Abstract
Background:
Senile depression patients in China usually present with a higher risk of coronary heart disease that may trigger cardiac death. Selective serotonin reuptake inhibitors (SSRIs) were the most prescribed antidepressants in China; the cardiovascular safety of SSRIs when used in Chinese senile depression patients has not been evaluated.
Methods:
A network of meta-analysis was conducted to fill the objectives. PubMed, Embase databases, and 2 Chinese language electronic databases WANFANG and CNKI were searched for the related articles. The primary outcome of the present study was the number of cardiovascular reactions when each SSRI drug was used among senile depression patients in China. Odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) were calculated within pairwise and network meta-analysis.
Results:
Fifteen trials were identified, including 1432 patients; the network meta-analysis showed that Chinese senile depression patients treated by Escitalopram were associated with a lower risk of cardiovascular reaction (CDR) than Paroxetine (ORs 0.37, 95% CI 0.14–0.37). Escitalopram also exhibited distinct advantages compared with other SSRIs. The rank of SSRIs with respect to cardiovascular safety was Escitalopram > Sertraline > Citalopram > Paroxetine > Fluoxetine, respectively.
Conclusion:
Escitalopram exhibited distinct advantages compared with other SSRIs, while Fluoxetine had the biggest cardiovascular reaction probability.
Keywords: cardiovascular safety, network meta-analysis, senile depression patients, SSRIs
1. Introduction
Depression has become an important risk factor, which not only damages the health of elders but also decreased their quality of life greatly.[1] According to the estimate of previous study, the prevalence of depression among elders in China has been increased in recent years, which was higher than other common group.[2] Without appropriate treatment, depression in the elderly can easily lead to or aggravate some serious diseases such as coronary heart disease (CHD),[3] hypertension,[4] diabetes,[5] and tumors,[6] severe depression, and even result in suicide.[7]
For many years, selective serotonin reuptake inhibitors (SSRIs) are recommended by the National Institute for Health and Care Excellence (NICE) as the first-line treatment of depression,[8] and were the most prescribed antidepressants in many countries, including China.[9]
The efficacy of SSRIs, which include citalopram, fluvoxamine, fluoxetine, sertraline, paroxetine, escitalopram, has been proved in different groups.[10] A previous study has concluded that SSRIs did not affect the risk of CHD in those without a previous diagnosis of CHD,[11] and have not reported the electrocardiography (ECG) changes for the patients without known pre-existing heart disease, which was associated with SSRIs.[12] van Haelst et al[13] have conducted a research about the occurrence of QTc interval prolongation in an elderly surgical population after they used SSRIs; the result indicated that use of an SSRI by elderly surgical patients was not associated with the occurrence of QTc interval prolongation. However, Beach et al[14] have conducted a meta-analysis to evaluate the association between SSRIs and correlated QT interval prolongation, and concluded that SSRIs were associated with a modest but statistically significant increase in the QTc interval. So, there has been controversy regarding the cardiovascular safety of SSRIs in treating depression.
And, the US Food and Drug Administration (FDA) has issued a warning stating that patients older than 60 years are not recommended to receive doses of citalopram exceeding 20 mg daily due to concerns of corrected QT (QTc) prolongation.[15]
In addition, the aged people of China have a high prevalence of CHD,[16] and much important is that the drug tolerance of old people has decreased due to lower metabolism and organ dysfunction.[17] Thus, although it is relatively safe for the general population as previous studies indicated,[11–13] there may be cardiovascular reaction when used in elders with the general therapeutic dose, which was verified by another study.[18]
In the current study, a network meta-analysis (NMA) was conducted to combine both direct and indirect evidences in order to provide ranking of the cardiovascular safety of SSRIs, and so can provide good evidence for clinicians to make the best choice for elder patients in China.
2. Method
2.1. Ethical approval
This study was not applicable of review approval because it used data from published papers, and the study did not involve patient consent.
2.2. Search strategy
Embase, Web of science, PubMed, and 2 Chinese language electronic databases WANFANG and CNKI were searched. Studies from onset up to September 2018 were identified to evaluate the cardiovascular safety of SSRIs.
Only English and Chinese language articles were searched. SSRIS or citalopram, fluoxetine, fluoxetine, sertraline, paroxetine, and escitalopram were combined with “Geriatric depression,” “old patients with depression,” “depression of elder,” and “randomized, controlled, trials.”
2.3. Bias assessment
According to the guideline of the Cochrane reviews, risk of bias of all included studies was assessed; two of our authors conducted the assessment independently for information such as blind, random, outcome, and defined as “low risk,” "unclear risk,” or “high risk” with respect to the degree of information integrity.
