To the Editor: Ureteral calculus is a common urological disease. In recent years, one of the main treatment methods is ureteroscopy with holmium laser lithotripsy. However, with the extensive application of holmium laser lithotripsy, the increasing incidence of postoperative ureteral stricture or even occlusion has attracted the attention of clinicians to its etiology. The issue of the intraoperative holmium laser thermal effect has also gradually gained attention. The holmium laser is a long-wavelength pulsed laser, which crushes calculi by an optomechanical/photoacoustic mechanism as well as a photothermal mechanism, and it is mainly based on the photothermal mechanism.[1] Currently, multiple in vitro studies worldwide have confirmed that the holmium laser did increase the water temperature in the working area. However, these studies only simulated the lithotripsy process of the holmium laser in vitro, which did not completely reflect the thermal effect of the holmium laser during an actual operation. Accordingly, this study monitored the temperature changes of the lavage fluid in the operative field during the actual holmium laser lithotripsy process under an ureteroscope, with the aim of providing more information for the etiology study of ureteral stricture after ureteroscopy with holmium laser lithotripsy.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Third Xiangya Hospital of Central South University (No. 2018-S235) and registered with the China Clinical Trial Registration Center (ChiCTR1800017830).
Patients who were suffering from ureteral calculi and were candidates for holmium laser lithotripsy under standard ureteroscopy (Fr8.0/9.8) were selected. An ureteroscope (WOLF Fr8.0/9.8; Richard Wolf GmbH, Knittlingen, Germany), holmium laser machine (Lumenis PowerSuite100W; Lumenis Inc., Santa Clara, CA, USA), endoscope perfusion pump (JR-03; Shenda Endoscope Co., Ltd., Liaoning, China), thermometer (Uni-T, UT321; Tian Cheng Electronics Co., Ltd., Shenzhen, China), thermocouple temperature measurement wire (WRNK-191; Shanghai Automation Instrumentation Co., Ltd., Shanghai, China), and laptop computer (ThinkPad New S2; Lenovo Group, Beijing, China) were utilized in this study.
The specific steps performed were as follows: (1) Combined spinal and epidural anesthesia was performed; the patient was placed in the lithotomy position, and routine disinfection and draping were conducted. (2) An ureteroscope (F8.0/9.8) was inserted into the ipsilateral ureter under the guidance of a safe guidewire and reached the location of the calculi. The temperature measurement guidewire was inserted into the ureteroscope, and the tip of the guidewire was extended slightly beyond the end of the ureteroscope. The position was suitable when the guidewire could be seen on the monitor [Figure 1]. The end of the temperature measurement wire was connected to a thermometer, and the temperature was measured at a frequency of once per second to monitor the temperature changes of the lavage solution around the calculi during the operation. The thermometer was connected to a computer, and the temperature data were transmitted to the computer in real time. (3) Clearance of the calculi: a 550-μm optical fiber was inserted through another operation channel of the ureteroscope, and the corresponding power was set to crush the calculi (the power setting and all operations in the surgery were determined by the surgeon according to the surgical situation and were not influenced by this study). (4) Indwelling of double J tube: after crushing the calculi, the ureteroscope and fiber were removed, a safe guidewire was inserted, and retrograde indwelling of a Fr6 double J tube was performed under the guidance of the guidewire for catheterization.
Figure 1.
