Abstract
Objective:
To review the application of radiomics in gastric cancer and its challenges as well as future prospects.
Data sources:
A research for relevant studies were performed in PubMed with the terms of “radiomics,” “texture analysis,” and “gastric cancer.” The search was updated until February 28th, 2019.
Study selection:
All original articles regarding the investigation of texture analysis or radiomics in gastric cancer were retrieved. Only papers written in English were included.
Results:
A total of 17 original articles were selected in final. It is shown that radiomics has yielded moderate to excellent performance in a spectrum of respects including differential diagnosis, assessment of histological differential degree, evaluation of tumor stage, prediction of response to therapy, and prognosis in gastric cancer. Yet, a number of challenges are facing both radiomics itself and its application in gastric cancer.
Conclusions:
Radiomics holds great potential in facilitating decision-making in gastric cancer. With the standardization of work-flow and advancement of machine learning methods, radiomics is expected to make great breakthroughs in precision medicine of gastric cancer.
Keywords: Gastric cancer, Radiology, Radiomics
Introduction
Gastric cancer is a major health burden, although its incidence has decreased worldwide in recent decades. It still serves as the third leading cause of malignancy-related death worldwide. It is estimated that there were over 1,000,000 new gastric cancer cases and about 783,000 gastric cancer deaths globally in 2018.[1] In China, the reported new gastric cancer cases and deaths were respectively 6,791,000 and 498,000 patients in 2015.[2] Imaging modalities play a crucial role in the diagnosis, staging, and risk stratification of gastric cancer for optimal therapeutic strategy selection and outcome improvement.[3] Radiomics is an emerging field using a non-invasive approach to extract numerous quantitative features from medical images, especially parameters not visible to the naked human eye or quantifiable by routine analysis.[4–6] Radiomics has shown promise for gene expression, pathological classification, tumor metastasis, treatment response, and clinical outcomes in variable cancers, such as lung cancer, breast cancer, rectal cancer, hepatocellular carcinoma, nasopharyngeal carcinoma, bladder cancer, and gastric cancer.[7–17] This article briefly reviews the application of radiomics in gastric cancer and challenges as well as future prospects.
Overview of Radiomics
The concept of radiomics was raised by Lambin et al[18] in 2012 and subsequently refined by Kumar et al[19] as the high-throughput extraction and analysis of large amounts of advanced quantitative imaging features from medical images obtained with computed tomography (CT), positron emission tomography (PET) or magnetic resonance imaging. The dominant advantage of radiomics is that it enables the acquisition of numerable quantitative features which could offer information on tumor phenotype and microenvironment which is unavailable by traditional radiology.[5,18] Another major strength of radiomics is the utilization of artificial intelligence or machine learning approaches, which will transform the mineable high-dimensional data to develop diagnostic, predictive or prognostic radiomic models or signatures to support personalized clinical decision making.[4,20] A radiomics study can be structured into the following four phases: (1) Image acquisition: obtaining large-scale medical images with standard scanning and reconstruction protocols is pivotal for eliminating unnecessary confounding variability in radiomics; (2) Image segmentation: regions of interest (ROIs) or volumes of interest (VOIs) of the tumor, metastatic lesions, and normal tissues can be segmented manually or semi-automatically for further analysis; (3) Feature extraction and selection: high-throughput extraction of quantitative imaging features from ROIs or VOIs is the essence of radiomics. Commonly used radiomics features can be categorized into shape and size features, first-order histograms, second-order histograms (textural), and fractal features.