Abstract
Background:
The effect and mechanism of Saccharomyces boulardii (Sb) in inflammatory bowel disease are unclear. The objective of the study was to evaluate the impact of Sb on intestinal mucosal barrier and intestinal flora in a colitis mouse model.
Methods:
Forty C57BL/6J male mice were randomly assigned to five groups: normal control group (A), pathologic control group (B), Sb treatment group (C), mesalazine treatment group (D), and Sb combined with mesalazine treatment group (E). Colitis was induced by the addition of 2.5% (wt/vol) dextran sodium sulfate (DSS) in the drinking water ad libitum for 7 days. The general condition, weight change, stool property, and bloody stool level of mice were observed to evaluate the disease activity index. The expression of zona occludens-1 (ZO-1) and occludin in intestinal tissue were measured by immunohistochemistry. The level of tumor necrosis factor-α (TNF-α) and interleukin (IL)-8 in plasma was measured by enzyme linked immunosorbent assay. Inter-cellular tight junctions were observed by transmission electron microscopy. The feces and intestinal contents were collected sterilely, and intestinal flora was analyzed by 16S rRNA sequencing.
Results:
Compared with group B, Sb reduced the disease activity index and histological score of group C (disease activity index: group B 2.708 ± 0.628, group C 1.542 ± 0.616, PBC = 0.005; histological score: group B 9.875 ± 3.271, group C 4.750 ± 1.832, PBC = 0.005) in DSS-induced colitis in mice. Sb exerted a protect effect on the expression of ZO-1 (group B 2.075 ± 1.176, group C 4.225 ± 1.316, PBC = 0.019) and occludin (group B 2.200 ± 0.968, group C 3.525 ± 1.047, PBC = 0.023). Compared with group B, Sb decreased the level of TNF-α and IL-8 of group C (TNF-α: group B 716.323 ± 44.691 ng/L, group C 521.740 ± 90.121 ng/L, PBC = 0.001; IL-8: group B 128.992 ± 11.475 pg/mL, group C 106.283 ± 15.906 pg/mL, PBC = 0.012). Treatment with Sb preserved the tight junctions and ameliorated microvilli and inter-cellular space. Treatment with Sb also showed its own characteristics: a higher percentage of Bacteroidetes and a lower percentage of Firmicutes, with significant differences or a significant trend. The proportion of the S24-7 family was increased significantly in the Sb treatment group.
Conclusions:
Sb shows an anti-inflammatory effect and has a protective effect on the intestinal mucosal mechanical barrier. Sb may up-regulate the abundance of family S24-7 specifically, and maybe a mechanism underlying its function.
Keywords: Inflammatory bowel disease, Saccharomyces boulardii, Intestinal mucosal barrier
Introduction
Inflammatory bowel disease (IBD) is a chronic and recurrent intestinal inflammatory disease, which includes ulcerative colitis (UC) and Crohn's disease (CD). UC is characterized by mucosal inflammation and is limited to the colon. Unlike UC, CD can cause transmural inflammation and affect any part of the gastrointestinal tract and is commonly associated with complications such as abscesses, fistulas, and strictures.[1]
Although the pathogenesis of IBD remains unclear, intestinal microbial flora is thought to play a pivotal role.[2,3] To prevent direct exposure of immune cells from the gut microbiota, the bowel wall is coated with a single layer of epithelial cells that provide an efficient barrier preventing the uncontrolled access of bacteria from the bowel wall.[4] When the intestinal mucosal barrier was disrupted, bacteria can penetrate the bowel wall evoking a deregulated immune response, which is involved in the pathophysiology of IBD.[5] Intestinal homeostasis disturbance through intestinal barrier disruption presumably plays a key role in IBD development.[6]
Probiotics are extensively used in the treatment of IBD. It should be noted that yeast probiotics are different from bacterial probiotics, and the role of yeast probiotics is unclear. Saccharomyces boulardii (Sb) is the only yeast probiotics widely used in clinical practice, and previous studies have revealed that Sb can interfere with the inflammatory reaction of infection and diarrhea[7] and re-establish normal microbiota in antibiotic-shocked mice or patients with diarrhea.[8,9] However, the effect and mechanism of Sb in IBD remain unclear. Therefore, the objective of this study was to evaluate the effects of Sb on colitis and intestinal flora.