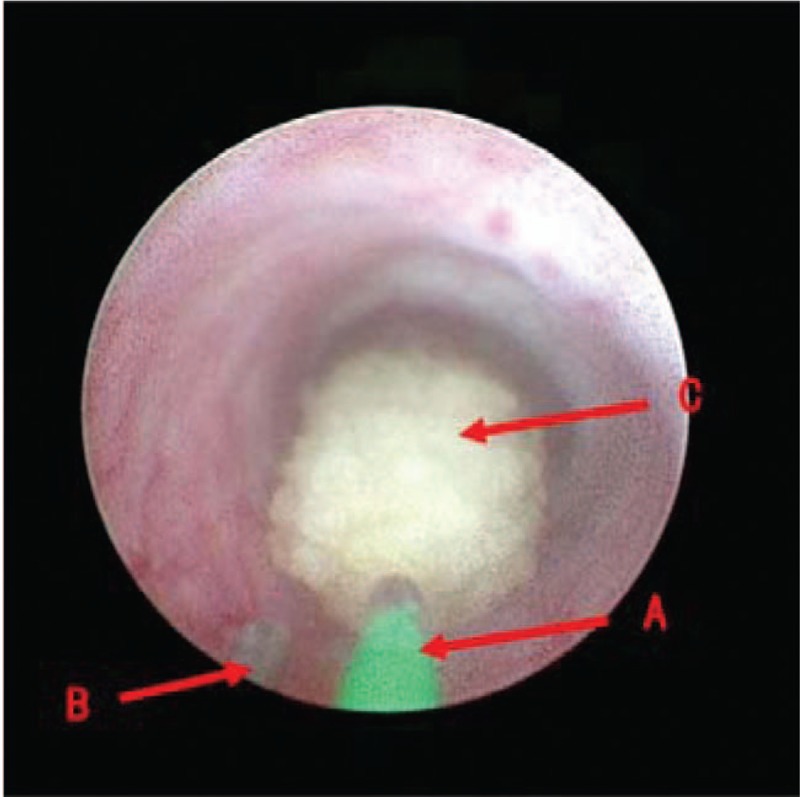
Reteroscope display screen: (A) the 550-μm holmium laser fiber; (B) the temperature guidewire; the top of the guidewire extends slightly out of the ureteroscope, the display can see the top of the guidewire; and (C) a stone.
The measurement data were expressed as mean ± standard deviation. The comparison between groups was performed by an unpaired t test. The statistical software SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the experimental data. A P < 0.05 was considered statistically significant.
The initial temperature of the lavage solution was the temperature of the operating room (25.41 ± 2.82°C). The intraoperative measurement of the lavage solution temperature of a total of 27 patients with ureterolithiasis was completed, and 30 sets of data were obtained [Table 1], including one patient with bilateral ureteral calculi, one patient with giant calculus in the lower ureter, and one patient with multiple ureterolithiasis; two datasets were obtained from each of these three patients. There were 16 males and 14 females; 17 lesions were on the left side and 13 were on the right side. The mean age was 47.4 ± 13.7 years, and the mean calculi diameter was 14.16 ± 8.11 mm. There were 14 cases of incarcerated calculi and 16 cases of nonincarcerated calculi.
Table 1.
Baseline characteristics of all patients in this study.
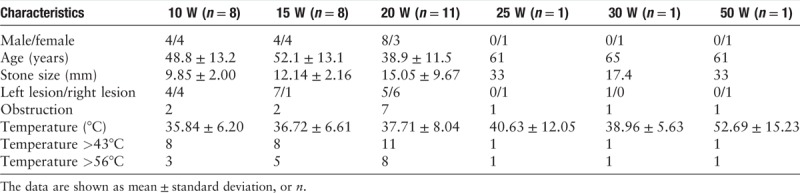
As shown in Table 1, the highest temperatures in the 30 sets of data were all higher than 43°C (100%), with 19 sets (63.3%) having peak temperatures higher than 56°C. As the power increased, the measured temperature generally increased.
In the 20 W dataset when comparing the seven cases of incarcerated calculi (38.56 ± 8.51°C) with the four cases of nonincarcerated calculi (34.92 ± 5.35°C), the results showed that the mean intraoperative temperature of the lavage solution in the incarcerated calculi group was significantly higher than that in the nonincarcerated calculi group, with statistical significance (P < 0.001).
During the follow-up, a total of 27 patients, one case (30 W) lost and seven cases had symptoms of low back pain (two cases in 10 W group, one case in 15 W group, three cases in 20 W group, the same case in 25 W and 50 W groups). In four of the seven patients, the temperature exceeded the threshold (56°C) during the operation. Among them, three cases (two cases in 20 W group, and one case in 25 W group) reported “hydronephrosis” by color Doppler ultrasonography, suggesting that ureteral obstruction might be possible, and the intraoperative perfusion temperature of the three patients all exceeded the threshold (56°C).