[6,21] Features that are redundant or may not correlated with the given tasks should be excluded for model construction. The least absolute shrinkage and selection operator (LASSO), maximum relevance and minimum redundancy, and principal component analysis are frequently used feature selection methods; (4) Model construction and validation: identification of optimal machine-learning models based on the clinical information and selected features is the pivotal step. Support vector machine (SVM), random forest, artificial neural networks (ANNs) and bootstrapping are widely used machine-learning methods. The selected model should be validated prior to its application in scientific and clinical communities. Excellent models should exhibit statistical consistency between the training and validation sets.[5,6,19,21]
Application of Radiomics Approaches in Gastric Cancer
Data sources, study selection, and analysis
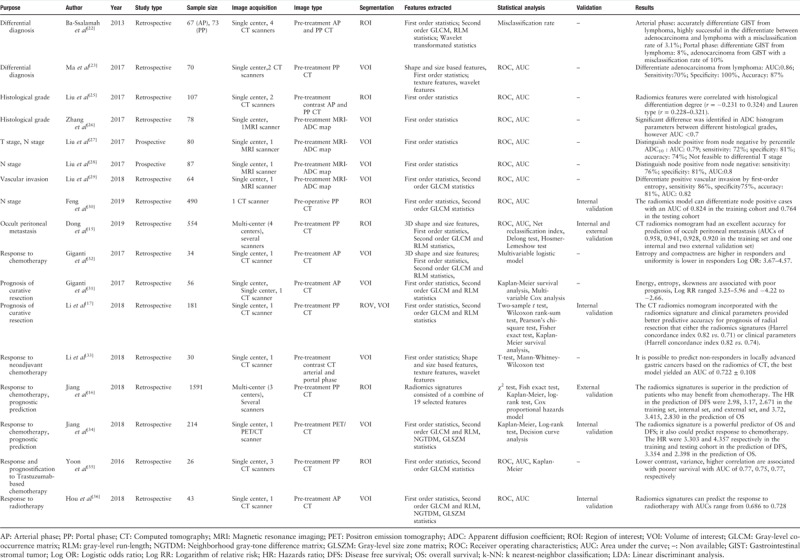
A research for relevant studies was performed in PubMed databases with index terms of “radiomics,” “texture analysis,” and “gastric cancer.” The search was updated until February 28th, 2019. All original articles regarding the investigation of texture analysis or radiomics in gastric cancer were retrieved. Only papers written in English were included. A total of 17 studies were selected in final. These investigations have found that radiomics may be attributable to the differential diagnosis (two studies), assessment of histological differential degree (two studies), pathological N stage (three studies), M stage (occult peritoneal metastasis, one study), vascular invasion (one study), response to chemotherapy (five studies) or radiotherapy (one study), and prognosis of surgery (two studies). A summary of these works was presented in Table 1.
Table 1.
Radiomics study in gastric cancer.
Differential diagnosis
Primary gastric lymphoma, gastrointestinal stromal tumor, and adenocarcinoma can frequently mimic each other, yet with remarkably different management strategies and prognoses.[22] The differential diagnosis remains challenging based on routine CT characteristics. Two studies have investigated the ability of radiomics for differential diagnosis of gastric cancer. Quantitative radiomics analysis is shown to be promise to supplement conventional CT in the distinction of gastric cancer. The work conducted by Ba-Ssalamach et al[22] analyzed the texture features derived from pre-operative arterial phase (47 patients) and portal phase (48 patients) images. They found that VOI-based texture features from arterial phase CT images can differentiate gastrointestinal stromal tumor from lymphoma with 100% accuracy and can distinguish adenocarcinoma from lymphoma with a misclassification rate of 3.1%; the corresponding misclassification rate was 8% and 10% based on portal phase images, respectively. Ma et al[23] collected the pre-operative portal phase images of 40 patients with Bormann IV type gastric cancer and 30 cases with gastric lymphoma to carry out radiomics analysis, and they reported that whole-lesion-based texture features from portal phase CT images can differentiate adenocarcinoma from lymphoma with an accuracy of 87%.
Prediction of histological grade
The histopathological features of gastric cancer significantly influences treatment and prognosis of patients.[24] Two studies have been performed to explore the values of radiomics in the assessment of histological grade of gastric cancer. The study by Liu et al[25] segmented the whole lesions on the pre-operative arterial and portal phase images of 107 patients. They identified that the radiomic features were correlated with the histological grade (r = −0.231 to −0.324) and Lauren type (r = 0.228–0.321). Apparent diffusion coefficient (ADC) maps of 78 patients were collected by Zhang et al[26] and the whole lesions were segmented. The extracted histogram parameters were found to be significantly different in lesions with disparate histological grades. Nevertheless, the role of these histogram parameters is likely to be limited in clinical practice because the area under the curve (AUC) was less than 70%.