Methods
Animals
The study was approved by the Peking University First Hospital Laboratory Animal Welfare and Ethics Committee (No. J201635). Forty six-week-old male C57BL/6J mice were provided by the Center of Experimental Animals of Peking University First Hospital. The mice were acclimatized under specific pathogen-free conditions for 7 days prior to the experiment. All the mice were housed in a 12 h light/dark cycle.
The mice were assigned to five groups randomly (n = 8/group): normal control (group A), colitis model group (group B), Sb treatment group (group C), mesalazine treatment group (group D), and combination treatment group (group E). Group A mice were given purified water to drink freely. Colitis was induced by the addition of 2.5% (wt/vol) dextran sodium sulfate (DSS) (molecular weight of 36,000–50,000; MP Biomedicals, Santa Ana, CA, USA) in the drinking water ad libitum for 7 days in the other four groups. Groups A and B were gavaged by normal saline, and the remaining groups were treated with Sb (0.46 mg/g per day), mesalazine (0.605 mg/g per day) and combination treatment twice a day, respectively; the dosage was calculated according to the conversion between humans and mice.
Evaluation of the disease activity index (DAI)
The DAI was determined by scoring the extent of body weight loss (0: none, 1: 1%–5%, 2: 6%–10%, 3: 11%–15%, 4: >15%), stool consistency (0: normal, 2: loose stool, 4: diarrhea), and the presence of occult or gross blood (0: negative, 2: fecal occult blood test positive, 4: gross bleeding).[10]
At the end of the experiment, all mice were anesthetized by pentobarbital, the blood was collected from the peri-orbital vein; the entire colon was removed, and all mice were administered euthanasia by cervical dislocation. Using sterile scissors, the colon was cut open longitudinally along the mesenteric line, and then fecal samples were collected sterilely and immediately frozen in liquid nitrogen. The colon was rolled around to form a Swiss roll, and then was fixed in 10% formalin, embedded in paraffin cut into 5 μm sections, and stained with hematoxylin and eosin. The severity of inflammation was evaluated by two authors independently and graded according to previous evaluation criteria. Histological assessment was graded as follows: inflammation: none (0), mild (1), severe (2); infiltration depth was graded as follows: none (0), sub-mucosa layer (1), muscular layer (2), serosal layer (3); crypt loss was graded as follows: none (0), 1/3 of crypts lost (1), 2/3 of crypts lost (2), loss of entire crypt with the surface epithelium remaining intact (3), loss of both the entire crypt and surface epithelium (4); the percentage involvement of disease process was graded as follows: 1% to 25% (1), 26% to 50% (2), 51% to 75% (3), 76% to 100% (4).[11]
Enzyme-linked immunosorbent assay
The levels of tumor necrosis factor α (TNF-α) and interleukin (IL)-8 in the plasma of each group were measured by enzyme-linked immunosorbent assay using commercial kits (Fangcheng Biology, Beijing, China) according to the manufacturer's instructions.
Immunohistochemistry
Four-micrometer-thick tissues were deparaffinized in xylene and passed through graded alcohols. Next, the tissues were microwaved twice for 11 min in 10 mmol/L sodium citrate pH 6.0 to induce epitope retrieval. The tissues were incubated with antibodies against Occludin (ab168986; Abcam, UK) and zona occludens-1 (ZO-1) (PA5-28858; Thermo Fisher, Massachusetts, USA) at 4°C overnight and used at a dilution of 1:100. Endogenous peroxidase activity was quenched with 3% H2O2 in distilled H2O. Next, the tissues were washed with phosphate buffered saline (PBS) and incubated with secondary antibody (P0448; Dako, Denmark) for 90 min. After washing with PBS, the tissues were incubated with 3, 3-diamino-benzidine for 30 s and counterstained with hematoxylin. Finally, the samples were observed under an Olympus CX31 Light Microscope (Olympus, Tokyo, Japan) and blindly reviewed by two authors. The following system was employed to estimate the percentage of positive cells and intensity of positive staining at 400× magnification: extent: 0% to 5% = 0; 6% to 25% = 1; 26% to 50% = 2; 51% to 75% = 3; >75% = 4; intensity: absent or faint blush = 0; weak = 1; moderate = 2; strong = 3.[12]
Electron microscopy
Transmission electron microscopy (TEM) was used to examine the morphology of tight junctions between epithelial cells. The colon tissue samples were exposed to 3% glutaraldehyde in 0.2 mol/L PBS for at least 2 h at 4°C. After rinsing three times with sucrose-PBS, the samples were fixed in 1% osmium acid at 4°C for 2 h, and then dehydrated through a graded series of acetone and embedded in epon resin. Ultrathin sections were prepared by an ultramicrotome and stained with uranyl acetate and lead citrate and then were observed under TEM (JEM-1230; JEOL, Japan).