Ureteral calculus is a relatively common urological disease. With the continuous development of minimally invasive techniques, ureteroscopy with holmium laser lithotripsy has been increasingly used more extensively in the treatment of this disease. However, the corresponding incidence of postoperative ureteral stricture is also increasing. Therefore, how to effectively prevent the occurrence of ureteral stricture after ureteroscopy with holmium laser lithotripsy is an issue that is attracting increasing attention by clinical physicians. There are many possible factors leading to postoperative ureteral stricture, and the holmium laser thermal effect is one of the possible factors that is attracting attention. The holmium laser emits a high-energy pulse wave that can cause an increase in ambient temperature during its operation, which has been confirmed in many in vitro experiments. However, in the operative setting, how the temperature of the lavage solution around the calculi changes and whether “boiling of the ureter” truly exists have not been clarified by studies.
It has been found that when a holmium laser fiber with a diameter of 550 μm was excited in the air with an output energy of 1.5 J and output frequency of 20 Hz, the temperature measured at 2 mm from its end was 750°C.[2] When the frequency is increased to 30 Hz, the temperature at the measurement point is much higher than 1400°C, which is too high. However, when it is excited in the lavage fluid, as the wavelength of the holmium laser is very close to the absorption peak of water, most of the laser energy can be absorbed by the lavage solution during the holmium laser lithotripsy process. At the same time, when performing ureteroscopy with holmium laser lithotripsy, the operative field space between the end of the ureteroscope and the ureteral calculus is very small; if the diameter of the cross-section of the calculus is 10 mm and the distance between the end of the ureteroscope and the ureteral calculus is 10 mm, the volume of the operative field is only approximately 0.8 mL. In particular, for the incarcerated calculi, before the calculi are crushed and the fluid can get through, the fluidity of the lavage solution is poor. According to the specific heat capacity of water, 1 J of energy can raise the temperature of 1 mL of water by 0.24°C.[3] Therefore, in such a relatively small closed space, repetitive excitation of the holmium laser is very likely to cause the water temperature in the working area to increase sharply, thereby resulting in the possibility of thermal damage to the ureteral wall.
Some in vitro studies have also shown that the holmium laser could indeed cause an increase in lavage solution temperature during the excitation process.[4–7] Molina et al[5] found that the high temperature of 112.4°C was measured when the parameter of 1.0 J × 10 Hz was set. In in vitro experiments, lowering the power of the holmium laser could lower the temperature of the lavage solution.[8] Gong et al[9] also confirmed that when the normal saline temperature rose to 57°C, the renal cortex of rabbits was white, the cortex and medulla boundary was not clear, the color of renal medulla was rosy, pathological examination showed that most of the cortical renal unit was necrotic, and the degeneration of medulla was not obvious. The ureteral degeneration was obvious. This indicates that the normal saline heated by holmium laser does cause thermal damage to the fresh kidney and ureter in rabbits. However, in these two experiments, the holmium laser was operating in physiological saline that was not being exchanged. During the actual operation, to obtain a clear visual field, the surgeon will continuously perfuse the field with physiological saline and intentionally open the water outlet of the ureteroscope to reinforce the exchange of the lavage solution in the operative field. Obviously, such an exchange can remove some of the heat, reducing the actual increase in the temperature of the lavage solution in the operative field. Wollin et al[10] proposed that as long as there is sufficient drainage (100 mL/min), even if the power of the holmium laser is increased (1 J × 20 Hz), a relatively stable temperature can be maintained. With the decrease of the perfusion speed (0 mL/min) of the lavage solution, even a lower power (0.6 J × 6 Hz) can still cause a significant increase in temperature. Aldoukhi et al[11] excited a holmium laser in the presence of perfusion with saline at different flow rates and found that at a higher flow rate, even a high power of 40 W did not cause thermal damage to the renal pelvis in pigs. Buttice et al[12] showed that compared with normal-temperature perfusion, the use of a low-temperature lavage solution can significantly reduce the thermal effect of the holmium laser. However, in surgery, hypothermic perfusion is generally not recommended because it may lead to intraoperative hypothermia and shivering, affecting the successful performance of the surgery and the postoperative recovery of the patient.