Prediction of tumor stage
The accurate evaluation of tumor stage is a pre-requisite for the selection of an appropriate therapeutic approach and have prognostic significance.[24] Altogether five studies were carried out to evaluate the role of radiomics in the prediction of lymph node status, vascular invasion, and occult peritoneal metastasis. Traditional method to evaluate the lymph node is based on the size of the lymph node. Diagnostic uncertainty frequently occurs as normal-size nodes can be malignant yet inflammatory nodes may be enlarged. The radiomics approach was shown to be promising in assessment of tumor stage. Liu et al[27,28] segmented the VOIs of lesions on ADC maps in approximately 80 cases and found that whole-lesion-based radiomic features can identify patients with positive lymph node metastases with an accuracy ranging between 74% and 81%, but these signatures were not capable to predict the T stage. Liu et al[29] evaluated the whole-volume ADC-based entropy parameters in the pre-operative assessment of gastric cancer's aggressiveness in 64 patients. They found that four entropy related parameters were obviously differed between patients with and without perineural invasion. Feng et al[30] collected the pre-operative portal phase images of 490 patients. A total of 93 features were derived from the segmented ROIs. A radiomics model was built using the modified recursive feature selection SVM method, which yielded an AUC of 0.824 in the training cohort and 0.764 in the test cohort in prediction of lymph node metastasis. Early detection of peritoneal metastasis is pivotal for optimal treatment selection and avoidance of unnecessary surgical procedures. However, the specificity of conventional CT in the detection of peritoneal metastasis is unsatisfactory only around 50%. Dong et al[15] carried out a multi-center study in which 554 subjects with occult peritoneal metastasis were retrospectively analyzed. ROIs of both the tumor and the peritoneal region nearest to the center of the primary tumor were segmented. A total of 133 features were extracted on each ROI of each patient. A nomogram was constructed incorporating radiomics features extracted from the tumor and the peritoneal region as well as clinical factors. The nomogram yielded an excellent performance in the prediction of occult peritoneal metastasis with an AUC of 0.958 in the training set and 0.941, 0.928, and 0.920 in an internal and two external validation sets.
Prediction of response to therapy and patient prognosis
The identification of pre-therapeutic predictive markers for response and prognosis would be invaluable in individualized patient treatment.
Prognosis of surgical resection
Tumor-node-metastasis staging systems are primary prognostic factors, yet it is not uncommon that patients with same stage exhibit heterogeneous outcomes. Two studies evaluated the values of radiomics in the prediction of prognosis after surgical resection. Giganti et al[31] investigated the association between CT texture-derived parameters and the overall survival (OS) in 56 patients with resectable gastric cancer. In total, 107 features were extracted from each VOI on pre-operative arterial phase images. The study identified that features including energy, entropy, maximum Hounsfield unit value, skewness, root mean square, and mean absolute deviation were significantly associated with a negative prognosis with logistic relative risk ranged 3.25 to 5.96 and −4.22 to −2.66. The work carried out by Li et al[17] included pre-operative portal phase CT images of 181 patients. They segmented both the ROI and VOI of the tumor with the purpose to compare the performance between two-dimensional and three-dimensional segmentation. A total of 273 features were extracted from each ROI and 485 features were extracted from each VOI. LASSO method was used for feature selection and a LASSO Cox regression model was built for the prediction of OS. Both two-dimensional and three-dimensional features were associated with OS in the training set; however, no significant association for prediction of OS was found by three-dimensional features in the test set. A nomogram incorporated with the ROI-based radiomics signature and clinical parameters was built which provided better predictive accuracy for prognosis of radial resection than either the radiomics signatures (Harrel concordance index 0.82 vs. 0.71) or clinical parameters (Harrell concordance index 0.82 vs. 0.74).