DNA extraction
Total DNA was extracted from fecal samples using the fecal DNA extraction kit (BGI Co., Ltd., Wuhan, China), according to the manufacturer's protocol. The concentrations of metagenomics DNA samples were tested using a qubit fluorometer, and integrity was tested by agarose gel electrophoresis. Next, all the qualified DNA was used to construct a library with primers (forward 515F: 5′-GTGCCAGCMGCCGCGGTAA-3′ and reverse 806R: 5′-GGACTACHVGGGTWTCTAAT-3′) targeting the V4 region of the 16S rRNA gene. Sequencing of the library was conducted using a MiSeq system (BGI Co., Ltd.). The raw data were filtered to eliminate the adapter pollution and low quality to obtain clean reads, and then paired-end reads with overlap were merged to tags. Thereafter, the tags were clustered to optical transform unit (OTU) at 97% sequence similarity. OTU representative sequences were taxonomically classified using ribosomal database project Classifier v.2.2 trained on the Greengenes database, using 0.80 confidence values as the cut-off.
Statistical analyses
Statistical analyses were performed using SPSS software version 20.0 (IBM, NY, USA). Quantitative variables were expressed as means ± standard deviation. Differences were analyzed by the Mann-Whitney U test. P-values < 0.05 were considered to be statistically significant.
Results
Sb reduces the DAI in DSS-induced colitis in mice
The DAI was scored every day for each group. Compared with group A (0.458 ± 0.173), the DAI score of group B (2.708 ± 0.628) was considerably increased (PAB = 0.001). Compared with group B (2.708 ± 0.628), treatment with Sb and mesalazine significantly reduced the DAI score (group C: 1.542 ± 0.616, PBC = 0.005; group D: 1.833 ± 0.713, PBD = 0.031). However, combination treatment showed a trend of improvement in the DAI without statistical significance (group B 2.708 ± 0.628, group E 2.292 ± 0.825, PBE = 0.392). No mortality was observed during the experiment.
Sb reduce the histological score in DSS-induced colitis in mice
Group A mice showed normal epithelium. Group B showed severe colitis characterized by numerous inflammatory cells accumulated in inflamed regions and crypt distortion. By contrast, groups C, D, and E showed ameliorated inflammatory reactions. The histological score for group B (9.875 ± 3.271) was significantly higher than that for group A (1.000, PAB < 0.001), while groups C, D, and E significantly attenuated the score (group C: 4.750 ± 1.832, PBC = 0.005; group D: 4.125 ± 0.991, PBD = 0.001; group E: 6.250 ± 2.550, PBE = 0.033) compared with that for group B. However, there was no significant difference among the three groups (PCD = 0.510, PCE = 0.199, PDE = 0.077) [Figure 1].
Figure 1.
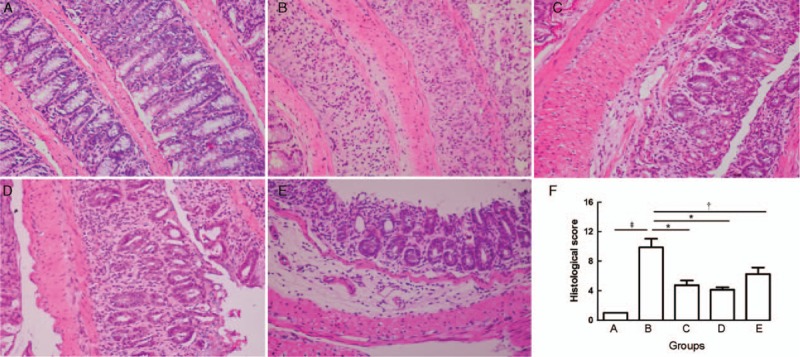
Hematoxylin and eosin-stained sections of colons from normal control group (A), colitis model group (B), Sb treatment group (C), mesalazine treatment group (D), and combination treatment group (E) (Original magnification ×400). The mean histological score from group A to group E (F). ∗P < 0.01, †P < 0.05, ‡P < 0.001. Sb: Saccharomyces boulardii.
