Abstract
Objective
Ultrasound imaging is well known to play an important role in the detection of thyroid disease, but the management of thyroid ultrasound remains inconsistent. Both standardized diagnostic criteria and new ultrasound technologies are essential for improving the accuracy of thyroid ultrasound. This study reviewed the global guidelines of thyroid ultrasound and analyzed their common characteristics for basic clinical screening. Advances in the application of a combination of thyroid ultrasound and artificial intelligence (AI) were also presented.
Data sources
An extensive search of the PubMed database was undertaken, focusing on research published after 2001 with keywords including thyroid ultrasound, guideline, AI, segmentation, image classification, and deep learning.
Study selection
Several types of articles, including original studies and literature reviews, were identified and reviewed to summarize the importance of standardization and new technology in thyroid ultrasound diagnosis.
Results
Ultrasound has become an important diagnostic technique in thyroid nodules. Both standardized diagnostic criteria and new ultrasound technologies are essential for improving the accuracy of thyroid ultrasound. In the standardization, since there are no global consensus exists, common characteristics such as a multi-feature diagnosis, the performance of lymph nodes, explicit indications of fine needle aspiration, and the diagnosis of special populations should be focused on. Besides, evidence suggests that AI technique has a good effect on the unavoidable limitations of traditional ultrasound, and the combination of diagnostic criteria and AI may lead to a great promotion in thyroid diagnosis.
Conclusion
Standardization and development of novel techniques are key factors to improving thyroid ultrasound, and both should be considered in normal clinical use.
Keywords: Thyroid, Ultrasound, Guideline, Artificial Intelligence, Image Classification
Introduction
In recent years, the high incidence of thyroid nodules has become particularly significant, primarily because of its gradually increasing annual prevalence and the increasing use of ultrasound.[1] Pathologically, most thyroid nodules are benign, with a malignancy rate of approximately 5% to 7%. Studies have shown that undifferentiated cancers that lead to a high mortality rate account for 1% to 2% of malignant nodules.[2,3] The majority of malignant nodules (especially those that are <1 cm) often have indolent behavior with positive prognoses.[4] Surgical resection is the primary treatment for thyroid nodules, but post-operative complications (eg, hypoparathyroidism, recurrent laryngeal nerve paralysis, etc) have adverse effects on patient quality of life.[5] Therefore, many researchers have suggested that patients with thyroid nodules receive excessive care, and the significance of ultrasound has been questioned. For example, a review article,[6] “Whether ultrasound examination should continue to be used in the thorough examination of thyroid nodules” the author argued that pointless ultrasound diagnoses of tiny malignant thyroid nodules did not have practical significance and might be one of the reasons for excessive care. Standardizing ultrasound diagnosis of thyroid nodules and its management and evaluating the ultrasound diagnosis values associated with thyroid nodules are crucial.
Based on the above reasons, the development of ultrasound diagnosis in the thyroid has not only focused on new technologies but also on diagnostic standardization. Since the Society of Radiologists in Ultrasound first proposed a consensus on the management of thyroid nodules identified with thyroid ultrasound in 2005,[7] various thyroid classification guidelines have successively emerged to improve the diagnostic performance and reduce the mental and economic burdens on patients. However, the diversified diagnostic criteria also lead to inconsistent risk prediction in the diagnosis. A rational understanding of the characteristics of various diagnostic criteria, as well as their similarities and differences, will help standardize thyroid ultrasound diagnoses.
Although each guideline provides a detailed explanation of the ultrasound diagnosis of thyroid nodules, the complexity of sonography leads to unavoidable deviation in diagnosis. Even though the application indications for fine needle aspiration (FNA), which is currently widely used, are clearly defined,[8] this procedure still provides false negatives. These false negatives not only reduce the diagnostic accuracy but also generate many unnecessary invasive tests. On the other hand, the growing demand for diagnosis also creates difficulties with regard to standard implementation, data analysis, and processing; thus, a more optimal method is urgently needed. With the development of technology, artificial intelligence (AI), a branch of computer science that trains computers to simulate human minds and cognitive functions, might be one solution for reducing the diagnostic differences and improving accuracy. Based on the integration of big data across multiple disciplines, an efficient intelligent diagnostic system will be helpful for promoting and generalizing thyroid ultrasound diagnostic standards in the future.
This review mainly focused on the popular guidelines of thyroid ultrasound worldwide, from the aspect of suspicious ultrasound features, assessment of cervical lymph nodes, indication for FNA, use of other diagnostic techniques and diagnostic criteria for special populations. The generality, specialty, and limitations are compared among the guidelines. These common characteristics and parts of the acceptable rules that we established cannot replace the guidelines, but they can provide a concise reference for practical application. Thus, physicians may no longer confuse how to choose the suitable guidelines. Furthermore, we summarize the application of computer-aided diagnosis in thyroid ultrasound, especially for diagnosis using image classification techniques. This technique can be a supplement to traditional ultrasound diagnosis to promote diagnostic value, stability, and efficiency.