Prognosis of neoadjuvant chemotherapy (NAC)
NAC is the mainstay for locally advanced cases, as it can facilitate downgrading of the lesion and improve the radical resection rate. However, not all patients could benefit from the schemes. Individuals who were insensitive to NAC may experience unnecessary drug-toxicity. Four studies were published regarding the value of radiomics in the prediction of response and prognosis of NAC until now, the results of which indicated that radiomics may provide incremental values in selection of appropriate candidates for NAC. Giganti et al[32] included the pre-treatment arterial phase images of 43 patients and manually segmented the whole tumor. They found that 14 features were significantly different between the responders and non-responders at univariate analysis. Multivariate analysis revealed that entropy, range, root mean square were independent predictors of responders and non-responders with logistic odds ratio of 4.11, 3.67, and 4.57, respectively. In another study with inclusion of the pre-treatment arterial and portal phase images of 30 patients, Li et al[33] analyzed the values of ROI-based radiomics features with 32 combinations of feature selection and machine-learning methods. A total of 19,985 radiomics features were extracted in the arterial and portal phase images of each patient. One machine-learning method showed AUC >0.6 using features from arterial phase images and 12 algorithms displayed AUCs >0.6 based on features from portal phase images in predicting the response to NAC. The largest study was carried out by Jiang et al,[16] which was a multi-center retrospective analysis with inclusion of the pre-operative portal phase CT images of 1591 patients. A total of 269 features were extracted from each ROI. A LASSO Cox regression model was used to build a prognostic classifier and 19 potential predictors were selected. The study revealed that the nomogram based on the combination of clinical factors and radiomics signatures would facilitate the prediction of disease-free survival (DFS) with hazard ratios (HRs) of 2.98, 3.17, and 2.671 in the training set, internal test set, and external test set, while the corresponding HRs for predicting OS were 3.72, 3.415, and 2.830, respectively. Additionally, a multi-center study included PET-CT images of 214 patients was conducted by Jiang et al.[34] Each ROI derived 80 features. A multiple-feature-based radiomics signature was constructed for predicting DFS. A nomogram was built based on the radiomics signature and clinical predictors which were shown to be a powerful predictor of OS (HRs were 3.354 and 2.398 in the training set and test set, respectively) and DFS (HRs were 3.303 and 4.357 in the training and test sets, respectively).
Prognosis of targeted chemotherapy with trastuzumab
Although a survival gain was observed for targeted therapy with trastuzumab in patients with human epidermal receptor 2 (HER2) over-expression, there are still patients who are insensitive to this approach. There has been only one study regarding radiomics in the prediction of targeted chemotherapy with trastuzumab, which was conducted by Yoon et al.[35] They enrolled 26 cases with HER2 over-expression aimed at predicting the response to trastuzumab treatment. A number of histogram and gray-level co-occurrence matrices (GLCM) features were derived from the manually delineated ROIs on portal phase CT images. It is reported that GLCM features including angular second moment, contrast, variance, and correlation can differentiate responders from non-responders with AUCs ranging from 0.75 to 0.77. The results supported that radiomics markers may provide additional prognostic information for patient selection.
Prognosis of radiotherapy
Radiotherapy has been proved as effective treatment strategy across a range of cancer, including gastric cancer. Nevertheless, the response to radiotherapy is highly individual.[35] Cases received yet insensitive to radiotherapy may lead to a delay of the modification of treatment plan. The works investigated the value of radiomics in the differentiation between responder and non-responders to radiotherapy were rare. Only Hou et al[36] segmented the VOIs of the pre-treatment arterial phase images of 43 patients. A total of 1117 features were extracted from each VOI. The study revealed that these signatures can predict the response to radiotherapy with AUCs of 0.714 and 0.749 using the ANN and k-nearest neighbor methods in the training set, respectively; the AUCs in the validation set were both 0.816. The study suggested that radiomics may serve as a valuable tool for early prediction of response to radiotherapy in gastric cancer.
In a nutshell, it is evident that radiomics hold great promise in facilitating differentiation diagnosis, evaluation of histological degree and tumor stage, as well as response to therapy and prognosis in gastric cancer.