2.4. Study selection
We included such studies that meet all the follow criteria: comparison among citalopram, fluoxetine, fluoxetine, sertraline, paroxetine, and escitalopram; inclusion of individuals with depression; sample comes from Mainland China, and the age ≥60 years; language was English or Chinese; clear diagnostic criteria such as DSM-IV or DSM-V or ICD-10 or CCMD-3. In this research, cardiovascular safety was defined as without the abnormal changes of ECT, or without symptoms associated with impaired cardiac function; otherwise, we assume that there is some cardiovascular reaction. We excluded such studies in which samples have been diagnosed as cardiovascular diseases, renal function, or other associated comorbidities, because these factors can interfere with the results.
2.5. Data extraction
Two authors independently extracted relevant data from the included articles. Name of first author, year of publication, study design, duration of treatment, number of patients, average age, and medications were documented. The number of cardiovascular reaction cases was considered as the clinical outcome of the study.
2.6. Statistical analysis
The safety of SSRI drugs was compared by NMA. We conducted a Bayesian model NMA to combine both direct and indirect evidences into 1 single comparison. Odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) were calculated. The heterogeneity was assessed with I2 test, with an I2 >50% indicating the existence of heterogeneity. P values were calculated to identify the difference between direct and indirect evidences. In addition, the consistency was checked by node-splitting plot. Funnel plot and Egger test were conducted to analysis the publication bias. All statistical analyses were conducted using R version 3.4.4 (R Project for Statistical Computing, Vienna, Austria). P < .05 was considered to be significant.
3. Result
3.1. Study selection
We searched the relative database according to the search strategy, and first got 546 potentially eligible trials; 95 duplicates were removed. Another 350 studies were excluded after screening titles and abstracts; then, among the remaining 101 full-text studies, 86 studies were ruled out due to missing valuable outcome, which we wanted for research. So, a total of 15[19–34] articles with 1432 patients were included in the present study to evaluate cardiovascular safety of SSRIs drugs among elders in China.
The flow chart is schematically shown in Fig. 1.
Figure 1.
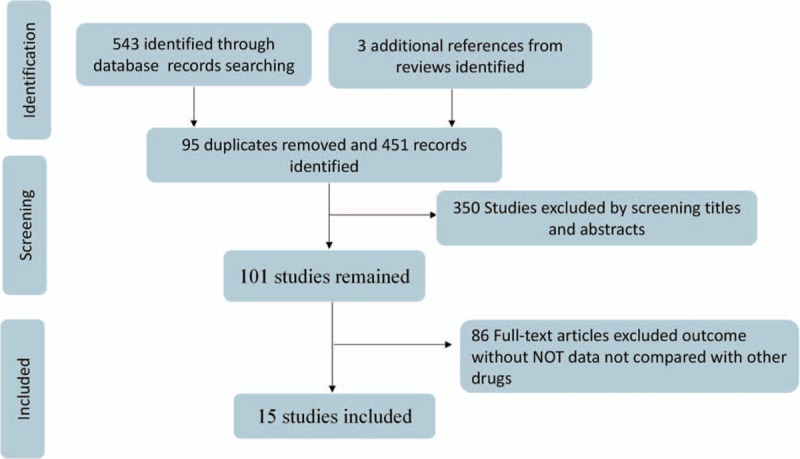
Flowchart presenting the steps of the literature search and selection.
3.2. Study characters
The general characters of included studies are summarized in Table 1. Among the identified articles, there were 15 two-arm studies, and 9 trials compared the cardiovascular safety of paroxetine and escitalopram, which contain 987 patients; two studies aimed to compare the cardiovascular safety of paroxetine and citalopram; another comparison also included 1 trial of escitalopram and citalopram, 1 trial of escitalopram and fluoxetine, and 1 trial of comparison between combination of fluoxetine and sertraline. A total of 1432 elder depression patients in China were involved in this study; among of them, 591 (41.27%) patients received treatment of Escitalopram, 112 patients (7.82%) were treated by Citalopram, 78 (5.45%) patients received Fluoxetine, 571 (39.87%) patients received Paroxetine, and 80 (5.59%) patients received treatment of Sertraline. The risk of bias assessment of included studies is summarized in Table 2. Network plots of SSRIs are shown in Fig. 2.
Table 1.
The basic information and data of all included studies in the meta-analysis.
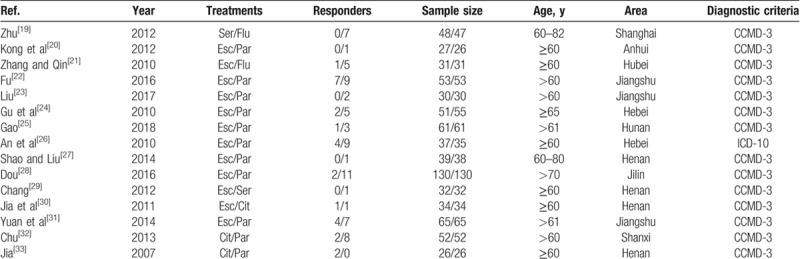
Table 2.
Risk of bias assessment.