Guidelines and Consensus on Thyroid Ultrasound
Common diagnostic standards
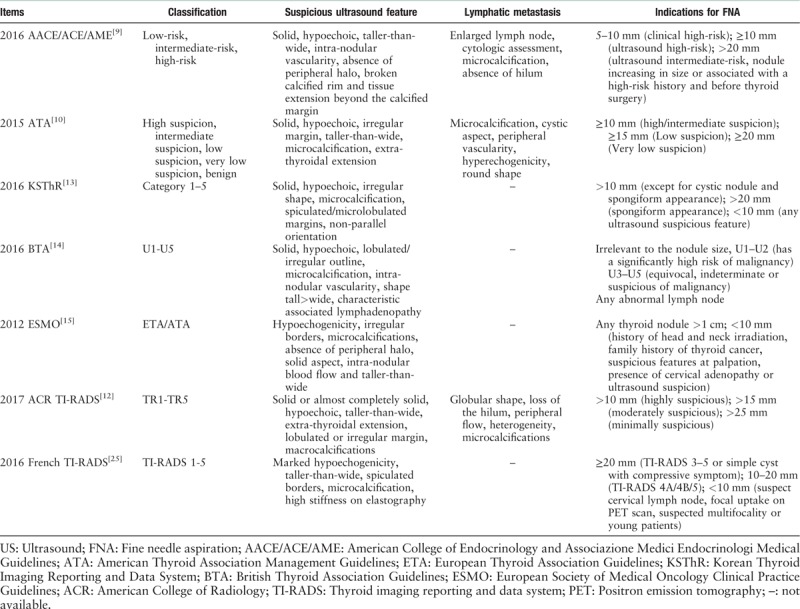
The general diagnostic criteria for thyroid ultrasound include the 2016 American Association of Clinical Endocrinologists, American College of Endocrinology and Associazione Medici Endocrinologi Medical (AACE/ACE/AME) Guidelines,[9] 2015 American Thyroid Association Management (ATA) Guidelines,[10] 2017 American College of Radiology (ACR) Thyroid imaging reporting and data system (TI-RADS),[11,12] 2016 Korean Thyroid Imaging Reporting and Data System (KSThR),[13] 2016 British Thyroid Association Guidelines (BTA),[14] and 2012 European Society of Medical Oncology (ESMO) Clinical Practice Guidelines.[15] All the above guidelines or consensuses are the latest updated editions.
Suspicious ultrasound features
Regarding the suspicious ultrasound features, the guidelines arrive at a consensus that solid nodule structure, hypoechogenicity, taller-than-wide shape, irregular margin, microcalcification, and invasion of surrounding tissue are associated with malignancy [Figure 1]. However, a single ultrasound feature is insufficient to make a diagnosis.[14,15] In addition, the AACE/ACE/AME guidelines suggest that for multiple nodules, radiologists should prioritize nodules with suspicion ultrasound findings rather than size. These two-dimensional ultrasound features are the main factors in thyroid ultrasound diagnosis.
Figure 1.
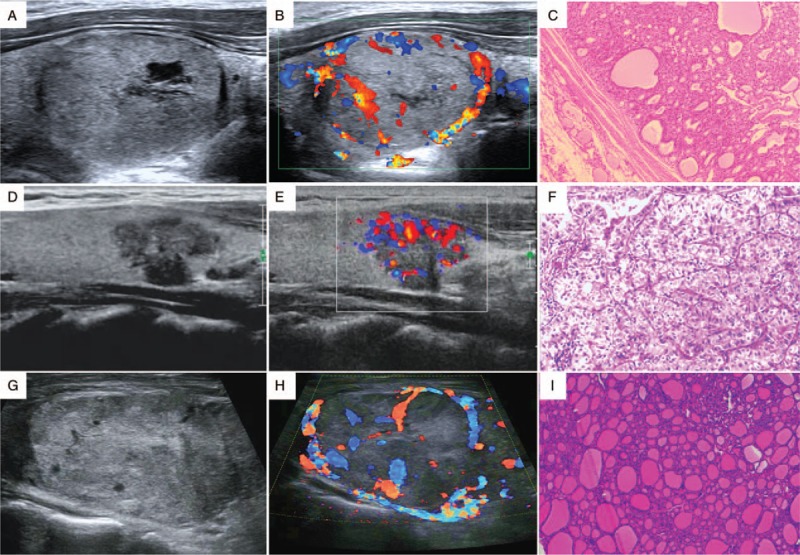
Benign and suspicious ultrasound features and pathological findings (HE staining, original magnification ×200) of thyroid nodules. (A–C) A nodular goiter. The two-dimensional image, color Doppler image, and pathologic image are shown. (D–F) A papillary thyroid carcinoma. The ultrasound image shows a hypoechoic nodule with an irregular margin and microcalcification. (G) (H), and (I) A follicular carcinoma demonstrating a large isoechoic nodule without microcalcification. HE: Hematoxylin and eosin.
Assessment of metastatic cervical lymph nodes
Lymphatic metastasis is common in thyroid papillary carcinoma.[16] Ultrasound has high specificity and sensitivity values for the assessment of lymphatic metastasis, as it can be used to detect the size, structure, and blood flow, making it an important method. The signs of malignancy used to detect metastatic lymph nodes include microcalcification, cystic degeneration, peripheral blood flow, hyperecho, and morphological rounding. When the above signs are present, we recommend that a cytological examination be conducted.[10] If a suspicious lymph node is detected via ultrasound, a description of its zoning, number, shape, size, margin, internal echo, the presence or absence of hilum, and the signs of blood flow is necessary.[9]
As ultrasound is a popular method for diagnosing metastatic cervical lymph nodes, an increasing number of atypical and latent thyroid carcinomas will be detected in the early stages, which shows the high clinical value of standardization in ultrasound assessment of metastatic cervical lymph nodes.
