Abstract
Supplemental Digital Content is available in the text
To the Editor: Since avian influenza A(H7N9) was first identified in Shanghai, China, in March 2013, there have been a total of five epidemics. These have amounted to 1564 laboratory-confirmed cases up to September 2017, with a fatality rate of about 40%.[1] In the fifth wave, 4.09% of cases (31/758) were infected with the highly pathogenic avian influenza (HPAI) A(H7N9). This indicated that the pathotype of the A(H7N9) had switched from low pathogenic avian influenza (LPAI) to HPAI.
Guangxi is located in southwest China, adjacent to Guangdong province. Guangdong is one of three provinces with the highest cumulative numbers of reported incidences of human infection with A(H7N9) since 2013.[2] There were few A(H7N9) cases in Guangxi before 2017, but the number of cases rapidly increased in 2017. Some of the cases were even HPAI infections. This study was to explore the cause of the first A(H7N9) outbreak in Guangxi and compare it with other regions. We investigated the epidemiological characteristics of patients infected with A(H7N9) and the detection of H7 using live poultry markets (LPMs) surveillance; the relationship between detection of H7 and the increased number of cases is also explored.
The collection of data from influenza A(H7N9) cases was decided to be a part of a public health investigation of emerging outbreak, which was exempt from institutional review board assessment.
All laboratory-confirmed cases of human infection with A(H7N9) in Guangxi in the 2016 to 2017 epidemic were included. The local Center for Disease Prevention and Control (CDC) was responsible for detecting A(H7N9) in respiratory samples from suspected human A(H7N9) cases. Real-time polymerase chain reaction was used in the laboratory to test for A(H7N9).
Active surveillance on avian influenza was increased from February 2017 until the outbreak was over. The surveillance sites covered all 14 cities of Guangxi. All the samples, including swabs from cages, chopping boards, avian feces, and drinking water of poultry, were collected and tested for avian influenza virus by the local CDC.
Fisher exact test was used to compare frequencies of demographic categorical variables and the Mann-Whitney U test was used to compare time-to-event distributions. Spearman correlation test was used to calculate the correlation between the positive rate of H7 detection and the number of A(H7N9) cases.
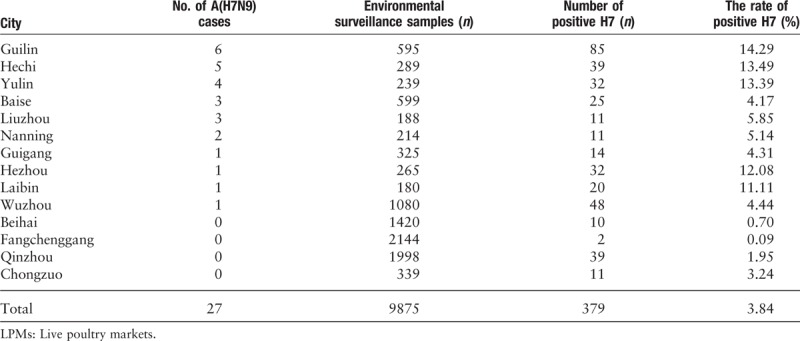
From October 1st, 2016 to June 9th, 2017, a total of 27 cases of human infection with A(H7N9) were reported in Guangxi. Human A(H7N9) cases were detected in ten of 14 cities [Table 1]. The first laboratory-confirmed case was imported from neighboring Guangdong Province. This patient contracted the illness on January 27, 2017 and died on February 15, 2017. The first indigenous case was reported on February 18, 2017 and the last case was reported on June 9, 2017. The median age of all cases was 53 years, the male-to-female ratio was 2.86, 22 (81%) lived in rural areas, and all but one of the cases had clear poultry exposure within 10 days of illness onset [Supplementary Table 1;]. Sixteen human A(H7N9) cases (59%) had been exposed to backyard poultry, while nine cases (33%) were exposed due to their occupation. In 63% of cases, the first hospital-level visited was a private or village clinic. The median number of days between illness onset and first medical visit was 1 day, whereas the median number of days from illness onset to taking anti-viral therapy was 5 days.
Table 1.
Geographical distribution of human A(H7N9) cases and the rate of positive H7 RNA test in LPMs in Guangxi, China, 2016 to 2017.
Fourteen (52%) of these infections resulted in death. The demographic characteristics were similar for both the death cases and the recovery cases. All reported human A(H7N9) cases were severe and required hospitalization. We found a higher proportion of cases resulted in death for patients who had backyard poultry exposure (71% died vs. 46% recovered; Fisher exact test, P = 0.252) or a longer time before receiving anti-viral treatment (6 days died vs. 4 days recovered; Mann-Whitney U test, P = 0.128). Significantly more of the cases that resulted in death involved patients who had a chronic disease compared with cases that resulted in recovery (57% vs. 8%; Fisher exact test, P = 0.013). Underlying chronic conditions were associated with an increased risk of the death (adjusted odds ratio, 15.34; 95% confidence interval, 1.50–156.91).
Due to missing samples or failed amplification, only 11 HPAI cases and six LPAI cases were identified. No significant differences were observed in the demographic characteristics between these two groups. The exposure to sick or dead poultry was significantly higher in patients with HPAI than with LPAI (7 vs. 0; Fisher exact test, P = 0.035). Other clinical factors such as the time between illness onset and hospitalization, anti-viral treatment, and clinic outcomes, were comparable (data not shown).