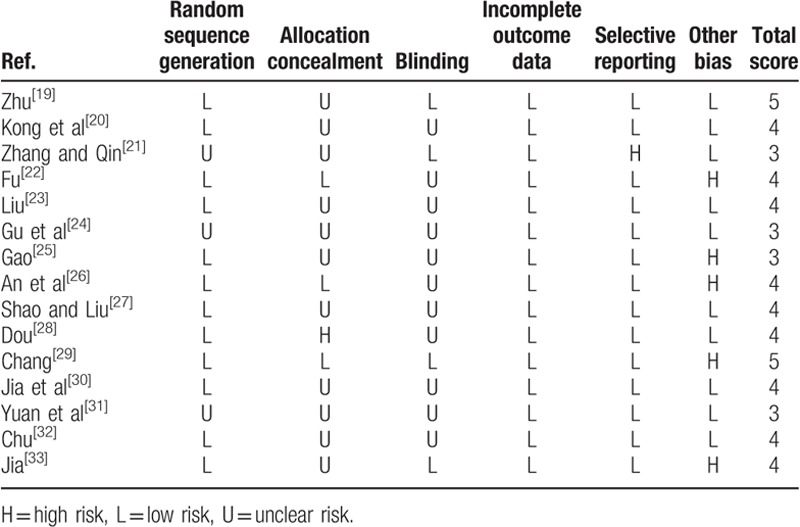
Figure 2.
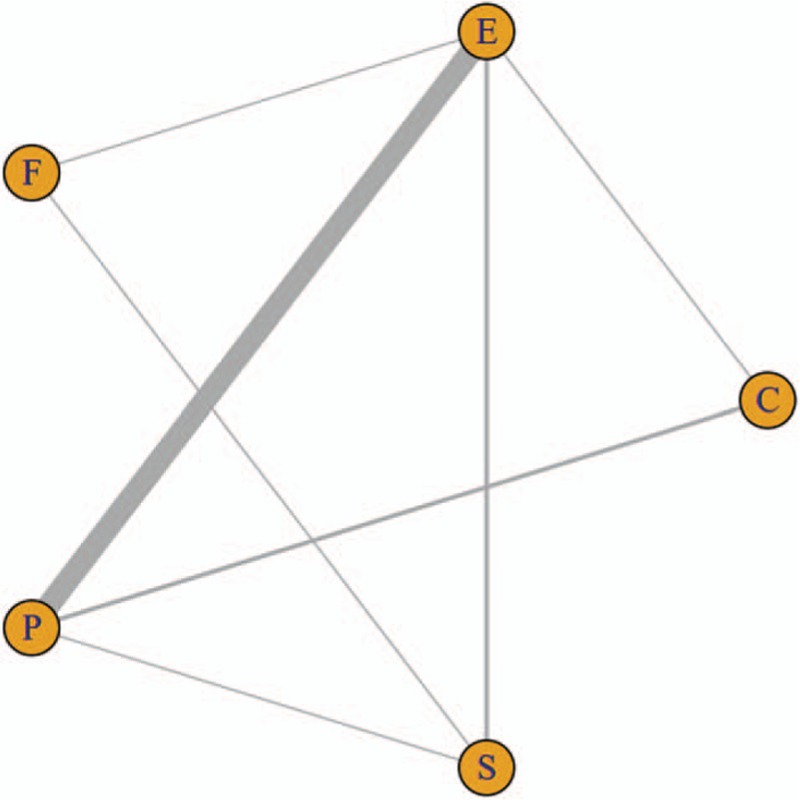
Network plots of SSRIs. The width of the lines represents the total number of trials for each comparison. C = Citalopram, E = Escitalopram, F = Fluoxetine, P = Paroxetine, S = Sertraline.
3.3. Incidence of cardiovascular reaction
As summarized in Table 3, Escitalopram showed a significant decline in the incidence of cardiovascular side effects compared with Paroxetine (OR 0.37, 95% CI 0.14–0.77), and Escitalopram was also associated a lower risk of cardiovascular reaction than Fluoxetine (OR 0.06, 95% CI 0.00–0.74); Sertraline was also associated with a lower risk of cardiovascular reaction than Fluoxetine (OR 0.02, 95% CI 0.00–0.31). In other comparisons, alternative assumptions are not acceptable (P > 0.05). The forest plot of network results is illustrated in Fig. 3.
Table 3.
Results of SSRIs for the incidence of cardiovascular reaction (CDR) from network meta-analysis.
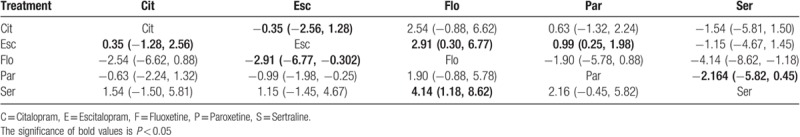
Figure 3.