Assessment of nodule blood flow
The growth of thyroid nodules is associated with blood supply.[17] According to a previous research, an abundant microvascular blood flow has a strong correlation with malignant thyroid nodules.[18] However, whether the assessment of thyroid nodule blood supply by ultrasound can be a diagnostic index remains controversial. ATA, KSThR guidelines, and the ACR TI-RADS classification system suggest that an increase in blood flow may be associated with malignancy, but it is unreliable.[10] This viewpoint is in contrast to the AACE/ACE/AME, BTA, and ESMO guidelines, which suggest that abundant blood flow and intra-nodular vascularization are an expression of malignancy.[14,15]
This discrepancy might be caused by individual differences and variations in accuracy of diverse blood flow detection methods. Anatomically, the thyroid is an organ with abundant blood flow, and it adjoins the esophagus and carotid artery. Thyroid ultrasound is susceptible to respiration and vascular pulsation; therefore, the requirements of blood flow detection techniques for thyroid nodules are high. Early techniques, such as color Doppler flow imaging and Power Doppler imaging, are unable to accurately detect the microvasculature. Therefore, no consensus can be reached on the application of ultrasound blood flow detection.[19] In recent years, new ultrasound techniques with improved detection of microvasculature (including contrast-enhanced ultrasonography and superb microvascular imaging) have enhanced the level of detection.[20–22] Big data analysis is needed for further assessment of the reliability of ultrasound blood flow indexes.
Indications for FNA
FNA has been widely accepted as a minimally invasive diagnostic technique that can obtain biopsy results conveniently and safely. Generally, the nodule size and risk of malignancy are identified as indications for FNA. The 2017 updated edition of the ACR TI-RADS classification system expanded the scope of the indications for FNA from “mildly suspicious and moderately suspicious nodules (2.0 and 1.0 cm, respectively)” to “mildly suspicious and moderately suspicious nodules (2.5 and 1.5 cm, respectively).”[12] In this way, some unnecessary FNA procedures could be avoided. In addition, there are a few recommended supplements to the guidelines, such as “aspiration of at least two sites within the nodule” and “for multiple nodules, prioritize nodules to be sampled according to ultrasound findings.”[9] These new recommendations offer better guidance during the FNA procedure.
Other ultrasound techniques
The development of ultrasound elastography and three-dimensional ultrasound provides new research directions for thyroid ultrasound diagnosis.[23] However, these new technologies are not highly accepted among the different guidelines. For instance, the AACE/ACE/AME and ATA guidelines and the ACR TI-RADS classification system consider the performance of ultrasound elastography to be variable and operator dependent; moreover, it is not suitable for obese patients or those with multiple nodules. Thus, ultrasound elastography may prove to be an additional diagnostic criterion instead of an independent diagnostic method.[9,10,12,24] Although evidences show that the quantitative parameters of contrast-enhanced ultrasonography (CEUS) (e.g., the maximum intensity of peak) have great value, CEUS provides only ancillary data for the diagnosis of malignant thyroid nodules according to the guideline.[9] Comparisons of the guidelines are listed in Table 1.
Table 1.
Comparison of different ultrasound diagnostic criteria in the thyroid.
Diagnostic criteria for thyroid nodules in special populations
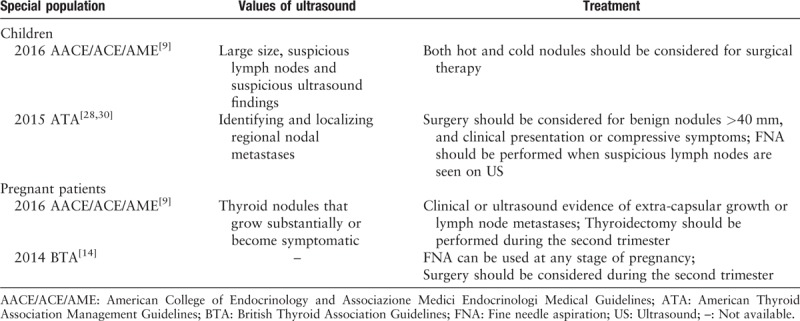
The development of diagnostic criteria for special populations, including children and pregnant women, is necessary. Therefore, the ATA revised their guideline in 2015, adding criteria for thyroid nodules in children. The guideline primarily focuses on children ≤18 years of age. Although the incidence of thyroid disease in children is relatively low, its malignancy rate is 3 to 5 times higher than that in adults.[26] Furthermore, it also has a high rate of lymphatic and pulmonary metastasis (19–25%).[27] Ultrasound imaging plays an important role in FNA guidance in the identification of cervical diseases and long-term follow-up. High-frequency ultrasound is more likely to monitor recurrence after thyroid cancer surgery.[28,29] Notably, the size of the nodules cannot predict malignancy because children's thyroid volume always changes with age. Similarly, hot nodules identified by nuclear scintigraphy have malignancy risks in children. As a result, the guidelines suggest that even when the FNA result is benign, nodules over 40 mm in children should be removed by surgery.[30]
The thyroid nodules of most pregnant patients existed prior to conception, while others are initially discovered during pregnancy. Gestation increases the risk of newly developed thyroid nodules and causes the volumes of pre-existing nodules to increase. For these patients, gestational age is not an absolute contraindication of FNA. Ultrasound examination is applicable for any pregnant woman with thyroid nodules. If necessary, FNA can be used at any stage of pregnancy.[9]Table 2 shows the guidelines for thyroid nodules in children and pregnant women.