Sapareto and Dewey[13] proposed a “time–temperature relationship” for tissue thermal damage, namely, thermal damage will occur when the biological tissue is in an environment of 43°C for more than 120 min. Depending on this relationship, many scholars have carried out hyperthermia of tumor cells, that is, killing tumor cells with high temperature. Tumor tissue at high temperature (50°C) causes protein denaturation and direct tissue ablation. Generally referred to as tumor hyperthermia is heating in an acceptable range of 40–45°C.[14] In general, there is no inherent difference between the hyperthermia sensitivity of normal cells and tumor cells except for hematological malignancy.[15]
According to the relationship of the tissue thermal damage mentioned earlier, for every 1°C exceed, the “threshold (43°C).” The time required to cause damage to the same number of cells will decrease by half. For example, when the tissue is in a 45°C environment for 15 s, the degree of thermal damage to cells is similar to that at 44°C for 30 s or 43°C for 1 min. When the temperature reaches 56°C, it takes only 1 s to cause thermal damage to the tissue cells. Therefore, in this experiment, whether the lavage solution temperature of the operative field reached 56°C was considered a criterion for judging whether there was ureteral thermal injury.
Our observations showed that during the actual holmium laser lithotripsy process, the temperature of the lavage solution of the operation field was increased, and with the increase in the power of the holmium laser, the increase in the temperature of the lavage solution in the operative field increased. Among the 30 sets of data, the highest temperatures in 19 sets (63.3%) exceeded the abovementioned 56°C standard, suggesting that in more than half of the cases, the temperature of the lavage solution in the operative field reached and exceeded the standard that may cause thermal damage to the ureter during actual surgery.
When comparing the changes in the temperature of lavage solution during lithotripsy between incarcerated and nonincarcerated calculi, it was obvious that the temperature increase of the former was higher than that of the latter. Regarding the reasons for this difference, for the incarcerated calculi, the lavage solution was difficult to inject into the ureter lumen and the renal pelvis at the proximal end of the calculi, and it could only be exchanged in the narrow space at the distal end of the calculi; therefore, the increase in the temperature of the lavage solution was more obvious. When the calculi were partially or completely crushed, the lavage solution could enter the ureter, renal pelvis, and renal calyces at the proximal end of the calculi. In patients with obvious water build-up, the buffering capacity was even larger. At this time, the increase in the temperature of the lavage solution around the calculi would be reduced.
This study showed that the temperature of the lavage solution increased significantly during ureteral holmium laser lithotripsy. With the increase in the power of the holmium laser, or with incarcerated calculi, the increase in the temperature of the lavage solution around the calculi was even greater. Moreover, its increase often exceeded the temperature that could be safely tolerated by the tissue; therefore, temperature might be one of the factors that caused ureteral thermal injury and resulted in postoperative stricture.
In our follow-up results, seven patients had symptoms of low back pain, three of whom received the color Doppler ultrasound showing “hydronephrosis, ureteral obstruction may be”; the temperature of the perfused fluid in these three patients exceeded 56°C. Since follow-up time was not long, it was uncertain whether it would further develop into ureteral stricture and remained to be observed. After all, ureteral stricture is determined by a combination of factors. However, we should pay more attention to the thermal effect of holmium laser on ureter.