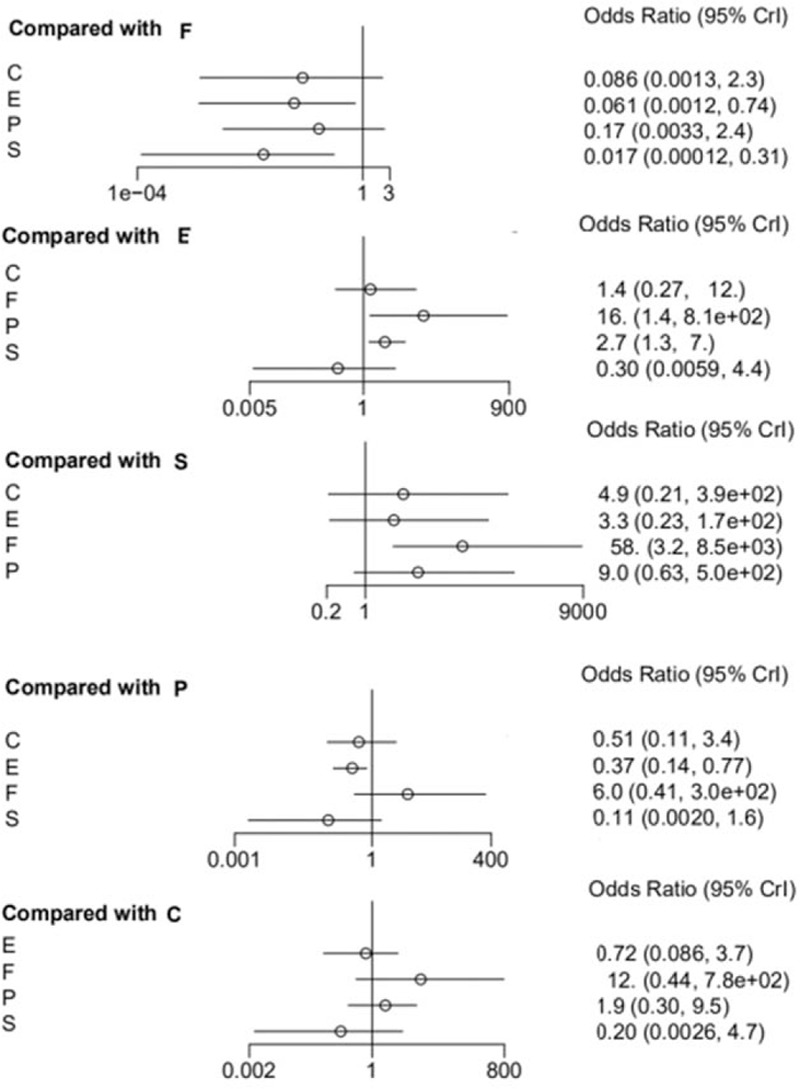
The forest plot of network results on the incidence of CDR. C = Citalopram, E = Escitalopram, F = Fluoxetine, P = Paroxetine, S = Sertraline.
3.4. The cardiovascular side effects rank of SSRIs
An advantage of Bayesian model can provide ranks of different drugs; the ranking diagram and rank probability are shown in Table 4 and Fig. 4. The drugs were ranked according to the number of cardiovascular reaction cases from higher to lower, and the larger the number was, the safety of the drug was indicated poor.
Table 4.
Rank probability of caused CDR among SSRIs.
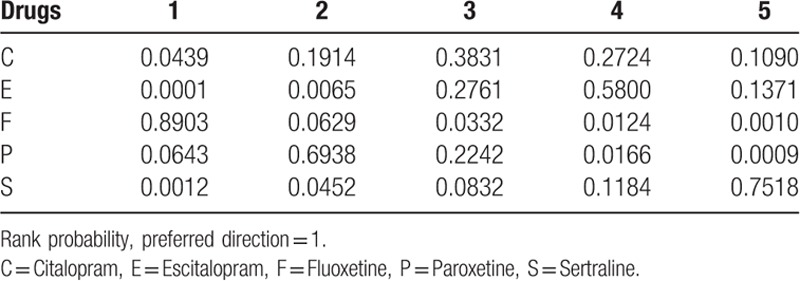
Figure 4.