Table 2.
Ultrasound guidelines of special populations.
The standardization of thyroid ultrasound diagnosis is based on the standardization of the data reporting system and its management.[31] Currently, no global consensus exists for ultrasound diagnosis of the thyroid and might be associated with factors such as anatomic properties, hormones, and the development of detection technology. We developed a concise list of common characteristics as follows:
-
1.
A single ultrasound feature is insufficient to make a diagnosis.
-
2.
Microcalcification, cystic degeneration, peripheral blood flow, hyperecho, and morphological rounding are highly revealing of metastatic lymph nodes.
-
3.
Nodule blood flow is not a reliable diagnostic index in thyroid ultrasound diagnosis.
-
4.
FNA is necessary when a mildly suspicious nodule is larger than 2.5 cm.
-
5.
Other ultrasound techniques (such as ultrasound elastography and CEUS) can be only supplementary methods.
-
6.
Since the malignancy rate of thyroid nodules in children is relatively high, when suspicious ultrasound characteristics are found, indications for FNA and surgery should be broadened.
-
7.
FNA should be used when suspicious ultrasound evidence, such as extra-capsular growth or lymph node, is found in any period of pregnancy.
Even so, further promotion of the accuracy and stability in thyroid ultrasound diagnosis is difficult, as is reducing the examination time. Therefore, searching for methods that can improve diagnostic efficiency and optimize the diagnostic process is equally important when establishing ultrasound diagnostic criteria. In the early 21st century, AI provides a new development in medical imaging; it can compensate for the unavoidable limitations of traditional ultrasound, and the combination of diagnostic criteria and AI may lead to considerable improvements in thyroid diagnosis.
Image Classification Techniques in Thyroid Ultrasound
Conventional ultrasound has limitations. Compared with other imaging methods, ultrasound is susceptible to the patient's position and imaging artifacts. The specificity of ultrasound is not acceptable for the identification of some diseases, such as adenoma and nodular goiter. The diagnostic results depend on the examiner, and a strong level of subjectivity exists, which in turn leads to differences among physicians at different diagnostic levels. Moreover, given the increase in the number of patients and time-consuming scanning, the burden on physicians who perform thyroid ultrasound scanning is also increasing. With the development of computer science, AI has been used for medical imaging diagnosis. Image classification, the most common technique, develops an intelligent classification model based on the different features in ultrasound images and achieves an accurate diagnosis. This technology generally consists of several steps, which may include image pre-processing, feature extraction, and data classification.
Feature extraction of thyroid ultrasound image
A good selection of features is beneficial for simplifying the complicated data and obtaining the most important information.[32] The most intuitive features come from the original ultrasound images, such as the texture feature.[33] In a study of 70 cases, 270 texture features were analyzed for the identification of benign and malignant thyroid nodules.[34] Likewise, Chen's study[35] used texture analysis and hierarchical support vector machine (SVM) to classify the follicle base and fibrosis base thyroid nodules according to the corresponding pathologic findings. The results showed that the diagnostic accuracy of the classification system is 96.34% to 100%. The decision on features is the basic factor of system efficiency; well-chosen features were accepted in image segmentation, image diagnostic classification, and image registration and fusion.
Pre-processing of thyroid ultrasound image
Since the original ultrasound images (especially the low-quality images) contain large amounts of imprecise and incomplete information, pre-processing is essential for data consistency and accuracy. Systems using pre-processed images show better diagnostic performance than those using the original images. Image segmentation is the most common of the pre-processing methods. Segmentation of thyroid ultrasound images is always used to detect nodules, estimate the volume automatically, and perform guided interventions.[36] A system using a region of interest has superior classification efficiency compared with those using the whole image.[37,38] The segmentation methods in thyroid ultrasound imaging include conventional methods (such as region segmentation, edge segmentation, and contour segmentation) and deep learning methods. Table 3 summarizes the characteristics of different segmentation methods.
Table 3.
Characteristics of different methods of thyroid ultrasound image segmentation.
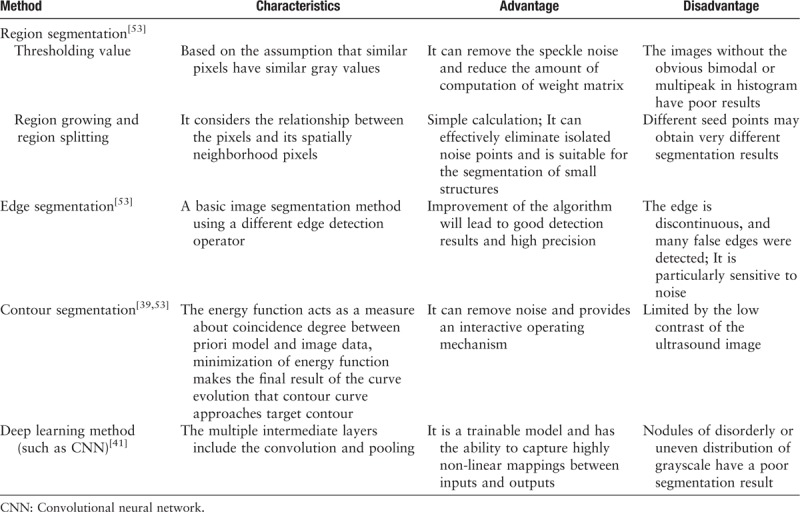
The thyroid echo is complex, frequently leading to low performance of segmentation using conventional methods.[39] The emergence of deep neural network learning in the early 21th century provided a better choice for segmentation.[40] Based on a multilayer neural network, feature learning is added to deep learning to achieve the goal of automatic feature extraction and improve classification accuracy. However, for thyroid nodules with a complex background, the accuracy of deep learning segmentation should be optimized by expanding the data size and adding training layers.[41] In general, quantitative metrics, namely, Dice coefficient, Jaccard coefficient, Boundary displacement error, and global consistency error are adopted to validate the segmentation.[42,43] A quantitative analysis is convenient for the horizontal comparison of segmentation efficiency using different algorithm methods.