This study had some limitations. First, according to the current research, the thermal effect of the holmium laser was associated with the pulse energy, frequency, pulse-width modulation, fiber diameter, perfusion flow rate, lavage solution temperature, and lithotripsy method. This study only explored the relationship between thermal effect and power (energy × frequency). More complete experiments are still needed. Second, the operation was not completed by the same doctor, but rather by doctors with similar experience. Due to the difference in individual surgical techniques and intraoperative conditions, the use of the holmium laser power, the time of continuous excitation of the holmium laser, and excitation intervals differed, which may have impacted the experimental data. Moreover, the time for lithotripsy for each patient was different, which influenced the data analysis. Finally, animal experiments and pathological results are needed to further confirm the ureteral wall damage caused by the thermal effect of the holmium laser.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Funding
This study was supported by a grant from the Hunan Provincial Health and Family Planning Commission (No. B2017031).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang XK, Jiang ZQ, Tan J, Yin GM, Huang K. Thermal effect of holmium laser lithotripsy under ureteroscopy. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000300
References
- 1.Vassar GJ, Chan KF, Teichman JM, Glickman RD, Weintraub ST, Pfefer TJ, et al. Holmium: YAG lithotripsy: photothermal mechanism. J Endourol 1999; 13:181.doi: 10.1089/end.1999.13.181. [DOI] [PubMed] [Google Scholar]
- 2.Cecchetti W, Zattoni F, Nigro F, Tasca A. Plasma bubble formation induced by holmium laser: an in vitro study. Urology 2004; 63:586–590. doi: 10.1016/j.urology.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Jacques SL. Laser-tissue interactions. Photochemical, photothermal, and photomechanical. Surg Clin North Am 1992; 72:531–558. doi: 10.1016/s0039-6109(16)45731-2. [DOI] [PubMed] [Google Scholar]
- 4.Jens Cordes FNKS. First intraluminal temperature measurement during Ho_YAG-laser exposure at an in-vitro URS. OJU 2015; 5:5.doi: 10.4236/oju.2015.51001. [Google Scholar]
- 5.Molina WR, Silva IN, Donalisio Da Silva R, Gustafson D, Sehrt D, Kim FJ. Influence of saline on temperature profile of laser lithotripsy activation. J Endourol 2015; 29:235–239. doi: 10.1089/end.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallidonis P, Kamal W, Panagopoulos V, Vasilas M, Amanatides L, Kyriazis I, et al. Thulium laser in the upper urinary tract: does the heat generation in the irrigation fluid pose a risk? Evidence from an in vivo experimental study. J Endourol 2016; 30:555–559. doi: 10.1089/end.2015.0768. [DOI] [PubMed] [Google Scholar]
- 7.Hein S, Petzold R, Schoenthaler M, Wetterauer U, Miernik A. Thermal effects of Ho: YAG laser lithotripsy: real-time evaluation in an in vitro model. World J Urol 2018; 36:1469–1475. doi: 10.1007/s00345-018-2303-x. [DOI] [PubMed] [Google Scholar]
- 8.Gong CY, Qu R, Deng HZ, Luo XR, Yang L, He RX, et al. Preliminary study on the thermal effects of common power in holmium laser surgery (in Chinese). China Health Industry 2017; 55–56. doi: 10.16659/j.cnki.1672-5654.2017.08.055. [Google Scholar]
- 9.Gong CY, Li L, Qu R, Yang L, Feng YY, Deng HZ, et al. Preliminary study of domestic holmium laser surgery on rabbit kidney injury (in Chinese). Huaxi Med 2017; 32:984–987. doi: 10.7507/1002-0179.201601009. [Google Scholar]
- 10.Wollin DA, Carlos EC, Tom WR, Simmons WN, Preminger GM, Lipkin ME. Effect of laser settings and irrigation rates on ureteral temperature during holmium laser lithotripsy, an in vitro model. J Endourol 2018; 32:59–63. doi: 10.1089/end.2017.0658. [DOI] [PubMed] [Google Scholar]
- 11.Aldoukhi AH, Hall TL, Ghani KR, Maxwell AD, MacConaghy B, Roberts WW. Caliceal fluid temperature during high-power holmium laser lithotripsy in an in vivo porcine model. J Endourol 2018; 32:724–729. doi: 10.1089/end.2018.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buttice S, Sener TE, Proietti S, Dragos L, Tefik T, Doizi S, et al. Temperature changes inside the kidney: what happens during holmium:yttrium-aluminium-garnet laser usage? J Endourol 2016; 30:574–579. doi: 10.1089/end.2015.0747. [DOI] [PubMed] [Google Scholar]
- 13.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 14.Mallory M, Gogineni E, Jones GC, Greer L, Ii CBS. Therapeutic hyperthermia: the old, the new, and the upcoming. Crit Rev Oncol Hematol 2016; 97:56–64. doi: 10.1016/j.critrevonc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 15.van der Zee J. Heating the patient: a promising approach? Ann Oncol 2002; 13:1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]