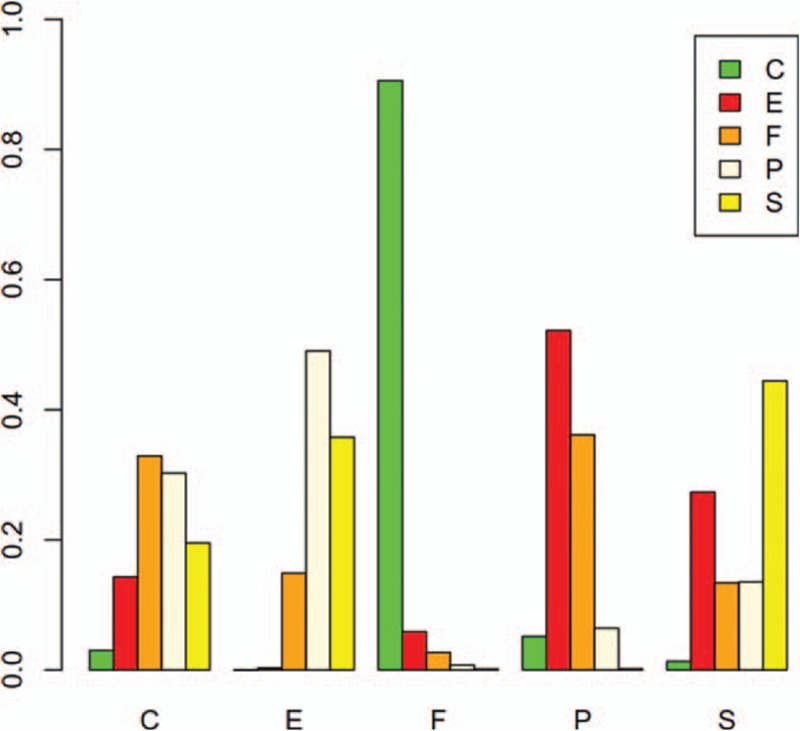
Probabilistic ranking of cardiovascular safety among Chinese senile depression patients. C = Citalopram, E = Escitalopram, F = Fluoxetine, P = Paroxetine, S = Sertraline.
The result showed that Fluoxetine had the highest cardiovascular side effects or adverse reaction probability (probability = 0.89), the second was Paroxetine (probability = 0.06), and then was Citalopram (probability = 0.04). Escitalopram (probability = 0.00%) exhibited the best reliable performance in comparison with other medications with respect to cardiovascular adverse reaction in the elder. The rank of SSRIs with respect to cardiovascular safety was Escitalopram > Sertraline > Citalopram > Paroxetine > Fluoxetine, respectively.
3.5. Heterogeneity, consistency, and publication bias
The heterogeneity was assessed with I2 test, with an I2 >50% indicating the existence of heterogeneity. According to the result displayed in Fig. 5, there was no significant heterogeneity among the included studies.
Figure 5.
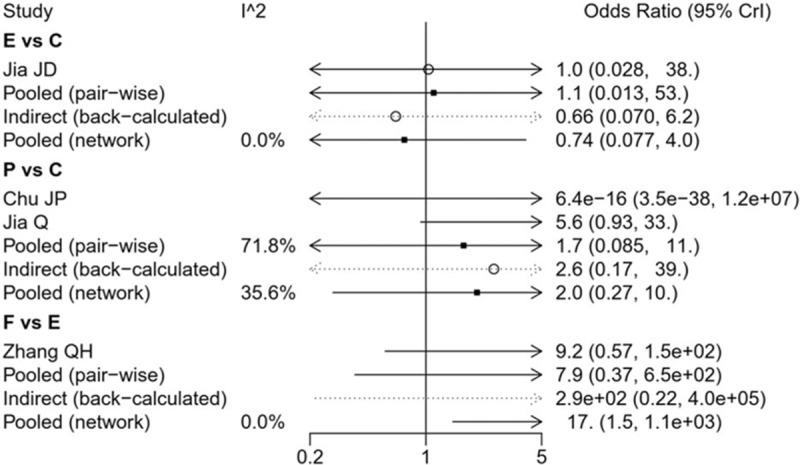
The heterogeneity of the included studies. C = Citalopram, E = Escitalopram, F = Fluoxetine, P = Paroxetine, S = Sertraline.
The node-splitting method was used to assess the consistency of direct and indirect evidences; the forest plots are shown in Fig. 6. A P value of less than .05 suggests potentially significant inconsistency; the detailed result could also be seen in Table 5. No significant difference was observed in the present study. The result of publication bias analysis is presented in Fig. 7. No significant publication bias was identified. Thus, the validity and credibility of this meta-analysis was confirmed.
Figure 6.
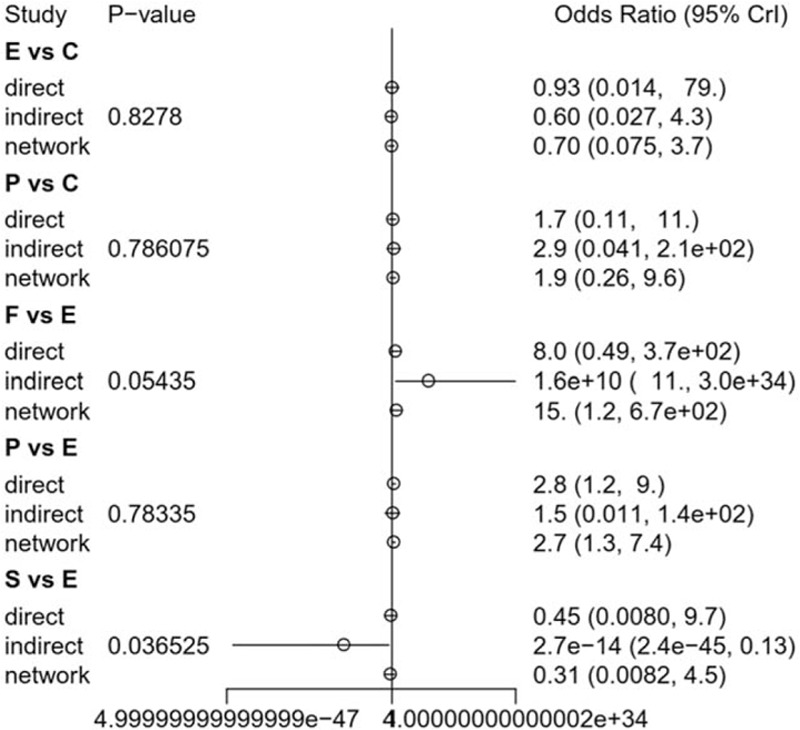
Summarized results of direct and indirect comparisons between different SSRIs. C = Citalopram, E = Escitalopram, F = Fluoxetine, P = Paroxetine, S = Sertraline.