Development of an image classification algorithm model
The early detection of thyroid cancer is of great importance for successful treatment. Currently, FNA is widely used to obtain cytological results, and it is rapid, convenient, and minimally invasive. However, 5% to 20% of FNA biopsy diagnoses is undetermined and requires further pathological examinations.[44] Compared with FNA, image classification is non-invasive and can effectively decrease the use of unnecessary invasive detection.
Classifiers are the basic components of image classification; in other words, the automatic classification model of unknown images is developed through steps of data input, supervised learning, training, and feedback. At present, many classifiers are used for thyroid ultrasound images. The two most common classifiers are artificial neural network (ANN) and SVM. In addition, the Gaussian mixture model, decision tree, and Bayesian classifier are likewise used.[44,45] Unique classifiers have different diagnostic accuracies and must be selected based on the actual situation. In addition to the target classifier, other classifiers can also be used for comparative analyses to verify diagnostic performance.[45] However, such comparisons should be made within the same data set but not among different data sets.
The ANN model consists of three components, including the input layer, hidden layer, and output layer. This model simulates neurons and classifies new individuals through learning and training processes. In 2006, based on the ANN, Hinton et al proposed the concept of deep learning, which was represented by the convolutional neural network (CNN). This network reconstructs high-dimensional data through the middle layer and trains multilayer neural networks to reduce data dimensionality. Because of the low training speed and poor performance, ANN is rarely used alone in recent studies. Instead, using an algorithm that applies deep learning (including new algorithms and improvements of existing algorithms) or joint evaluation via multiple classifiers is more common.[46,47] Since the collection of medical images is difficult, these methods mentioned above will contribute to a more precise model.[48] Deep learning technology has the advantages of extracting and classifying features automatically and reducing subjectivity and has a low diagnosis and treatment cost. Chi et al[49] used thyroid ultrasound images after pre-processing (including movement of annotations and resizing the images) to improve the fine-tuning of the deep CNN based on GoogleNet. This method showed good classification performance with an accuracy of 98.29%, a sensitivity of 99.10%, and a specificity of 93.90%. In another study, 21,532 images from 5842 patients were collected to develop a cascade CNN-based model. A better result was obtained in the experimental group, in which the area under the curve (AUC) of cascade CNN was 98.51%, while the AUCs of K-nearest neighbor (KNN) and radial basis function neural network was 64.68% and 78.56%, respectively.[50]
SVM is a common type of classifier for high-dimensional data. A multidimensional hyperplane is constructed to obtain the optimal solution for classification using statistical methods. Tsantis et al[51] developed an SVM-based image analysis system with the highest classification accuracy of 96.7%. Compared with the quadratic least squares minimum distance and the quadratic Bayesian classifiers, the system based on SVM algorithm had better performance in avoiding unnecessary thyroid nodule biopsies. Chang et al[52] used features in combination with an SVM for classification, which had an accuracy ranging from 78.0% to 83.1% and showed improvement in a multivariate analysis (98.3%).
The selection of a classifier depends on multiple factors. SVM is more suitable than deep neural network for small sample sizes, while the opposite is true for large sample sizes. A 50-patient study compared the diagnostic accuracy of different classifiers, including KNN, probabilistic neural network, and decision tree. The result indicated that the neural network did not have the best accuracy in that data set.[53] Similar conclusions were drawn in another study of Hashimoto thyroiditis, in which SVM had the best diagnostic efficiency.[54] However, in a study of 970 cases, the radial basis function neural network had the best classification accuracy.[55] In one of the latest reports, researchers used 8148 thyroid ultrasound images to develop a hybrid method of two pre-trained CNNs. The classification performance of the hybrid method was superior to that of the histogram method and SVM.[56] Furthermore, the results of Yu's study demonstrated that in a method combining ANN and SVM, the sensitivity increased, but the specificity decreased.[57]
Deep learning in neural networks is highly efficient and accurate and can effectively improve the accuracy of ultrasound diagnosis in the thyroid, and the increase in sample size is helpful for the classification performance. The two-dimensional features along with the blood flow and the hardness of thyroid nodules are gradually being incorporated into the deepening exploration of ultrasound technology. Intelligent diagnostic systems based on multimode imaging technology will become the developmental trend of thyroid ultrasound diagnosis.