Sb exerts a protect effect on the expression of ZO-1
Immunohistochemical staining showed decreased ZO-1 expression in the cells of colons in group B. By contrast, group C, D, and E showed ameliorated expression of ZO-1. The immunohistochemical score for group B (2.075 ± 1.176) was significantly lower than that of group A (6.375 ± 0.590, PAB = 0.001), while group C, D, and E showed a significantly increased score compared with those of group B (group C: 4.225 ± 1.316, PBC = 0.019; group D: 4.475 ± 0.848, PBD = 0.002; group E: 3.800 ± 0.968, PBE = 0.006). However, there was no significant difference among groups C, D, and E (PCD = 0.750, PCE = 0.958, PDE = 0.187) [Figure 2].
Figure 2.
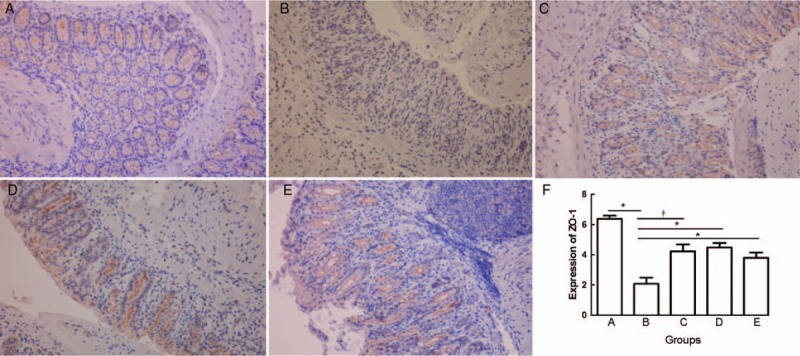
Immunohistochemical staining of ZO-1 from normal control group (A), colitis model group (B), Sb treatment group (C), mesalazine treatment group (D), and combination treatment group (E) (Original magnification ×400). The mean ZO-1 score from group A to group E (F). ∗P < 0.01, †P < 0.05. Sb: Saccharomyces boulardii; ZO-1: Zona occludens.
Sb exerts a protect effect on the expression of Occludin
Immunohistochemical staining showed decreases in Occludin expression in the cells of colons in group B. By contrast, groups C, D, and E showed ameliorated expression of Occludin. The immunohistochemical score for group B was significantly lower than that of group A (group A 5.025 ± 0.618, group B 2.200 ± 0.968, PAB = 0.001), while the scores for groups C, D, and E were significantly increased compared with group B (group B 2.200 ± 0.968, group C 3.525 ± 1.047, group D 4.000 ± 0.741, group E 3.825 ± 0.871, PBC = 0.023, PBD = 0.004, PBE = 0.005). However, there was no significant difference between groups C, D, and E (PCD = 0.247, PCE = 0.460, PDE = 0.712) [Figure 3].
Figure 3.
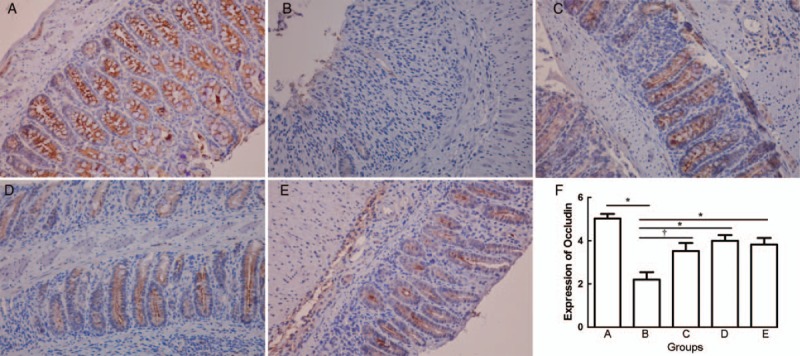
Immunohistochemical staining of Occludin from normal control group (A), colitis model group (B), Sb treatment group (C), mesalazine treatment group (D), and combination treatment group (E) (Original magnification ×400). The mean Occludin score from group A to group E (F). †P < 0.05 and ∗P < 0.01. Sb: Saccharomyces boulardii.