Table 5.
Results of consistency analysis by node-splitting plot.
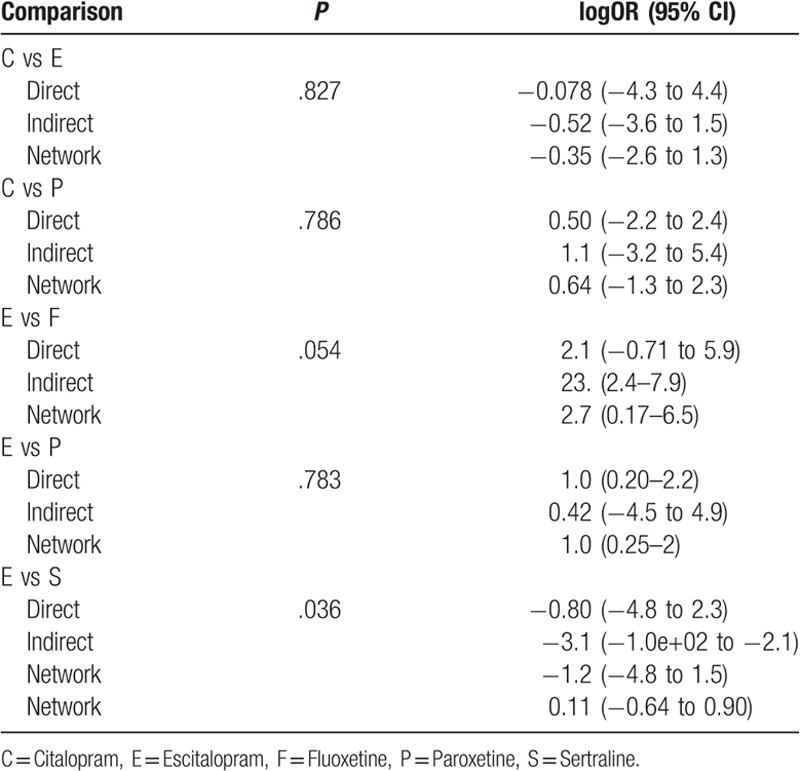
Figure 7.
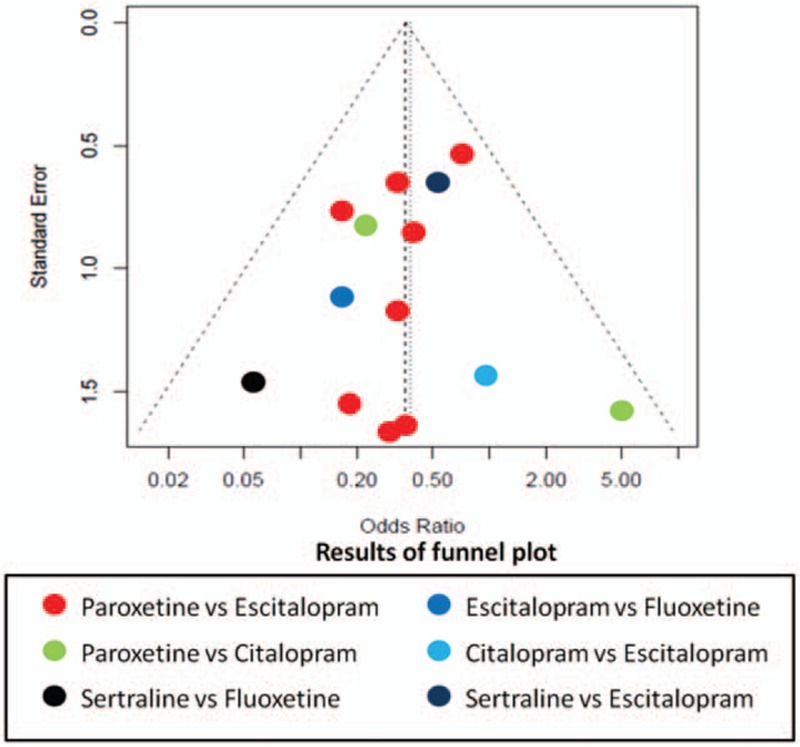
Funnel plot of publication bias.