Limitations in clinical application
Despite the advantages described above, clinical application of AI in medical diagnosis still needs patience and is a long-term process. First, sufficient data are required for system validation and verification. The collection process will take a long time, especially when the department lacks a normalized diagnostic standard. Moreover, physicians need time to become familiar with the complicated formulas and algorithms used during the modeling process. At present, in clinical applications, one algorithm model may be suitable only for a unique database. Adjustment of parameters and algorithms is necessary for changing the database or preparing to improve the diagnostic concordance rate. In addition, there are unavoidable subjective problems in the clinical application. For instance, some nodules have irregular shape and unclear boundaries only in some sections but not in all the sections. An atypical section may be the cause of misdiagnose. The solutions are further standardization (to define specific sections by human) and scan with multiple sections (to reduce the probability of misdiagnose caused by single section). With regard to the objectivity, three-dimensional imaging may be another better choice.
Conclusions
The development of ultrasound thyroid diagnoses lies in standardization and technological innovation, and technological development leads to a continuous improvement in diagnostic criteria. A systematic understanding of the various guidelines can make thyroid ultrasound diagnosis more practical and evidence-based. As an advanced technology, the development of AI corrects the variability and subjectivity in conventional ultrasound diagnosis. Furthermore, AI can shorten the diagnosis time and reduce the burden on physicians. In the context of the increasing data volume, AI will become the main trend in the development of thyroid ultrasound diagnoses in the future.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81671707), Natural Science Foundation of Guangdong Province (No. 2016A030311054), Research Projects of Guangzhou Science Technology and Innovation Commission (No. 201607010201), Research Fund for Lin He's Academician Workstation of New Medicine and Clinical Translation, the Youth Foundation of Scientific Research of the Third Affiliated Hospital of Guangzhou Medical University (NO. 2018Q18).
Conflicts of interest
None.
Footnotes
How to cite this article: Liang XW, Cai YY, Yu JS, Liao JY, Chen ZY. Update on thyroid ultrasound: a narrative review from diagnostic criteria to artificial intelligence techniques. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000346
References
- 1.Kim TY, Shong YK. Active surveillance of papillary thyroid microcarcinoma: a mini-review from Korea. Endocrinol Metab (Seoul) 2017; 32:399–406. doi: 10.3803/EnM.2018.33.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory A, Bayat M, Kumar V, Gregory A, Bayat M, Kumar V, et al. Differentiation of benign and malignant thyroid nodules by using comb-push ultrasound shear elastography: a preliminary two-plane view study. Acad Radiol 2018; 25:1388–1397. doi: 10.1016/j.acra.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendes GF, Garcia MR, Falsarella PM, Rahal A, Cavalcante Junior FA, Nery DR, et al. Fine needle aspiration biopsy of thyroid nodule smaller than 1.0 cm: accuracy of TIRADS classification system in more than 1000 nodules. Br J Radiol 2018; 91:20170642.doi: 10.1259/bjr.20170642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 2001; 130:1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 5.Lin S, Huang H, Liu X, Li Q, Yang A, Zhang Q, et al. Treatments for complications of tracheal sleeve resection for papillary thyroid carcinoma with tracheal invasion. Eur J Surg Oncol 2014; 40:176–181. doi: 10.1016/j.ejso.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Cronan JJ. Thyroid nodules: is it time to turn off the US machines. Radiology 2008; 247:602–604. doi: 10.1148/radiol.2473072233. [DOI] [PubMed] [Google Scholar]
- 7.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005; 237:794–800. doi: 10.1097/01.ruq.0000226877.19937.a1. [DOI] [PubMed] [Google Scholar]
- 8.Zahir ST, Vakili M, Ghaneei A, Sharahjin NS, Heidari F. Ultrasound assistance in differentiating malignant thyroid nodules from benign ones. J Ayub Med Coll Abbottabad 2016; 28:644–649. [PubMed] [Google Scholar]
- 9.Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. American Association of clinical endocrinologists, American college of endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 UPDATE. Endocr Pract 2016; 22:622–639. doi: 10.4158/EP161208.GL. [DOI] [PubMed] [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoang JK, Langer JE, Middleton WD, Wu CC, Hammers LW, Cronan JJ, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR incidental thyroid findings committee. J Am Coll Radiol 2015; 12:143–150. doi: 10.1016/j.jacr.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017; 14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Ha EJ, Moon WJ, Na DG, Lee YH, Choi N, Kim SJ, et al. A multicenter prospective validation study for the Korean thyroid imaging reporting and data system in patients with thyroid nodules. Korean J Radiol 2016; 17:811–821. doi: 10.3348/kjr.2016.17.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014; 81 suppl 1:1–122. doi: 10.1111/cen.12515. [DOI] [PubMed] [Google Scholar]