Sb decreases the level of TNF-α in DSS-induced colitis in mice
A significant increase in TNF-α was observed in group B mice (716.323 ± 44.691 ng/L) compared with that in group A (509.776 ± 99.409 ng/L, PAB = 0.001). By contrast, the level of TNF-α in groups C, D, and E was significantly lower than that in group B (group C: 521.740 ± 90.121 ng/L, PBC = 0.001; group D: 476.752 ± 40.052 ng/L, PBD = 0.001; group E: 526.493 ± 126.657 ng/L, PBE = 0.016), while no significant difference was found among groups A, C, D, and E (PAC = 1.000, PAD = 0.600, PAE = 1.000, PCD = 0.294, PCE = 0.916, PDE = 0.916) [Figure 4A].
Figure 4.
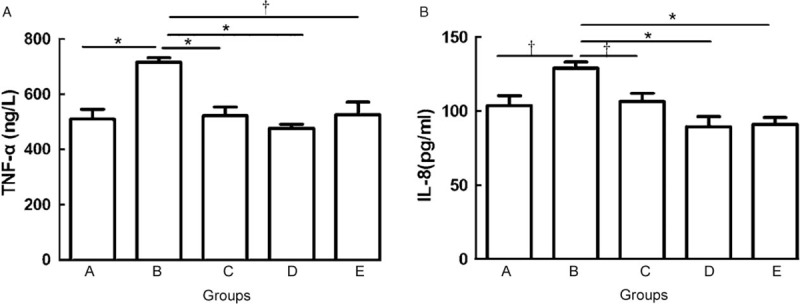
The mean level of TNF-α (A) and IL-8 (B) from group A to group E. ∗P < 0.01, †P < 0.05. IL-8: Interleukin-8; TNF-α: Tumor necrosis factor-α.
Sb decreases the level of IL-8 in DSS-induced colitis in mice
Significantly increased IL-8 was observed in group B mice (128.992 ± 11.475 pg/mL) compared with that in group A mice (103.734 ± 18.646 pg/mL, PAB = 0.016). By contrast, the level of IL-8 in groups C, D, and E was significantly lower than that in group B (group C: 106.283 ± 15.906 pg/mL, PBC = 0.012; group D: 89.471 ± 19.117 pg/mL, PBD = 0.005; group E: 90.900 ± 13.324 pg/mL, PBE = 0.001), while no significant difference was found compared with that in groups A, C, D, and E (PAC = 0.916, PAD = 0.115, PAE = 0.141, PCD = 0.066, PCE = 0.093, PDE = 0.834) [Figure 4B].
Sb ameliorates DSS-induced ultra-structural changes
TEM revealed intact tight junctions in group A. By contrast, severely disrupted tight junctions and irregular microvilli and an enlarged inter-cellular space were seen in group B. Treatment with Sb preserved the tight junctions and ameliorated the microvilli and inter-cellular space. Treatment with mesalazine also preserved the tight junctions and ameliorated microvilli, but the inter-cellular space was enlarged. Treatment with the combination preserved the tight junctions and microvilli, but the inter-cellular space was also enlarged [Figure 5].
Figure 5.
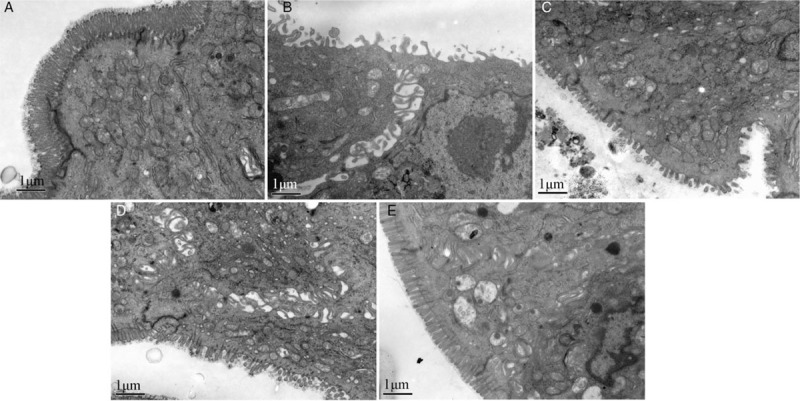
Tight junctions and inter-cellular space from normal control group (A), colitis model group (B), Sb treatment group (C), mesalazine treatment group (D), and combination treatment group (E) under transmission electron microscope. Scale bar = 1 μm. Sb: Saccharomyces boulardii.