Challenges and Future Prospects
Although radiomics holds the promise to empower the next major breakthrough in precision medicine of gastric cancer, it is still in its infancy. Challenges are facing both radiomics itself and its application in gastric cancer.[5,6,37]
Each of the four process of radiomics has its unique challenges
Image acquisition
The power of radiomic models is dependent on sufficient patient population. Extracting a large number of imaging features from a small dataset is likely to reduce its power and increase the risk of overfitting. Robert et al[21] recommended that at least ten patients are needed for each feature in binary classifiers. Given the number of radiomic features derived, patient population was relatively small for the majority of the previous publications. In addition, most of the investigations were retrospective analyses based on images from more than one scanner, variable slice thickness as well as multiple reconstruction algorithms. Variations in image acquisition parameters and reconstruction is likely to introduce alteration of the features that are not caused by the underlying biologic mechanisms, resulting in redundant or less reproducible features.[38] Mackin et al[39] reported that the bias of features derived from multiple scanners was comparable to those extracted from one scanner. Kim et al[40] identified that compared with inter-reader variability, bias caused by variable reconstruction algorithms weights more. The analysis by Midya et al[41] revealed that image acquisition parameters such as tube current, noise index, and reconstruction technique had strong influence in the reproducibility of radiomics features. Smoother reconstruction algorithms and thinner slices are considered favorable factors for improving the reproducibility of the extracted features.[42] Given that non-standardized imaging protocols are inevitable at the moment, extensive disclosure of the imaging protocols is recommended to facilitate reproducibility and comparability of radiomics studies.[6]
Image segmentation
Variable manual and semi-automatic segmentation methods were used to derive ROI- or VOI-based features among the radiomics investigations of gastric cancer. The segmentation determines which voxels within an image are analyzed and serves as one of the most critical steps in the radiomics workflow. The variability in segmentation can introduce bias into the derived features. Computer-aided edge detection followed by manual curation is currently considered the optimum segmentation method for reproducibility. A migration towards deep learning and advanced neural network approaches may be more useful and can compensate for the variability of manual segmentation.[5] Although two-dimensional ROI-based features are easier to obtain with less labor consumption and faster calculations, it is assumed that these features were unable to accurately reflect the heterogeneity of an entire tumor.[43] The efficiency of three-dimensional VOI-based features will be compromised due to larger partial volume artifacts along the z-direction.[42] The work by Li et al[17] reported that VOI based features pales in comparison with ROI based features in predicting of the prognosis of radical resection. The perspectives on whether ROI-based features are superior in reproducibility and implementation compared with VOI-based features requires further studies.[42,44]
Feature extraction and selection
Various software and programs were utilized for feature extraction in the radiomics studies of gastric cancer. Filograna et al[45] called for the software used for feature extraction to be open-sourced to facilitate external validation and further optimize the constructed models. What's more, not all features extracted will be useful for classifiers. The derived features should be exploited, and those that lack robustness should be eliminated. The performance of the radiomics model can be variable based on different feature selection methods.[46] Avanzo et al[6] advocated that the process of feature reduction or exclusion needs to be documented clearly.
Model construction and validation
Different techniques are associated with distinct inherent limitations.[47] The choice of modeling technique has been shown to affect prediction performance.[48] Nevertheless, the prediction model is often a single technique selected according to the preference and experience of the performers in almost all existing studies. The key point of model selection is that the work is entirely reproducible.[42] Although validation is an indispensable component of a complete radiomics analysis, only a sub-set of prior studies was internally or externally validated. Researchers must assess whether the model is predictive for the target patient population or only for sub-sets of the samples analyzed. Ideally, the models should be externally validated.[19]
Future direction of radiomics in gastric cancer
Further development of radiomics in gastric cancer should be focused on the following three respects. First, although NAC is widely recommended, evidence-based studies of its role in the improvement of long-term prognoses are still absent.[49] Radiomics has been applied to predict the short-term efficacy of NAC, yet its role in the prediction of long-term survival after NAC has not been investigated and future researches will be warranted. Second, the identification of specific cancer sub-groups, such as cases of HER2 overexpression or programmed cell death-1 ligand 1-positive cases, is of great clinical significance in the selection of candidates for targeted chemotherapy or immunotherapy.[24,50] Last but no least, nearly all published radiomics studies focused on single modalities, yet hybrid images are likely to hold more information and can develop a more complete picture of the tumor.[19] Multimodality imaging-based radiomics merits future study.