- 15.Pacini F, Castagna MG, Brilli L, Pentheroudakis G. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; suppl 7:vii110–vii119. doi: 10.1093/annonc/mds230. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Gan X, Shen F, Cai W, Xu B. The role of two tumor foci for predicting central lymph node metastasis in papillary thyroid carcinoma: a meta-analysis. Int J Surg 2018; 52:166–170. doi: 10.1016/j.ijsu.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Lu R, Meng Y, Zhang Y, Zhao W, Wang X, Jin M, et al. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med Imaging 2017; 17:65.doi: 10.1186/s12880-017-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhang MB, Luo YK, Li J, Wang ZL, Tang J. The value of peripheral enhancement pattern for diagnosing thyroid cancer using contrast-enhanced ultrasound. Int J Endocrinol 2018; 2018:1625958.doi: 10.1155/2018/1625958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med 2004; 23:1455–1464. [DOI] [PubMed] [Google Scholar]
- 20.Kong J, Li JC, Wang HY, Wang YH, Zhao RN, Zhang Y, et al. Role of superb micro-vascular imaging in the preoperative evaluation of thyroid nodules. J Ultrasound Med 2017; 36:1329–1337. doi: 10.7863/ultra.16.07004. [DOI] [PubMed] [Google Scholar]
- 21.Ahn HS, Lee JB, Seo M, Park SH, Choi BI. Distinguishing benign from malignant thyroid nodules using thyroid ultrasonography: utility of adding superb microvascular imaging and elastography. Radiol Med 2018; 123:260–270. doi: 10.1007/s11547-017-0839-2. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Huang L, Zhang H, Ma W, Shang X, Zhou Q, et al. Contrast-enhanced sonography of thyroid nodules. J Clin Ultrasound 2015; 43:153–156. doi: 10.1002/jcu.22240. [DOI] [PubMed] [Google Scholar]
- 23.Xue J, Cao XL, Shi L, Lin CH, Wang J, Wang L. The diagnostic value of combination of TI-RADS and ultrasound elastography in the differentiation of benign and malignant thyroid nodules. Clin Imaging 2016; 40:913–916. doi: 10.1016/j.clinimag.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Russ G, Royer B, Bigorgne C, Rouxel A, Bienvenu-Perrard M, Leenhardt L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol 2013; 168:649–655. doi: 10.1530/EJE-12-0936. [DOI] [PubMed] [Google Scholar]
- 25.Russ G. Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: description and reflections. Ultrasonography 2016; 35:25–38. doi: 10.14366/usg.15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mussa A, De Andrea M, Motta M, Mormile A, Palestini N, Corrias A. Predictors of malignancy in children with thyroid nodules. J Pediatr 2015; 167:886–892. doi: 10.1016/j.jpeds.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Diesen DL, Skinner MA. Pediatric thyroid cancer. Semin Pediatr Surg 2012; 21:44–50. doi: 10.1053/j.sempedsurg.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Tracy ET, Roman SA. Current management of pediatric thyroid disease and differentiated thyroid cancer. Curr Opin Oncol 2016; 28:37–42. doi: 10.1097/CCO.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 29.Vali R, Rachmiel M, Hamilton J, El Zein M, Wasserman J, Costantini DL, et al. The role of ultrasound in the follow-up of children with differentiated thyroid cancer. Pediatr Radiol 2015; 45:1039–1045. doi: 10.1007/s00247-014-3261-0. [DOI] [PubMed] [Google Scholar]
- 30.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015; 25:716–759. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe ME, Osorio M, Likhterov I, Urken ML. Evaluation of ultrasound reporting for thyroid cancer diagnosis and surveillance. Head Neck 2017; 39:1756–1760. doi: 10.1002/hed.24825. [DOI] [PubMed] [Google Scholar]
- 32.Mao Q, Tsang IW. A feature selection method for multivariate performance measures. IEEE Trans Pattern Anal Mach Intell 2013; 35:2051–2063. doi: 10.1109/TPAMI.2012.266. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Q, Shi CZ, Luo LP. Role of the texture features of images in the diagnosis of solitary pulmonary nodules in different sizes. Chin J Cancer Res 2014; 26:451–458. doi: 10.3978/j.issn.1000-9604.2014.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbasian Ardakani A, Gharbali A, Mohammadi A. Application of texture analysis method for classification of benign and malignant thyroid nodules in ultrasound images. Iran J Cancer Prev 2015; 8:116–124. [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SJ, Chang CY, Chang KY, Tzeng JE, Chen YT, Lin CW, et al. Classification of the thyroid nodules based on characteristic sonographic textural feature and correlated histopathology using hierarchical support vector machines. Ultrasound Med Biol 2010; 36:2018–2026. doi: 10.1016/j.ultrasmedbio.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Narayan NS, Marziliano P, Kanagalingam J, Hobbs CG. Speckle patch similarity for echogenicity-based multiorgan segmentation in ultrasound images of the thyroid gland. IEEE J Biomed Health Inform 2017; 21:172–183. doi: 10.1109/JBHI.2015.2492476. [DOI] [PubMed] [Google Scholar]