There were 9875 environmental surveillance samples from LPMs collected from January to July 2017. Of these, 379 samples (3.84%) were found to be positive for H7 using RNA testing. The rate of positive H7 detection in LPMs varied from 0.09% to 14.29% in different cities [Table 1]. We found a steep increase in the number of avian influenza A(H7N9) cases between February 10 and March 21, 2017. Meanwhile, the rate of positive H7 RNA tests in LPMs was higher from 5.20% to 11.82%. There was a positive correlation between the case reports and the H7 detection rate (R = 0.793, P < 0.05).
In this study, we described the prevalence of human infection with influenza A(H7N9) in Guangxi during 2016 to 2017 season and compared the demographical, epidemiological, and clinical characteristics between death and recovery cases. Middle-aged men living in rural areas accounted for the majority of human A(H7N9) cases. This characteristic was consistent with the fifth epidemic in mainland China,[2] but different from the first epidemic.
The case-fatality was higher than those reported in other provinces and previous epidemic. It has been proven that initiation of anti-viral therapy within 48 h of the onset of the illness, could reduce the risk of death.[2] However, the mean time between illness onset and anti-viral treatment in our study was 5 days, which was more than 48 h. Furthermore, for the death cases in our study, the times from illness onset to first medical service, to initiation of anti-viral treatment, and to diagnosis, were longer compared to the recovery cases (though, these differences were not significant). Therefore, enhancing early identification and timely initiation of anti-viral treatment is crucial to reduce the mortality of patients infected with A(H7N9). Another possible reason for the higher case-fatality rate observed was that six of the 14 cases that resulted in death in our study were patients infected with HPAI A(H7N9).
Overall, compared with the outbreaks in other Chinese provinces, in Guangxi, we observed a higher proportion of backyard poultry exposure, which could lead to an increase in the risk of avian influenza infection. This difference was mainly due to the fact that most of the patients we studied lived in rural areas. Preference for fresh chicken meat results in poultry farming in rural areas, while the raising and slaughtering increased the frequency of exposure. Our findings were consistent with previous research in that touching sick or dead poultry was the most important risk factor for HPAI A(H7N9) infection.[3] HPAI A(H7N9) may accelerate disease progression and severity,[4] and has been shown to exhibit multi-drug resistance to neuraminidase inhibitors.
The increased H7 detection rate in LPMs was strongly associated with the increase in A(H7N9) cases reported. The reported number of human A(H7N9) cases was much higher in Guangdong than in Guangxi. The lower positive rate of H7 detection in Guangxi than in Guangdong may offer a partial explanation. Additionally, asymptomatic patients may have been ignored in Guangxi due to the lack of clinical experience, as all reported cases were severe and resulted in hospitalization. Further study will be required to elucidate the full explanation for this difference.
In response to the rapid increase of human cases in Guangxi, local authorities temporarily closed LPMs, which was effective in reducing the risk of human infection. In addition, intensive media reports had strengthened public awareness of the risk of a A(H7N9) pandemic, particularly in rural areas. However, due to the traditional demand for fresh chicken meat among people living in the southern China, asymptomatic infection in poultry caused by the LPAI A(H7N9), and exposure pattern shift[2], extensive efforts are needed to prevent and control A(H7N9) infection. These efforts could include active LPM surveillance and strengthening the immunization of poultry, which is considered to an extremely effective way to control the outbreak of avian influenza.[5]
In conclusion, highlighting the importance of avoiding touching sick or dead poultry, closure of LPMs, and ongoing active environmental surveillance are critical in preventing and controlling future influenza A(H7N9) epidemics. Underlying chronic conditions and delayed treatment accelerate the progression of clinical severity of the illness. Therefore, strengthening awareness of the importance of early diagnosis and administration of anti-viral therapy will be helpful in reducing the risk of fatality.
Acknowledgements
The authors thank staff in the local Center for Disease Control and Prevention (CDC) for providing assistance with data collection.
Conflicts of interest
None.
Footnotes
How to cite this article: Wang J, Jiang LN, Ning CY, Yang YP, Chen M, Zhang C, He WT, Tan Y. First outbreak of human infection with avian influenza A(H7N9) virus in Guangxi, China, 2016 to 2017. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000376
References
- 1.Influenza at the Human-animal Interface. World Health Organization, 2017. Available from: https://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_10_30_2017.pdf?ua=1 Accessed June 3, 2019. [Google Scholar]
- 2.Wang X, Jiang H, Wu P, Uyeki TM, Feng L, Lai S, et al. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013-17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis 2017; 17:822–832. doi: 10.1016/S1473-3099(17)30323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang M, Lau EHY, Guan W, Yang Y, Song T, Cowling BJ, et al. Epidemiology of human infections with highly pathogenic avian influenza A(H7N9) virus in Guangdong, 2016 to 2017. Euro Surveill 2017; 22:30568.doi: 10.2807/1560-7917.ES.2017.22.27.30568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Belser JA, Pappas C, Pulit-Penaloza JA, Brock N, Zeng H, et al. Risk assessment of fifth-wave H7N9 influenza A viruses in mammalian models. J Virol 2018; 93:e01740–e1818. doi: 10.1128/JVI.01740-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, Ke C, Lau EHY, Song Y, Cheng KL, Zou L, et al. Influenza H5/H7 virus vaccination in poultry and reduction of zoonotic infections, Guangdong Province, China, 2017-18. Emerg Infect Dis 2019; 25:116–118. doi: 10.3201/eid2501.181259. [DOI] [PMC free article] [PubMed] [Google Scholar]