4. Discussion
Patients with depression usually have a higher risk of cardiovascular disease, which may trigger cardiogenic death.[3] The elderly people in China have a high prevalence of depression, and among them, many have pre-existing heart disease; thus, when antidepressants are prescribed for the special group, cardiovascular safety should be consider first. For a long time, SSRIs have been the most prescribed antidepressants in many countries for different groups. In this study, we collected data from 15 trials, which investigated 5 SSRIs in order to assess their cardiovascular safety when used among the Chinese senile depression patients. We also ranked these medicines with respect to their cardiovascular safety in elder depression patients, so as to provide potential guidance to clinicians. In the present study, the result of NMA showed that patients treated by Escitalopram were associated with a reduced risk of CDR compared with other SSRIs drugs. Although the results indicate that in elderly Chinese patients with depression, the cardiovascular safety of sertralin was superior to that of escitalopram. Among other comparisons, fluoxetine showed the worst performance with respect to CDR, while another study has reported that fluoxetine may cause sinus tachycardia and myocardial infarction.[34] And, Citalopram exhibited a better performance than Paroxetine.
As the most widely prescribed antidepressants, dispute about the efficacy of SSRIs existed especially in mild to moderate cases, and most concerns were their side effects.[35] Although CDR was not the most common side effect of SSRIs, the US FDA had given guideline in order to ensure cardiovascular safety when using them.[36] As the first NMA study, it can provide valuable information to reduce the huge burden of mental illness and improve the mental health of elderly patients with depression. However, some limitations of this study should be noted, which may affect the results. First, though we had conducted a thorough literature search, all included studies were still published in Chinese, this may be exist publication bias. Second, drug safety was not a predefined outcome, and therefore may not have been accurately evaluated, and among the included studies, there were 2 diagnostic criteria, that is, ICD-10 and CCMD-3, which may influence the result. Third, significant variation existed in the number of studies with respect to each comparison, for example, there were 9 trials that compared the efficacy of Paroxetine and Escitalopram, only 1 trial of Paroxetine and Fluoxetine, and 1 trial of comparison between Escitalopram and sertraline. So, this may result in a wide CI for summary statistics.
For summary, Escitalopram exhibited the least probability of CDR when used in the Chinese senile depression patients, and sertraline showed the second best cardiovascular safety, while Fluoxetine exhibited the highest probability that caused CDR among the Chinese senile depression patients.
Acknowledgment
The authors thank all the reviewers for their helpful comments.
Author contributions
Conceived and designed the analysis: SG, HX. Performed the analysis: SG, LC, SC. Wrote the paper: GS, LC.
Conceptualization: Shengyu Guo, Huilan Xu.
Data curation: Huilan Xu.
Formal analysis: Shengyu Guo.
Funding acquisition: Shengyu Guo.
Methodology: Shengyu Guo, Ling Chen.
Software: Sixiang Cheng.
Supervision: Huilan Xu.
Writing – original draft: Shengyu Guo.
Writing – review & editing: Shengyu Guo, Sixiang Cheng, Ling Chen, Huilan Xu.
Footnotes
Abbreviations: CDR = cardiovascular reaction, CHD = coronary heart disease, CIs = confidence intervals, ECG = electrocardiography, NICE = National Institute for Health and Care Excellence, NMA = network meta-analysis, ORs = odds ratios, SSRIs = selective serotonin reuptake inhibitors.
This work was supported by Education fund of Hunan province (Number:18B409, http://jyt.hunan.gov.cn/sjyt/xxgk/tzgg/201903/t20190318_5297301.html).
The authors have declared that no competing interests exist.