Conclusions
As a product of cooperation between medicine and engineering, radiomics serves as the frontier of decision making in gastric cancer by using advanced algorithms. While radiomics is still in the early phases, a number of details of its workflow need to be refined, and a large amount of researches are urgently needed to be carried out. It is convinced that with the continuous accumulation of data and standardization of work-flow and improvement of artificial techniques, radiomics will make great breakthroughs in precision medicine of gastric cancer.
Funding
The study was funded by National Public Welfare Basic Scientific Research Program of Chinese Academy of Medical Sciences (No. 2018PT32003 and No. 2017PT32004).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang Y, Jin ZY. Radiomics approaches in gastric cancer: a frontier in clinical decision making. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000360
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Giganti F, Tang L, Baba H. Gastric cancer and imaging biomarkers: part 1 – a critical review of DW-MRI and CE-MDCT findings. Eur Radiol 2018; 29:1743–1753. doi: 10.1007/s00330-018-5732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodalal Z, Trebeschi S, Beets-Tan R. Radiomics: a critical step towards integrated healthcare. Insights Imaging 2018; 9:911–914. doi: 10.1007/s13244-018-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 6.Avanzo M, Stancanello J, Naqa IEI. Beyond imaging: the promise of radiomics. Phys Med 2017; 38:122–139. doi: 10.1016/j.ejmp.2017.05.071. [DOI] [PubMed] [Google Scholar]
- 7.Liang CH, Tian J, Sun YS, Liu ZY. Accelerating the progression and clinical translation of radiomics (in Chinese). Chin J Radiol 2017; 51:897–898. doi: 10.3760/cma.j.issn.1005-1201.2017.12.001. [Google Scholar]
- 8.He L, Huang YQ, Ma ZL, Liang CS, Huang XM, Cheng ZX, et al. A CT-based radiomics analysis for clinical staging of non-small cell lung cancer (in Chinese). Chin J Radiol 2017; 51:906–911. doi: 10.3760/cma.j.issn.1005-1201.2017.12.004. [Google Scholar]
- 9.Ma WJ, Zhao YM, Ji Y, Hao YJ, Liu JJ, Liu PF. Triple-negative and non-triple-negative breast cancer prediction by mammographic radiomics features (in Chinese). Chin J Radiol 2018; 52:842–846. doi: 10.3760/cma.j.issn.1005-1201.2018.11.006. [Google Scholar]
- 10.Liu LL, Yang H, Shao GL, Fan LY, Yang YB, Pang PP, et al. CT radiomics model for predicting the three-year survival time of primary hepatocellular carcinoma (in Chinese). Chin J Radiol 2018; 52:681–686. doi: 10.3760/cma.j.issn.1005-1201.2018.09.007. [Google Scholar]
- 11.Shu ZY, Fang SH, Shao Y, Mao DW, Chai R, Chen YJ, et al. A radiomic nomogram based on T2WI for predicting synchronous liver metastasis of rectal cancer (in Chinese). Chin J Radiol 2019; 53:205–211. doi: 10.3760/cma.j.issn.1005-1201.2019.03.009. [Google Scholar]
- 12.Zhang B, Tian J, Dong D, Gu D, Zhang L, Lian Z, et al. Radiomics features of multiparametric MRI as novel prognostic factors in advanced nasopharyngeal carcinoma. Clin Cancer Res 2017; 23:4259–4269. doi: 10.1158/1078-0432.CCR-16-2910. [DOI] [PubMed] [Google Scholar]
- 13.Wu PQ, Zhao K, Wu L, Liu ZY, Liang CH. Correlation of radiomic features based on diffusion weighted imaging and dynamic contrast-enhancement MRI with molecular subtypes of breast cancer (in Chinese). Chin J Radiol 2018; 52:338–343. doi: 10.3760/cma.j.issn.1005-1201.2018.05.004. [Google Scholar]
- 14.Fan L, Fang MJ, Dong D, Tu WT, Wang Y, Li Q, et al. Subtype discrimination of lung adenocarcinoma manifesting as ground glass nodule based on radiomics (in Chinese). Chin J Radiol 2017; 51:912–917. doi: 10.3760/cma.j.issn.1005-1201.2017.12.005. [Google Scholar]
- 15.Dong D, Tang L, Li ZY, Fang MJ, Gao JB, Shan XH, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol 2019; 30:431–438. doi: 10.1093/annonc/mdz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Chen C, Xie J, Wang W, Zha X, Lv W, et al. Radiomics signature of computed tomography imaging for prediction of survival and chemotherapeutic benefits in gastric cancer. EBioMedicine 2018; 36:171–182. doi: 10.1016/j.ebiom.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Zhang L, Tian C, Song H, Fang M, Hu C, et al. Prognostic value of computed tomography radiomics features in patients with gastric cancer following curative resection. Eur Radiol 2018; 29:3079–3089. doi: 10.1007/s00330-018-5861-9. [DOI] [PubMed] [Google Scholar]
