the manuscript. P.M.: collected the data, interpreted the data, critically revised the manuscript. I.O.H.: collected the data, interpreted the data, critically revised the manuscript. A.C.: analyzed the data, interpreted the data, critically revised the manuscript. Y.Z.: analyzed the data, interpreted the data, critically revised the manuscript. I.G.: analyzed the data, interpreted the data, critically revised the manuscript. M.L.: designed the study, interpreted the data, critically revised the manuscript. S.F.: designed the study, collected the data, interpreted the data, critically revised the manuscript. K.A.B.: designed the study, interpreted the data, wrote and critically revised the manuscript. G.G.: designed the study, collected the data, interpreted the data, critically revised the manuscript. All authors approved the final draft submitted.
Financial support: The study was funded by Eli Lilly and Company. Employees and contractors of Eli Lilly and Company participated in the study design, collection, analysis, and interpretation of the data and in the writing of the report.
Potential competing interests: I.G. and Y.Z. are employees and stockholders of Eli Lilly and Company. A.C., K.A.B., and M.L. are former employees and stockholders of Eli Lilly and Company. E.A. received research funding from Bayer, Bristol-Myers Squibb, Incyte, Ipsen, Novartis, AMGEN, Sanofi, and Servier. P.R.G. received honoraria from Bayer, Eli Lilly, BMS, MSD, Sirtex, AstraZeneca, SillaJen, Blueprint Medicines, Ipsen, and Eisai. P.M. received consulting and/or research funding from Bayer, Onxeo, Ipsen/Exelixis, Eli Lilly, MSD, BTG, and Roche. I.O.H. received research funding from AbbVie, MSD, Eli Lilly, and Bayer, and served on the speaker bureau for Bayer. S.F. received consulting and research funding from BeiGene, Blueprint Medicines, Bristol-Myers Squibb, Bayer Pharma, Eli Lilly, Incyte, Ipsen, Merck Serono, MSD, and Novartis. R.K.K. received research funding from Adaptimmune, Agios, AstraZeneca, Bayer, BMS, Eli Lilly, Exelixis, Novartis, QED, Taiho, Merck, and Medimmune, and he is an advisor to Genentech/Roche and Target Pharma Solutions. All remaining authors have declared no conflicts of interest.
Study Highlights.
WHAT IS KNOWN
✓ The TGF-β pathway is active in HCC, and its inhibition potentiates the action of sorafenib in preclinical models.
✓ Galunisertib is a small-molecule selective inhibitor of TGF-β receptor I.
✓ In second-line patients with advanced HCC, galunisertib had an acceptable safety profile and was associated with longer survival in patients with lower baseline AFP or a decrease in AFP or TGF-β1 during treatment.
WHAT IS NEW HERE
✓ Galunisertib/sorafenib combination was tested in first-line patients with advanced HCC.
✓ The combination was well tolerated and extended survival compared to historical results with sorafenib alone, although time to progression was similar.
TRANSLATIONAL IMPACT
✓ In future studies, galunisertib should be tested in a combination strategy with other agents whose activity may be augmented by inhibition of TGF-β signaling.
✓ The results merit future evaluation of how to best combine galunisertib with other standard treatments in HCC.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Eli Lilly and Company, Indianapolis, IN, USA. We thank the patients and their caregivers for participating in this trial. We also thank the investigators and their support staff who generously participated in this work and the trial personnel. Writing assistance was provided by Caryl J. Antalis, PhD, Eli Lilly and Company. Parts of this data were previously presented at ASCO 53rd Annual Meeting in June 2017 in Chicago, IL, USA.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A55, http://links.lww.com/CTG/A56, and http://links.lww.com/CTG/A57
REFERENCES
- 1.International Agency for Research on Cancer, World Health Organization GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. (http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx). Accessed October 30, 2017.