Sb up-regulates the percentage of S24-7 specifically in DSS-induced colitis in mice
Differences in the microbiota among the groups were compared at the phylum level. Bacteroidetes and Firmicutes constituted the major phylum of bacteria in group A. DSS treatment greatly increased the levels of Proteobacteria and decreased the levels of Firmicutes compared with those in the normal control group. Interestingly, treatment with Sb showed its own characteristics: a higher percentage of Bacteroidetes and a lower percentage of Firmicutes, with a significant difference or a significant trend [Figure 6].
Figure 6.
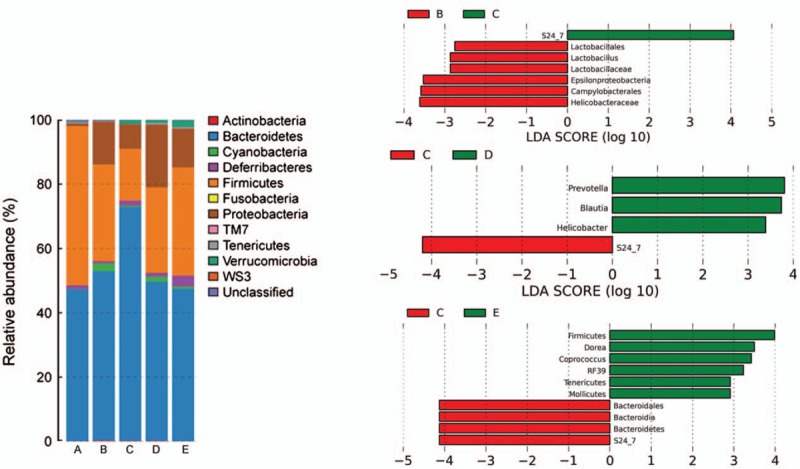
Differences in the microbiota from group A to group E at the phylum level (Left). Differentially abundant bacterial taxa between group B and group C, group C and group D, group C and group E (Right).
Linear discriminant analysis effect size (LEfSe) was utilized to determine differentially abundant bacterial taxa between the five groups. The LEfSe analysis revealed that Sb treatment could up-regulate the percentage of S24-7 specifically [Figure 6].
Discussion
In this study, we showed three important aspects when considering the usefulness of Sb to treat DSS-induced colitis. First, Sb could decrease inflammatory cytokine levels and exerted a positive effect on histological presentation. Second, Sb showed a protective effect on intestinal tight junctions. Third, Sb induced an increase in the number of S24-7 in intestinal flora.
Immunological abnormalities play an important role in the pathogenesis of IBD, in which multiple cytokines are directly involved in the process of inflammation.[13–16] The imbalance between pro-inflammatory cytokines (IL-1, IL-2, IL-6, IL-8, TNF-α, INF-γ) and anti-inflammatory cytokines (IL-4, IL-10) is the main cause of mucosal injury. The process of cytokine secretion is regulated by the NF-κB and mitogen activated protein kinases (MAPK) pathways.[17,18] Multiple cytokines influence the expression of tight junction proteins. Interferon-γ (IFN-γ) has been found to affect claudin-2 and Occludin expression and induces the loss of barrier integrity.[19] TNF-α decreases the number of strands that form the tight junctions and that is induced in intestinal epithelial cells, thus leading to increased transepithelial ion permeability.[20]
Sb is a type of non-pathogenic yeast administered to prevent and treat several of diseases. Sb may act as an immune regulator, which offers a promising adjunctive therapy for IBD. In a trinitrobenzene sulfonic acid (TNBS) colitis model, the administration of Sb reduces the histologic damage and levels of some pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and elevates the colonic level of peroxisome proliferator activated receptor-γ (PPAR-γ).[21] In T84 colonocytes infected by Shigella flexneri, Sb decreased extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and nuclear factor-kappa B (NF-κB) activation, as well as the transepithelial migration of polymorphonuclear leukocytes across T-84 monolayers.[22] These studies reveal that the anti-inflammatory mechanism of Sb involves blocked NF-κB and MAPK-mediated signal transduction pathways, which stimulate pro-inflammatory cytokine production.[7]
The tight junction is built up by both transmembrane proteins such as Occludin, tricellulin, different claudins, and junctional adhesion molecules, as well as peripheral membrane proteins such as ZO-1, -2, -3, and cingulin.[23] Inflammatory cytokines that are associated with gut inflammation alter epithelial permeability through their effects on the junctional complexes.[24,25] The administration of Sb significantly increases the barrier integrity through the role of anti-inflammatory factors. In this study, we found that Sb showed a positive effect on tight junctions, a finding that is in accordance with the level of inflammatory cytokines.