- 18.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magn Reson Imaging 2012; 30:1234–1248. doi: 10.1016/j.mri.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panth KM, Leijenaar RT, Carvalho S, Lieuwes NG, Yaromina A, Dubois L, et al. Is there a causal relationship between genetic changes and radiomics-based image features? An in vivo preclinical experiment with doxycycline inducible GADD34 tumor cells. Radiother Oncol 2015; 116:462–466. doi: 10.1016/j.radonc.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ba-Ssalamah A, Muin D, Schernthaner R, Kulinna-Cosentini C, Bastati N, Stift J, et al. Texture-based classification of different gastric tumors at contrast-enhanced CT. Eur J Radiol 2013; 82:e537–e543. doi: 10.1016/j.ejrad.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, Fang M, Huang Y, He L, Chen X, Liang C, et al. CT-based radiomics signature for differentiating Borrmann type IV gastric cancer from primary gastric lymphoma. Eur J Radiol 2017; 91:142–147. doi: 10.1016/j.ejrad.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Liu S, Ji C, Zheng H, Pan X, Zhang Y, et al. Application of CT texture analysis in predicting histopathological characteristics of gastric cancers. Eur Radiol 2017; 27:4951–4959. doi: 10.1007/s00330-017-4881-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Chen J, Liu S, Shi H, Guan W, Ji C, et al. Assessment of histological differentiation in gastric cancers using whole-volume histogram analysis of apparent diffusion coefficient maps. J Magn Reson Imaging 2017; 45:440–449. doi: 10.1002/jmri.25360. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Zhang Y, Chen L, Guan W, Guan Y, Ge Y, et al. Whole-lesion apparent diffusion coefficient histogram analysis: significance in T and N staging of gastric cancers. BMC Cancer 2017; 17:665.doi: 10.1186/s12885-017-3622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Zhang Y, Xia J, Chen L, Guan W, Guan Y, et al. Predicting the nodal status in gastric cancers: the role of apparent diffusion coefficient histogram characteristic analysis. Magn Reson Imaging 2017; 42:144–151. doi: 10.1016/j.mri.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Zheng H, Zhang Y, Chen L, Guan W, Guan Y, et al. Whole-volume apparent diffusion coefficient-based entropy parameters for assessment of gastric cancer aggressiveness. J Magn Reson Imaging 2018; 47:168–175. doi: 10.1002/jmri.25752. [DOI] [PubMed] [Google Scholar]
- 30.Feng QX, Liu C, Qi L, Sun SW, Song Y, Yang G, et al. An intelligent clinical decision support system for preoperative prediction of lymph node metastasis in gastric cancer. J Am Coll Radiol 2019; 16:952–960. doi: 10.1016/j.jacr.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Giganti F, Antunes S, Salerno A, Ambrosi A, Marra P, Nicoletti R, et al. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol 2017; 27:1831–1839. doi: 10.1007/s00330-016-4540-y. [DOI] [PubMed] [Google Scholar]
- 32.Giganti F, Marra P, Ambrosi A, Salerno A, Antunes S, Chiari D, et al. Pre-treatment MDCT-based texture analysis for therapy response prediction in gastric cancer: comparison with tumour regression grade at final histology. Eur J Radiol 2017; 90:129–137. doi: 10.1016/j.ejrad.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Zhang D, Dai Y, Dong J, Wu L, Li Y, et al. Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: a pilot study. Chin J Cancer Res 2018; 30:406–414. doi: 10.21147/j.issn.1000-9604.2018.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Y, Yuan Q, Lv W, Xi S, Huang W, Sun Z, et al. Radiomic signature of (18)F fluorodeoxyglucose PET/CT for prediction of gastric cancer survival and chemotherapeutic benefits. Theranostics 2018; 8:5915–5928. doi: 10.1016/j.ebiom.