References
- [1].Chan SW, Chiu HF, Chien WT, et al. Quality of life in Chinese elderly people with depression. Int J Geriatr Psychiatry 2010;4:312–8. [DOI] [PubMed] [Google Scholar]
- [2].Ma LLL, Chang W, Xin YM, et al. Depression in Chinese elderly populations. Asia-Pacific Psychiatry 2011;3:46–53. [Google Scholar]
- [3].Rutledge T, Redwine LS, Linke SE, et al. A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom Med 2013;75:335–49. [DOI] [PubMed] [Google Scholar]
- [4].Bogner HR, de Vries HF. Depression, hypertension. Ann Fam Med 2008;6:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prestele S, Aldenhoff J, Reiff J. The HPA-axis as a possible link between depression, diabetes mellitus and cognitive dysfunction. Fortschr Neurol Psychiatr 2003;71:24–36. [DOI] [PubMed] [Google Scholar]
- [6].Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry 2003;54:269–82. [DOI] [PubMed] [Google Scholar]
- [7].Clark L, Dombrovski AY, Siegle GJ, et al. Impairment in risk-sensitive decision-making in older suicide attempters with depression. Psychol Aging 2011;26:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Veale D. National Collaborating Centre for Mental Health[M]. 2006. [Google Scholar]
- [9].Geddes JR, Cipriani A. Selective serotonin reuptake inhibitors. BMJ Br Med J 2004;329:809–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oh SW, Kim J, Myung SK, et al. Antidepressant use and risk of coronary heart disease: meta-analysis of observational studies. Br J Clin Pharmacol 2015;78:727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goldberg RJ. Selective serotonin reuptake inhibitors: infrequent medical adverse effects. Arch Fam Med 1998;7:78–84. [DOI] [PubMed] [Google Scholar]
- [13].van Haelst IM, van Klei WA, Doodeman HJ, et al QT interval prolongation in users of selective serotonin reuptake inhibitors in an elderly surgical population: a cross-sectional study. J Clin Psychiatry 2014;75:15–21. [DOI] [PubMed] [Google Scholar]
- [14].Beach SR, Kostis WJ, Celano CM, et al. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry 2014;75:e441. [DOI] [PubMed] [Google Scholar]
- [15].Fabo KR, Nye AM, Gagliardi JP, et al. Evaluation of changes in citalopram prescribing patterns following a US Food and Drug Administration alert: a retrospective cohort study. Prim Care Companion CNS Disord 2015;17: doi:10.4088/PCC.14m01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cheng TO. Coronary heart disease in China. Hosp Med 1999;60:1485. [PubMed] [Google Scholar]
- [17].Wang XZ, Zhang P, Wang YS, et al. Clinical effect and safety of carvedilol in elderly patients with diabetes mellitus and chronic heart failure. J Guangdong Med College 2008;26: [Google Scholar]
- [18].Gorp FV, Whyte IM, Isbister GK. Clinical and ECG effects of escitalopram overdose. Ann Emerg Med 2009;54:404–8. [DOI] [PubMed] [Google Scholar]
- [19].Zhu XH. Clinical study of sertraline and fluoxetine in the treatment of depression in elderly patients. World Health Digest Med Period 2012;9:215–1215. [Google Scholar]
- [20].Kong XM, Zhu DF, Zhang Xl, et al. The effect of escitalopram in the treatment of geriatric depression. Chin J Clin Health 2012;15:123–4. [Google Scholar]
- [21].Zhang QH, Qin S. Esdtalopram and fluoxeline in the treatment of geriatric depression. Med Innovat China 2010;7:49–51. [Google Scholar]
- [22].Fu D. Comparison of effect and safety between escitalopram and paroxetine among elder in China. Chin J Clin Ration Drug Use 2016;9:67–8. [Google Scholar]
- [23].Liu L. Comparative analysis of escitalopram and paroxetine in treatment of senile depression. J Syst Med 2017;8:1–3. [Google Scholar]
- [24].Gu Y, Jiang T, Guo JB, et al. Efficacy and safety of escitalopram in elderly patients with major depression: a randomized and control study. Chin Mental Health J 2010;24:445–9. [Google Scholar]
- [25].Gao J. Comparison of the efficacy of escitalopram and paroxetine in the treatment of senile depression. Chin J Clin Ration Drug Use 2018;11:67–8. [Google Scholar]
- [26].An YM, Zhao HF, Liu YL, et al. A comparative study of escitalopram and paroxetine in the treatment of senile depression. HeBei Med J 2011;33:711–3. [Google Scholar]
- [27].Shao GY, Liu J. Efficacy and safety of escitalopram and paroxetine in the treatment of senile patients with depression. China J Health Psychol 2014;22:19–20. [Google Scholar]
- [28].Dou FZ. Efficacy of escitalopram and paroxetine in the treatment of elderly patients with depression. Chin Med J 2016;16:135–6. [Google Scholar]
- [29].Chang SH. Efficacy and safety of escitalopram and sertraline in the treatment of senile patients with depression. China J Health Psychol 2012;20:1296–7. [Google Scholar]
- [30].Jia JD, Yang JZ, Yu Y, et al. Clinical study on efficacy and safety of escitalopram vs citalopramin the treatment of first episode geriatric depression. Med J Chin People Health 2011;23:2112–6. [Google Scholar]
- [31].Yuan P, zhang LH, Li RH. Analysis and comparison of curative effect of escitalopram and paroxetine in the treatment of senile depression patients. China Prac Med 2014;27:11–2. [Google Scholar]
- [32].Chu JP. Comparison of citalopram and paroxetine in elder. China J Health Psychol 2007;15:582–3. [Google Scholar]
- [33].Jia Q. A comparative study of citalopram and paroxetine in the treatment of senile depressive disorder. Chin Remed Clin 2013;13:914–5. [Google Scholar]
- [34].Pacher P, Ungvari Z, Nanasi PP, et al. Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any? Curr Med Chem 1999;6:469–80. [PubMed] [Google Scholar]
- [35].Jakobsen JC, Katakam KK, Schou A, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and trial sequential analysis. BMC Psychiatry 2017;17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].And C F D E. Drug Safety and Availability - FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses [J]. [Google Scholar]