Interestingly, we found that combination therapy did not show advantages compared with monotherapy. The causes could be the following: 1, the course of combination treatment in the acute colitis model was too short to show any advantage on monotherapy. This may be verified through a longer course, such as a chronic colitis model; 2, the mesalazine and Sb were mixed in the combination therapy group. It is unclear whether mesalazine inhibits the activity of Sb; 3, 5-aminosalicylic acid (5-ASA) is easily broken under low pH condition, and Sb may change the pH of the intestine, thus decreasing the efficiency of mesalazine.
Importantly, Sb specifically induced an increase in the number of S24-7 in intestinal flora. S24-7 is an uncultured family of the order Bacteroidales, which was named after one of the earliest environmental clones belonging to the lineage.[26] S24-7 distributes almost exclusively in the guts of homeothermic animals and plays a vital role in the degradation of polysaccharides, such as α-glucans, hemicellulose, pectin, and host-derived glycans.[26] A key question related to the members of S24-7 is whether they are friends or foes, and the relationship between S24-7 and sIgA may provide a clue. Palm et al found the proportion of intestinal bacteria that are coated with IgA was significantly increased in patients with CD and UC compared with that in healthy controls. IgA+ and IgA− bacteria from patients with IBD were transplanted to germ-free mice to evaluate their effects on intestinal inflammation. No influence on intestinal inflammation was observed. However, in mice with induced colitis by DSS, more severe intestinal inflammation was observed with colonization by bacteria with high levels of IgA coating as compared to than with colonization by bacteria with low levels of IgA coating. This revealed that IgA coating identifies inflammatory commensals that preferentially drive intestinal disease.[27] However, both pathogenic bacteria and commensal bacterial can be coated by IgA; the difference between them is that the IgA responses to pathogenic bacteria are T dependent and is thought to yield high-affinity antigen-specific IgA, and that IgA responses to commensal bacterial are thought to be derived from T-independent responses, which generate low-affinity IgA.[28] The members of S24-7 contain both IgA+ and IgA−, but the process of IgA coating is T independent.[29] This may reveal that S24-7 tends to be commensal bacteria. Sb specifically elevates the number of S24-7 in intestinal flora, which may be a mechanism of Sb.
In conclusions, Sb is as effective as mesalazine in decreasing serum inflammatory factors and protecting histological structure and tight junctions between epithelial cells. Combination therapy is not superior to monotherapy. Furthermore, Sb specifically elevates the number of S24-7 in intestinal flora, which tends to be a protective mechanism.
Funding
This work was supported by grants from the Beijing Natural Science Foundation (No. 7174358) and Youth Clinical Research Project of Peking University First Hospital (No. 2017CR08) awarded to Gui-Gen Teng.
Conflicts of interest
None.