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon SH, Kim YH, Lee YJ, Park J, Kim JW, Lee HS, et al. Tumor heterogeneity in human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer assessed by CT texture analysis: association with survival after trastuzumab treatment. PLoS One 2016; 11:e0161278.doi: 10.1371/journal.pone.0161278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou Z, Yang Y, Li S, Yan J, Ren W, Liu J, et al. Radiomic analysis using contrast-enhanced CT: predict treatment response to pulsed low dose rate radiotherapy in gastric carcinoma with abdominal cavity metastasis. Quant Imaging Med Surg 2018; 8:410–420. doi: 10.21037/qims.2018.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip SS, Aerts HJWL. Applications and limitations of radiomics. Phys Med Biol 2016; 61:R150–R166. doi: 10.1088/0031-9155/61/13/R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berenguer R, Pastor-Juan MDR, Canales-Vazquez J, Castro-Garcia M, Villas MV, Mansilla Legorburo F, et al. Radiomics of CT features may be nonreproducible and redundant: influence of CT acquisition parameters. Radiology 2018; 288:407–415. doi: 10.1148/radiol.2018172361. [DOI] [PubMed] [Google Scholar]
- 39.Mackin D, Fave X, Zhang L, Fried D, Yang J, Taylor B, et al. Measuring computed tomography scanner variability of radiomics features. Invest Radiol 2015; 50:757–765. doi: 10.1097/RLI.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H, Park CM, Lee M, Park SJ, Song YS, Lee JH, et al. Impact of reconstruction algorithms on CT radiomic features of pulmonary tumors: analysis of intra- and inter-reader variability and inter-reconstruction algorithm variability. PLoS One 2016; 11:e0164924.doi: 10.1371/journal.pone.0164924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Midya A, Chakraborty J, Gonen M, Do RKG, Simpson AL. Influence of CT acquisition and reconstruction parameters on radiomic feature reproducibility. J Med Imaging (Bellingham) 2018; 5:011020.doi: 10.1117/1.JMI.5.1.011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao B, Tan Y, Tsai WY, Qi J, Xie C, Lu L, et al. Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep 2016; 6:23428.doi: 10.1038/srep23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng F, Kozarski R, Ganeshan B, Goh V. Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol 2013; 82:342–348. doi: 10.1016/j.ejrad.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Zhao B, Tan Y, Bell DJ, Marley SE, Guo P, Mann H, et al. Exploring intra- and inter-reader variability in uni-dimensional, bi-dimensional, and volumetric measurements of solid tumors on CT scans reconstructed at different slice intervals. Eur J Radiol 2013; 82:959–968. doi: 10.1016/j.ejrad.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filograna L, Lenkowicz J, Cellini F, Dinapoli N, Manfrida S, Magarelli N, et al. Identification of the most significant magnetic resonance imaging (MRI) radiomic features in oncological patients with vertebral bone marrow metastatic disease: a feasibility study. Radiol Med 2018; 124:50–57. doi: 10.1007/s11547-018-0935-y. [DOI] [PubMed] [Google Scholar]
- 46.Degenhardt F, Seifert S, Szymczak S. Evaluation of variable selection methods for random forests and omics data sets. Brief Bioinform 2017; 20:492–503. doi: 10.1093/bib/bbx124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thanh Noi P, Kappas M. Comparison of random forest, k-nearest neighbor, and support vector machine classifiers for land cover classification using sentinel-2 imagery. Sensors (Basel) 2017; 18: doi: 10.3390/s18010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parmar C, Grossmann P, Bussink J, Lambin P, Aerts HJ. Machine learning methods for quantitative radiomic biomarkers. Sci Rep 2015; 5:13087.doi: 10.1038/srep13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines (ver. 5). Tokyo: Kanehara; 2018. [Google Scholar]
- 50.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Gastric Cancer (Ver. 2): NCCN, 2018. Available from: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf Accessed October 11, 2018. [Google Scholar]