- 37.Bibicu D, Moraru L, Biswas A. Thyroid nodule recognition based on feature selection and pixel classification methods. J Digit Imaging 2013; 26:119–128. doi: 10.1007/s10278-012-9475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayan NS, Marziliano P, Hobbs CG. Automatic removal of manually induced artefacts in ultrasound images of thyroid gland. Conf Proc IEEE Eng Med Biol Soc 2013; 2013:3399–3402. doi: 10.1109/EMBC.2013.6610271. [DOI] [PubMed] [Google Scholar]
- 39.Zhao J, Zheng W, Zhang L, Tian H. Segmentation of ultrasound images of thyroid nodule for assisting fine needle aspiration cytology. Health Inf Sci Syst 2013; 1:5.doi: 10.1186/2047-2501-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki K. Overview of deep learning in medical imaging. Radiol Phys Technol 2017; 10:257–273. doi: 10.1007/s12194-017-0406-5. [DOI] [PubMed] [Google Scholar]
- 41.Ma J, Wu F, Jiang T, Zhu J, Kong D. Ultrasound image-based thyroid nodule automatic segmentation using convolutional neural networks. Int J Comput Assist Radiol Surg 2017; 12:1895–1910. doi: 10.1007/s11548-017-1649-7. [DOI] [PubMed] [Google Scholar]
- 42.Wu S, Yu S, Zhuang L, Wei X, Sak M, Duric N, et al. Automatic segmentation of ultrasound tomography image. Biomed Res Int 2017; 2017:2059036.doi: 10.1155/2017/6258395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prabusankarlal KM, Thirumoorthy P, Manavalan R. Segmentation of breast lesions in ultrasound images through multiresolution analysis using undecimated discrete wavelet transform. Ultrason Imaging 2016; 8:384–402. doi: 10.1177/0161734615615838. [DOI] [PubMed] [Google Scholar]
- 44.Acharya UR, Swapna G, Sree SV, Molinari F, Gupta S, Bardales RH, et al. A review on ultrasound-based thyroid cancer tissue characterization and automated classification. Technol Cancer Res Treat 2014; 13:290–301. doi: 10.7785/tcrt.2012.500381. [DOI] [PubMed] [Google Scholar]
- 45.Xia J, Chen H, Li Q, Zhou M, Chen L, Cai Z, et al. Ultrasound-based differentiation of malignant and benign thyroid Nodules: An extreme learning machine approach. Comput Methods Programs Biomed 2017; 147:37–49. doi: 10.1016/j.cmpb.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Wu L, Cheng JZ, Li S, Lei B, Wang T, Ni D. FUIQA: fetal ultrasound image quality assessment with deep convolutional networks. IEEE Trans Cybern 2017; 47:1336–1349. doi: 10.1109/TCYB.2017.2671898. [DOI] [PubMed] [Google Scholar]
- 47.Chen H, Wu L, Dou Q, Qin J, Li S, Cheng JZ, et al. Ultrasound standard plane detection using a composite neural network framework. IEEE Trans Cybern 2017; 47:1576–1587. doi: 10.1109/TCYB.2017.2685080. [DOI] [PubMed] [Google Scholar]
- 48.Kermany DS, Goldbaum M, Cai W, Valentim CCS, Liang H, Baxter SL, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 2018; 172:1122–1131. doi: 10.1016/j.cell.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Chi J, Walia E, Babyn P, Wang J, Groot G, Eramian M. Thyroid nodule classification in ultrasound images by fine-tuning deep convolutional neural network. J Digit Imaging 2017; 30:477–486. doi: 10.1007/s10278-017-9997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma J, Wu F, Jiang T, Zhu J, Kong D. Cascade convolutional neural networks for automatic detection of thyroid nodules in ultrasound images. Med Phys 2017; 44:1678–1691. doi: 10.1002/mp.12134. [DOI] [PubMed] [Google Scholar]
- 51.Tsantis S, Cavouras D, Kalatzis I, Piliouras N, Dimitropoulos N, Nikiforidis G. Development of a support vector machine-based image analysis system for assessing the thyroid nodule malignancy risk on ultrasound. Ultrasound Med Biol 2005; 31:1451–1459. doi: 10.1016/j.ultrasmedbio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Chang Y, Paul AK, Kim N, Baek JH, Choi YJ, Ha EJ, et al. Computer-aided diagnosis for classifying benign versus malignant thyroid nodules based on ultrasound images: a comparison with radiologist-based assessments. Med Phys 2016; 43:554–567. doi: 10.1118/1.4939060. [DOI] [PubMed] [Google Scholar]
- 53.Acharya UR, Faust O, Sree SV, Molinari F, Garberoglio R, Suri JS. Cost-effective and non-invasive automated benign & malignant thyroid lesion classification in 3D contrast-enhanced ultrasound using combination of wavelets and textures: a class of ThyroScan™ algorithms. Technol Cancer Res Treat 2011; 10:371–380. doi: 10.7785/tcrt.2012.500214. [DOI] [PubMed] [Google Scholar]
- 54.Acharya UR, Vinitha Sree S, Mookiah MR, Yantri R, Molinari F, Zieleźnik W, et al. Diagnosis of Hashimoto's thyroiditis in ultrasound using tissue characterization and pixel classification. Proc Inst Mech Eng H 2013; 227:788–798. doi: 10.1177/0954411913483637. [DOI] [PubMed] [Google Scholar]
- 55.Wu H, Deng Z, Zhang B, Liu Q, Chen J. Classifier model based on machine learning algorithms: application to differential diagnosis of suspicious thyroid nodules via sonography. AJR Am J Roentgenol 2016; 207:1–6. doi: 10.2214/AJR.15.15813. [DOI] [PubMed] [Google Scholar]
- 56.Ma J, Wu F, Zhu J, Xu D, Kong D. A pre-trained convolutional neural network based method for thyroid nodule diagnosis. Ultrasonics 2017; 73:221–230. doi: 10.1016/j.ultras.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 57.Yu Q, Jiang T, Zhou A, Zhang L, Zhang C, Xu P. Computer-aided diagnosis of malignant or benign thyroid nodes based on ultrasound images. Eur Arch Otorhinolaryngol 2017; 274:2891–2897. doi: 10.1007/s00405-017-4562-3. [DOI] [PubMed] [Google Scholar]