Footnotes
How to cite this article: Dong JP, Zheng Y, Wu T, He Q, Teng GG, Wang HH. Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000364
References
- 1.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol 2014; 20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Invest 2014; 124:4190–4196. doi: 10.1172/JCI72330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loddo I, Romano C. Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol 2015; 6:551.doi: 10.3389/fimmu.2015.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker C, Neurath MF, Wirtz S. The intestinal microbiota in inflammatory bowel disease. ILAR J 2015; 56:192–204. doi: 10.1093/ilar/ilv030. [DOI] [PubMed] [Google Scholar]
- 5.Cario E. Commensal-innate immune miscommunication in IBD pathogenesis. Dig Dis 2012; 30:334–340. doi: 10.1159/000338120. [DOI] [PubMed] [Google Scholar]
- 6.Buchheister S, Buettner M, Basic M, Noack A, Breves G, Buchen B, et al. CD14 plays a protective role in experimental inflammatory bowel disease by enhancing intestinal barrier function. Am J Pathol 2017; 187:1106–1120. doi: 10.1016/j.ajpath.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 7.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 2010; 16:2202–2222. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S, Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology 2008; 135:568–579. doi: 10.1053/j.gastro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Barc MC, Charrin-Sarnel C, Rochet V, Bourlioux F, Sandré C, Boureau H, et al. Molecular analysis of the digestive microbiota in a gnotobiotic mouse model during antibiotic treatment: influence of Saccharomyces boulardii. Anaerobe 2008; 14:229–233. doi: 10.1016/j.anaerobe.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Hamamoto N, Maemura K, Hirata I, Murano M, Sasaki S, Katsu K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)). Clin Exp Immunol 1999; 117:462–468. doi: 10.1046/j.1365-2249.1999.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993; 69:238–249. [PubMed] [Google Scholar]
- 12.Fromowitz FB, Viola MV, Chao S, Oravez S, Mishriki Y, Finkel G, et al. Ras p21 expression in the progression of breast cancer. Hum Pathol 1987; 18:1268–1275. doi: 10.1016/S0046-8177(87)80412-4. [DOI] [PubMed] [Google Scholar]
- 13.Schoultz I, Keita AV. Cellular and molecular therapeutic targets in inflammatory bowel disease-focusing on intestinal barrier function. Cells 2019; 8:1–24. doi: 10.3390/cells8020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan Q, Zhang J. Recent advances: the imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediators Inflamm 2017; 2017:1–8. doi: 10.1155/2017/4810258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014; 14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 16.Bevivino G, Monteleone G. Advances in understanding the role of cytokines in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2018; 12:907–915. doi: 10.1080/17474124.2018.1503053. [DOI] [PubMed] [Google Scholar]
- 17.Schmid RM, Adler G. NF-kappaB/rel/IkappaB: implications in gastrointestinal diseases. Gastroenterology 2000; 118:1208–1228. doi: 10.1016/S0016-5085(00)70374-X. [DOI] [PubMed] [Google Scholar]
- 18.Hollenbach E, Neumann M, Vieth M, Roessner A, Malfertheiner P, Naumann M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J 2004; 18:1550–1552. doi: 10.1096/fj.04-1642fje. [DOI] [PubMed] [Google Scholar]
- 19.Willemsen LE, Hoetjes JP, van Deventer SJ, van Tol EA. Abrogation of IFN-gamma mediated epithelial barrier disruption by serine protease inhibition. Clin Exp Immunol 2005; 142:275–284. doi: 10.1111/j.1365-2249.2005.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gitter AH, Bendfeldt K, Schmitz H, Schulzke JD, Bentzel CJ, Fromm M. Epithelial barrier defects in HT-29/B6 colonic cell monolayers induced by tumor necrosis factor-alpha. Ann N Y Acad Sci 2000; 915:193–203. doi: 10.1111/j.1749-6632.2000.tb05242.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee SK, Kim YW, Chi SG, Joo YS, Kim HJ. The effect of Saccharomyces boulardii on human colon cells and inflammation in rats with trinitrobenzene sulfonic acid-induced colitis. Dig Dis Sci 2009; 54:255–263. doi: 10.1007/s10620-008-0357-0. [DOI] [PubMed] [Google Scholar]
- 22.Mumy KL, Chen X, Kelly CP, McCormick BA. Saccharomyces boulardii interferes with Shigella pathogenesis by postinvasion signaling events. Am J Physiol Gastrointest Liver Physiol 2008; 294:G599–G609. doi: 10.1152/ajpgi.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol 2018; 10:a029314.doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol 2016; 22:3117–3126. doi: 10.3748/wjg.v22.i11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigmann B, Neurath MF. Th9 cells in inflammatory bowel diseases. Semin Immunopathol 2017; 39:89–95. doi: 10.1007/s00281-016-0603-z. [DOI] [PubMed] [Google Scholar]
- 26.Ormerod KL, Wood DL, Lachner N, Gellatly SL, Daly JN, Parsons JD, et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016; 4:36.doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014; 158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol 2012; 12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 29.Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, et al. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 2015; 43:541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