- 2.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016;122(9):1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn RS, Zhu AX, Farah W, et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: A systematic review and meta-analysis. Hepatology 2018;67(1):422–35. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10(1):25–34. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl J Med 2018;379(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 8.Zhu AX, Galle PR, Kudo M, et al. A study of ramucirumab (LY3009806) versus placebo in patients with hepatocellular carcinoma and elevated baseline alpha-fetoprotein (REACH-2). J Clin Oncol 2018;36(4 Suppl):TPS538. [Google Scholar]
- 9.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19(7):940–52. [DOI] [PubMed] [Google Scholar]
- 10.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389(10088):2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31(28):3517–24. [DOI] [PubMed] [Google Scholar]
- 13.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov 2004;3(12):1011–22. [DOI] [PubMed] [Google Scholar]
- 14.Fransvea E, Angelotti U, Antonaci S, et al. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology 2008;47(5):1557–66. [DOI] [PubMed] [Google Scholar]
- 15.Mazzocca A, Fransvea E, Lavezzari G, et al. Inhibition of transforming growth factor beta receptor I kinase blocks hepatocellular carcinoma growth through neo-angiogenesis regulation. Hepatology 2009;50(4):1140–51. [DOI] [PubMed] [Google Scholar]
- 16.Fransvea E, Mazzocca A, Antonaci S, et al. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology 2009;49(3):839–50. [DOI] [PubMed] [Google Scholar]
- 17.Bergamini C, Sgarra C, Trerotoli P, et al. Laminin-5 stimulates hepatocellular carcinoma growth through a different function of alpha6beta4 and alpha3beta1 integrins. Hepatology 2007;46(6):1801–9. [DOI] [PubMed] [Google Scholar]
- 18.Sacco R, Leuci D, Tortorella C, et al. Transforming growth factor beta1 and soluble Fas serum levels in hepatocellular carcinoma. Cytokine 2000;12(6):811–4. [DOI] [PubMed] [Google Scholar]
- 19.Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 2009;69(18):7385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungerleider N, Han C, Zhang J, et al. TGFbeta signaling confers sorafenib resistance via induction of multiple RTKs in hepatocellular carcinoma cells. Mol Carcinog 2017;56(4):1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannelli G, Bergamini C, Fransvea E, et al. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology 2005;129(5):1375–83. [DOI] [PubMed] [Google Scholar]
- 22.Serova M, Tijeras-Raballand A, Dos Santos C, et al. Effects of TGF-beta signalling inhibition with galunisertib (LY2157299) in hepatocellular carcinoma models and in ex vivo whole tumor tissue samples from patients. Oncotarget 2015;6(25):21614–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gueorguieva I, Cleverly AL, Stauber A, et al. Defining a therapeutic window for the novel TGF-beta inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J Clin Pharmacol 2014;77(5):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara Y, Nokihara H, Yamada Y, et al. Phase 1 study of galunisertib, a TGF-beta receptor I kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2015;76(6):1143–52. [DOI] [PubMed] [Google Scholar]
- 25.Herbertz S, Sawyer JS, Stauber AJ, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther 2015;9:4479–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacs RJ, Maldonado G, Azaro A, et al. Cardiac safety of TGF-beta receptor I kinase inhibitor LY2157299 monohydrate in cancer patients in a first-in-human dose study. Cardiovasc Toxicol 2015;15(4):309–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodon J, Carducci M, Sepulveda-Sanchez JM, et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-beta receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs 2015;33(2):357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faivre SJ, Santoro A, Gane E, et al. A phase 2 study of galunisertib, a novel transforming growth factor-beta (TGF-β) receptor I kinase inhibitor, in patients with advanced hepatocellular carcinoma (HCC) and low serum alpha fetoprotein (AFP). J Clin Oncol 2016;34(15 Suppl):4070. [Google Scholar]
- 29.Faivre S, Santoro A, Kelley RK, et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liv Int. [Epub ahead of print April 8, 2019.] [DOI] [PubMed] [Google Scholar]
- 30.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 31.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Personeni N, Bozzarelli S, Pressiani T, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol 2012;57(1):101–7. [DOI] [PubMed] [Google Scholar]
- 33.Rodon J, Carducci MA, Sepulveda-Sanchez JM, et al. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res 2015;21(3):553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Yu T, Yuan Y, et al. Sorafenib reduces hepatic infiltrated regulatory T cells in hepatocellular carcinoma patients by suppressing TGF-beta signal. J Surg Oncol 2013;107(4):422–7. [DOI] [PubMed] [Google Scholar]
- 35.Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol 2016;34(4, Suppl):192. [Google Scholar]
- 36.Koeberle D, Dufour JF, Demeter G, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): A randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann Oncol 2016;27(5):856–61. [DOI] [PubMed] [Google Scholar]
- 37.Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33(6):559–66. [DOI] [PubMed] [Google Scholar]
- 38.Holmgaard RB, Schaer DA, Li Y, et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer 2018;6(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin TH, Shao YY, Chan SY, et al. High serum transforming growth factor-beta1 levels predict outcome in hepatocellular carcinoma patients treated with sorafenib. Clin Cancer Res 2015;21(16):3678–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.